Abstract
Background
FMS-like tyrosine kinase 3 (FLT3) is a class III receptor tyrosine kinase involved in hematopoietic progenitor cell development. Mutations of FLT3 have been reported in about a third of patients with acute myeloid leukemia (AML), and inhibitors of FLT3 are of clinical interest. Sorafenib is an orally active multikinase inhibitor with potent activity against FLT3 and the Raf/ERK/MEK kinase pathway.
Methods
We studied the patterns of molecular response and relapse in 18 patients with mutated FLT3 treated with the combination of sorafenib, idarubicin, and cytarabine.
Results
The median follow-up was 9 months. Sixteen patients achieved complete remission (CR), and the other 2 patients achieved CR but lacked platelet recovery for an overall response rate of 100%. Ten patients had their FLT3-mutated clone eradicated, with 6 patients who showed some residual FLT3-mutated cells, and 2 patients who showed persistent FLT3-mutated cells. The elimination of FLT3-mutated population at the time of morphologic CR, however, was not predictive of relapse. After a median follow-up of 9 months (range, 1–16 months), 10 (55%) patients had relapsed, with a median CR duration of 8.8 months (range, 1–9.5 months). By DNA sequencing, there was no evidence of an acquired FLT3 point mutation at the time of relapse in 7 patients tested, which suggested the presence of other mechanisms of sorafenib resistance.
Conclusion
Sorafenib, combined with chemotherapy, is effective in attaining CR, but relapses still occur.
Keywords: Acute Myeloid Leukemia AML, FLT3 mutation, Sorafenib
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia, and its effective therapy remains challenging. Induction with cytarabine and an anthracycline remains the standard of care. New regimens are incorporating target-specific agents to improve both complete remission (CR) and survival rates. These novel agents typically target protein products of mutated genes and balanced translocations, such as upregulated or constitutively activated tyrosine kinases, in leukemia cells.
FMS-like tyrosine kinase-3 (FLT3) is a member of class III receptor tyrosine kinase and is involved in normal hematopoietic progenitor cell development.1–3 It is a membrane-bound receptor with an intrinsic tyrosine kinase domain.4 Autocrine signaling by the FLT3 ligand through wild-type (WT) FLT3 can lead to enhanced kinase activity.5,6 Mutations of FLT3, such as an internal tandem duplication (ITD) or tyrosine kinase domain (TKD) point mutations, occur in >30% of patients with AML and lead to constitutive activation of the FLT3 kinase.6,7 The presence of FLT3-ITD is associated with reduced disease-free and overall survival (OS).7–9 Whether FLT3-TKD has a similar effect on the outcome has been the subject of conflicting reports.10–13
FLT3-ITD occurs in approximately 25% of younger patients with AML, with a variable length of duplicated DNA (between 3 and >400 base pairs in the juxtamembrane domain).14 The juxtamembrane domain is a negative regulatory domain that inhibits the activation loop from adopting the active conformation. Several studies have documented the prognostic impact of these genetic alterations with patients with the ITD having a poor prognosis.7–9,15,16 High mutant to WT allele burden, the number of mutants of different size in the same patient, and the size of DNA insertions, have all been linked to a worse prognosis.17–20
Sorafenib is an oral, small-molecule, multikinase inhibitor that has been approved for the treatment of renal and hepatocellular cancers at a dose of 400 mg twice daily. It inhibits FLT3 and its downstream Raf/ERK/MEK pathway.21 In preclinical studies, it caused dephosphorylation of MEK1/2 and ERK, and induced apoptosis in AML cells.22 This effect was seen preferentially in FLT3-mutated cells compared with cells with WT FLT3 by >1000 –3000-fold. Sorafenib has demonstrated clinical activity in phase I studies in patients with FLT3-ITD AML.23–25
Several potential mechanisms of resistance have been entertained in patients treated with FLT3 inhibitors.26,27 These mechanisms include plasma protein binding, bypass activation of downstream STAT5 and MAP kinase signaling, limited specificity against target FLT3, and secondary TKD mutations that interfere with drug activity.28–32 In in vitro culture studies, exposure of AML cell lines to continuous FLT3 inhibitors (including sorafenib) induces kinase domain mutations that confer resistance.30 The objective of this report was to determine the pattern of molecular response and relapse in patients with previously untreated AML who received induction therapy with the combination of sorafenib and chemotherapy, and to determine potential mechanisms of resistance. An earlier report of this clinical trial, including patients treated on the phase I portion of the study, has been previously published.33 In this report, we focus only on the 18 patients with FLT3 mutation (including 3 patients not included in the previous report) treated on the phase II portion of the study.
