Abstract
Introduction: Retinitis pigmentosa (RP), an inherited disease characterized by progressive loss of photoreceptors and retinal pigment epithelium, is a leading genetic cause of blindness. Cell transplantation to replace lost photoreceptors is a potential therapeutic strategy, but technical limitations have prevented clinical application. Adult human peripheral blood mononuclear cells (hPBMCs) may be an ideal cell source for such therapies. This study examined the survival and migration of pre-induced hPBMCs three months after subretinal transplantation in the retinal degeneration slow (rds) mouse model of RP. Materials and Methods: Freshly isolated adult hPBMCs were pre-induced by co-culture with neonatal Sprague-Dawley (SD) rat retinal tissue for 4 days in neural stem cell medium. Pre-induced cells were labeled with CM-DiI for tracing and injected into the right subretinal space of rds mice by the trans-scleral approach. After two and three months, right eyes were harvested and transplanted cell survival and migration examined in frozen sections and whole mountretinas. Immunofluorescence in whole-mount retinas was used to detect the expression of human neuronal and photorece ptorsprotein markers by transplanted cells. Results: Pre-induced adult hPBMCs could survive in vivo and migrate to various parts of the retina. After two and three months, transplanted cells were observed in the ciliary body, retinal outer nuclear layer, inner nuclear layer, ganglion cell layer, optic papilla, and within the optic nerve. The neuronal and photoreceptor markers CD90/Thy1, MAP-2, nestin, and rhodopsin were expressed by subpopulations of CM-DiI-positive cells three months after subretinal transplantation. Conclusion: Pre-induced adult hPBMCs survived for at least three months after subretinal transplantation, migrated throughout the retina, and expressed human protein markers. These results suggest that hPBMCs could be used for cell replacement therapy to treat retinal degenerative diseases.
Keywords: Adult stem cells, hPBMCs, migration, retinal degeneration, subretinal transplantation, survival.
INTRODUCTION
Retinitis pigmentosa (RP) is a leading cause of inherited blindness, and there is currently no effective treatment to slow the progressive loss of photoreceptors and retinal pigment epithelium (RPE) characteristic of this disease. Replacement of lost cells by transplantation of pluripotent stem cells is considered a possible therapy [1, 2], but the efficacy of this strategy is predicated on the survival, differentiation, and integration of stem cells in vivo.
A number of recent studies have indeed demonstrated the potential of stem cells to replace damaged retinal cells and improve visual function [3-12]. In 2010, the FDA approved the first clinical trial using human embryonic stem cell-derived retinal pigment epithelial (hESC-RPE) cells for the treatment of dry age-related macular degeneration (AMD) and Stardgart’s disease (STGD). However, ethical issues related to harvesting human fetal stem cells and serious potential dangers such as xenotransplant rejection and teratoma have limited clinical application. Moreover, human retinal progenitors are difficult to harvest and maintain, greatly limiting the supply. In contrast, adult induced pluripotent stem cells (iPSC) possess unlimited capacity for self-renew, can be harvested from patients to eliminate the risk of rejection, and theoretically can be pretreated (pre-induced) to follow specific lineages. However, the reprogramming of non-pluripotent cells to iPSCs and the preparation of specific iPSC-derived lineages is complex. Thus, further investigation is required to identify the optimal pluripotent stem cell type(s) for transplantation. Induced PSCs are not likely ideal for this purpose [13]. Bone mesenchymal stem cells (BMSCs) have regeneration potential [14], can secrete trophic factors [15-18], and inhibit immunological reactions [19-22], but the capacity for integration into the retina is limited [23], and harvesting from bone marrow is neither safe nor convenient. In contrast, peripheral blood mononuclear cells (PBMCs) meet many of the conditions of an ideal cell source [24]. In addition to ease of isolation from patients, previous studies have shown that PBMCs can differentiate into neurons [24-29].
