Abstract
Necrotizing enterocolitis (NEC) is an inflammatory disease with evidence of increased production of proinflammatory cytokines in the intestinal mucosa. Lactobacillus reuteri DSM 17938 (LR17938) has been shown to have anti-inflammatory activities in an experimental model of NEC. Activated effector lymphocyte recruitment to sites of inflammation requires the sequential engagement of adhesion molecules such as CD44. The phenotype of CD44+CD45RBlo separates T effector/memory (Tem) cells from naive (CD44−CD45RBhi) cells. It is unknown whether these Tem cells participate in the inflammation associated with NEC and can be altered by LR17938. NEC was induced in 8- to 10-day-old C57BL/6J mice by gavage feeding with formula and exposure to hypoxia and cold stress for 4 days. Survival curves and histological scores were analyzed. Lymphocytes isolated from mesenteric lymph nodes and ileum were labeled for CD4, CD44, CD45RB, intracellular Foxp3, and Helios and subsequently analyzed by flow cytometry. LR17938 decreased mortality and the incidence and severity of NEC. The percentage of Tem cells in the ileum and mesenteric lymph nodes was increased in NEC but decreased by LR17938. Conversely, the percentage of CD4+Foxp3+ regulatory T (Treg) cells in the intestine decreased during NEC and was restored to normal by LR17938. The majority of the Treg cells preserved by LR17938 were Helios+ subsets, possibly of thymic origin. In conclusion, LR17938 may represent a useful treatment to prevent NEC. The mechanism of protection by LR17938 involves modulation of the balance between Tem and Treg cells. These T cell subsets might be potential biomarkers and therapeutic targets during intestinal inflammation.
Keywords: probiotics, intestine, inflammation, regulatory T cells, necrotizing enterocolitis
necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in premature infants and a leading cause of death in the neonatal intensive care unit (42). Although the pathogenesis of NEC remains incompletely defined, low birth weight, early enteral feedings, intestinal ischemic injury, and the resident bacterial flora have been identified as major risk factors in this severe inflammatory condition of the neonatal intestine (41, 42). Evidence for unregulated inflammation in NEC includes increased tissue and serum levels of a wide range of proinflammatory mediators, including TNF-α, IL-1β, IL-6, IL-8, IL-12, and IL-18, and platelet-activating factor and increased intestinal expression of Toll-like receptor (TLR) 4 (TLR4) in patients and animals with NEC (4, 30). These data suggest that the immature neonatal intestine is prone to an exaggerated immune response to pathogenic injury.
In humans and mice, regulatory T (Treg) cells expressing the transcription factor Foxp3 are critical for immune homoeostasis in the intestinal tract. In humans, patients with X-linked immune dysregulation, polyendocrinopathy, and enteropathy (IPEX syndrome), a rare condition resulting from Treg cell deficiency due to mutations in the Foxp3 gene, develop severe gastrointestinal inflammation (35), demonstrating an essential role in suppression of innate and adaptive host responses. Treg cell development can be interrupted by a local proinflammatory cytokine milieu (34, 61). A previous study attempted to investigate whether there were inadequate Treg cells in the intestine of infants with NEC. However, because of technical limitations, investigators were unable to detect a difference in the quantities of Treg cells between preterm and full-term infants when intestinal tissues were examined by immunohistochemistry (64). Recently, the same investigators performed a more detailed study using flow cytometry to quantify Treg cells in the lamina propria of resected ileum from gestational age-matched infants with and without NEC. The ratio of Treg to effector T cells was found to be significantly decreased in premature infants with NEC (63). Recently, we characterized T cell subsets in the ileum of neonatal rats with NEC by comparing these sick animals with dam-fed control rat pups. We reported that the frequency of Foxp3+ Treg cells was significantly diminished in NEC (27). Subsequently, we used adoptive transfer of Treg cells in this NEC model to show that increasing the number of Treg cells in the intestine improved survival and decreased the incidence and severity of NEC (11).
Postnatal gastrointestinal exposure in preterm infants differs from that in full-term infants. For example, restricted breast feeding and treatment with antibiotics may lead to abnormal bacterial colonization and altered immunological development in the gut, thereby increasing the susceptibility to NEC (7, 38, 48). T cells are present in the human fetal ileum in early gestation and accumulate during chorioamnionitis (53). Single-nucleotide polymorphism studies of genetic risk factors for NEC suggest that an enhanced Th1-mediated immune response is associated with more severe disease (60). In the pathogenesis of NEC, the balance of immune regulation in the intestinal mucosa might be disrupted not only by a deficiency of Treg cells, but also by the presence of an expanded effector T cell population that reacts to commensals or pathogens.
Activated effector lymphocyte recruitment to sites of inflammation requires the sequential engagement of adhesion molecules such as CD44. Adhesion receptor CD44 is associated with diverse biological processes involving the migration of cells, including inflammation, angiogenesis, bone metabolism, and wound healing (2). CD44 is upregulated concomitant with the activation of naive T lymphocytes during invasive microbial infection (2). Recent studies showed that CD44 deficiency attenuates murine ileitis (8). The adhesion molecule CD44, as a marker for activated effector CD4+ T cells in inflamed tissues, potentiates T cell activation and maintenance of T cell memory function (3). The CD45 isoform CD45RB [restricted to exon 5 (B)] (33) has been shown to be highly expressed on naive T cells, with loss of expression when cells become activated. Therefore, the phenotype of CD44+CD45RBlo separates effector memory T (Tem) from naive (CD44−CD45RBhi) cells in the non-Treg cell population (57). It is not clear whether Tem cells residing in the intestinal mucosa play a role in NEC.
Probiotic supplementation has been associated with a significantly decreased risk of NEC in preterm very-low-birth-weight infants (62). Lactobacillus reuteri DSM 17938 (LR17938) was derived from L. reuteri ATCC 55730, a component of a Peruvian mother's breast milk. Two plasmids harboring antibiotic resistance genes were removed from strain 55730 to obtain strain 17938 (46), which inhibits pathogen growth and modulates the immune system. We have shown that feeding LR17938 to newborn rats produced a strong anti-inflammatory effect (28, 29), reducing the incidence and severity of NEC (29) while increasing the frequency of Treg cells in the intestinal mucosa (27).
The aim of this study was to identify changes in Tem and Treg cells in the intestine of mice with NEC, thereby clarifying the immunomodulatory mechanism of LR17938 in a mouse model of NEC. We determined if the frequency of Tem or Foxp3+ Treg cells changes in the intestine and mesenteric lymph nodes (MLNs) of mice with NEC, and we determined if changes could be reversed by LR17938 supplementation.
