Abstract
The hypoxic response is mediated by two transcription factors, hypoxia-inducible factor (HIF)-1α and HIF-2α. These highly homologous transcription factors are induced in hypoxic foci and regulate cell metabolism, angiogenesis, cell proliferation, and cell survival. HIF-1α and HIF-2α are activated early in cancer progression and are important in several aspects of tumor biology. HIF-1α and HIF-2α have overlapping and distinct functions. In the intestine, activation of HIF-2α increases inflammation and colon carcinogenesis in mouse models. Interestingly, in ischemic and inflammatory diseases of the intestine, activation of HIF-1α is beneficial and can reduce intestinal inflammation. HIF-1α is a critical transcription factor regulating epithelial barrier function following inflammation. The beneficial value of pharmacological agents that chronically activate HIF-1α is decreased due to the tumorigenic potential of HIFs. The present study tested the hypothesis that chronic activation of HIF-1α may enhance colon tumorigenesis. Two models of colon cancer were assessed, a sporadic and a colitis-associated colon cancer model. Activation of HIF-1α in intestinal epithelial cells does not increase carcinogenesis or progression of colon cancer. Together, the data provide proof of principle that pharmacological activation of HIF-1α could be a safe therapeutic strategy for inflammatory bowel disease.
Keywords: hypoxia, hypoxia-inducible factor-1, colon cancer, prolyl hydroxylase domain proteins, colitis
inflammatory bowel disease (IBD) is a chronic inflammatory disease of the intestine with an unclear etiological mechanism. Currently, the paradigm proposed is a feed-forward synergistic dysregulation of the mucosal epithelial barrier, the mucosal immune system, and gut microbiota (2, 6, 52). Metagenomic and genome-wide association studies have clearly confirmed the role of all three in the progression of IBD (12, 20, 24, 35, 46, 50a, 52). Moreover, hypoxia is present in the inflammatory foci, and through its transcription factors hypoxia-inducible factor (HIF)-1α and HIF-2α play an important role in inflammation and resolution of IBD (26, 27, 44, 53). Currently, mechanisms leading to hypoxia in inflammatory foci are not clearly known and most likely involve vasculitis and edema (6). Moreover, neutrophil migration into epithelial cells is critical in depleting local oxygen and activating HIF-1α (3). HIF-1α and HIF-2α in normoxic cells are rapidly degraded through oxygen-sensing enzymes prolyl hydroxylase domain proteins (PHD) (21, 22). PHDs use oxygen as a substrate for hydroxylation (37). In a cellular environment with sufficient oxygen, PHDs hydroxylate HIF-1α and HIF-2α, leading to von Hippel-Lindau gene (VHL)-induced proteasomal degradation (21, 22). Knockout mouse models and chemical inhibitors of PHDs demonstrate a robust activation of HIF signaling and underscore the importance of PHDs in the regulation of HIFs (37).
HIF-1α and HIF-2α have distinct functions in intestinal inflammation. HIF-2α has been shown to increase inflammation through activation of epithelial inflammatory response (44, 53). Several proinflammatory mediators are activated by HIF-2α. Mice overexpressing HIF-2α specifically in the intestinal epithelial cells demonstrate spontaneous colitis and are significantly more susceptible to intestinal injury in several mouse models of colitis (53). However, HIF-1α in intestinal epithelial cells is a potent protective factor through regulation of a battery of genes important in maintaining the epithelial tight barrier and mucosal immune response (3, 13, 26, 28, 34, 39, 48). Currently no known adherens or tight junction proteins have been shown to be direct targets of HIF-1α in the intestine. However, several direct target genes of HIF-1α are expressed on the apical surface of intestinal epithelial cells and are critical in maintaining barrier integrity. Disruption of intestinal HIF-1α clearly demonstrates the central role of HIF-1α-induced barrier function in IBD (26).
PHD inhibitors or PHD knockout mice, which potently activate HIF-1α, have been shown to be beneficial in several mouse models of colitis (8, 28, 39, 49). However, the tumor-promoting effect of HIF-1α activators poses a major concern and limitation for their therapeutic use in the treatment of IBD. The colon tumor microenvironment shares several similar characteristics as the inflammatory foci in IBD. HIF-1α and HIF-2α are robustly increased in cancer (29, 42). Chronic activation of HIF-2α in intestinal epithelial cells potentiates tumorigenesis in mouse models of colon cancer (54, 55). Currently it is not known whether activation of HIF-1α can also increase colon carcinogenesis in mouse models. In the present study, we tested the hypothesis that activation of HIF-1α in intestinal epithelial cells would increase colon carcinogenesis. A mouse model of constitutively expressed HIF-1α in intestinal epithelial cells was characterized by whole-genome mRNA expression analysis. Moreover, the activation of HIF-1α in colon cancer was assessed in a sporadic intestinal tumor model and a colitis-associated colon cancer model. HIF-1α significantly increased glycolytic genes. However, constitutive activation of HIF-1α did not significantly alter tumor multiplicity, size, proliferation, or apoptosis. The data demonstrate that increase in HIF-1α in intestinal epithelial cells does not result in spontaneous tumor formation or further potentiate the progression of colon cancer.
MATERIALS AND METHODS
Animals and treatments.
The Hif-1αLSL/LSL and littermate controls were previously described (53). The Adenomatous polyposis coli (Apc)min/+ were purchased from The Jackson Laboratory. To investigate the role of HIF-1α in colorectal cancer, Hif-1αLSL/LSL mice were crossed with Apcmin/+ mice to generate Hif-1αLSL/LSL/Apcmin/+ and Hif-1α+/+/Apcmin/+ mice. All mice are on C57BL/6 background, maintained in standard cages in a light- and temperature-controlled room, and were allowed standard chow and water ad libitum. For the azoxymethane (AOM)/dextran sulfate sodium (DSS) study, 6-wk-old Hif-1αLSL/LSL and Hif-1α+/+ mice were injected intraperitoneally with AOM (10 mg/kg body wt). It should be noted that no dysplasia was observed in mice receiving a single AOM dose. Seven days following AOM injection, the Hif-1αLSL/LSL mice and littermate controls received water with 1.5% DSS for 7 days (inflammatory phase). The mice were placed on regular drinking water for 14 days (recovery phase), and two more inflammatory and recovery cycles were performed. All animal studies were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan (UCUCA approval number: 10299).
Mucosal cell isolation.
The jejunum and proximal large intestine were flushed with 1× PBS and cut open longitudinally. Mucosal scraping was performed to enrich for epithelial cells using a glass slide. It should be noted that isolated mucosal cells contain stromal cells as well as intraepithelial lymphocytes. The cells were used for Western blot or gene expression analysis as detailed below.
