Abstract
Background
In a single-center study published more than a decade ago involving patients presenting to the emergency department with severe sepsis and septic shock, mortality was markedly lower among those who were treated according to a 6-hour protocol of early goal-directed therapy (EGDT), in which intravenous fluids, vasopressors, inotropes, and blood transfusions were adjusted to reach central hemodynamic targets, than among those receiving usual care. We conducted a trial to determine whether these findings were generalizable and whether all aspects of the protocol were necessary.
Methods
In 31 emergency departments in the United States, we randomly assigned patients with septic shock to one of three groups for 6 hours of resuscitation: protocol-based EGDT; protocol-based standard therapy that did not require the placement of a central venous catheter, administration of inotropes, or blood transfusions; or usual care. The primary end point was 60-day in-hospital mortality. We tested sequentially whether protocol-based care (EGDT and standard-therapy groups combined) was superior to usual care and whether protocol-based EGDT was superior to protocol-based standard therapy. Secondary outcomes included longer-term mortality and the need for organ support.
Results
We enrolled 1341 patients, of whom 439 were randomly assigned to protocol-based EGDT, 446 to protocol-based standard therapy, and 456 to usual care. Resuscitation strategies differed significantly with respect to the monitoring of central venous pressure and oxygen and the use of intravenous fluids, vasopressors, inotropes, and blood transfusions. By 60 days, there were 92 deaths in the protocol-based EGDT group (21.0%), 81 in the protocol-based standard-therapy group (18.2%), and 86 in the usual-care group (18.9%) (relative risk with protocol-based therapy vs. usual care, 1.04; 95% confidence interval [CI], 0.82 to 1.31; P = 0.83; relative risk with protocol-based EGDT vs. protocol-based standard therapy, 1.15; 95% CI, 0.88 to 1.51; P = 0.31). There were no significant differences in 90-day mortality, 1-year mortality, or the need for organ support.
Conclusions
In a multicenter trial conducted in the tertiary care setting, protocol-based resuscitation of patients in whom septic shock was diagnosed in the emergency department did not improve outcomes. (Funded by the National Institute of General Medical Sciences; ProCESS ClinicalTrials.gov number, NCT00510835.)
There are more than 750,000 cases of severe sepsis and septic shock in the United States each year.1 Most patients who present with sepsis receive initial care in the emergency department, and the short-term mortality is 20% or more.2,3 In 2001, Rivers et al. reported that among patients with severe sepsis or septic shock in a single urban emergency department, mortality was significantly lower among those who were treated according to a 6-hour protocol of early goal-directed therapy (EGDT) than among those who were given standard therapy (30.5% vs. 46.5%).4 On the basis of the premise that usual care lacked aggressive, timely assessment and treatment, the protocol for EGDT called for central venous catheterization to monitor central venous pressure and central venous oxygen saturation (Scvo2), which were used to guide the use of intravenous fluids, vasopressors, packed red-cell transfusions, and dobutamine in order to achieve prespecified physiological targets. In the decade since the publication of that article, there have been many changes in the management of sepsis, raising the question of whether all elements of the protocol are still necessary.5–7
To address this question, we designed a multicenter trial comparing alternative resuscitation strategies in a broad cohort of patients with septic shock. Specifically, we tested whether protocol-based resuscitation was superior to usual care and whether a protocol with central hemodynamic monitoring to guide the use of fluids, vasopressors, blood transfusions, and dobutamine was superior to a simpler protocol that did not include these elements.
Methods
Study Oversight
We conducted the multicenter, randomized Protocolized Care for Early Septic Shock (ProCESS) trial at 31 hospitals in the United States. The institutional review board at the University of Pittsburgh and at each other participating site approved the registered study protocol, which is available with the full text of this article at NEJM.org. The National Institute of General Medical Sciences funded the study and convened an independent data and safety monitoring board (see the Supplementary Appendix, available at NEJM.org). The Scvo2 monitoring equipment for the study was loaned to the sites by Edwards Lifesciences, but the company had no other role in the study. Study coordinators at each site entered data into a secure Web-based data-collection instrument. The University of Pittsburgh Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center managed all the data and generated blinded and un-blinded reports for the data and safety monitoring board. We reported the statistical analysis plan before the data were unblinded.8 The clinical coordinating team and investigators at the participating sites remained unaware of the study-group outcomes until the data were locked in December 2013. The writing committee vouches for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Sites and Patients
All the participating sites were academic hospitals with more than 40,000 emergency department visits yearly. To be eligible, the study sites had to use the measurement of serum lactate levels as the method for screening for cryptogenic shock and had to adhere to the Surviving Sepsis Campaign guidelines9,10 for nonresuscitation aspects of care but could have no routine resuscitation protocols for septic shock and could not routinely use continuous Scvo2 catheters. We recruited patients in the emergency department in whom sepsis was suspected according to the treating physician, who were at least 18 years of age, who met two or more criteria for systemic inflammatory response syndrome11 (see the Methods section in the Supplementary Appendix), and who had refractory hypotension or a serum lactate level of 4 mmol per liter or higher. We defined refractory hypotension as a systolic blood pressure that either was less than 90 mm Hg or required vasopressor therapy to maintain 90 mm Hg even after an intravenous fluid challenge. We initially required the fluid challenge to be 20 ml or more per kilogram of body weight, administered over the course of 30 minutes, but in April 2010, we simplified the requirement to a challenge of 1000 ml or more administered over the course of 30 minutes. Patients did not have to be in shock on arrival in the emergency department but had to be enrolled in the study in the emergency department within 2 hours after the earliest detection of shock and within 12 hours after arrival. The exclusion criteria are listed in the Methods section in the Supplementary Appendix. All patients or their legally authorized representatives provided written informed consent. Randomization was performed with the use of a centralized Web-based program in variable block sizes of 3, 6, or 9, with stratification according to site and race.