MATERIALS AND METHODS
Probiotic LR17938 Preparation
Human breast milk-derived LR17938 was provided by Biogaia (Stockholm, Sweden). Lactobacillus acidophilus DDS (La DDS; kindly provided by Dr. David R. Mack, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada) was used as the control bacterium for immune cell analysis. Lactobacillus organisms were anaerobically cultured in deMan-Rogosa-Sharpe (MRS) medium (Difco, Detroit, MI) at 37°C for 24 h, plated in MRS agar at specific serial dilutions, and grown anaerobically at 37°C for 48–72 h. For quantitative analysis of bacteria in culture medium, a photometer (Eppendorf, Hamburg, Germany) was used to compare absorbance (at 600 nm) of culture at known concentrations using a standard curve of bacterial colony-forming units (CFU) per milliliter grown on MRS agar. Bacteria in the culture medium were harvested by centrifugation at 1,500 g for 15 min and resuspended in formula before feeding.
Experimental NEC Model and Experimental Design
Animal studies were approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston (HSC-AWC-11-063).
Experimental NEC model.
We developed a protocol for NEC induction in mouse pups that included formula feeding + exposure to stress (FS); this model was not associated with excessive early mortality. We modified the techniques of Jilling et al. (23), Nadler et al. (40), and Sodhi et al. (51). Neonatal (8- to 10-day-old) C57BL/6J mice from breeding pairs of adult male and female animals (Jackson Laboratory) were separated from their dams, housed in an incubator, and starved for 12 h before the initiation of orogastric feeding with 0.2 ml of formula via a sterile Solomon 22-gauge 35-mm feeding needle (Instech Laboratories) four times daily for 4 days. To induce NEC, mouse pups were exposed to 5% O2-95% N2 for 10 min in a hypoxia chamber (Billups-Rothenberg, Del Mar, CA) and then to cold stress at 4°C for 5 min twice daily for 4 days. The formula consisted of 15 g of Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 ml of Esbilac canine milk replacement (PetAg, Hampshire, IL), which contains 1.86 kcal/ml. The calculated calorie intake met the maintenance energy requirement of newborn mice (∼200 kcal·kg−1·day−1). Animals were monitored every 3 h during the 4-day study period. No analgesia was necessary for mice or rats in this study or in previous publications by other groups (1, 17, 23, 45). Live animals were counted on each day, and pups were euthanized on day 5 after NEC induction (FS) for tissue collection. In some cases, pups in the FS group were euthanized on day 3 or 4, for example, if they were in pain, demonstrated labored respirations, exhibited abdominal distension, or had gastrointestinal bleeding. After euthanization, we collected tissues for histological analysis.
Experimental groups.
Mouse pups were randomly divided into four groups: 1) dam-fed mouse pups [normal control (dam), n = 23] were left with their mothers to breast-feed; 2) dam + 17938 (106 CFU·g body wt−1·day−1) mouse pups (n = 16) were left with their mothers to breast-feed and were given LR17938 by gavage for 7 days; 3) formula + stress (FS) mouse pups (n = 36) were separated from their mothers, housed in an incubator, fed formula, and exposed to stress (hypoxia and hypothermia) for 4 days to induce NEC; and 4) formula + stress + 17938 (FS + 17938) mouse pups (n = 20) were treated as described for the FS group, except the formula contained LR17938 (106 CFU·g body wt−1·day−1). For characterization of Tem and Treg cells, we also added one group of FS + La DDS mouse pups (n = 4), which were treated as described for the FS group, except the formula contained La DDS (106 CFU·g body wt−1·day−1).
Tissue Harvest and NEC Evaluation
After incision of the abdomen, the gastrointestinal tract was carefully removed. The small intestine was evaluated visually for typical gross signs of NEC, such as intestinal distension, wall hemorrhage, or necrosis. The terminal 5 cm of small intestine (ileum) were excised. The terminal 1 cm of each sample was fixed with formalin and processed by the Cellular and Molecular Morphology Core Lab (Texas Medical Center Digestive Diseases Center, Houston, TX). The sections of ileal tissues were prepared longitudinally and stained with hematoxylin and eosin for histological evaluation. The remaining 4 cm of small intestine were used for isolation of lymphocytes. The severity of disease was determined by histological NEC scores according to previous studies (1, 4, 5) as follows: 0, normal intestine; 1, epithelial lifting or separation; 2, sloughing of epithelial cells to the midvillous level; and 3, necrosis of the entire villus. Animals with histological scores ≥2 were defined as having NEC.
Tissue Preparation for Cytokine IL-1β Assay
Ileal tissues were weighed and then homogenized in 0.4 ml of lysis buffer containing protease inhibitors with 20 mmol/l Tris·HCl (pH 7.5), 150 mmol/l NaCl, 1 mmol/l Na2EDTA, 1 mmol/l EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mmol/l Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/l PMSF. The homogenates were centrifuged at 14,000 g for 10 min at 4°C after incubation on ice for 30 min. A mouse cytokine kit (Meso Scale Discovery, Gaithersburg, MD) was used according to the manufacturer's protocol to measure IL-1β in the collected supernatant. The detection range from the standard curve of this assay is 1.32–10,000 pg/ml. Final cytokine levels were normalized by the weight of collected ileal tissues and reported as picograms of cytokine per gram of ileal tissue weight.
Preparation of Single-Cell Suspension for Flow Cytometry
Ileal tissues were gently fragmented and digested in RPMI 1640 (Sigma) complete medium containing collagenase V (0.1 mg/ml) from Clostridium histolyticum (Sigma) at 37°C for 30 min. After the samples were vigorously vortexed for 1 min, single-cell suspensions were obtained by filtration through 40-μm cell strainers (BD Biosciences, San Jose, CA). Single-cell suspensions from MLNs were obtained by gentle fragmentation and filtration of the tissues through 40-μm cell strainers into MACS buffer consisting of phosphate-buffered saline, 0.5% bovine serum albumin (Hyclone Laboratories), and 2 mM EDTA (Lonza). Cells were washed with MACS buffer and finally resuspended in MACS buffer for surface and intracellular staining using specific antibodies for further flow cytometric analysis.