Western blot analysis.
For Western blot analysis, nuclear fractions were prepared from the epithelial cells of the small intestine and colon using a hypertonic buffer (200 mM sucrose, 10 Tris·HCl, and 2 mM EDTA with protease inhibitors). Nuclear proteins were then extracted using RIPA buffer, and proteins were resolved on SDS-PAGE. Proteins were then transferred onto a nitrocellulose membrane and probed with specific antibodies against HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA) and histone H3 (Cell Signaling Technology, Danvers, MA).
Histology, immunohistochemistry, and immunofluorescence.
Mice were killed following a 2-h treatment of 100 mg/kg of 5-bromo-2-deoxyuridine (BrdU) (Sigma, St Louis, MO). Paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene and rehydrated in ethanol gradient. Immunohistochemical analysis was performed with antibodies for BrdU (Bu20a; eBioscience, San Diego, CA) and cleaved caspase 3 (cCasp3) (Cell Signaling Technology) as previously described (55). Histological analysis was done on hematoxylin and eosin-stained paraffin sections.
Quantitative real-time RT-PCR.
RNA was isolated and reverse transcribed as previously described (55). For the AOM/DSS-treated normal tissue and tumor tissue following isolation, the RNA was further purified by lithium chloride precipitation. cDNA was quantified using SYBR green dye and run on a 7900HT Fast Real-Time RT-PCR system (Life Technologies, Carlsbad, CA) (primers listed in Table 1). Ct values were normalized to β-actin and expressed as fold difference from controls.
Table 1.
Primers list
| qPCR Primer | Sequence (5′-3′) |
|---|---|
| β-actin F | TATTGGCAACGAGCGGTTCC |
| β-actin R | GGCATAGAGGTCTTTACGGATGT |
| Pgk1 F | CAAATTTGATGAGAATGCCAAGACT |
| Pgk1 R | TTCTTGCTGCTCTCAGTACCACA |
| Plod2 F | AGAACTTCTTCAGGCCCACC |
| Plod2 R | GCTATGGAGCACTACGCCAG |
| Pdk1 F | TTACTCAGTGGAACACCGCC |
| Pdk1 R | GTTTATCCCCCGATTCAGGT |
| Mif F | AAAAGTCATCAGCTGGTCCG |
| Mif R | AGAGGGGTTTCTGTCGGAG |
| P4 ha1 F | CGCCATCATTAAAACAACCC |
| P4 ha1 R | GACAGGACTGACCCGAGC |
| Shmt2F | ATGCAGACCAGCTGACCAC |
| Shmt2R | AGTGGCTAGTCCTCCTGTGC |
| Egln3 F | GACCGGCTGGTCCTGTACTG |
| Egln3 R | CTTGGACCGCTCCTTGACA |
| Bnip3 F | TGAAGTGCAGTTCTACCCAGG |
| Bnip3 R | CCTGTCGCAGTTGGGTTC |
| Ndufa4l2 F | AGTCTAGGGACCCGCTTCTAC |
| Ndufa4l2 R | TGTACTGGTCATTGGGACTCA |
| Myl7 F | AACTTGTCTGCCTGGGTCAT |
| Myl7 R | TCTTCCTCACACTCTTCGGG |
| Slc16a3 F | TTCACCAAGAACTGAGCTGC |
| Slc16a3 R | CTGCCACAGCCACACAATAG |
| Nrn1 F | GCCCTTAAAGACTGCATCACA |
| Nrn1 R | GGGACTTAAGTTGAACGGCA |
| Fam162a F | AATGCTTCCTCGTCTCTAAGGT |
| Fam162a R | CACCCCGTGTCTGTAAGACTG |
| Aldoc F | ACCATATTGGGCTTGAGCAG |
| Aldoc R | CCTCAAACGTTGCCAGTATGT |
| Stc2 F | TCTGTTCACACTGAGCCTGG |
| Stc2 R | TTTCAAGGATCTCCTGCTGC |
| Car9 F | GGTGTGGTCAGAGACCCTTC |
| Car9 R | TGGAAGAAATCTCGGAGGAA |
| Ak4 F | GGTACTGTTTTGCCACGTCA |
| Ak4 R | AAAGGATCGCCCAGAACTTT |
| Greb1 F | CTCTGCTCAGCACCAGACAG |
| Greb1 R | CACCTAGCCCACAGGATGAC |
cDNA microarray analysis.
RNA was extracted from colon mucosal scrapings from 8-wk-old Hif-1αLSL/LSL and littermate control mice and reverse transcribed. cDNA was hybridized to the Mouse Gene 1.1 ST Array Strips (Affymetrix, Santa Clara, CA), and data were analyzed as previously described (55) and compared with gene expression analysis from the colons of intestine-specific Vhl knockout (VhlΔIE) mice. The full data set is available on the GEO database accession number GSE55915 for the Hif-1αLSL/LSL mice and accession number GSE36091 for the VhlΔIE mice.
Statistics.
Results are expressed as means ± SD. P values were calculated by independent t-test, one-way ANOVA, Dunnett's t-test, and two-way ANOVA. P < 0.05 was considered significant.
RESULTS
Activation of intestinal HIF-1α does not lead to gross histological changes in the small intestine and colon.