Study Interventions
We randomly assigned patients, in a 1:1:1 ratio, to one of three groups: protocol-based EGDT, protocol-based standard therapy, or usual care. The same trained and dedicated physician-led team implemented both the protocol-based EGDT and the protocol-based standard-therapy interventions. The team consisted of at least one available physician who was trained in the protocolguided resuscitation interventions, a study coordinator who monitored adherence to protocol instructions and provided timed prompts, and a bedside nurse. All study physicians were trained in emergency medicine or critical care medicine and had completed a Web-based certification examination. The protocol-based care began in the emergency department but could be continued elsewhere. Details regarding the training and conduct of the personnel are provided in the Methods section in the Supplementary Appendix. In cases in which a team physician was the bedside provider before enrollment, care was transferred to a nonstudy physician before enrollment.
For patients randomly assigned to protocol-based EGDT, the resuscitation team followed the protocol outlined in Figure S1 in the Supplementary Appendix, which mimics that used by Rivers et al.4 The protocol prompted placement of a central venous catheter to monitor pressure and Scvo2 and to administer intravenous fluids, vasopressors, dobutamine, or packed red-cell transfusions, as directed. We did not require placement of an arterial catheter for blood-pressure monitoring. The protocol in our study, like the protocol in the study by Rivers et al., specified the amount and timing, but not the type, of resuscitation fluid. Similarly, the protocol in our study specified thresholds for vasopressor use but not the specific choice of vasopressor. The protocol guided only resuscitation, with all other aspects of care, including the choice of antimicrobial agents, given at the discretion of the treating physician.
Protocol-based standard therapy also used a team approach with a set of 6-hour resuscitation instructions, but the components were less aggressive than those used for protocol-based EGDT (Fig. S2 in the Supplementary Appendix). ProCESS investigators designed the protocol-based standard-therapy approach on the basis of a review of the literature, two independent surveys of emergency physician and intensivist practice worldwide,5,12 and consensus feedback from investigators. Protocol-based standard therapy required adequate peripheral venous access (with placement of a central venous catheter only if peripheral access was insufficient) and administration of fluids and vasoactive agents to reach goals for systolic blood pressure and shock index (the ratio of heart rate to systolic blood pressure) and to address fluid status and hypoperfusion, which were assessed clinically at least once an hour. In contrast to the triggers in the EGDT protocol, protocol-based standard therapy recommended packed red-cell transfusion only if the hemoglobin level was less than 7.5 g per deciliter. The protocol for standard therapy mandated administration of fluids until the team leader decided that the patient’s fluids were replete. The standard-therapy protocol, like the EGDT protocol, did not specify the type of fluid or vasopressor and did not specify nonresuscitation aspects of care, which were provided by the treating physician. We assessed adherence to the EGDT and standard-therapy protocols using an algorithm that screened for decision prompts and actions at 2, 4, and 6 hours (Fig. S3 and S4 in the Supplementary Appendix).
For patients in the usual-care group, the bedside providers directed all care, with the study coordinator collecting data but not prompting any actions. Lead investigators at a site could not serve as the bedside treating physician for patients in the usual-care group.