Few studies have examined the potential of PBMCs for ophthalmology applications [30]. Our previous studies demonstrated that freshly isolated adult hPBMCs can differentiate into cells with the morphological characteristics and protein expression patterns of neurons, astrocytes, and photoreceptors when cultured in medium conditioned by rat retinal tissue [31, 32]. However, the capacity for migration and integration was limited at one month after intravitreal injection [31]. In contrast, transplanted cells demonstrated good migration and survival one month after subretinal injection [32]. Transplanted cell survival and migration in the damaged retina are a prerequisite for cell-based repair, so whether these transplanted cells continue to survive, migrate, and differentiate over longer periods within the rds mouse retina after subretinal transplantation requires further observation. To study the longer-term survival and migratory capacity of pre-induced hPBMCs, we labeled these cells, injected them into the subretinal space of rds mice, and examined cell location and protein expression phenotype two and three months after injection.
MATERIALS AND METHODS
Animals
The retinal degeneration slow (rds) mouse, a model of recessive RP, carries a homozygous mutation of the peripherin/rds gene that restricts formation of the outer segment disk membrane and eventually leads to photoreceptor apoptosis. One and a half-month-old rds mice (n=160) were divided into three groups: a treatment group injected with pre-induced adult hPBMCs (n=108, 36 for each human PBMC source), a sham control group injected with serum-free culture medium (n=36), and an untreated blank control group (n=16). Treatment group mice were injected with 2 µl serum-free culture medium containing 2×105/µl CM-DiI-labeled cell into the right subretinal space, while sham control group rds mice were injected with 2 µl serum-free culture medium. Two and three months after sham injection or cell transplantation, rds mice were sacrificed by cervical dislocation and the right eyes removed. The study was approved by the Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China (NO [2008]15). All procedures with animals were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Institutional Animal Ethics Committee (Animal Welfare Assurance NO.2011-015).
Isolation, Pre-induction, and Labeling of Adult hPBMCs
The methods employed for adult hPBMC isolation, pre-induction, and labeling were described previously [31, 32]. Briefly, peripheral blood was collected from 3 healthy individuals (ages 24, 27, and 51 years). Donors were healthy as defined by normal blood and urine test results, normal liver and lung function, no history of genetic disease, and no current infectious diseases. Written informed consent was obtained from the participants. Each 30 ml peripheral blood sample was separated by Ficoll–Hypaque (Chuanye Biotech, China) density gradient centrifugation at 1500 rpm for 20 min and hPBMCs obtained from the interface between the plasma layer and the Ficoll-Hypaque layer. Cells were seeded into the bottom wells of 6-well transwell plates (Corning, USA) at 3–4 × 106 cells per well. The top wells had been pre-seeded 24 h earlier with 4 retinal tissue samples from one-day-old SD rats to create a special conditioned neural stem cell medium. These co-cultures were incubated in neural stem cell medium at 37ºC under a 5% CO2 atmosphere for 4 days. The raw neural stem cell medium was composed of Dulbecco’s modified Eagle’s medium (DMEM)/F12 (90 ml/100 ml; Gibco, USA), human basic fibroblast growth factor (hbFGF, 103 ng/100 ml; PeproTech, USA), human stem cell factor (hSCF, 200 ng/100 ml; PeproTech, USA), human epidermal growth factor (hEGF, 103 ng/100 ml; PeproTech, USA), B27 stem cell culture supplement (50×, 2 ml/100 ml; Gibco, USA), L-glutamine (3% solution, 1 ml/100 ml, Weijia Technology, China), fetal bovine serum (FBS,7 ml/100 ml; SiJiQing Biotech, China), and Gentamicin (150 ml/100 ml, Tianxin Pharmaceuticals, China). The medium was changed as needed (as indicated by pH indicator yellowing).
After 4 days’ pre-induction, hPBMCs were collected and resuspended in PBS at 106/ml. Untreated cell suspensions were used as blank controls for flow cytometry, while other samples were resuspended in serum-free culture medium at 106/ml and labeled by adding 5 μl of a 25 μg/ml CM-DiI solution to each 1 ml of the cell suspension. The mixture was incubated at 37 ºC for 5 min, then at 4ºC for 15 min with resuspension of cells every five min. Labeled cells were washed twice in PBS, resuspended in PBS at 106/ml, and then analyzed by flow cytometry. The remaining CM-DiI-labeled cells were suspended at 2×105 cells/µl in serum-free culture medium and used for subretinal injection.