Patients and Methods
Patient Eligibility
Patients 18–60 years old with previously untreated AML (based on the World Health Organization [WHO] criteria) were eligible for treatment on this phase II study. Patients 61–65 years old also were eligible if they had a low probability of 8-week mortality with intensive chemotherapy based on adverse risk factors (cytogenetics, ECOG PS [Eastern Cooperative Oncology Group performance status], antecedent hematologic diseases, and organ function).34 All the patients had to have adequate cardiac, renal, and hepatic function, with an ECOG PS of 0, 1, 2, and 3 (left ventricular ejection fraction ≥50%, creatinine ≤ 2.0 mg/dL, bilirubin ≤ 2.0 mg/dL, and liver transaminases < 3 times the institutional upper limit of normal). All the patients signed an informed consent approved by the institutional review board. Only patients with FLT3 mutations (ITD, TKD, or both) were included in this report.
Treatment Regimen
Induction consisted of sorafenib 400 mg orally (p.o.) twice daily for 7 days combined with cytarabine 1.5 g/m2 by continuous intravenous (I.V.) infusion daily for 4 days (patients > 60 years of age received 3 days only) in addition to idarubicin 12 mg/m2 I.V. over 1 hour daily for 3 days. The patients who did not achieve CR after 1 course could receive another induction course. For consolidation, patients in CR received up to 5 cycles of idarubicin 8 mg/m2 I.V. daily for 2 days with cytarabine 0.75 g/m2 I.V. over 24 hours for 3 days in addition to sorafenib 400 mg p.o. twice daily for 28 days. The cycles were repeated every 4–6 weeks based on toxicity and recovery of counts. Patients who completed consolidation received up to 1 year of sorafenib as maintenance therapy unless they underwent stem cell transplantation. The dose of all agents could be reduced during consolidation and maintenance based on the available guidelines related to the various adverse effects.
Response Criteria
CR was defined by the presence of <5% blasts in the bone marrow (BM) with >1 × 109/L neutrophils and >100 × 109/L platelets in the peripheral blood. CR duration was calculated from the time of CR until relapse. Relapse was defined by the recurrence of > 5% blasts in BM aspirate not related to recovery or the development of extramedullary disease. OS was calculated from the time of diagnosis until death.
FLT3 Mutation Detection
FLT3-ITD and codon 835/836 TKD mutation status were determined in DNA from initial, postinduction, follow-up, and relapsed unsorted BM aspirate samples by a polymerase chain reaction (PCR) based method, with an analytical sensitivity of 1%–2% mutation-bearing cells. FLT3 allele burden was determined by the ratio of the area under the mutated and unmutated PCR amplicon peaks as detected software calls after capillary electrophoresis on a 3100 or 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). A manual 400-cell differential performed on smears and multicolor flow cytometry on aspirate samples was used to track the levels of residual leukemia blasts. When leukemic blasts were detected, ratios of FLT3 mutation were normalized to blast count. Relapsed BM samples (or skin biopsy in 1 case) were analyzed for kinase domain mutations in exons 16, 17, 20, and 21 by using PCR-based Sanger sequencing of genomic DNA (sensitivity level of 20% mutation-bearing cells). In diagnostic BM samples, exon 1 and 2 KRAS and NRAS point mutations were assessed by using PCR-based pyrosequencing of genomic DNA (sensitivity level of 5% mutation-bearing cells) and NPM1 exon 12 mutation and/or duplications were assessed by using a PCR-based capillary electrophoresis method (sensitivity level of 2% mutation-bearing cells) as previously described.35,36
Statistical Analysis
The main objective of this trial was to provide an assessment of the efficacy of adding sorafenib to combination chemotherapy. Survival was calculated by the Kaplan-Meier method, and different categories were compared by the log-rank test. Differences in subgroups by different covariates were evaluated by using the χ2 test for nominal values (high vs. low FLT3 mutation burden) and the Mann-Whitney U test and Fisher exact test for continuous variables (FLT3 burden and outcome).