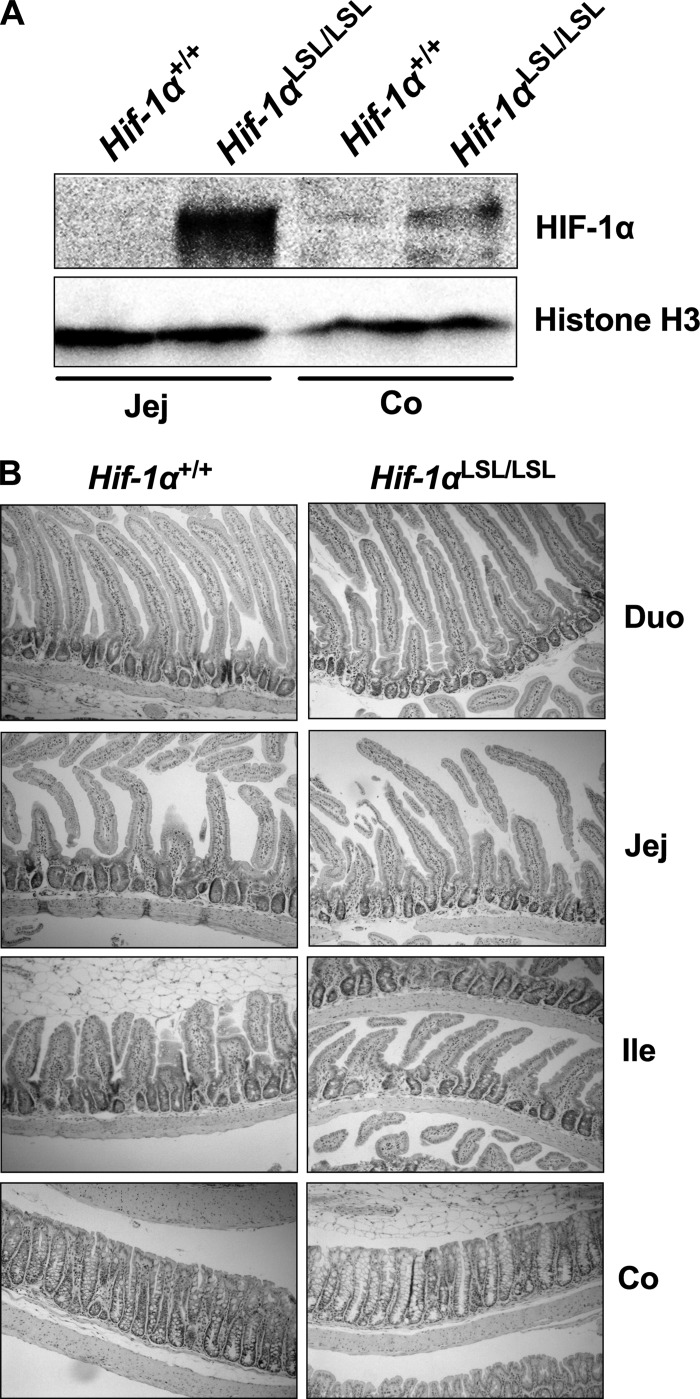
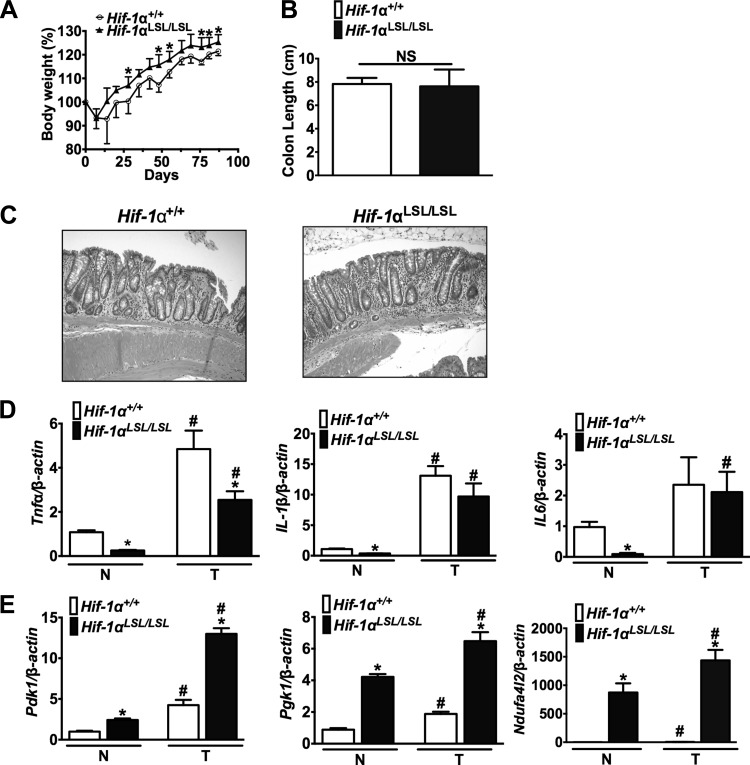
To directly understand the role of HIF-1α in the intestinal epithelium, the Hif-1αLSL/LSL mouse model was assessed (53). These mice overexpress an oxygen-stable HIF-1α in intestinal epithelial cells (Fig. 1A). The Hif-1αLSL/LSL mice do not have any overt phenotypes compared with littermate controls (53). Histological analysis of the small intestine and the colon also demonstrated no obvious difference compared with wild-type mice (Fig. 1B).
Fig. 1.
Activation of hypoxia-inducible factor (HIF)-1α in intestinal epithelial cells. A: Western blot analysis for the expression of HIF-1α in the jejunum (Jej) or colon (Co) in nuclear extracts of scraped mucosal cells from Hif-1αLSL/LSL or littermate control Hif-1α+/+ mice. Loading was assessed by histone H3. B: hematoxylin and eosin (H&E) staining in the duodenum (duo), jejunum (Jej), ileum (Ile), and colon (Co) of Hif-1αLSL/LSL or Hif-1α+/+ mice.
Global gene expression analysis in the colons of Hif-1αLSL/LSL mice.