Flow Cytometric Analysis
Cells were detected by immunofluorescent staining with flow cytometric analysis. Cell surfaces were stained by fluorochrome-conjugated anti-mouse antibodies, including CD4 (GK1.5) conjugated with peridinin-chlorophyll proteins (PerCP/Cy5.5), CD8a (53-6.7) conjugated with phycoerythrin (PE), CD44 (IM7) conjugated with fluorescein isothiocyanate (FITC), and CD45RB conjugated with PE (C363-16A) from Biolegend (San Diego, CA). For intracellular staining, the anti-mouse antibodies Foxp3 (FJK-16a) conjugated with Alexa Fluor 647 (AF647, eBioscience, San Diego, CA) and Helios (22F6) conjugated with FITC (Biolegend) were used. Fixation and permeabilization were performed using a fixation/permeabilization kit according to the manufacturer's protocol (eBioscience). All samples were analyzed with FACSCalibur (BD Biosciences) and processed with FlowJo (TreeStar, Ashland, OR).
Statistics
Experimental results are expressed as means ± SE. Statistical analysis was performed using one-way ANOVA (Prism 4.0, GraphPad Software, San Diego, CA). Dunnett's and Tukey's multiple-comparison tests were used for comparison of multiple groups or multiple groups with a control group. Kaplan-Meier survival curves were performed, and χ2 test was used for comparison of survival rate between groups. P < 0.05 was considered statistically significant.
RESULTS
L. reuteri DSM 17938 Increased Survival of Mouse Pups With NEC
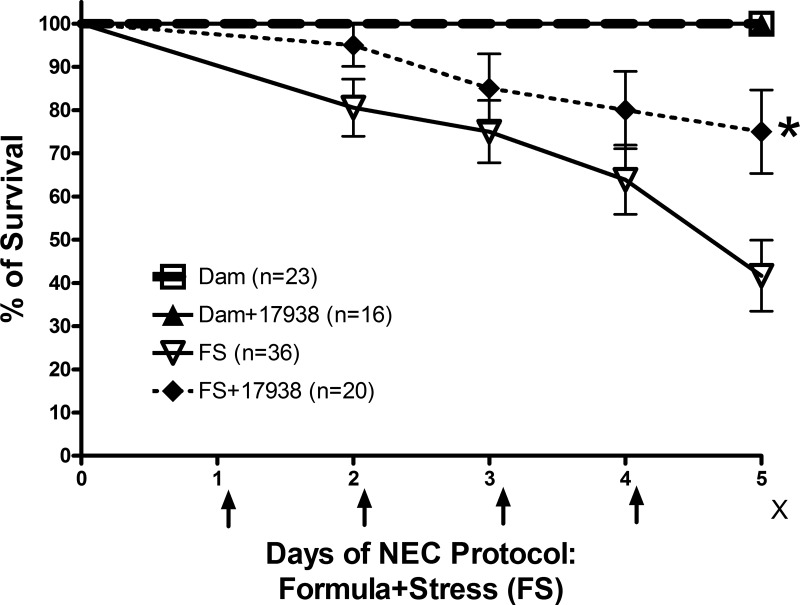
Feeding LR17938 to neonatal mice subjected to the NEC-inducing protocol (FS) significantly increased survival compared with mice given FS without probiotic. Survival on day 5 was 75 ± 9.7% in the FS + LR17938 group compared with 42 ± 8.2% in the FS group (P = 0.0277). Survival on day 5 was 100% in dam-fed and dam-fed + LR17938 mice (Fig. 1). Even though LR17938 significantly increased the survival rate of mice subjected to the NEC protocol, the survival rate of mice subjected to the NEC protocol and treated with LR17938 on day 5 was lower than that of dam-fed or dam-fed + 17938 mice (P < 0.05).
Fig. 1.
Lactobacillus reuteri DSM 17938 (LR17938) increased survival rate of mouse pups with necrotizing enterocolitis (NEC). Mice (8–10 days old) were separated from their mothers and housed in an incubator. NEC was induced by formula feeding + stress (FS) with hypoxia (5% O2-95% N2 for 10 min) and cold exposure (4°C for 5 min) twice daily for 4 days (indicated by arrows). Live animals were counted on each day for calculation of survival rate. Mice were euthanized on day 5 after NEC protocol (indicated by ×), and tissues were collected. In some cases, pups in the FS group were euthanized on day 3 or 4, if they were in pain, demonstrated labored respirations, or exhibited abdominal distension or gastrointestinal bleeding, to collect tissues for histological analysis and to calculate survival rate. LR17938 [106 colony-forming units (CFU)·g body wt−1·day−1] was added to the formula for treatment (FS + 17938). LR17938 reduced mortality rate on day 5 compared with FS without probiotic. *P = 0.027.
L. reuteri 17938 Reduced the Incidence and Severity of NEC
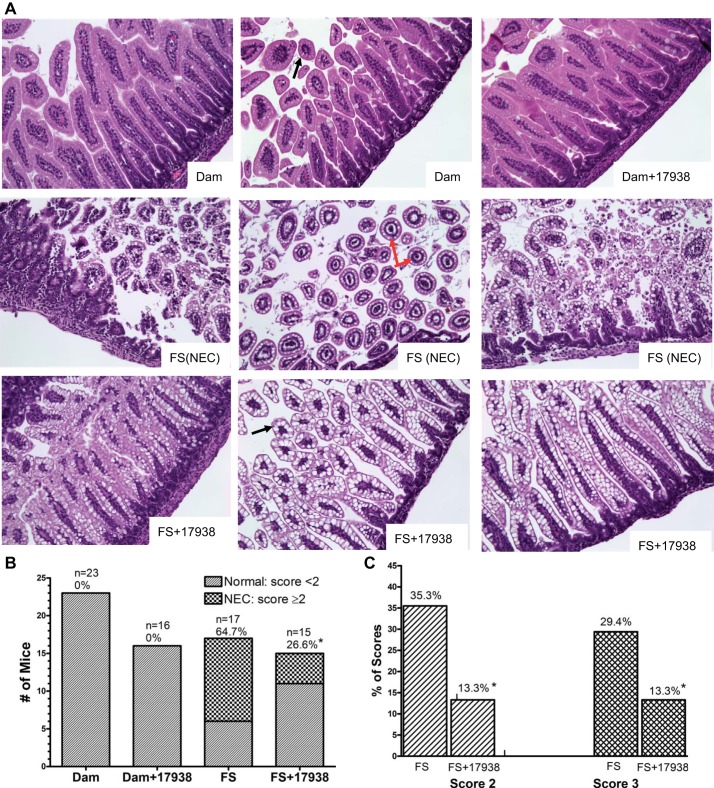
The severity of NEC was evaluated by histological NEC scoring of ileal tissues on a scale of 1–3 (1, 5). Animals with histological scores ≥2 were defined as having NEC. Intestinal tissues for evaluation of histological changes were obtained from living animals on day 5. However, in some groups with severe NEC, tissues were collected on day 3 or 4 for the purpose of comparison. Morphological analysis of ileal segments from mice subjected to the NEC protocol revealed various degrees of inflammatory change, ranging from sloughing of epithelial cells to the midvillous level to necrosis of the entire villus (Fig. 2A), and villous core separation was also seen (Fig. 2A, red arrow). For comparison, Fig. 2A shows the histological features of ileal mucosa from dam-fed and FS + 17938 animals. In contrast, dam-fed and dam-fed + LR17938 mice had normal intestinal architecture and rarely showed inflammatory changes. The incidence of histological NEC was significantly higher in the FS group (11 of 17, 65%) than in dam-fed (0 of 23) or dam-fed + 17938 (0 of 16) mice (P < 0.01).