Results
Patient Characteristics
From October 2007 to September 2009, 64 patients with AML were enrolled into this phase II clinical study; 18 had mutated FLT3 and are subjects of this report (Table 1). These patients are the patients who participated in the phase II portion of the study previously reported, including 3 patients not reported in the previous publication.33 Their median age was 54 years (range, 20–65 years); 10 were women. Fifteen patients had FLT3-ITD, 2 had FLT3-TKD, and one had mutations at both sites. Five patients had a low FLT3 mutation burden (ie, blast-normalized mutation ratio of <25%) consistent with presence in only a subset of the blast population. Two patients also had RAS mutations (both were NRAS exon 12), whereas 5 others had NPM1 mutations. Most patients (83%) had a PS of 0 and 1 compared with only 3 (17%) patients with a PS of 2 and 3. Ten (55%) of 18 patients had diploid cytogenetics, and 8 had various abnormalities (deletions or additions but no translocations). The most common FAB subtype was M1 in 8 (44%) patients and M4 in 4 (22%) of them.
Table 1.
Patient Characteristics
| Patient Characteristics | |
|---|---|
| Median (Range) Age (y) | 54 (20–65) |
| FLT3 Mutation Type | |
| ITD | 15 (83%) |
| TKD | 2 (11%) |
| ITD/TKD | 1 (6%) |
| FLT3 Mutation Burden | |
| Higha | 13 (72%) |
| Low | 5 (28%) |
| Eastern Cooperative Oncology Group Performance Status | |
| 0 | 5 (28%) |