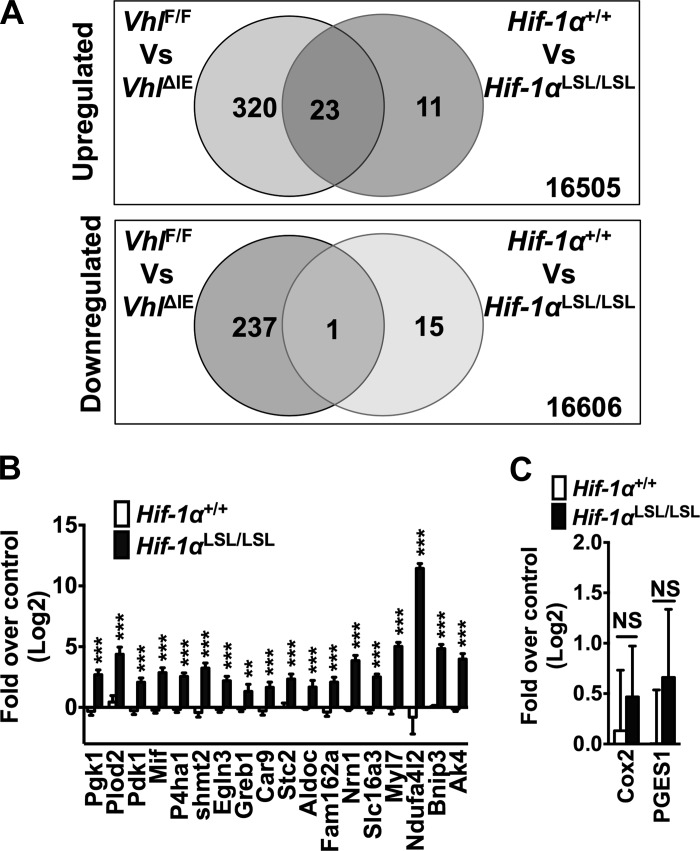
To better characterize the changes in the Hif-1αLSL/LSL mice, whole-genome mRNA analysis was performed on scraped colonic mucosal cells from the Hif-1αLSL/LSL and age-matched wild-type littermates. The microarray analysis using a twofold cutoff demonstrated that 34 genes were upregulated and 16 genes were downregulated in Hif-1αLSL/LSL mice compared with littermate controls (Tables 2 and 3). As expected, several well-characterized target genes were induced, including genes involved in glycolysis, autophagy, and mitochondrial metabolism. The microarray data were compared with whole-genome mRNA analysis obtained from colons of VhlΔIE mice (55). Disruption of VHL led to a robust activation of HIF-1α and HIF-2α signaling, and the Hif-1αLSL/LSL mice demonstrated significant overlap with the VhlΔIE mice with respect to genes that are increased (Fig. 2A and Table 4). Interestingly, only one gene demonstrated overlap in genes that were downregulated from the Hif-1αLSL/LSL and VhlΔIE mice (Fig. 2A and Table 4). qPCR was performed on known HIF-1α target genes for verification that HIF-1α signaling was activated in the Hif-1αLSL/LSL mice (Fig. 2B). Several known HIF-1α target genes were significantly induced (phosphoglycerate kinase 1, Pgk1; procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2, Plod2; pyruvate dehydrogenase kinase, isozyme 1, Pdk1; macrophage migration inhibitory factor, Mif; prolyl 4-hydroxylase, alpha polypeptide I, P4ha1; egl nine family homolog 3, Egln3; carbonic anhydrase 9, Car9; stanniocalcin 2, Stc2; aldolase c, Aldoc; family with sequence similarity 162; member A, Fam162a; NADH dehydrogenase 1 alpha subcomplex, 4-like 2, Ndufa4l2; and BCL2/adenovirus E1B 19kDa interacting protein 3, Bnip3) (1, 4, 15, 16, 23, 30, 31, 33, 43, 45, 50, 51). Moreover, novel putative HIF-1α-dependent genes that may impact tumor metabolism (serine hydroxymethyltransferase 2; growth regulation by estrogen in breast cancer 1; neuritin 1; adenylate kinase 4; myosin, light polypeptide 7, regulatory; and solute carrier family 16 member 3) were also assessed by qPCR analysis to confirm the results of the microarray (Fig. 2B). Together, these data provide evidence that Hif-1αLSL/LSL mice are an appropriate model to assess the role of HIF-1α activation in colon cancer. Interestingly, prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2, COX-2) a HIF-1α target gene critical in the growth of colon cancer through the production of prostaglandin E2 (PGE2), was not induced in the Hif-1αLSL/LSL mice (25). Similarly, PGE synthase (PGES1), the downstream gene of COX-1 important in the production of PGE2, was also not induced in the colon of Hif-1αLSL/LSL mice (Fig. 2B). These data further confirm a previous study demonstrating a critical and specific role of HIF-2α in the regulation of the COX-2-mPGES1-PGE2 axis in colon cancer (54).
Table 2.
List of upregulated genes from the colons of Hif-1αLSL/LSL vs. Hif-1α+/+ mice
| Symbol | Description |
|---|---|
| Bnip3 (45) | Bcl2/adenovirus E1B-interacting protein 3 |
| Ndufa4l2 (50) | NADH dehydrogenase (ubiquinone) 1 α-subcomplex, 4-like 2 |
| Slc16a3 | Solute carrier family 16 (monocarboxylic acid transporters), member 3 |
| Shmt2 | Serine hydroxymethyltransferase 2 (mitochondrial) |
| Ak4 | Adenylate kinase 4 |
| Myl7 | Myosin, light polypeptide 7, regulatory |
| Pfkfb3 (36) | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 |
| Nrn1 | Neuritin 1 |
| P4 ha1 (15) | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), α1 polypeptide |
| Stc2 (33) | Stanniocalcin 2 |
| Lpo | Lactoperoxidase |
| Greb1 | Gene regulated by estrogen in breast cancer protein |
| Mif (1) | Macrophage migration inhibitory factor |
| Pdk1 (30) | Pyruvate dehydrogenase kinase, isoenzyme 1 |
| Plod2 (16) | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 |
| Car9 (51) | Carbonic anhydrase 9 |
| Grin1 | Glutamate receptor, ionotropic, NMDA1 (ζ1) |
| Itga7 | Integrin α7 |
| Plod1 (16) | Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1 |
| Rgs11 | Regulator of G protein signaling 11 |
| Aldoc (23) | Aldolase C, fructose-bisphosphate |
| Eltd1 | EGF, latrophilin 7 transmembrane domain containing 1 |
| Higd1a | HIG1 domain family, member 1A |
| Fam162a (31) | Family with sequence similarity 162, member A |
| Egln3 (4) | Egl 9 homolog 3 (C. elegans) |
| Pgk1 (43) | Phosphoglycerate kinase 1 |
| Hmox1 (32) | Heme oxygenase (decycling) 1 |
| P4 ha2 (15) | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), αII polypeptide |
| Cmah | Cytidine monophospho-N-acetylneuraminic acid hydroxylase |
| Krt84 | Krt84 keratin 84 |
| Eno1 (43) | Enolase 1, α nonneuron |
| 5730507C01Rik | RIKEN cDNA 5730507C01 gene |
| 1700057G04Rik | RIKEN cDNA 1700057G04 gene |
| Ero1l (14) | ERO1-like (S. cerevisiae) |
Bold font indicates known hypoxia-inducible factor (HIF)-1α target genes with the subsequent reference.
Table 3.
List of downregulated genes from the colons of Hif-1αLSL/LSL vs. Hif-1α+/+ mice
| Symbol | Description |
|---|---|
| Igh-VJ558 | Immunoglobulin heavy chain (J558 family) |
| Ighg | Immunoglobulin heavy chain (γ polypeptide) |
| Gm7120 | Gm7120 predicted gene 7120 |
| Duoxa2 | Dual oxidase maturation factor 2 |
| Igj | Immunoglobulin joining chain |
| Iglv1 | Immunoglobulin λ variable 1 |
| Myl9 | Myosin, light polypeptide 9, regulatory |
| Ifit3 | Interferon-induced protein with tetratricopeptide repeats 3 |
| Actg2 | Actin, γ2, smooth muscle, enteric |
| Apoa4 | Apolipoprotein A-IV |
| Des | Desmin |
| Acta2 | Actin, α2, smooth muscle, aorta |
| Igk | Immunoglobulin κ chain complex |
| Myh11 | Myosin, heavy polypeptide 11, smooth muscle |
| Fabp1 | Fatty acid binding protein 1, liver |
| Cnn1 | Calponin 1 |
Fig. 2.