Outcome Measures
The primary outcome of the study was the rate of in-hospital death from any cause at 60 days. Secondary mortality outcomes included the rate of death from any cause at 90 days and cumulative mortality at 90 days and 1 year. Other outcomes included the duration of acute cardiovascular failure (defined as the duration of the need for vasopressors), acute respiratory failure, and acute renal failure (defined as the duration of mechanical ventilation or dialysis during the acute hospitalization, truncated at 60 days, in patients who had not had a long-term need for ventilation or dialysis before enrollment); the duration of the stay in the hospital and intensive care unit; and hospital discharge disposition (i.e., discharge to a long-term or other acute care facility, a nursing home, a private home, or other). We collected information on serious adverse events using standard federal guidelines.13
Statistical Analysis
We analyzed all data according to the intention-to-treat principle. For the primary outcome, our design tested sequentially whether protocol-based resuscitation (EGDT or standard therapy) was superior to usual care and, if it was, whether protocol-based EGDT was superior to protocol-based standard therapy. We initially calculated that with a sample of 1950 patients, the study would have at least 80% power to detect a reduction in mortality of 6 to 7 percentage points, at an alpha level of 0.05 for both hypotheses, assuming mortality of 30 to 46% with usual care; interim analyses were planned after 650 patients and 1300 patients had been enrolled. The trial did not meet the stopping criteria at the first planned interim analysis (after the enrollment of 650 patients). Before the second interim analysis, we observed that the overall mortality was approximately 20%, which was much lower than anticipated but consistent with the results of a recent study involving similar patients.14 After consultation with the data and safety monitoring board and the National Institute of General Medical Sciences, and with the group assignments still concealed, we calculated that we would need to enroll a total of 1350 patients to preserve the same power for the same absolute risk reduction.
After spending 0.0005 alpha for the first interim analysis, and after recalculation of the sample size (which removed the requirement for a second interim analysis), the alpha level required for the sequential hypotheses was 0.0494, with no adjustment for multiple testing. We tested for between-group differences in the primary outcome using Fisher’s exact test. In the event that protocol-based care (EGDT and standard therapy combined) was not superior to usual care, all other analyses were to be specified as secondary. Because of possible site heterogeneity, we also conducted a secondary analysis using a generalized linear mixed model in which we allowed for a random effect of study site, with treatment group as a covariate; assessed significance with the use of type 3 tests; and used compound symmetry for the covariance structure.
For other end points, we used Fisher’s exact test for categorical outcomes and an analysis of variance for continuous outcomes. For survival analyses, we generated Kaplan–Meier estimates, assessed between-group differences using the log-rank test, and expressed the data as cumulative mortality curves. In prespecified subgroup analyses, we used the Breslow–Day test to assess interactions between treatment assignment and subgroups defined according to age, sex, race, source of infection, and enrollment criterion (refractory hypotension or elevated serum lactate level). We also conducted post hoc subgroup analyses according to thirds of values for the Acute Physiology and Chronic Health Evaluation (APACHE) II score, for the baseline serum lactate level, and for the time from detection of shock until randomization, using logistic regression to test for an interaction between treatment assignment and subgroups. Unless otherwise specified, analyses are for tests of differences across the three study groups, with P values of less than 0.05 considered to indicate statistical significance. We used SAS software, version 9.3, for all analyses.