| 1 | 10 (55%) |
| 2 | 2 (11%) |
| 3 | 1 (6%) |
| Median (Range) White Blood Cell Count (×109/L) | 18 (1.4–196) |
| Median (Range) Hemoglobin (g/dL) | 8.5 (6.9–10.8) |
| Median (Range) Platelet (×109/L) | 51.5 (18–189) |
| Cytogenetics | |
| Diploid | 10 (55%) |
| Other | 8 (45%) |
≥25% blast-normalized mutation ratio (see Methods section).
Response and Outcome
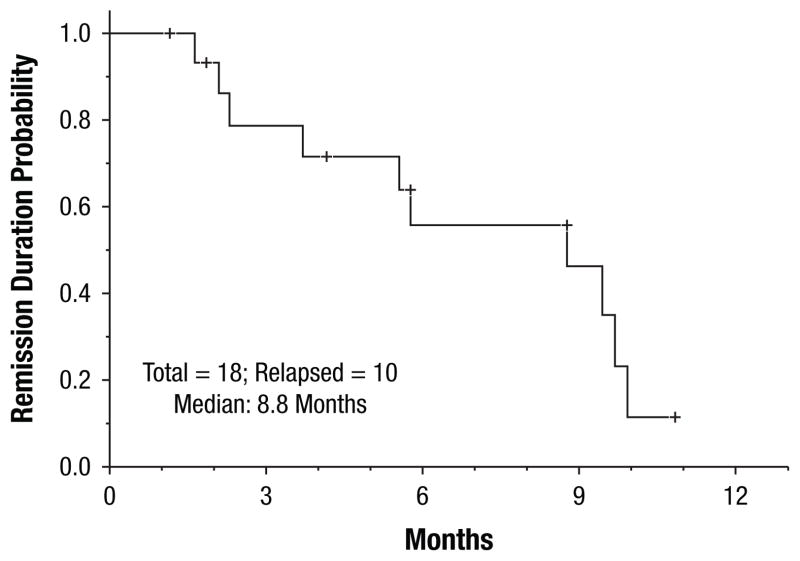
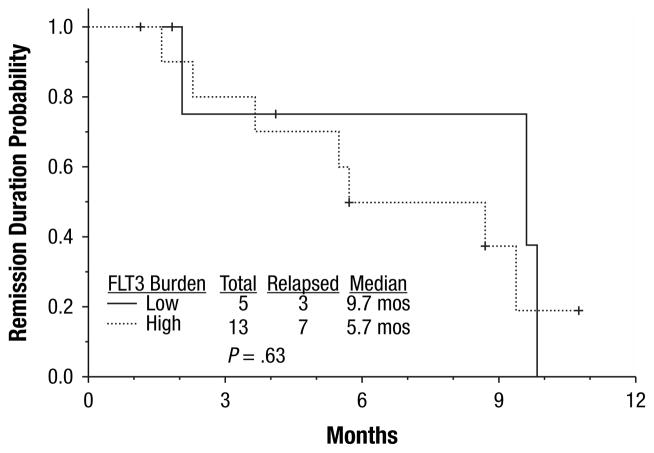
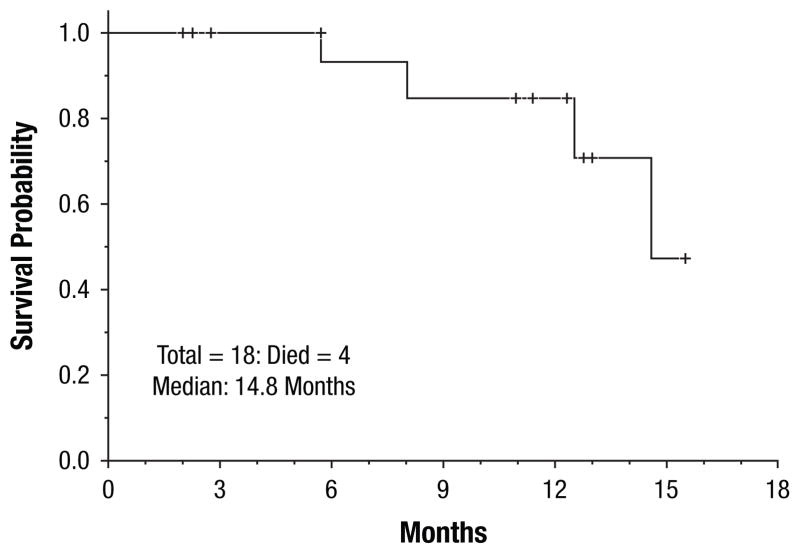
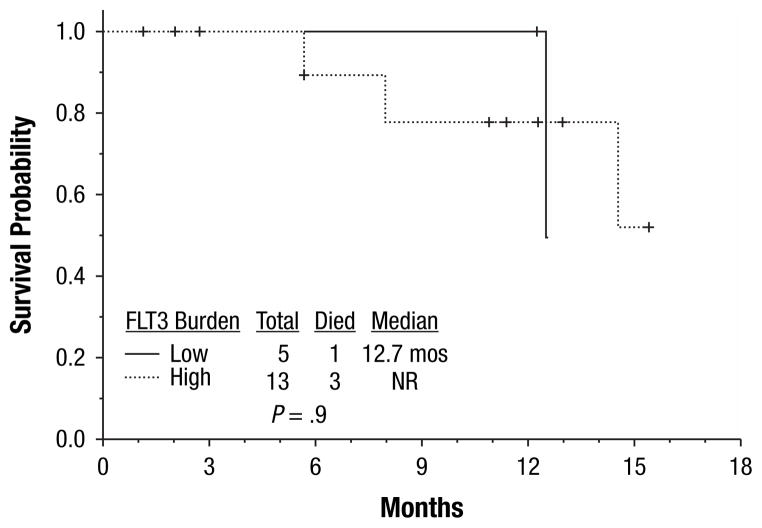
All 18 (100%) patients achieved CR or CR but lacked platelet recovery (CRp) (16 CR and 2 CRp). All but 2 patients responded after one cycle of induction; 2 patients achieved CRp after 2 cycles of induction. After a median follow-up of 9 months (range, 1–16 months), 10 (55%) patients had relapsed, with a median CR duration of 8.8 months (range, 1–9.5 months) The CR duration was independent of FLT3 mutation burden at diagnosis (P = .63) (Figures 1, 2). Twelve (67%) patients proceeded to allogeneic stem cell transplantation; 5 in CR1, and 7 after relapse. Four patients died (3 of whom had matched unrelated allogeneic donor stem cell transplantation in either CR1 [n = 1] or CR2 [n = 2]), with a median OS of 14.8 months (range, 1–16 months). Survival duration was independent of FLT3 mutation burden at diagnosis (P = .9) (Figures 3, 4). Three of the 4 deceased patients had a high mutant allele burden.