Gene expression analyses in the colon from Hif-1αLSL/LSl mice. A: global gene expression profiling in colon RNAs isolated from Hif-1αLSL/LSL mice compared with Hif-1α+/+ and VhlΔIE mice. B: qPCR analysis for selected genes from the microarray data set in colons from Hif-1αLSL/LSL and littermate control mice. C: qPCR analysis of cyclooxygenase (COX)-2 and prostaglandin E synthase 1 (PGES1). 6 mice were assessed per each group. Gene expression was normalized to β-actin, and each bar represents the mean value ± SD. **P < 0.05, ***P < 0.01, compared with Hif-1α+/+ mice.
Table 4.
List of common changed genes from the colons of Hif-1αLSL/LSL and VhlΔIE mice
| Symbol | Description | Expression |
|---|---|---|
| Bnip3 | Bcl2/adenovirus E1B interacting protein 3 | Up |
| Egln3 | Egl 9 homolog 3 (C. elegans) | Up |
| Ndufa4l2 | NADH dehydrogenase (ubiquinone) 1α subcomplex, 4-like 2 | Up |
| Slc16a3 | Solute carrier family 16 (monocarboxylic acid transporters), member 3 | Up |
| Shmt2 | Serine hydroxymethyltransferase 2 (mitochondrial) | Up |
| Ak4 | Adenylate kinase 4 | Up |
| Myl7 | Myosin, light polypeptide 7, regulatory | Up |
| Pfkfb3 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | Up |
| Nrn1 | Neuritin 1 | Up |
| P4 ha1 | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), α1 polypeptide | Up |
| Stc2 | Stanniocalcin 2 | Up |
| Greb1 | Gene regulated by estrogen in breast cancer protein | Up |
| Mif | Macrophage migration inhibitory factor | Up |
| Pdk1 | Pyruvate dehydrogenase kinase, isoenzyme 1 | Up |
| Plod2 | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | Up |
| Car9 | Carbonic anhydrase 9 | Up |
| Itga7 | Integrin α7 | Up |
| Aldoc | Aldolase C, fructose-bisphosphate | Up |
| Eltd1 | EGF, latrophilin 7 transmembrane domain containing 1 | Up |
| Higd1a | HIG1 domain family, member 1A | Up |
| Hmox1 | Heme oxygenase (decycling) 1 | Up |
| P4 ha2 | Procollagen-proline, 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase), αII polypeptide | Up |
| Ero1l | ERO1-like (S. cerevisiae) | Up |
| Fabp1 | Fatty acid binding protein 1, liver | Down |
Activation of intestinal HIF-1α does not increase sporadic colon tumorigenesis.
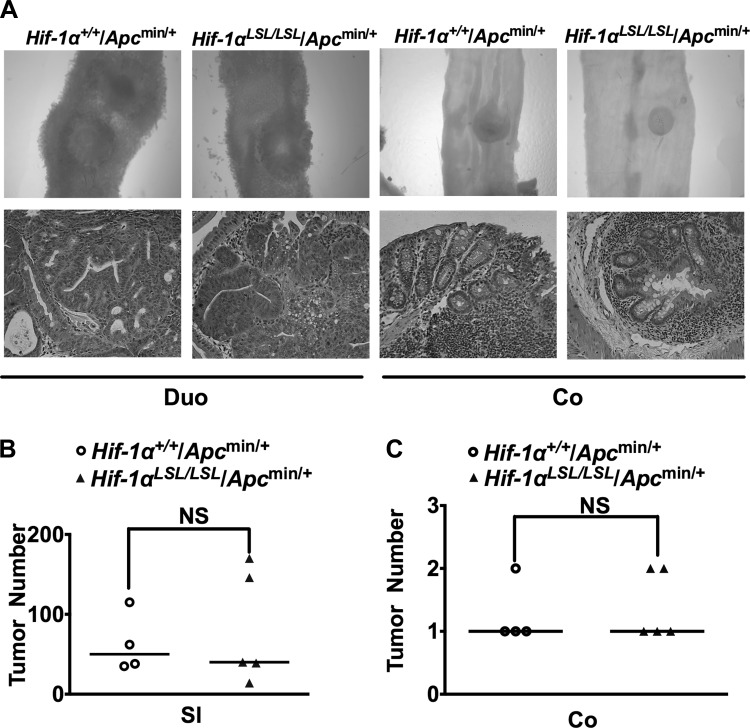
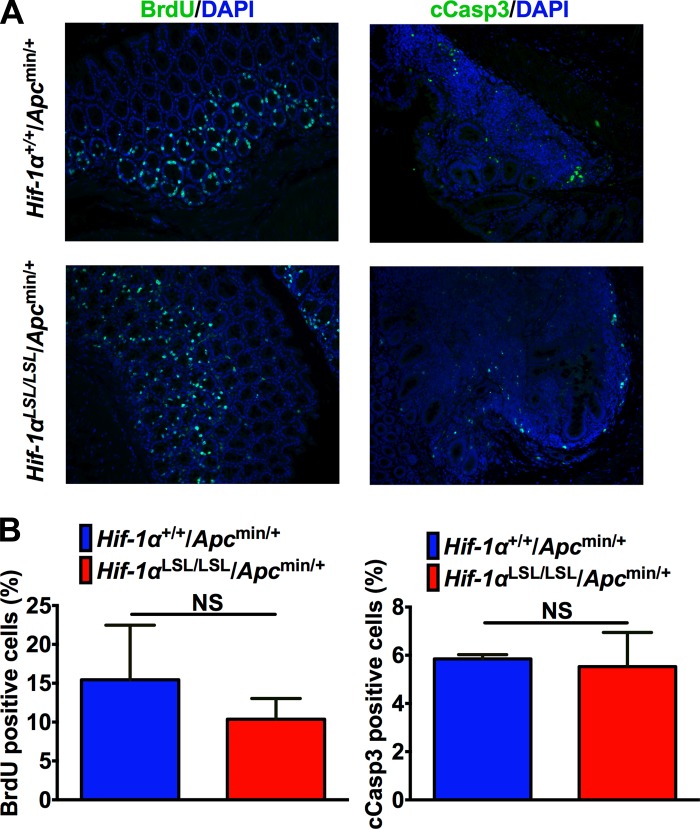
Hif-1αLSL/LSL and littermate controls were crossed to the Apcmin/+ mice. Apcmin/+ mice have a truncated mutation at codon 850 of the murine Apc gene (47). APC mutations are observed in over 80% of patients with sporadic colon cancer (10). The Apcmin/+ mice develop mostly small intestinal adenomas (47). Consistent with these data, Hif-1αLSL/LSL/Apcmin/+ mice developed predominantly small intestinal tumors. However, no significant increase in small intestinal tumors were observed in Hif-1αLSL/LSL/Apcmin/+ compared with littermate control mice (Hif-1α+/+/Apcmin/+) (Fig. 3, A and B). Similarly, HIF-1α activation did not lead to an increase in colon tumors in the Apcmin/+ mice (Fig. 3, A and C). Also the tumors in the small intestine and colon were all adenomas, and no increase in progression was observed in Hif-1αLSL/LSL/Apcmin/+ mice compared with littermate controls (Fig. 3A). The tumors were further analyzed for proliferation and apoptosis by BrdU and cCASP3 staining, respectively. However, no significant difference in tumor proliferation or apoptosis was observed in the Hif-1αLSL/LSL/Apcmin/+ compared with the littermate control mice (Fig. 4).