Results
Patients
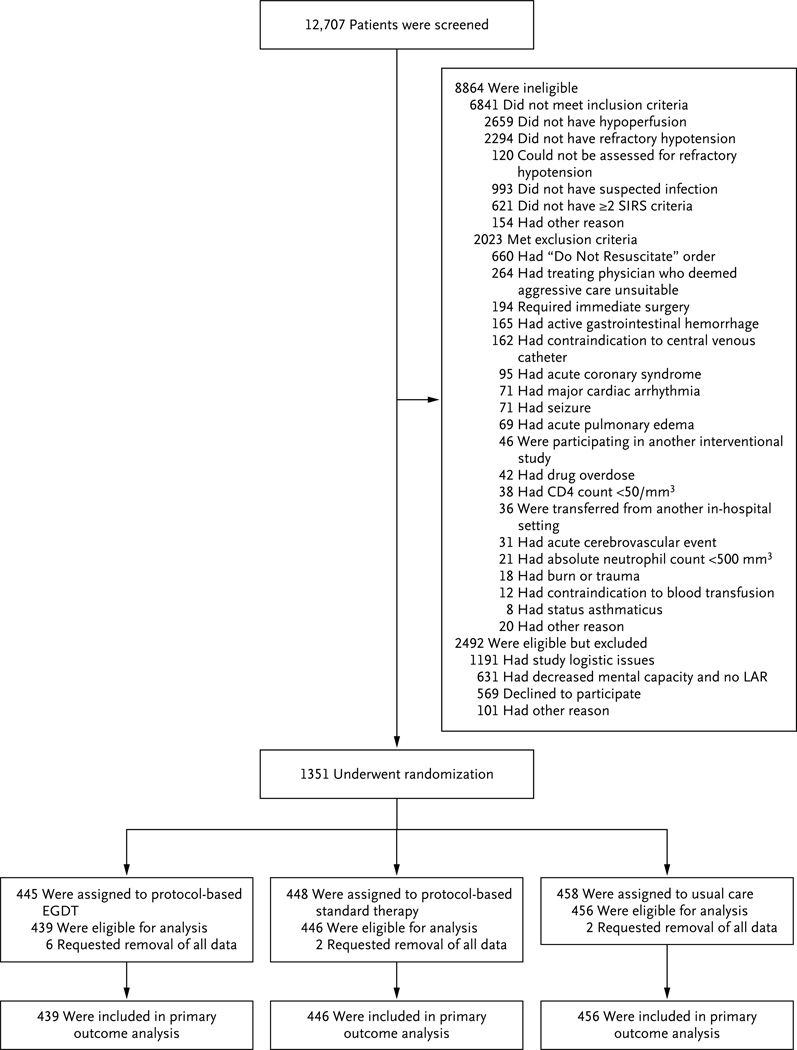
From March 2008 through May 2013, we enrolled 1351 patients (Fig. 1, and Fig. S5 in the Supplementary Appendix). Ten patients who provided informed consent later requested complete withdrawal from the study, leaving a final cohort of 1341 patients for the analysis: 439 in the protocol-based EGDT group, 446 in the protocol-based standard-therapy group, and 456 in the usual-care group. The three groups were well matched at baseline with respect to demographic and clinical characteristics, as well as the care received before randomization (Table 1, and Tables S1, S2, and S4 in the Supplementary Appendix).
Figure 1. Screening, Randomization, and Follow-up.
EGDT denotes early goal-directed therapy, LAR legally authorized representative, and SIRS systemic inflammatory response syndrome.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Protocol-Based EGDT (N = 439) |
Protocol-Based Standard Therapy (N = 446) |
Usual Care (N = 456) |
|---|---|---|---|
| Age — yr† | 60±16.4 | 61±16.1 | 62±16.0 |
| Male sex — no. (%) | 232 (52.8) | 252 (56.5) | 264 (57.9) |
| Residence before admission — no. (%)‡ | |||
| Nursing home | 64 (14.6) | 72 (16.1) | 73 (16.0) |
| Other | 373 (85.0) | 373 (83.6) | 382 (83.8) |
| Charlson comorbidity score§ | 2.6±2.6 | 2.5±2.6 | 2.9±2.6 |
| Source of sepsis — no. (%) | |||
| Pneumonia | 140 (31.9) | 152 (34.1) | 151 (33.1) |
| Urinary tract infection | 100 (22.8) | 90 (20.2) | 94 (20.6) |
| Intraabdominal infection | 69 (15.7) | 57 (12.8) | 51 (11.2) |
| Infection of unknown source | 57 (13.0) | 47 (10.5) | 66 (14.5) |
| Skin or soft-tissue infection | 25 (5.7) | 33 (7.4) | 38 (8.3) |
| Catheter-related infection | 11 (2.5) | 16 (3.6) | 11 (2.4) |
| Central nervous system infection | 3 (0.7) | 3 (0.7) | 4 (0.9) |
| Endocarditis | 1 (0.2) | 3 (0.7) | 3 (0.7) |
| Other | 28 (6.4) | 31 (7.0) | 26 (5.7) |
| Determined after review not to have infection | 5 (1.1) | 14 (3.1) | 12 (2.6) |
| Positive blood culture — no. (%) | 139 (31.7) | 126 (28.3) | 131 (28.7) |
| APACHE II score¶ | 20.8±8.1 | 20.6±7.4 | 20.7±7.5 |
| Entry criterion — no. (%) | |||
| Refractory hypotension | 244 (55.6) | 240 (53.8) | 243 (53.3) |
| Hyperlactatemia‖ | 259 (59.0) | 264 (59.2) | 277 (60.7) |
| Physiological variables | |||
| Systolic blood pressure — mm Hg | 100.2±28.1 | 102.1±28.7 | 99.9±29.5 |
| Serum lactate — mmol/liter** | 4.8±3.1 | 5±3.6 | 4.9±3.1 |
| Time to randomization — min | |||
| From arrival in the emergency department†† | 197±116 | 185±112 | 181±97 |
| From meeting entry criteria | 72±77 | 66±38 | 69±45 |
Plus–minus values are means ±SD. There were no significant differences in baseline characteristics across groups (P values range from 0.10 to 0.96). EGDT denotes early goal-directed therapy.
Information on age was missing for one patient in the usual-care group.
Information on residence before admission was missing for four patients. The category of nursing home included personal-care homes, skilled or unskilled assisted-living facilities, and extended-care facilities.
The Charlson comorbidity index15 measures the effect of coexisting conditions on mortality, with scores ranging from 0 to 33 and higher scores indicating a greater burden of illness.