Figure 1.
Complete Remission Duration
Figure 2.
Complete Remission Duration for Patients with High- and Low-FLT3 Mutation Burden
Figure 3.
Overall Survival
Figure 4.
Overall Survival for Patients with High and Low FLT3 Mutation Burden
FLT3 Mutation Levels After Initial Therapy
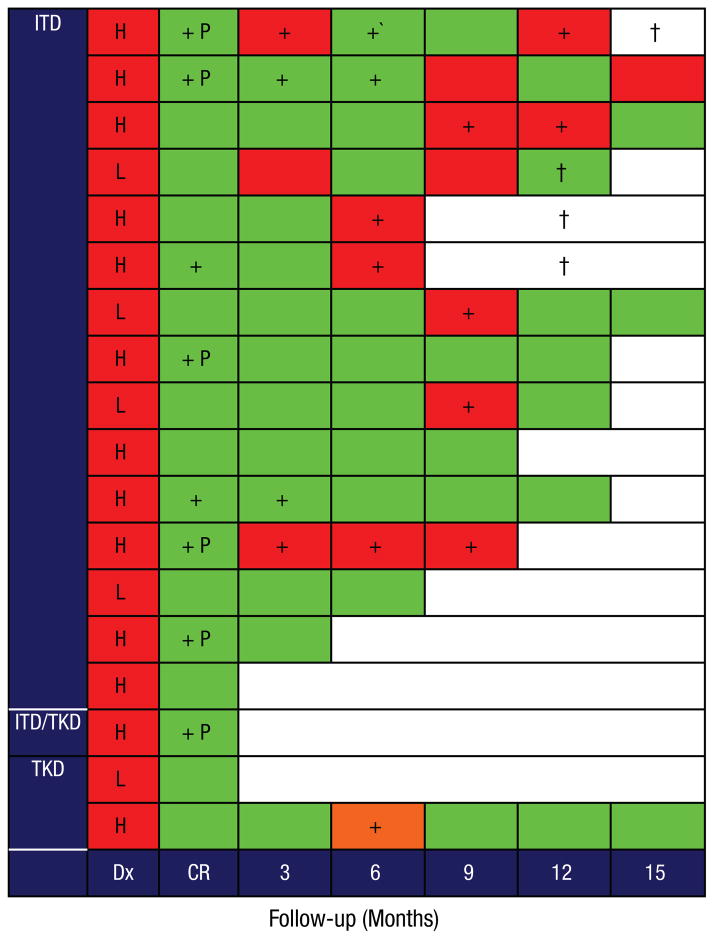
Samples from all the patients after induction (approximately day 30) were collected and analyzed for molecular response (Figure 5). Ten (56%) patients showed no evidence of the mutated FLT3 clone by PCR analysis, whereas 6 (33%) showed partial regression of the FLT3 mutated clone, and 2 (11%) patients had persistent molecular evidence of the disease, with no preferential regression of the mutated clone. The degree of regression of the mutated FLT3 clone did not predict relapse (the relapse rate in patients with complete regression was 6/10 [60%] vs. 5/8 [63%] in the rest). We also compared the regression of FLT3 mutated clone in the study group with 67 patients with FLT3-ITD treated at our institution with standard anthracycline-cytarabine regimens without sorafenib. Overall, partial or complete FLT3 regression after the first cycle of therapy was evident in 89% of the study patients compared with 6% among patients treated with regimens that lacked sorafenib (P < .001, χ2 test).
Figure 5.