Fig. 3.
Activation of HIF-1α does not increase tumor formation in Apcmin/+ mice. A: representative duodenum (Duo) and colon (Co) and H&E staining from Hif-1αLSL/LSL/Apcmin/+ and Hif-1α+/+/Apcmin/+ mice. B and C: tumor counting in the small intestine (SI) and colon (Co) from 3-mo-old Hif-1αLSL/LSL/Apcmin/+ and Hif-1α+/+/Apcmin/+ mice. 4–5 mice were assessed per each group; NS; not significant.
Fig. 4.
Activation of HIF-1α does not alter proliferation or apoptosis in the colon tumors from Apcmin/+ mice. 5-Bromo-2-deoxyuridine (BrdU) and cleaved caspase 3 (cCasp3) staining (A) and quantitation (B) in colonic tumor tissues from 3-mo-old Hif-1αLSL/LSL/Apcmin/+ and Hif-1α+/+/Apcmin/+ mice. 4–5 mice were assessed per each group; NS; not significant.
Activation of intestinal HIF-1α does not increase colitis-associated colon cancer.
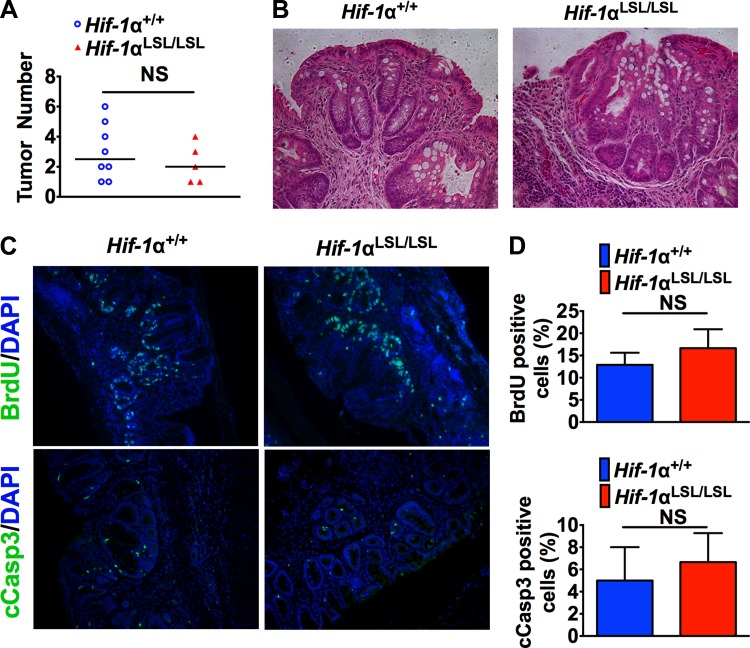
The Apcmin/+ mice are not a robust model for colon cancer, and therefore a colitis-associated colon cancer (CAC) mouse model was assessed (5). Body weight is a good index of systemic inflammation following DSS, and the littermate control mice had decreased body weight throughout the inflammatory and recovery phase compared with Hif-1αLSL/LSL mice (Fig. 5A). These results demonstrated that activation of intestinal HIF-1α significantly protected from DSS-induced injury compared with wild-type littermates. However, repeated exposures to DSS led to no significant difference in local inflammation in Hif-1αLSL/LSL and wild-type mice, as assessed by colon length and histology (Fig. 5, B and C). The expression of cytokines that are critical in the progression of colon cancer such as TNF-α, IL-1β, and IL-6 were assessed by qPCR (18, 38). There was a significant decrease in TNF-α, IL-1β, and IL-6 in normal tissue from Hif-1αLSL/LSL mice compared with wild-type mice. In the tumors, there was a significant decrease in TNF-α, but not IL-1β and IL-6 (Fig. 5D). Moreover, HIF-1α-dependent glycolytic and mitochondrial respiration genes important in cancer were significantly increased in tumor and normal tissue from Hif-1αLSL/LSL mice compared with littermate control mice (Fig. 5E). Despite the increase in genes critical in tumor metabolism in the Hif-1αLSL/LSL mice, no difference in tumor number and tumor histology was observed compared with littermate controls (Fig. 6, A and B). Moreover, tumor proliferation and apoptosis were similar in the Hif-1αLSL/LSL mice and littermate controls (Fig. 6C).
Fig. 5.
Activation of HIF-1α in mouse models of colitis-associated colon cancer (CAC). Body weight (A), colon length (B), representative H&E-stained colon section (C), and qPCR analysis of inflammatory (D) or metabolic genes (E) from Hif-1αLSL/LSL and Hif-1α+/+ mice in normal (N) and tumor (T) tissue following azoxymethane (AOM)/dextran sulfate sodium (DSS) model of colon cancer. Gene expression analysis is normalized to β-actin, and each bar represents the mean value ± SD. *P < 0.01, compared with normal or tumor tissues from Hif-1α+/+ mice. #P < 0.01, compared with normal tissues from Hif-1αLSL/LSL and Hif-1α+/+ mice. 5–8 mice were assessed per each group.
Fig. 6.
Activation of HIF-1α does not increase tumor formation in a mouse model of CAC. Tumor counting in the colons (A), representative colon tumor H&E staining (B), and representative colon tumor BrdU and cCasp3 staining (C) and quantitation (D) from Hif-1αLSL/LSL and Hif-1α+/+ mice following AOM/DSS model of colon cancer. 5–8 mice were assessed per each group; NS; not significant.