Flow Cytometry
Flow cytometry was performed to quantify the expression of cell-specific markers in both freshly isolated and pre-induced hPBMCs. Both direct and indirect immunolabeling methods were used. Antibodies used for direct labeling (FITC- or PerCP-conjugated) were specific for human CD3, CD16, CD19, or CD45 (Invitrogen, USA), and each staining trail was compared to a specific isotype control. Primary antibodies used for indirect labeling were specific for human nestin (Millipore, USA), vimentin, MAP2, GFAP, synapsin, rhodopsin (all from Abcam, England), or β-tubulin III (Sigma-Aldrich, USA). The secondary antibodies were FITC- conjugated goat anti-rabbit (Cell Signaling Technology, USA) or PE-conjugated goat anti-rabbit (SouthernBiotech, USA). Fix and Cell Permeabilization reagents (Invitrogen, USA) were used for labeling intracellular antigens. Cell suspensions were stained and counted by flow cytometry according to the manufacturer’s directions. Cell suspensions treated with secondary antibodies only were measured as the isotype controls for indirect labeling. After staining, cell suspensions were tested immediately by flow cytometry (Becton, Dickinson Company, USA) using FCS Express V3 software for data analysis. The positive value of the isotype controls ranged from 0% to 1%.
Subretinal Transplantation
The specific method used for subretinal transplantation was the same as in our previous work [32]. Briefly, after weighing, treatment and sham group rds mice were anesthetized with 4.3% chloral hydrate (0.43 mg/g body weight) by intraperitoneal injection. Right eyes were flushed with saline, dilated using tropicamide eye drops (Shenyang xing qi Pharmaceutical, China), and fixed in position toward the top right. After topical anesthesia, bulbar conjunctiva and fascia in the 2 o'clock position were blunt dissected, the sclera exposed, and a 10-0 corneal suture (Alcon Company, USA) preset through the shallow sclera 2 mm posterior to the limbus under a surgical microscope. The sclera within the suture boundary was pierced with a corneal needle at 15 degrees into the anterior chamber toward 6 o'clock. Anterior chamber aqueous humor was partially discharged to reduce intraocular pressure. Then a 33-gauge microscopic needle (Hamilton Company, Switzerland) connected to a Hamilton microsyringe containing media (sham treatment) or the CM-DiI-labeled cell suspension was inserted into the subretinal space, and a 2 μl volume slowly injected. A successful subretinal transplantation had to meet the following conditions: (1) bullous retinal detachment was observed on the injection site, (2) the bullous retinal detachment reattached around 3 days after the operation, and (3) no cataract, vitreous hemorrhage, or endophthalmitis associated with subretinal injection was observed after injection. Tobramycin and dexamethasone eye drops were administered locally to the operative eye three times a day during the week after transplantation.
Frozen Sections and Nucleus Staining
To investigate the migration of transplanted cells through retinal cell layers, frozen sections were prepared and stained. Briefly, 40 rds mice (24 from the treatment group, 8 from the sham group, and 8 from the blank control group) were sacrificed by cervical dislocation two or three months after injection and the right eye removed and embedded in O.C.T. compound (Sakura Finetek, USA) at -20ºC. Sections (4 μm thick) were cut using a cryostat microtome (Leica, Germany) and mounted on polylysine-coated glass slides. Mounted sections were fixed in 4% paraformaldehyde for 5 min, washed three times with PBS, stained by Hoechst 33342 (1: 1000, Sigma-Aldrich, USA) for 5 min, washed three times with PBS, treated with anti-fade agent, and sealed under coverslips. Finally, the slides were visualized under a laser scanning confocal microscope (Carl Zeiss, Germany).