Fig. 2.
LR17938 reduced incidence and severity of NEC. A: representative intestinal histological changes in dam-fed, dam-fed + 17938, FS (NEC protocol), and FS + 17938 mice. Ileal tissues were stained with hematoxylin and eosin. Magnification ×200. Red arrows, villous core separations in animals with NEC; black arrows, normal villi in ileal tissue from dam-fed or FS + 17938 mice. B: number of animals with normal histology (NEC scores <2) and with NEC (NEC scores ≥2). n, Number of live animals from which tissue samples could be collected. Terminal ileum of each mouse was harvested at day 5 (or day 3 or 4 for some samples in the FS group). %, Incidence of NEC. *P < 0.05 vs. FS. C: FS and FS + 17938 groups with NEC scores of 2 and 3. *P < 0.05 vs. FS. LR17938 reduced NEC histological injury.
Feeding LR17938 to mice subjected to the NEC protocol significantly reduced the incidence of histological NEC to 27% (4 of 15) compared with 65% in the FS group (11 of 17, P < 0.05; Fig. 2B). In addition, the severity of NEC also decreased with administration of LR17938, demonstrating that probiotic administration shifted intestinal damage from severe to mild, as indicated by a change in the NEC score from 2 or 3 to 1. Specifically, 29% (5 of 17) had a NEC score of 3 in the FS group compared with 13% (2 of 15) in the FS + 17938 group (P < 0.05); 35% (6 of 17) had a NEC score of 2 compared with 13% (2 of 15) in the FS + 17938 group (P < 0.05; Fig. 2C). Conversely, a score of 0 or 1 was seen in 73% (11 of 15) of animals in the FS + 17938 group compared with 35% (6 of 17) of pups in the FS group.
L. reuteri DSM 17938 Decreased Inflammatory Cytokine IL-1β Levels in the Intestine of Mice With NEC
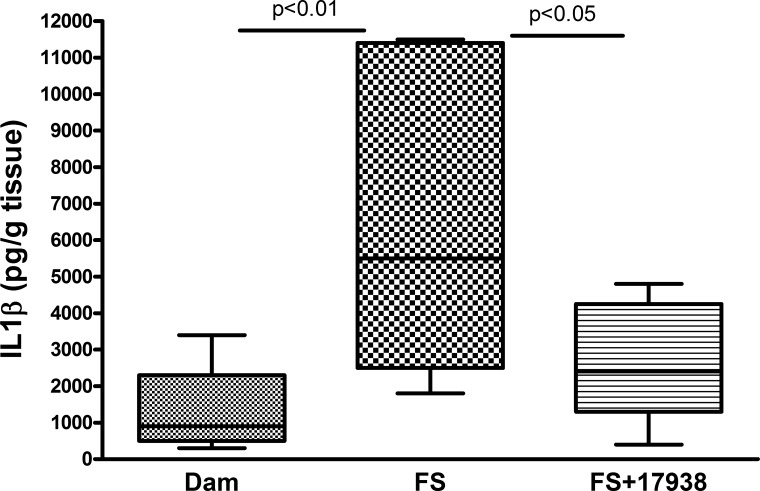
Cytokines are key mediators in inflammation, and several cytokines, including IL-1β, are dysregulated in this disease (30, 50). Analysis of intestinal protein levels of IL-1β indicated that this proinflammatory cytokine significantly increased in the intestines of mouse pups given the NEC-inducing FS treatment compared with normal dam-fed mice (P < 0.01), while feeding LR17938 to mice with FS significantly decreased IL-1β levels compared with FS without probiotic treatment (P < 0.05; Fig. 3).
Fig. 3.
Increased IL-1β levels in intestine of mice subjected to the NEC protocol and the modifying effect of LR17938 treatment. Cytokine levels in tissue lysates were examined using Meso Scale Discovery cytokine assay; n = 8–12 mice per group. Comparisons showed significant differences: P < 0.01 (FS vs. dam) and P < 0.05 (FS + 17938 vs. FS).
Frequency of Treg Cells in the Intestinal Mucosa Was Decreased in NEC and Could be Reversed by Feeding L. reuteri DSM 17938
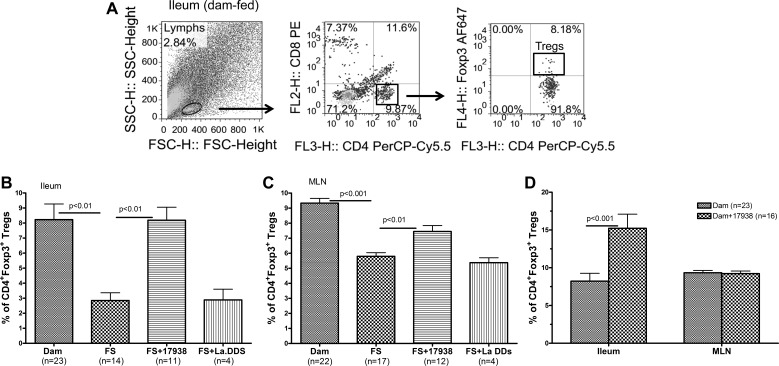
Treg cells maintain intestinal homeostasis by controlling inflammation and inducing tolerance. In a previous study we proposed that there are insufficient numbers of Treg cells to control inflammation in NEC on the basis of a decreased frequency of Treg cells in the intestines of rat pups with NEC (27). In the present study we further validated this observation in the mouse model of NEC by analyzing the Treg cell counts by gating CD4+ T cells to calculate the frequency of Foxp3+ Treg cells within the CD4+ T cell population (Fig. 4A).
Fig. 4.