DISCUSSION
HIF-1α and HIF-2α are the two major transcription factors critical for hypoxic-mediated transcriptional and translational cellular response. In the intestine, HIF-2α has recently been shown to increase inflammation and colon cancer (53–55). HIF-2α can directly regulate several proinflammatory genes. TNF-α is a critical HIF-2α target gene in hypoxic inflammation (53). HIF-1α activation in the intestine leads to barrier protection and a decrease in several proinflammatory cytokines in mouse models of colitis (26, 28). However, the role of HIF-1α in cancer has led to concerns for the therapeutic use and efficacy of HIF-1α activators in IBD. The present data clearly demonstrate that, in mouse models of colon cancer, chronic activation of HIF-1α does not increase sporadic or inflammation-induced colon cancer. HIF-1α through a battery of target genes maintains barrier homeostasis and decreases inflammation in an acute colitis models (13, 26, 28, 34, 48). Consistent with these data, a significant decrease in proinflammatory mediators and a protection in body weight loss following DSS treatment in Hif-1αLSL/LSL mice compared with littermates control mice were observed. Tumor-elicited inflammation in sporadic colon cancer and inflammation in CAC are due to dysregulation of the intestinal epithelial barrier and activation of proinflammatory response (19). PHDs are critical in the activation of basal NF-κB and also in limiting IL-1β-induced NF-κB (11, 40). PHD inhibitors repress NF-κB activity, which is a critical signaling pathway in the progression of sporadic and colitis-associated colon cancer (17). PHD inhibitors may decrease tumor inflammation through two mechanisms, barrier protection and inhibition of NF-κB signaling, and therefore would be beneficial in patients with colon cancer.
HIF-1α has been shown to have a major role in many aspects of tumor biology. A key function of hypoxia signaling in tumors is shifting the tumor metabolism to anaerobic glycolysis. HIF-1α is a direct regulator of glycolytic genes, which we confirm in vivo in the present study. Moreover, Ndufa4l2 is robustly increased in the colon of Hif-1αLSL/LSL mice, and this gene has been shown to be critical in limiting oxygen consumption and cellular adaptation of cells to hypoxia (41, 50). The data demonstrate that the increase in these genes in normal colonic epithelial cells does not lead to cancer. Moreover, the increase in glycolytic and mitochondrial metabolic genes in adenomas does not increase the progression of early-stage tumors. Although most mouse models of colon cancer do not progress further than adenomas, it is possible that, in later stages of colon cancer, chronic activation of HIF-1α may potentiate progression. The microarray data from the Hif-1αLSL/LSL mice also demonstrated a significant increase in several novel genes that will require further experiments to confirm mechanisms of regulation by HIF-1α. Several downregulated genes were also identified. However, unlike the genes that were induced, there was not a good correlation between the downregulated genes in the Hif-1αLSL/LSL mice and the VhlΔIE mice. This may be due to the role of HIF-1α as a transcription factor that can directly bind to promoters and activate gene expression, whereas, to date, no known direct repressed HIF-1α target genes have been confirmed. Interestingly, fatty acid binding protein-1 (Fabp1) was inhibited in both mouse models. Fabp1 is highly expressed in the intestine, and deletion results in significant protection in adenoma formation in the Apcmin/+ mice on 10% fat-containing diet (9). Although no protection was observed in adenoma formation in the Hif-1αLSL/LSL mice on standard chow, these data suggest that activation of HIF-1α may be beneficial in obesity-induced colon cancer.
The data provide strong evidence for the safe use of HIF-1α activators in colitis, without enhancing tumorigenesis. PHD inhibitors have been shown to be beneficial in mouse models of colitis and can activate the HIF response (8, 28, 39). In cancer-derived cell lines, PHD inhibitors can activate both HIF-1α and HIF-2α. In vivo in the intestine, PHD inhibitors primarily activate HIF-1α expression, thus limiting the proinflammatory role of HIF-2α (53). Moreover, three PHDs exist, PHD 1, 2, and 3 (also referred to as Egln-2, -1, and -3, respectively). The most commonly used inhibitors are pan antagonists of PHDs (37). However, through cell-based studies, it has been demonstrated that PHDs have different selectivity for HIF-1α and HIF-2α (37). Thus the pharmacological intervention to treat colitis using PHD inhibitors that can specifically activate either HIF-1α or HIF-2α response is feasible. Indeed, AKB-4924 is a HIF-1α-predominant PHD inhibitor, which is protective in mouse models of colitis (28).
In summary, the data demonstrate that in vivo activation of intestinal HIF-1α does not impact tumorigenesis in two mouse models of colon cancer. This provides evidence for the safe use of PHD inhibitors in the treatment of ischemic and inflammatory disorders of the intestine.
GRANTS
This work was supported by NIH grants (CA148828 and DK095201 to Y. Shah), The University of Michigan Gastrointestinal Peptide Center (Y. Shah), pilot grant from The University of Michigan GI Spore (CA130810 to Y. Shah), and Crohn's Colitis Foundation of America (Grant number 276556 to X. Xue).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.X., S.K.R., and Y.M.S. conception and design of research; X.X., S.K.R., and Y.M.S. performed experiments; X.X., S.K.R., and Y.M.S. analyzed data; X.X., S.K.R., and Y.M.S. interpreted results of experiments; X.X., S.K.R., and Y.M.S. prepared figures; X.X., S.K.R., and Y.M.S. drafted manuscript; X.X., S.K.R., and Y.M.S. edited and revised manuscript; X.X., S.K.R., and Y.M.S. approved final version of manuscript.