Radial Migration in Whole-mount Retinas
To assess radial migration of transplanted cells, whole-mount retinas were prepared. Briefly, 40 rds mice (24 from the treatment group, 8 from the sham group, and 8 from the blank control group) were sacrificed by cervical dislocation two or three months after injection. The injected eyes were removed, and a hole made in the cornea to allow 4% paraformaldehyde to enter the eyeball. The eyes were fixed in 4% paraformaldehyde for 1 h, the lens removed, and the remaining tissue fixed for an additional 2 h. The fixed retinas were carefully dissociated from the rest of the eyeball under a microscope (Carl Zeiss, Germany) and carefully placed on glass slides with the photoreceptor layer up. Radial incisions were made to allow the cup-shaped retinas to lie flat. Whole mounts were treated with anti-fade reagent, sealed under coverslips, and examined under a fluorescence microscope (Carl Zeiss, Germany). The distance from the injection site to the point of longest migration distance was measured using Axio Vision Rel. 4.8 software (Carl Zeiss, Germany). Other whole mounts were first immunolabeled before sealing (below).
Immunofluorescence of Whole-mount Retinas
To confirm that the red-stained cells (CM-DiI-labeled) were indeed those transplanted, whole mount retinas were stained with antibodies against human nestin, MAP-2, β-tubulin III, rhodopsin, or CD90/THY1. These antibodies showed no cross-reactivity to mouse (not shown).
At three months after injection, 80 rds mice (60 from the treatment group and 20 from the sham group) were sacrificed by cervical dislocation, and the right eyes removed. Eyes were fixed in 4% paraformaldehyde on ice for 30 min and retinas removed under a dissection microscope (Carl Zeiss, Germany). Fixed retinas were blocked for 1 h in PBS containing 0.5% Triton X-100 (PBST) plus 5% bovine serum albumin (BSA). After gently blotting the excess blocking buffer, retinas were incubated overnight at 4 ºC in PBST + BSA containing primary antibodies against rhodopsin (Abcam, England), MAP-2 (Abcam, England), CD90/THY1 (Abcam, England), nestin (Millipore, USA), and β-tubulin III (Sigma-Aldrich, USA). Immunolabeled retinas were washed three times with PBST (30 min/wash), incubated in PBST+BSA containing a secondary antibody (Cell Signaling Technology, USA) for 2 h, and washed three times in PBST (30 min/wash). Cell nuclei were stained by Hoechst 33342 (1:1000, Sigma-Aldrich, USA) for 30 min. Retinas were washed three times in PBST (30 min/wash) and radial incisions cut to allow them to lie flat. Whole mounts were treated with anti-fade reagent, sealed under coverslips, and examined under a confocal laser scanning microscope (Carl Zeiss, Germany).
Statistical Analysis
Group means (continuous variables) were compared by paired-sample T tests (analysis of variance). Statistical analysis was performed using SPSS 16.0 software. Data are expressed as mean ± S.D. P<0.05 was considered statistically significant.
RESULTS
Morphology and Phenotypic Expression of Adult hPBMCs Cultured In Vitro
Freshly isolated adult hPBMCs were round and dispersed after plating on the bottom wells of transwell plates in neural stem cell medium (Fig. 1A). After one day in transwell culture with rat neonatal retina tissue in the top wells, hPBMCs formed colonies (Fig. 1B) that gradually increased in number and volume over the next three days. There were no significant changes in cell morphology during this pre-induction culture (Fig. 1 B-E).