LR17938 reversed frequency of CD4+Foxp3+ regulatory T (Treg) cells in terminal ileum and mesenteric lymph nodes (MLNs) of mice with NEC. A: representative flow cytometric plots from terminal ileum of dam-fed mice with initial gating on lymphocyte population on the left forward scatter (FSC)-side scatter (SSC) plot (left), followed by gating on CD4+ T cells [middle; x-axis: CD4-peridinin-chlorophyll proteins (CD4-PerCP/Cy5.5); y-axis: CD–phycoerythrin (CD8-PE)], and Foxp3+ Treg cells within the group of CD8−CD4+ T cells (right; x-axis: CD4-PerCP/Cy5.5; y-axis: Foxp3-Alexa Fluor 647). B and C: frequency (%) of CD4+Foxp3+ Treg cells in terminal ileum and MLNs. n, Number of live animals from which sufficient cell numbers were obtained from tissues for analysis. FS vs. dam: P < 0.01 (B) or P < 0.001 (C); FS vs. FS + 17938: P < 0.01 (B and C); FS vs. FS + Lactobacillus acidophilus DDS (La DDS; B and C): P > 0.05. D: percentage of Treg cells in terminal ileum of dam-fed + LR17938 and dam-fed mice (P < 0.001).
The frequency of CD4+Foxp3+ Treg cells significantly decreased in the ileum (Fig. 4B) and MLNs of FS-exposed mice (Fig. 4C) compared with normal dam-fed mice (P < 0.01 in the ileum; P < 0.001 in the MLN). In response to LR17938, the frequency of CD4+Foxp3+ Treg cells returned to normal in the ileum (Fig. 4B) and MLNs (Fig. 4C) of FS-exposed mice compared with FS-exposed animals without LR17938 (P < 0.01). Similarly, in pups that were dam-fed for 1 wk and given LR17938, CD4+Foxp3+ Treg cells were increased in the ileum (P < 0.001), but not in the MLNs, compared with age-matched dam-fed control animals without probiotic (Fig. 4D). However, feeding La DDS, unlike L. reuteri (LR17938), could not increase the frequency of CD4+Foxp3+ Treg cells in the ileum or MLNs of mice subjected to NEC-inducing conditions (Fig. 4, B and C).
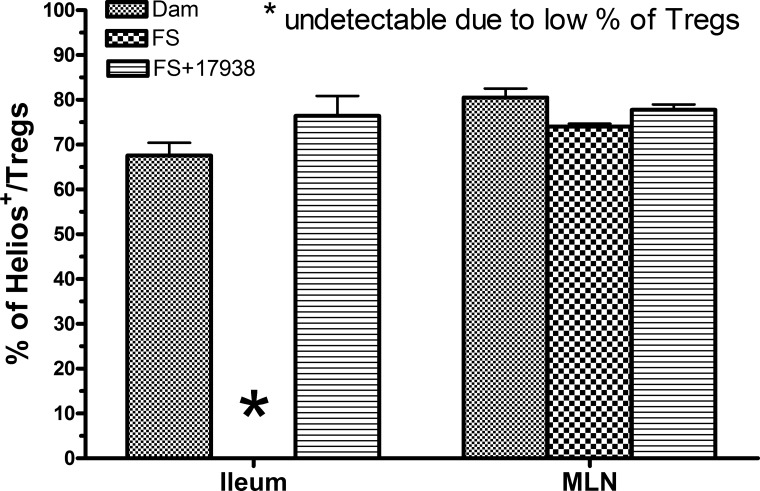
Helios+Foxp3+ Cells Were the Dominant Treg Cell Subset in the Ileum After LR17938 Administration to Mice With NEC
After demonstrating that CD4+Foxp3+ Treg cells in the ileum of mice exposed to the NEC-inducing procedure were augmented by feeding LR17938, we took advantage of a new marker called Helios, which is reported to detect Treg cells of thymic origin in mice (58). We found that 70% (68 ± 2.8%, n = 23) of CD4+Foxp3+ Treg cells in the ileum of dam-fed mice expressed Helios (Fig. 5). Helios was undetectable in the ileum of FS-exposed mice because of the significantly low levels of CD4+Foxp3+ Treg cells. However, ∼75% (76 ± 4.4%, n = 11) of Treg cells expressed Helios in the ileum of FS-exposed mice that were fed LR17938, indicating that the increased CD4+Foxp3+ Treg cells in mice could be of thymic origin. In the MLNs, too, 75–80% of CD4+Foxp3+ Treg cells showed Helios positivity. There were no significant differences between the groups.
Fig. 5.
Increased Treg cells induced by LR17938 during the NEC protocol in terminal ileum of mice were predominantly thymus-derived Treg cells with Helios expression. Percentage of Helios+ cells within CD4+Foxp3+ Treg cells is shown. *Undetectable due to low numbers of Treg cells in the ileum. There were no differences between FS + 17938 and dam-fed mice. All groups demonstrated high Helios expression in ∼75% of Treg cells.
Tem Cells in the Intestinal Mucosa Were Elevated in NEC and Decreased by Feeding LR17938
Tem cells express a different pattern of cell surface markers, and functionally they respond in several different ways compared with naive T cells. In mice, it has been noted that activation of lymphocytes and the transition to memory/effector phenotypes are associated with increased surface levels of CD44 (25). CD44, as an adhesion molecule, is required for recruitment of activated effector lymphocytes to sites of inflammation (2). In addition, naive T cells express a high level of the CD45 isoform restricted to exon 5 (B), named CD45RB in mice. Loss of CD45RB expression has been demonstrated when naive T cells change to Tem cells. Therefore, the phenotype of CD44+CD45RBlo separates Tem from naive (CD44−CD45RBhi) cells (59). The Tem cells are required for immunological protection, while Foxp3+ Treg cells are needed to restrain the immune system from excessive inflammation and/or autoimmunity (31). Thus both cell types are extremely important for the maintenance of immunological homeostasis in the host.