Follow-up, Morphologic and Molecular Relapse
Red Color = Positive for Leukemia by Flow Cytometry (FCM) and/or Morphology; Green Color = Negative for Leukemia by FCM and/or Morphology; Orange Color = Extramedullary Acute Myeloid Leukemia (AML); H = High Mutant FLT3 Burden, L = Low Mutant FLT3 Burden, † = Deceased, + = Positive Minimal Residual Disease by Polymerase Chain Reaction for Mutant FLT3; P = Partial Regression of Mutant FLT3 clone
Pattern of FLT3 Mutation After Relapse
Nine of the 10 patients who relapsed had recurrence of FLT3 mutated clone, although one had no evidence of mutated FLT3 in the sample obtained at relapse. The relapse rate was independent of the level of FLT3 mutation at diagnosis (P = .63). In 7 patients at relapse, sequencing of the TKD domain (which covers exons 16, 17, 20, and 21) revealed no additional mutation in FLT3 in BM specimens (n = 6) or skin biopsy (n = 1).
Discussion
Patients with FLT3-mutated AML have a worse prognosis than those with WT FLT3. Predictors of outcome such as the length of the tandem duplication mutation as well as the ratio of WT to mutant allele have been reported.17–20,37 Several novel agents targeted against FLT3 kinase have been evaluated in clinical trials.33,38–44 An improved response in patients with FLT3-mutated disease has been reported in these studies, but further studies are needed to demonstrate any effect on survival. Combining these agents with chemotherapy is preferred because a synergistic effect has been shown in in vitro studies.45 This inhibitory effect is present in both WT- and mutant-FLT3 cells in vitro but varies with dependence on FLT3 signaling.46
In this study, we observed an excellent response, with all patients achieving CR or CRp, which suggests a beneficial on-target effect. However, the majority of the patients relapsed suggesting the limited efficacy of the regimen to produce long-term remissions. Sorafenib was administered in induction and consolidation, and for maintenance for about 1 year. We were unable to identify any secondary mutations that, in 6 of 10 patients who relapsed, would confer resistance to sorafenib as has previously been reported and as has been demonstrated for BCR-ABL inhibitors,26,29,30,32 which suggests that other mechanisms of resistance may exist, although there are several limitations associated with this conclusion. For instance, when looking for secondary mutations, we only sequenced 4 exons and not the whole FLT3 gene. In addition, no pharmacokinetic data were collected in this phase II trial to demonstrate that adequate serum sorafenib levels were achieved. Finally, the small number of patients whose samples were sequenced further limits the validity of any conclusions.
The lack of correlation of molecular response with relapse makes any conclusions on the role of sorafenib and other kinase inhibitors in this disease difficult. For instance, the presence of minimal residual disease by PCR did not predict for relapse. Two patients relapsed with WT-FLT3 AML, which raises the possibility of clonal evolution (vs. selective inhibition of the mutated clone and the appearance of less competing clones). Achieving maximal or partial regression of the mutated clone did not predict relapse because 3 of 7 patients who achieved partial regression continued to be disease free until the date of this article. Monocytic phenotype (M4, M5) did not show any worse outcome compared with other FAB subtypes (3/5 patients were disease free) that did not support the report by Koh et al,15 which suggests clinical significance of FLT3 in monocytic subtype only.
In conclusion, induction therapy with sorafenib, idarubicin, and cytarabine is effective in attaining remission and in reducing the mutated clone in patients with mutated FLT3 but does not universally eradicate it. Continuous therapy with sorafenib in induction may be beneficial in further reducing the leukemic clone; however, the extent of suppression of the clone is not predictive of relapse. No secondary FLT3 mutations were found in the evaluated patients at relapse, which suggests that factors other than mutations that confer resistance to sorafenib may be important.