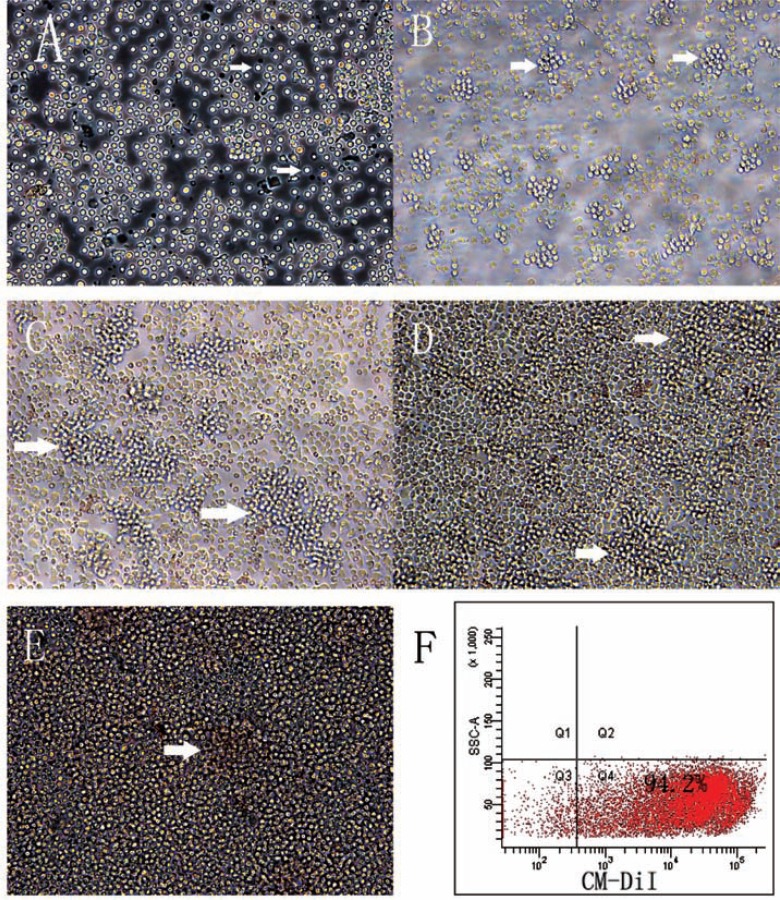
Fig. (1).
The morphology of adult hPBMCs in pre-induction culture. (A) Freshly isolated adult hPBMCs after seeding and exposure to the conditioned neural stem cell medium (x 200 Magnification). (B-E) Colonies (cell clusters, white arrows) increased in number and size overtime. (B) 24 h, (C)48 h, (D)three days, and (E) four days in conditioned medium (x 200 Magnification). (F) The labeling rate by CM-DiI as measured by flow cytometry.
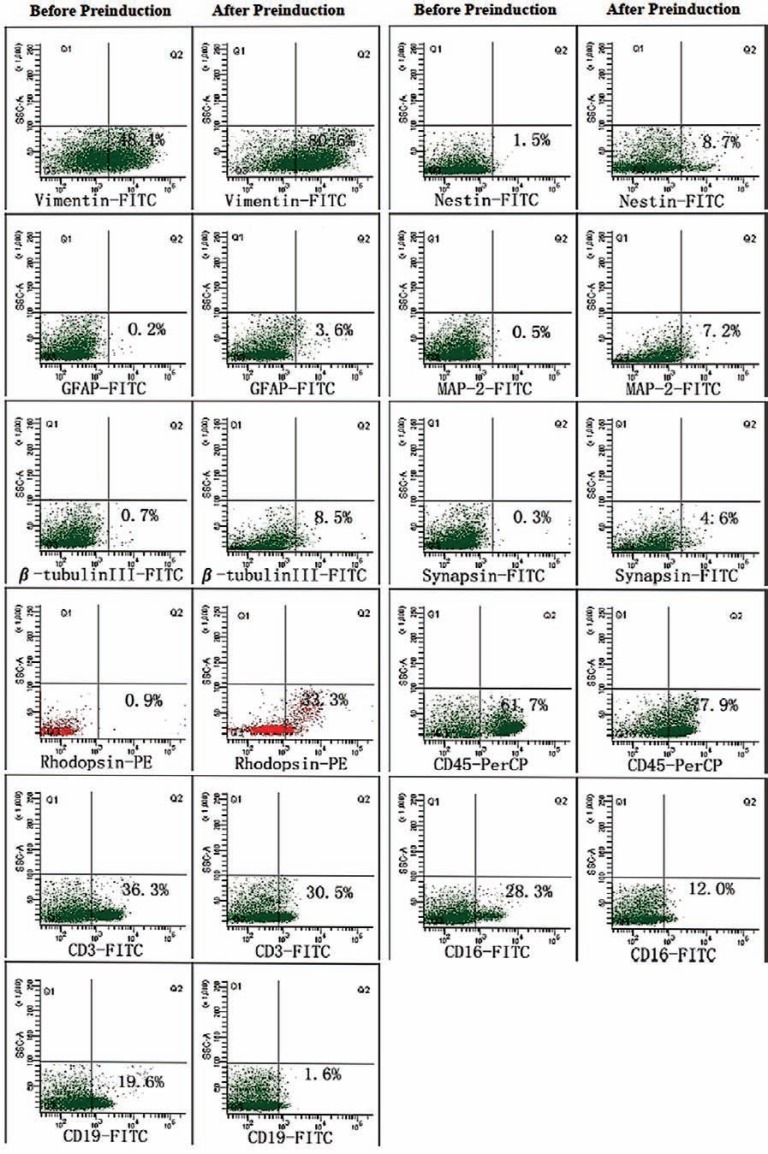
Phenotypic expression of adult hPBMCs cultured in vitro was consistent with previous results [27, 28]. In general, the expression levels of T cell, NK cell, and B cell markers (CD3, CD16, CD19) decreased, while expression levels of a neural stem cell marker (nestin), several neural cell markers (vimentin, MAP-2, β-tubulin III, synapsin, GFAP), and the retinal photoreceptor cell marker rhodopsin increased during pre-induction (Table 1 & Fig. 2). After 4 days of pre-induction culture, there was a significant increase in the number of rhodopsin-positive cells (P<0.05) (Table 1).
Table 1.
The expression of cell-specific markers in freshly isolated and pre-induced hPBMCs.
| Markers | Groups | T | P-value | |
|---|---|---|---|---|
| Before Pre-induction(mean±S.D.) | After Pre-induction(mean±S.D.) | |||
| Vimentin | 47.53±5.84 | 78.97±12.43 | -3.38 | 0.08 |
| Nestin | 1.73±1.86 | 5.23±3.03 | -3.50 | 0.07 |
| GFAP | 0.08±0.14 | 2.40±1.31 | -3.34 | 0.08 |
| MAP-2 | 0.20±0.26 | 5.70±3.04 | -3.29 | 0.08 |
| β-tubulin III | 0.63±0.50 | 7.53±7.32 | -1.75 | 0.22 |