Scores on the Acute Physiology and Chronic Health Evaluation (APACHE) II range from 0 to 71, with higher scores indicating greater severity of illness.
Hyperlactatemia was defined as a serum lactate level of 4 mmol per liter or higher. The serum lactate level was higher than 2 mmol per liter in 346 patients in the protocol-based EGDT group (78.8%), 340 in the protocol-based standard-therapy group (76.2%), and 359 in the usual-care group (78.7%).
Data on the baseline serum lactate level were available for 95.5% of the patients overall (1281 of 1341 patients).
Not all patients were eligible at the time of arrival in the emergency department.
Adherence to the Protocol
Adherence to the protocol was high in both protocol-based groups. At 6 hours, incomplete adherence was recorded in 48 of 404 patients in the EGDT group (11.9%) and 19 of 435 patients in the standard-therapy group (4.4%) who could be evaluated (Table S3 in the Supplementary Appendix). In most of the patients who had been randomly assigned to EGDT, a central venous catheter for monitoring of Scvo2 was placed promptly (Fig. S6A in the Supplementary Appendix). The reasons for failure to place a central venous catheter, which occurred in 30 of the 439 patients in that group (6.8%), included technical difficulties (10 patients), refusal by the treating clinician (9) or patient (5), the need for emergency surgery (1), and death (1); no reason was provided in the case of 4 patients). The mean (±SD) Scvo2 after catheterization was 71±13%. Although placement of central venous catheters was not required for patients in the protocol-based standard-therapy group or the usual-care group, central venous catheters were placed in 56.5% of the patients (252 patients) and 57.9% (264 patients) in the two groups, respectively; however, placement occurred later than in the EGDT group (P<0.001) (Fig. S6B in the Supplementary Appendix) and involved serial monitoring of Scvo2 in only a small proportion of patients (4.0% [18 patients] in the protocol-based standard-therapy group and 3.5% [16 patients] in the usual-care groups, vs. 93.6% [411 patients] in the EGDT group; P<0.001).
Resuscitation
During the first 6 hours, the volume of intravenous fluids administered differed significantly among the groups (2.8 liters in the protocol-based EGDT group, 3.3 liters in the protocol-based standard-therapy group, and 2.3 liters in the usual-care group (P<0.001) (Table S4 and Fig. S6C in the Supplementary Appendix). The volume of fluids administered decreased during the 6 hours in all the groups, but patients in the protocol-based standard-therapy group received the greatest volume initially and overall, patients in the usual-care group received the least volume of fluid, and patients in the protocol-based EGDT group received fluid at the most consistent rate (P<0.001 for differences in total volume and P = 0.007 for differences over time). Crystalloids were the predominant fluid used in all the groups, administered in 96% of the patients overall. More patients in the two protocol-based groups than in the usual-care group received vasopressors (54.9% in the protocol-based EGDT group and 52.2% in the protocol-based standard-therapy group vs. 44.1% in the usual-care group, P = 0.003) (Table S4 and Fig. S6D in the Supplementary Appendix). More patients in the protocol-based EGDT group than in the protocol-based standard-therapy group or the usual-care group received dobutamine and packed red-cell transfusions (dobutamine use, 8.0% vs. 1.1% and 0.9%, respectively; P<0.001; packed red-cell transfusions, 14.4% vs. 8.3% and 7.5%, respectively; P = 0.001) (Table S4, and Fig. S6D in the Supplementary Appendix). The use of antibiotics, glucocorticoids, and activated protein C was similar across the three groups (with P values ranging from 0.16 to 0.90) (Table S4 in the Supplementary Appendix).
Ancillary Care
The use of intravenous fluids, vasopressors, dobutamine, and blood transfusions between 6 and 72 hours did not differ significantly among the groups (Table S4 in the Supplementary Appendix). Patients in all three groups had mean values that were consistent with low-tidal-volume ventilation and moderate glycemic control (Table S4 in the Supplementary Appendix). In general, the condition of the patients in all three groups improved over time, with few differences among the groups. By 6 hours, the target mean arterial pressure of 65 mm Hg or higher had been achieved in more patients in each of the protocol-based groups than in the usual-care group (P = 0.02), but the mean heart rate did not differ significantly among the groups (P = 0.32) (Table S2 in the Supplementary Appendix). Patients in the protocol-based EGDT group had a higher mean international normalized ratio at 6 hours (2.2, vs. 1.7 in the protocol-based standard-therapy group and 1.6 in the usual-care group; P = 0.01), whereas patients in the usual-care group had slightly less acidosis at 6 hours and 24 hours (arterial pH, 7.31 in each protocol-based group vs. 7.34 in the usual-care group at 6 hours, and 7.34 in each protocol-based group vs. 7.36 in the usual-care group at 24 hours, P = 0.02), but these differences did not persist.