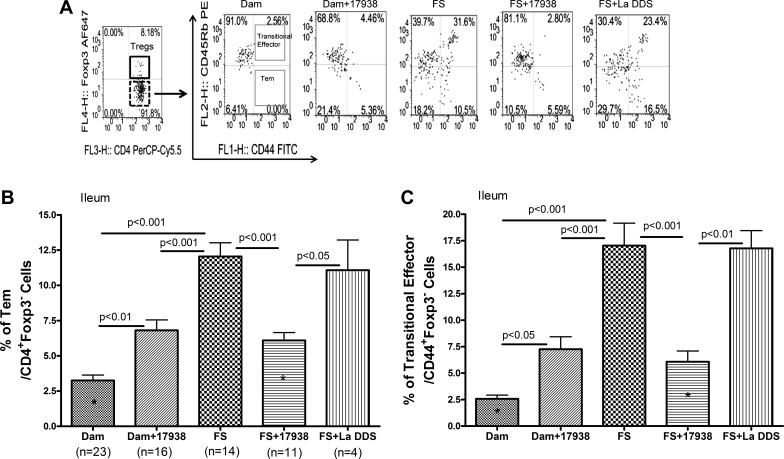
Because our studies indicated that the frequency of Foxp3+ Treg cells was decreased in NEC, we queried whether Tem cells could play a role in NEC. We stained immune cells isolated from the ileum and MLNs with antibodies to CD4, CD44, CD45RB, and Foxp3 and analyzed the cells by flow cytometry. After gating CD4+ T cells, populations of Foxp3+ Treg cells and Foxp3− non-Treg cells were defined, and the percentages of Tem cells (CD44+CD45RBlo) and transitional effector T (CD44+CD45RBhi) cells among the non-Treg cell population were analyzed (Fig. 6A). A significant increase in the percentage of Tem cells in the intestine was observed in mice exposed to NEC conditions (12.1 ± 0.9%, n = 14) compared with dam-fed controls (3.3 ± 1.9%, n = 23, P < 0.001). Feeding LR17938 to FS-exposed mice significantly decreased the Tem subset (6.1 ± 0.6%, n = 11) compared with samples from mice exposed to FS without probiotic (P < 0.001). We were surprised to discover that administration of LR17938 to healthy dam-fed mice also increased the Tem cell subset (6.8 ± 0.7%, n = 16) compared with dam-fed controls (P < 0.01). Feeding the “control” Lactobacillus La DDS could not change the increased percentage of Tem cells in the intestine induced by the NEC procedure (Fig. 6B). A similar pattern was observed in the percentage of transitional effector (CD44+CD45RBhi) cells in the intestine (Fig. 6C), with increased expression in FS-exposed animals and reduced expression in the FS + LR17938 group. There were no changes in these two T cell subsets in MLNs among the different groups, indicating that the changes in Tem cells were limited to the intestine.
Fig. 6.
LR17938 reduced the increased activated effector/memory T (Tem) cells in intestinal mucosa of mice with NEC. A: representative flow cytometric plots from terminal ileum of mice. Population of CD4+Foxp3− non-Treg cells was initially defined (dotted rectangle in 1st left plot) according to expression of CD4 and Foxp3 (x-axis: CD4-PerCP/Cy5.5; y-axis: Foxp3-Alexa Fluor 647). Two different effector T cells were further characterized in the non-Treg cell population on the basis of expression of CD44 and CD45RB (x-axis: CD44-FITC; y-axis: CD45RB-PE). Percentages of T cells with phenotypes of CD44+CD45RBlo (Tem) and CD44+CD45RBhi (transitional effector T) (rectangle in the 2nd left plot) are indicated. Representative plots show percentages of Tem and transitional effector T cells in dam-fed, dam-fed + 17938, FS, FS + 17938, and FS + La DDS mice. B and C: frequency (%) of Tem cells and transitional effector T cells in ileum of mice among the different groups. n, Number of live animals from which sufficient cell numbers were successfully obtained from tissues for analysis. Significant P values for multigroup comparisons are shown. *P < 0.05 FS + 17938 vs. dam.
DISCUSSION
NEC is a devastating disease of neonates associated with high morbidity and mortality (14). Over the past 15 years, a number of studies have investigated the effects of probiotics in preventing NEC (62). LR17938 inhibits pathogen growth and modulates the immune system. In this study the protective effects of the probiotic LR17938 on the survival, incidence, and severity of NEC in a mouse model further validated our previous observations in a rat NEC model (27, 29). In addition, previous studies indicated that the frequency of gut Foxp3+ Treg cells was decreased in NEC but reverted to normal following LR17938 treatment (27).
Central features of NEC pathophysiology include immaturity of the intestinal barrier and aberrant mucosal immunity, leading to bacterial invasion and markedly exaggerated inflammation in response to bacterial antigens. Supporting evidence includes higher serum and intestinal levels of several cytokines and chemokines during NEC (30, 50). Furthermore, increased TLR signaling activity in the intestine of rats with NEC has been demonstrated (16, 29–31, 52). We believe that LR17938 could prevent NEC via modulation of TLR4 and NF-κB signaling in the intestine (29).
Foxp3+ Treg cells are essential for intestinal immune homeostasis through suppression of innate and adaptive host responses. We proposed that disrupted immune regulation may be involved in perturbing the balance between Treg and Tem cells. CD4+ Tem cells express high levels of CD44, which is involved in Th1-driven inflammation (39), including recirculation of T cells to inflammatory sites and chemotaxis (8). CD44 extracted from Th1 cells was found to bind soluble E-selectin in vitro and cooperated with P-selectin glycoprotein ligand-1 in mediating a rolling interaction between T lymphocytes and the vascular endothelium. A combined absence of CD44 and P-selectin glycoprotein ligand-1 impairs inflammatory T cell recruitment (39). CD4+CD44+ T cells were found to have effector function and phenotype, to show enhanced reactivity to CD44 ligand hyaluronan, to produce inflammatory cytokines such as TNF-α and IL-2, and to be essential for the induction of ileitis in RAG−/− mice (8). Conversely, CD44 deficiency attenuated chronic murine ileitis (8). In addition to expressing CD44, the majority of effector/memory cells express low levels of CD45RB and L-selectin (59). The adoptive transfer of lamina propria CD44highCD62L−CD45RBlow Tem cells into severe combined immunodeficiency mice induced a severe colitis (15). However, it was not clear whether this Tem cell subset in the intestinal mucosa is involved in the pathogenesis of NEC.
On the opposite side of these changes, our previous study with adoptive Treg cells in a rat model of NEC demonstrated the ability of Treg cells to attenuate the development of NEC by inhibiting the activation of dendritic cells and the maturation of naive CD4+CD62L+ into effector CD4+CD62L− cells (11). Our current studies in a mouse model of NEC further confirm the loss of balance between these two T cell subsets in the intestinal mucosa, demonstrating increased intestinal Tem and transitional effector T cells in the face of decreased Foxp3+ Treg cells during NEC (compared with dam-fed controls). Treg cell development and function can be interrupted by a local proinflammatory cytokine milieu (9, 24). During the development of human or experimental NEC, formula feeding or stress may increase levels of endogenous TLR ligands (e.g., heat shock protein) in the intestinal inflamed tissue (20, 22), which, in combination with exogenous TLR ligands (e.g., bacterial lipopolysaccharide), would serve to activate TLRs and TLR-signaling pathways to produce proinflammatory cytokines by intestinal epithelial and immune cells (26, 30).