Footnotes
Disclosure
F. Ravandi received research support and honoraria from Bayer/Onyx, and is a member of the advisory board of Bayer/Onyx; J. Cortes received research support from Novartis, Bristol Myers Squibb, and Wyeth. H. Kantargian received research support from Novartis and Bristol Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravandi F, Talpaz M, Estrov Z. Modulation of cellular signaling pathways: prospects for targeted therapy in hematological malignancies. Clin Cancer Res. 2003;9:535–50. [PubMed] [Google Scholar]
- 2.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–9. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 4.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 5.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–74. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 7.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 8.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 9.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters—an analysis of 3082 patients. Blood. 2008;111:2527–37. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 11.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–9. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–70. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 13.Mead AJ, Gale RE, Hills RK, et al. Conflicting data on the prognostic significance of FLT3/TKD mutations in acute myeloid leukemia might be related to the incidence of biallelic disease. Blood. 2008;112:444–5. doi: 10.1182/blood-2008-04-150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 15.Koh Y, Park J, Ahn KS, et al. Different clinical importance of FLT3 internal tandem duplications in AML according to FAB classification: possible existence of distinct leukemogenesis involving monocyte differentiation pathway. Ann Hematol. 2009;88:1089–97. doi: 10.1007/s00277-009-0733-7. [DOI] [PubMed] [Google Scholar]
- 16.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–92. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 17.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–61. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 19.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–84. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 20.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–6. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 23.Delmonte J, Jr, Kantarjian HM, Andreeff M, et al. Update of a phase I study of sorafenib in patients with refractory/relapsed acute myeloid leukemia or high-risk myelodysplastic syndrome. ASH Annual Meeting Abstracts. 2007;110:893. [Google Scholar]
- 24.Mori S, Cortes J, Kantarjian H, Zhang W, Andreef M, Ravandi F. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–55. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–71. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 26.Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12:8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravandi F, Jilani I, Estey E, et al. Soluble phosphorylated fms-like tyrosine kinase III. FLT3 protein in patients with acute myeloid leukemia (AML) Leuk Res. 2007;31:791–7. doi: 10.1016/j.leukres.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109:1643–52. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagrintseva K, Geisenhof S, Kern R, et al. FLT3-ITD-TKD dual mutants associated with AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L) Blood. 2005;105:3679–85. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 30.Williams A, Nguyen B, Levis M, Brown P, Small D. Mutations in FLT3/ITD produce varying levels of resistance to FLT3 tyrosine kinase inhibitors. ASH Annual Meeting Abstracts. 2009;114:3776. [Google Scholar]
- 31.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 32.Bagrintseva K, Schwab R, Kohl TM, et al. Mutations in the tyrosine kinase domain of FLT3 define a new molecular mechanism of acquired drug resistance to PTK inhibitors in FLT3-ITD-transformed hematopoietic cells. Blood. 2004;103:2266–75. doi: 10.1182/blood-2003-05-1653. [DOI] [PubMed] [Google Scholar]
- 33.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–62. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 35.Lin P, Jones D, Medeiros LJ, Chen W, Vega-Vazquez F, Luthra R. Activating FLT3 mutations are detectable in chronic and blast phases of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am J Clin Pathol. 2006;126:530–3. doi: 10.1309/JT5BE2L1FGG8P8Y6. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Jones D, Medeiros LJ, Luthra R, Lin P. Acute myeloid leukaemia with FLT3 gene mutations of both internal tandem duplication and point mutation type. Br J Haematol. 2005;130:726–8. doi: 10.1111/j.1365-2141.2005.05666.x. [DOI] [PubMed] [Google Scholar]
- 37.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–9. [PubMed] [Google Scholar]
- 38.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 39.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–70. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 40.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for flt3 mutant aml patients in first relapse. ASH Annual Meeting Abstracts. 2009;114:788. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone RM, Fischer T, Paquette R, et al. A phase 1b study of midostaurin (PKC412) in combination with daunorubicin and cytarabine induction and high-dose cytarabine consolidation in patients under age 61 with newly diagnosed de novo acute myeloid leukemia: overall survival of patients whose blasts have FLT3 mutations is similar to those with wild-type FLT3. ASH Annual Meeting Abstracts. 2009;114:634. [Google Scholar]
- 42.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 43.O’Farrell AM, Yuen HA, Smolich B, et al. Effects of SU5416, a small molecule tyrosine kinase receptor inhibitor, on FLT3 expression and phosphorylation in patients with refractory acute myeloid leukemia. Leuk Res. 2004;28:679–89. doi: 10.1016/j.leukres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Yee KW, Schittenhelm M, O’Farrell AM, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104:4202–9. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- 45.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–50. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 46.Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]