Outcomes
By day 60, a total of 92 patients in the protocol-based EGDT group (21.0%), 81 in the protocol-based standard-therapy group (18.2%), and 86 in the usual-care group (18.9%) had died in the hospital (Table 2). The 60-day in-hospital mortality for the combined protocol-based groups (19.5% [173 of 885 patients]) did not differ significantly from that in the usual-care group (relative risk, 1.04; 95% confidence interval [CI], 0.82 to 1.31; P = 0.83), nor did mortality differ significantly when the groups were compared separately (with P values ranging from 0.31 to 0.89) (Table 2 and Fig. 2A). There were also no significant differences in 90-day mortality or in the time to death up to 90 days and 1 year (P = 0.66 for 90-day mortality and P = 0.70 and P = 0.92 for cumulative mortality at 90 days and 1 year, respectively) (Table 2 and Fig. 2B). Results were essentially unchanged when adjusted for potential site heterogeneity (odds of 60-day in-hospital death with protocol-based care vs. usual care, 1.08; 95% CI, 0.85 to 1.38; P = 0.54).
Table 2.
Outcomes.*
| Outcome | Protocol-based EGDT (N = 439) |
Protocol-based Standard Therapy (N = 446) |
Usual Care (N = 456) |
P Value† |
|---|---|---|---|---|
| Death — no./total no. (%) | ||||
| In-hospital death by 60 days: primary outcome | 92/439 (21.0) | 81/446 (18.2) | 86/456 (18.9) | 0.83‡ |
| Death by 90 days | 129/405 (31.9) | 128/415 (30.8) | 139/412 (33.7) | 0.66 |
| New organ failure in the first week — no./total no. (%) | ||||
| Cardiovascular | 269/439 (61.3) | 284/446 (63.7) | 256/456 (56.1) | 0.06 |
| Respiratory | 165/434 (38.0) | 161/441 (36.5) | 146/451 (32.4) | 0.19 |
| Renal | 12/382 (3.1) | 24/399 (6.0) | 11/397 (2.8) | 0.04 |
| Duration of organ support — days§ | ||||
| Cardiovascular | 2.6±1.6 | 2.4±1.5 | 2.5±1.6 | 0.52 |
| Respiratory | 6.4±8.4 | 7.7±10.4 | 6.9±8.2 | 0.41 |
| Renal | 7.1±10.8 | 8.5±12 | 8.8±13.7 | 0.92 |
| Use of hospital resources | ||||
| Admission to intensive care unit — no. (%) | 401 (91.3) | 381 (85.4) | 393 (86.2) | 0.01 |
| Stay in intensive care unit among admitted patients — days | 5.1±6.3 | 5.1±7.1 | 4.7±5.8 | 0.63 |
| Stay in hospital — days | 11.1±10 | 12.3±12.1 | 11.3±10.9 | 0.25 |
| Discharge status at 60 days — no. (%) | ||||
| Not discharged | 3 (0.7) | 8 (1.8) | 2 (0.4) | 0.82 |
| Discharged to a long-term acute care facility | 16 (3.6) | 22 (4.9) | 22 (4.8) | |
| Discharge to another acute care hospital | 8 (1.8) | 2 (0.4) | 5 (1.1) | |
| Discharged to nursing home | 71 (16.2) | 93 (20.9) | 88 (19.3) | |
| Discharged home | 236 (53.8) | 227 (50.9) | 235 (51.5) | |
| Other or unknown | 13 (3.0) | 13 (2.9) | 18 (3.9) | |
| Serious adverse events — no. (%)¶ | 23 (5.2) | 22 (4.9) | 37 (8.1) | 0.32 |
Plus–minus values are means ±SD.
Unless stated otherwise, P values are for a three-group comparison, with the use of Fisher’s exact test for categorical measures and linear models for continuous and normally distributed measures. Skewed outcomes were analyzed with the use of nonparametric alternatives.
The P value for the primary analysis was for a comparison between the two protocol-based groups combined and the usual-care group, with the use of Fisher’s exact test. The three-group comparison, with the use of Fisher’s exact test, was also nonsignificant (P = 0.55), as was each one of the two-way comparisons (with P values ranging from 0.31 to 0.89).
Included in the analysis were patients in whom new organ failure developed in the first week after randomization.
A detailed list of serious adverse events is provided in Table S5 in the Supplementary Appendix.
Figure 2. Cumulative Mortality.
Panel A shows cumulative in-hospital mortality, truncated at 60 days, and Panel B cumulative mortality up to 1 year after randomization.