Finally, we found that LR17938 ingestion produced a “rebound” of intestinal Foxp3+ Treg cells and decreased Tem cells not only in the intestine but also in the MLNs of mice with NEC. This differential modulation of LR17938 on Tem and Treg cells was not observed when animals were treated with the control Lactobacillus strain La DDS, most likely because La DDS has been shown to lack the properties of adherence to epithelial cells, induction of mucin expression by intestinal epithelial cells, inhibition of enteropathogenic Escherichia coli epithelial cell adherence (32), and inhibition of LPS-induced NF-κB activation (29). In contrast, L. reuteri ATCC 55730 (a parent strain of LR17938) has been shown to adhere to Caco-2 human intestinal epithelial cells (37). Recent studies indicated that this L. reuteri strain possesses a gene encoding a protein (MapA) or a collagen-binding protein (CnBP) (49). These findings further support adhesion mechanisms in the probiotic-host interaction. In addition, L. reuteri strains inhibit enteropathogenic E. coli by direct antimicrobial activity attributable to secreted reuterin (54) or to the repression of genes expressed by pathogenic E. coli (21).
We further studied the subset of these Foxp3+ Treg cells based on their expression of Helios. While controversial, it has been suggested that Helios can be a marker of thymus-derived Treg (tTreg) cells, and those lacking Helios would represent peripheral induced Treg (pTreg) cells (10). The tTreg and pTreg cells play nonredundant roles in immune regulation, but their functions are not easy to distinguish, in part because we lack markers to unambiguously discriminate between the two cell types. It has been proposed that only tTreg cells express Helios and neuropilin-1, which are expressed by most Foxp3+ Treg cells in blood and in the thymus (43). We therefore analyzed the expression of Helios within the CD4+Foxp3+ population in the ileum. We found that 75% of CD4+Foxp3+ Treg cells express Helios in the NEC-exposed group treated with probiotic LR17938, approximately the same level as seen in dam-fed controls. This finding indicates that the increased Treg cells in mice fed the probiotic were likely of thymic origin. In the MLNs, ∼70–80% of Treg cells were Helios+ tTreg cells in all studied groups. Our observations may be seen as contrasting with a previous report, in which the intestinal lamina propria Treg cell pool appeared to be enriched in pTreg cells (43). We speculate that the immunological difference might be due to observations in newborn vs. mature mice. Further study of molecules that mediate T cell homing to the gut, including the chemokines (e.g., CCL25), chemokine receptors (e.g., CCR9), integrins, and ligands (e.g., β7-integrin and mucosal addressin cell adhesion molecule-1) (44, 55), is needed in newborn subjects.
Finally, our current studies also showed that breast milk leads to increased intestinal Treg cells, which implies that some factor(s) in breast milk may enhance the immunomodulatory effects of LR17938. It has been known that breast milk contains bioactive milk proteins, which act against the inflammatory process, suggested by in vitro and in vivo studies (6). Breast milk contains casein and whey proteins (including α-lactalbumin, β-lactoglobulin, and osteopontin) that are believed to favor the growth of certain bacterial species (36), whereas whey proteins and IgA directly or indirectly modulate the immune system. Human milk contains up to 109 CFU/ml live bacteria, with a predominance of Bifidobacteria (18). The protective effect of maternal milk is associated with increased production of mucosal IL-10 in the site of injury, which indicates an immunomodulatory activity (12). Maternal milk is the major source of epidermal growth factor for neonates, which plays an important protective role against NEC development (13). Transforming growth factor (TGF)-β2, an immunomodulatory growth factor, is the most dominant form of the TGF-β superfamily in breast milk. The intestinal immune system in preterm neonates is immature, producing remarkably low amounts of endogenous TGF-β and TGF-β receptor (47). The restoration of these low levels of TGF-β through feeding breast milk is pivotal for provision of adequate protection against inflammation. Insulin-like growth factors (IGFs), such as IGF-I and IGF-II, important growth factors in breast milk (56), may initiate intracellular signaling, suppressing apoptosis and stimulating cell proliferation. The interaction of these factors with intraluminal LR17938 during immunomodulation should be further explored.
In summary, our data indicate that 1) NEC is an inflammatory condition with dysregulated immune activation based on increased Tem and decreased Treg cell activities; 2) L. reuteri DSM 17938 during NEC appears to promote Treg cell development and/or migration and to decrease Tem cells to dampen immune activation; and 3) the majority of the Treg cells associated with LR17938 treatment are of the Helios+ subset, which suggests a systemic effect on gut inflammation in NEC wherein thymus-derived circulating Treg cells might be attracted to the injured intestine. L. reuteri may directly affect the expression and activation of the chemoattractant molecules in the intestinal mucosa and/or may indirectly affect these molecules by inducing expression of higher levels of retinoic acid-producing enzymes on gut dendritic cells, which trigger the expression of gut-homing molecules (19). We acknowledge that a prophylactic, not a therapeutic, effect was demonstrated in these studies once the inflammation developed. The question of a therapeutic effect requires further studies. Our results support the concept that probiotic L. reuteri DSM 17938 may represent a useful treatment to prevent NEC. In addition, these T cell subsets might be potential biomarkers and therapeutic targets during intestinal inflammation.
GRANTS
This work was supported in part by the Department of Pediatrics of the University of Texas Health Science Center at Houston Medical School and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-56338, which funds the Texas Medical Center Digestive Diseases Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L., D.Q.T and J.M.R. are responsible for conception and design of the research; Y.L. and N.Y.F. performed the experiments; Y.L. and D.Q.T. analyzed the data; Y.L., D.Q.T. and J.M.R. interpreted the results of the experiments; Y.L. prepared the figures; Y.L. drafted the manuscript; Y.L., D.Q.T. and J.M.R. edited and revised the manuscript; J.M.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Syed S. Hashmi for performing the statistical analysis. We thank Drs. Elizabeth Donnachie and Miguel Escobar (Gulf States Hemophilia Center at the University of Texas) for generously providing access to their BD FACSCalibur and Dr. Eamonn Connolly (Biogaia, Stockholm, Sweden) for providing the probiotic L. reuteri DSM 17938.