REFERENCES
- 1. Baugh JA, Gantier M, Li L, Byrne A, Buckley A, Donnelly SC. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem Biophys Res Commun 347: 895–903, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut 62: 1653–1664, 2013 [DOI] [PubMed] [Google Scholar]
- 3. Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cioffi CL, Liu XQ, Kosinski PA, Garay M, Bowen BR. Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun 303: 947–953, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin 28: 1450–1459, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Dharmarajan S, Newberry EP, Montenegro G, Nalbantoglu I, Davis VR, Clanahan MJ, Blanc V, Xie Y, Luo J, Fleshman JW, Jr, Kennedy S, Davidson NO. Liver fatty acid-binding protein (L-Fabp) modifies intestinal fatty acid composition and adenoma formation in ApcMin/+ mice. Cancer Prev Res (Phila) 6: 1026–1037, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet 10: 721–733, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Fitzpatrick SF, Tambuwala MM, Bruning U, Schaible B, Scholz CC, Byrne A, O'Connor A, Gallagher WM, Lenihan CR, Garvey JF, Howell K, Fallon PG, Cummins EP, Taylor CT. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J Immunol 186: 1091–1096, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, Sina C, Onnie CM, Weersma RK, Stokkers PC, Wijmenga C, Gazouli M, Strachan D, McArdle WL, Vermeire S, Rutgeerts P, Rosenstiel P, Krawczak M, Vatn MH, group Is. Mathew CG, Schreiber S. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40: 1319–1323, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193: 1027–1034, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gess B, Hofbauer KH, Wenger RH, Lohaus C, Meyer HE, Kurtz A. The cellular oxygen tension regulates expression of the endoplasmic oxidoreductase ERO1-Lalpha. Eur J Biochem 270: 2228–2235, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem 288: 10819–10829, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res 11: 456–466, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15: 103–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491: 254–258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, Kugathasan S, Bradfield JP, Walters TD, Sleiman P, Kim CE, Muise A, Wang K, Glessner JT, Saeed S, Zhang H, Frackelton EC, Hou C, Flory JH, Otieno G, Chiavacci RM, Grundmeier R, Castro M, Latiano A, Dallapiccola B, Stempak J, Abrams DJ, Taylor K, McGovern D, Western Regional Alliance for Pediatric IBD. Silber G, Wrobel I, Quiros A, International IBD Genetics Consortium. Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmuda MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, NIDDK IBD Genetics Consortium. Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBD Consortium, Wellcome Trust Case Control Consortium. Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwillam R, Tremelling M, Delukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ, Heyman MB, Ferry GD, Kirschner B, Lee J, Essers J, Grand R, Stephens M, Levine A, Piccoli D, VanLimbergen J, Cucchiara S, Monos DS, Guthery SL, Denson L, Wilson DC, Grant SF, Daly M, Silverberg MS, Satsangi J, Hakonarson H. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet 41: 1335–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Jean JC, Rich CB, Joyce-Brady M. Hypoxia results in an HIF-1-dependent induction of brain-specific aldolase C in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L950–L956, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBD, GeneticsConsortium. Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491: 119–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res 66: 6683–6691, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle 4: 256–258, 2005 [PubMed] [Google Scholar]
- 28. Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, Kotsakis A, McNamee EN, Eltzschig HK, Kominsky DJ, Colgan SP. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol 7: 114–123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12: 9–22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Lee MJ, Kim JY, Suk K, Park JH. Identification of the hypoxia-inducible factor 1 alpha-responsive HGTD-P gene as a mediator in the mitochondrial apoptotic pathway. Mol Cell Biol 24: 3918–3927, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997 [PubMed] [Google Scholar]
- 33. Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem 278: 40296–40304, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627, 2006 [DOI] [PubMed] [Google Scholar]
- 35. McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagace C, Li C, Green T, Stevens CR, Beauchamp C, Fleshner PR, Carlson M, D'Amato M, Halfvarson J, Hibberd ML, Lordal M, Padyukov L, Andriulli A, Colombo E, Latiano A, Palmieri O, Bernard EJ, Deslandres C, Hommes DW, de Jong DJ, Stokkers PC, Weersma RK, NIDDK IBD Genetics Consortium. Sharma Y, Silverberg MS, Cho JH, Wu J, Roeder K, Brant SR, Schumm LP, Duerr RH, Dubinsky MC, Glazer NL, Haritunians T, Ippoliti A, Melmed GY, Siscovick DS, Vasiliauskas EA, Targan SR, Annese V, Wijmenga C, Pettersson S, Rotter JI, Xavier RJ, Daly MJ, Rioux JD, Seielstad M. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 42: 332–337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, Caro J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem 277: 6183–6187, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myllyharju J, Koivunen P. Hypoxia-inducible factor prolyl 4-hydroxylases: common and specific roles. Biol Chem 394: 435–448, 2013 [DOI] [PubMed] [Google Scholar]
- 38. Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118: 560–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scholz CC, Cavadas MA, Tambuwala MM, Hams E, Rodriguez J, von Kriegsheim A, Cotter P, Bruning U, Fallon PG, Cheong A, Cummins EP, Taylor CT. Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci USA 110: 18490–18495, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123: 3664–3671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9: 47–71, 2014 [DOI] [PubMed] [Google Scholar]
- 43. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269: 23757–23763, 1994 [PubMed] [Google Scholar]
- 44. Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134: 2036–2048; 2048; e2031–e2033, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61: 6669–6673, 2001 [PubMed] [Google Scholar]
- 46. Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, Li Y, Kurreeman FA, Zhernakova A, Hinks A, Guiducci C, Chen R, Alfredsson L, Amos CI, Ardlie KG, BIRAC Consortium. Barton A, Bowes J, Brouwer E, Burtt NP, Catanese JJ, Coblyn J, Coenen MJ, Costenbader KH, Criswell LA, Crusius JB, Cui J, deBakker PI, DeJager PL, Ding B, Emery P, Flynn E, Harrison P, Hocking LJ, Huizinga TW, Kastner DL, Ke X, Lee AT, Liu X, Martin P, Morgan AW, Padyukov L, Posthumus MD, Radstake TR, Reid DM, Seielstad M, Seldin MF, Shadick NA, Steer S, Tak PP, Thomson W, vander Helm-van Mil AH, van der Horst-Bruinsma IE, van der Schoot CE, van Riel PL, Weinblatt ME, Wilson AG, Wolbink GJ, Wordsworth BP, YEAR Consortium. Wijmenga C, Karlson EW, Toes RE, de Vries N, Begovich AB, Worthington J, Siminovitch KA, Gregersen PK, Klareskog L, Plenge RM. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42: 508–514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256: 668–670, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139: 2093–2101, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, Corral-Escariz M, Soro I, Lopez-Bernardo E, Perales-Clemente E, Martinez-Ruiz A, Enriquez JA, Aragones J, Cadenas S, Landazuri MO. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 14: 768–779, 2011 [DOI] [PubMed] [Google Scholar]
- 50a.UK IBD Genetics Consortium. Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Wellcome Trust Case Control Consortium 2. Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, McCarthy MI, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CC, Barrett JC, Bellenguez C, Davison D, Freeman C, Strange A, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Deloukas P, Peltonen L, Mathew CG, Blackwell JM, Brown MA, Corvin A, McCarthy MI, Spencer CC, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 41: 1330–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van den Beucken T, Koritzinsky M, Niessen H, Dubois L, Savelkouls K, Mujcic H, Jutten B, Kopacek J, Pastorekova S, van der Kogel AJ, Lambin P, Voncken W, Rouschop KM, Wouters BG. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J Biol Chem 284: 24204–24212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Limbergen J, Radford-Smith G, Satsangi J. Advances in IBD genetics. Nat Rev Gastroenterol Hepatol. In press [DOI] [PubMed] [Google Scholar]
- 53. Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145: 831–841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xue X, Shah YM. Hypoxia-inducible factor-2alpha is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis 34: 163–169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, Zimmermann EM, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2alpha activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 72: 2285–2293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]