The incidence of acute renal failure, as indicated by a new need for renal-replacement therapy, was higher in the protocol-based standard-therapy group than in the other two groups (6.0% in the protocol-based standard-therapy group vs. 3.1% in the protocol-based EGDT group and 2.8% in the usual-care group, P = 0.04), although the duration of therapy did not differ significantly across the groups (Table 2). The rate of admission to the intensive care unit was higher in the protocol-based EGDT group than in the other two groups, although among patients who were admitted, there were no significant between-group differences in the length of stay in the intensive care unit (Table 2). There were no significant differences in the incidence and duration of cardiovascular failure or respiratory failure, nor were there significant differences in the length of stay in the hospital or the discharge disposition (Table 2).
Reports of potentially serious adverse events (excluding death) were rare and did not differ significantly across groups (Table 2, and Table S5 in the Supplementary Appendix). There were no significant interactions between the assigned treatment and any prespecified subgroup with respect to the primary outcome of 60-day in-hospital mortality or with respect to the secondary mortality outcomes (Table S6 in the Supplementary Appendix). Similarly, in a post hoc analysis, there was no evidence of a treatment effect within ranges of values for the APACHE II score, serum lactate level, or time from meeting the criteria for shock to randomization (Table S7 in the Supplementary Appendix).
Discussion
In our study, adherence to the two experimental protocols was high, and, as expected, protocol-based care, as compared with usual care, resulted in increased use of central venous catheterization, intravenous fluids, vasoactive agents, and blood transfusions. The two protocol-based resuscitation approaches led to a small but transient improvement in blood pressure by the end of the resuscitation period but a higher requirement for intensive care and renal-replacement therapy. There were no significant differences in mortality, either overall or in a number of pre-specified and post hoc subgroups.
Our results differ from those of Rivers et al.4; however, our study was not a direct replication of that study, and there are probably several factors that contribute to the differences. Although the two trials used similar inclusion criteria, the enrolled populations differed. The study cohorts were similar with respect to many demographic and clinical characteristics, including the severity of illness (Table S8 in the Supplementary Appendix), but the cohort in the study by Rivers et al. was slightly older, had higher rates of preexisting heart and liver disease, and had a higher initial serum lactate level. Although we modified the minimum fluid bolus required to establish the presence of refractory hypotension, the mean volume of the bolus that was administered fell within the range used in the study by Rivers et al. (20 to 30 ml per kilogram). The mean initial Scvo2 reported by Rivers et al. was 49%, which was lower than that in the ProCESS trial. However, early central venous catheterization was considered to be part of usual care in that trial, allowing Scvo2 readings to be made before administration of the initial fluid bolus, the response to which was required to establish refractory hypotension. In contrast, for patients randomly assigned to the protocol-based EGDT group in our study, we measured Scvo2 only after the initial fluid bolus had been administered, making a direct comparison problematic. Nonetheless, the cohort in the study by Rivers et al. may have had, on average, more severe or persistent shock than the patients in our cohort. However, we were unable to show a benefit even when we restricted the analyses to the sickest third of our patients — those with the highest serum lactate levels and those with the highest APACHE II scores.
Both trials used the same EGDT protocol delivered by a trained, dedicated team at each site. Rivers et al. reported nearly perfect adherence but did not provide details regarding the assessment method. Although adherence to the protocol was high in our study, we cannot exclude the possibility that the outcome would have been better if adherence had been perfect. We believe that the rate of adherence in our study parallels the likely performance in any widespread effort targeting the care of patients with septic shock. Furthermore, changes during the past decade in the care of critically ill patients, including the use of lower hemoglobin levels as a threshold for transfusion, the implementation of lung-protection strategies, and the use of tighter control of blood sugar, may have helped lower the overall mortality and may have reduced the marginal benefit of alternative resuscitation strategies.9,10,16,17
In 2010, Jones et al. reported the results of a randomized trial involving a patient population similar to ours (Table S8 in the Supplementary Appendix). That trial showed that an EGDT protocol that was based on serial measurement of serum lactate levels was not inferior to an EGDT protocol that used Scvo2 monitoring.14 In-hospital mortality and the use of intravenous fluids, blood transfusions, and dobutamine were similar to those seen in the ProCESS trial. Other studies showing the benefit of EGDT in adults presenting to the emergency department with septic shock have been observational and open to potential confounding.18
There are important limitations to our study. First, although we took many steps to ensure close adherence to the resuscitation protocols, we cannot be sure that elements critical to the success of the protocol in the study by Rivers et al. were not lost during dissemination. Second, we enrolled patients who were recognized to be in septic shock. Our study does not address the extent to which any of these strategies offer advantages in settings where septic shock is not recognized promptly. Third, septic shock occurs in a heterogeneous population, and care before randomization can be variable. Fourth, we had limited power to address whether particular strategies were more effective in specific subgroups. Two ongoing multicenter trials of EGDT, the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial in Australia (ClinicalTrials.gov number, NCT00975793) and the Protocolised Management in Sepsis (ProMISe) trial in the United Kingdom (Current Controlled Trials number, ISRCTN36307479) may offer additional insight.19,20 Finally, in-hospital mortality among patients requiring life support is strongly influenced by varying practices regarding the withdrawal of care, which could have influenced our findings.