REFERENCES
- 1. Afrazi A, Sodhi CP, Good M, Jia H, Siggers R, Yazji I, Ma C, Neal MD, Prindle T, Grant ZS, Branca MF, Ozolek J, Chang EB, Hackam DJ. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol 188: 4543–4557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of T cells by CD44. Commun Integr Biol 3: 508–512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of antigen-experienced T cells: lessons from the quintessential memory marker CD44. Front Immunol 3: 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caplan MS, Sun XM, Hseuh W, Hageman JR. Role of platelet activating factor and tumor necrosis factor-α in neonatal necrotizing enterocolitis. J Pediatr 116: 960–964, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Cetin S, Leaphart CL, Li J, Ischenko I, Hayman M, Upperman J, Zamora R, Watkins S, Ford HR, Wang J, Hackam DJ. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am J Physiol Gastrointest Liver Physiol 292: G1347–G1358, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 45: 1730–1747, 2013 [DOI] [PubMed] [Google Scholar]
- 7. Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev 88 Suppl 1: S41–S49, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Collins CB, Ho J, Wilson TE, Wermers JD, Tlaxca JL, Lawrence MB, Solga M, Lannigan J, Rivera-Nieves J. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology 135: 1993–2002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Da Silva MM, Piccirillo CA. Functional stability of Foxp3+ regulatory T cells. Trends Mol Med 18: 454–462, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Peripheral and thymic foxp3+ regulatory T cells in search of origin, distinction, and function. Front Immunol 4: 253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dingle BM, Liu Y, Fatheree NY, Min J, Rhoads JM, Tran DQ. FoxP3+ regulatory T cells attenuate experimental necrotizing enterocolitis. PLos One 8: e82963, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196: 147–148, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Fujii R, Kanai T, Nemoto Y, Makita S, Oshima S, Okamoto R, Tsuchiya K, Totsuka T, Watanabe M. FTY720 suppresses CD4+CD44highC. Am J Physiol Gastrointest Liver Physiol 291: G267–G274, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol 83: 493–498, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 182: 636–646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gueimonde M, Laitinen K, Salminen S, Isolauri E. Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 92: 64–66, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 205: 2483–2490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hang P, Sangild PT, Sit WH, Ngai HH, Xu R, Siggers JL, Wan JM. Temporal proteomic analysis of intestine developing necrotizing enterocolitis following enteral formula feeding to preterm pigs. J Proteome Res 8: 72–81, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Jelcic I, Hufner E, Schmidt H, Hertel C. Repression of the locus of the enterocyte effacement-encoded regulator of gene transcription of Escherichia coli O157:H7 by Lactobacillus reuteri culture supernatants is LuxS and strain dependent. Appl Environ Microbiol 74: 3310–3314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang P, Siggers JL, Ngai HH, Sit WH, Sangild PT, Wan JM. The small intestine proteome is changed in preterm pigs developing necrotizing enterocolitis in response to formula feeding. J Nutr 138: 1895–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10: 595–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee WT, Vitetta ES. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell Immunol 132: 215–222, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res 11: 113–116, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads M. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLos One 8: e56547, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299: G1087–G1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 302: G608–G617, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21: 81–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52: 827–833, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McNeill L, Cassady RL, Sarkardei S, Cooper JC, Morgan G, Alexander DR. CD45 isoforms in T cell signalling and development. Immunol Lett 92: 125–134, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Moes N, Rieux-Laucat F, Begue B, Verdier J, Neven B, Patey N, Torgerson TT, Picard C, Stolzenberg MC, Ruemmele C, Rings EH, Casanova JL, Piloquet H, Biver A, Breton A, Ochs HD, Hermine O, Fischer A, Goulet O, Cerf-Bensussan N, Ruemmele FM. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology 139: 770–778, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD. Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol 28 Suppl 1: S11–S19, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Mountzouris KC, McCartney AL, Gibson GR. Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr 87: 405–420, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Moussavi M, Adams MC. An in vitro study on bacterial growth interactions and intestinal epithelial cell adhesion characteristics of probiotic combinations. Curr Microbiol 60: 327–335, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Mshvildadze M, Neu J, Mai V. Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutr Rev 66: 658–663, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Nacher M, Blazquez AB, Shao B, Matesanz A, Prophete C, Berin MC, Frenette PS, Hidalgo A. Physiological contribution of CD44 as a ligand for E-selectin during inflammatory T-cell recruitment. Am J Pathol 178: 2437–2446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLos One 6: e17776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pabst O. Trafficking of regulatory T cells in the intestinal immune system. Int Immunol 25: 139–143, 2013 [DOI] [PubMed] [Google Scholar]
- 44. Pabst O, Forster R, Lipp M, Engel H, Arnold HH. NKX2.3 is required for MAdCAM-1 expression and homing of lymphocytes in spleen and mucosa-associated lymphoid tissue. EMBO J 19: 2015–2023, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richardson WM, Sodhi CP, Russo A, Siggers RH, Afrazi A, Gribar SC, Neal MD, Dai S, Prindle T, Jr, Branca M, Ma C, Ozolek J, Hackam DJ. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology 139: 904–17, 917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol 74: 6032–6040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sangild PT, Mei J, Fowden AL, Xu RJ. The prenatal porcine intestine has low transforming growth factor-β ligand and receptor density and shows reduced trophic response to enteral diets. Am J Physiol Regul Integr Comp Physiol 296: R1053–R1062, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, Moelbak L, Buddington RK, Westrom BR, Holst JJ, Burrin DG. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130: 1776–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLos One 6: e18783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T, Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138: 185–196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spencer J, Dillon SB, Isaacson PG, MacDonald TT. T cell subclasses in fetal human ileum. Clin Exp Immunol 65: 553–558, 1986 [PMC free article] [PubMed] [Google Scholar]
- 54. Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14: 166–171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 107: 3447–3454, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Suikkari AM. Insulin-like growth factor (IGF-I) and its low molecular weight binding protein in human milk. Eur J Obstet Gynecol Reprod Biol 30: 19–25, 1989 [DOI] [PubMed] [Google Scholar]
- 57. Swain SL, Bradley LM, Croft M, Tonkonogy S, Atkins G, Weinberg AD, Duncan DD, Hedrick SM, Dutton RW, Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev 123: 115–144, 1991 [DOI] [PubMed] [Google Scholar]
- 58. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184: 3433–3441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tietz W, Hamann A. The migratory behavior of murine CD4+ cells of memory phenotype. Eur J Immunol 27: 2225–2232, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Treszl A, Tulassay T, Vasarhelyi B. Genetic basis for necrotizing enterocolitis—risk factors and their relations to genetic polymorphisms. Front Biosci 11: 570–580, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Veltkamp C, Ruhwald R, Giesem T, Autschbach F, Kaden I, Veltkamp R, Sartor RB, Stremmel W. CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm Bowel Dis 12: 437–446, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 47: 241–248, 2012 [DOI] [PubMed] [Google Scholar]
- 63. Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62: 73–82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weitkamp JH, Rudzinski E, Koyama T, Correa H, Matta P, Alberty B, Polk DB. Ontogeny of FOXP3+ regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr Dev Pathol 12: 443–449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]