In summary, in our multicenter, randomized trial, in which patients were identified early in the emergency department as having septic shock and received antibiotics and other nonresuscitation aspects of care promptly, we found no significant advantage, with respect to mortality or morbidity, of protocol-based resuscitation over bedside care that was provided according to the treating physician’s judgment. We also found no significant benefit of the mandated use of central venous catheterization and central hemodynamic monitoring in all patients.
Supplementary Material
Acknowledgments
Supported by a grant (P50 GM076659) from the National Institute of General Medical Sciences, National Institutes of Health.
Dr. Shapiro reports receiving consulting fees from Thermo Fisher Scientific, fees for serving on a data and safety monitoring board from Cumberland Pharmaceuticals, and grant support from Thermo Fisher Scientific, Rapid Pathogen Screening, Cheetah Medical, and Astute Medical. Dr. Angus reports receiving consulting fees from MedImmune, Ferring Pharmaceuticals, and Roche Diagnostics, lecture fees from Pfizer, fees for serving on a data and safety monitoring board from Eli Lilly, and grant support through his institution from Eisai.
Appendix
The members of the writing committee (Donald M. Yealy, M.D., John A. Kellum, M.D., David T. Huang, M.D., Amber E. Barnato, M.D., Lisa A. Weissfeld, Ph.D., and Francis Pike, Ph.D., University of Pittsburgh, Pittsburgh; Thomas Terndrup, M.D., Ohio State University, Columbus; Henry E. Wang, M.D., University of Alabama at Birmingham, Birmingham; Peter C. Hou, M.D., Brigham and Women’s Hospital, Boston; Frank LoVecchio, D.O., Maricopa Medical Center, Phoenix; Michael R. Filbin, M.D., Massachusetts General Hospital, and Nathan I. Shapiro, M.D., Beth Israel Deaconess Medical Center — both in Boston; and Derek C. Angus, M.D., M.P.H., University of Pittsburgh, Pittsburgh) assume responsibility for the content and integrity of the article. Address reprint requests to Dr. Angus at the Department of Critical Care Medicine, University of Pittsburgh, 3550 Terrace St., 614 Scaife Hall, Pittsburgh, PA 15261, or at angusdc@upmc.edu.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [Erratum, N Engl J Med 2013; 369:2069.] [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department — results of a national survey. Crit Care Med. 2007;35:2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 6.Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med. 2007;14:1072–1078. doi: 10.1197/j.aem.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reade MC, Huang DT, Bell D, et al. Variability in management of early severe sepsis. Emerg Med J. 2010;27:110–115. doi: 10.1136/emj.2008.070912. [DOI] [PubMed] [Google Scholar]
- 8.Pike F, Yealy DM, Kellum JA, et al. Protocolized Care for Early Septic Shock (Process) statistical analysis plan. Crit Care Resusc. 2013;15:301–310. [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [Errata, Crit Care Med 2004;32:1448, 2169 −70.] [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [Erratum, Crit Care Med 2008;36:1394–6.] [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Minneci PC, Eichacker PQ, Danner RL, Banks SM, Natanson C, Deans KJ. The importance of usual care control groups for safety monitoring and validity during critical care research. Intensive Care Med. 2008;34:942–947. doi: 10.1007/s00134-008-0999-6. [DOI] [PubMed] [Google Scholar]
- 13.A handbook for clinical investigators conducting therapeutic clinical trials supported by CTEP, DCTD, NCI. Bethesda, MD: National Cancer Institute CTEP; 2013. [Google Scholar]
- 14.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [Erratum, N Engl J Med 1999;340:1056.] [DOI] [PubMed] [Google Scholar]
- 17.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.Rivers EP, Katranji M, Jaehne KA, et al. Early interventions in severe sepsis and septic shock: a review of the evidence one decade later. Minerva Anestesiol. 2012;78:712–724. [PubMed] [Google Scholar]
- 19.Huang DT, Angus DC, Barnato A, et al. Harmonizing international trials of early goal-directed resuscitation for severe sepsis and septic shock: methodology of ProCESS, ARISE, and ProMISe. Intensive Care Med. 2013;39:1760–1775. doi: 10.1007/s00134-013-3024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reade MC, Delaney A, Bailey MJ, et al. Prospective meta-analysis using individual patient data in intensive care medicine. Intensive Care Med. 2010;36:11–21. doi: 10.1007/s00134-010-1761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.