| Synapsin | 0.43±0.31 | 4.13±4.65 | -1.39 | 0.30 |
| Rhodopsin | 1.50±1.78 | 26.43±6.83 | -5.68 | 0.03* |
| CD45 | 56.73±19.27 | 73.70±20.91 | -2.21 | 0.16 |
| CD3 | 37.70±11.56 | 29.33±14.39 | 4.01 | 0.06 |
| CD16 | 13.30±13.19 | 5.73±5.89 | 0.74 | 0.54 |
| CD19 | 13.53±8.05 | 1.23±0.32 | 2.55 | 0.13 |
Flow cytometry results are expressed as mean ± S.D. and group differences by T and P-values. *P< 0.05, significantly different between groups. Before Pre-induction: freshly iso-lated human peripheral blood mononuclear cells (hPBMCs). After Pre-induction: cells cultured for 4 days in a retinal tissue co-culture system with neural stem cell medium.
Fig. (2).
Flow cytometry results. After four days in pre-induced culture, the expression of all the neuronal markers tested rose while expression of PBMC markers decreased except CD45. There was a significant increase in rhodopsin immunoreactivity (P<0.05).
Survival and Migration of Pre-induced Adult hPBMCs in the Retinas of rds Mice
Adult hPBMCs were harvested after 4 days in transwell co-culture, resuspended in serum-free medium, and stained with CM-DiI prior to injection into rds mouse eyes as described. Two or three months after transplantation, frozen sections of retina and whole mount retinas were prepared and stained as described. Frozen sections were counterstained with Hoechst 33342 nuclear dye. Flow cytometry of the cell suspensions prior to injection revealed that 92.8% ± 5.3% of cells incubated in CM-DiI were labeled (Fig. 1F). Under a confocal laser scanning microscope, each rds retina in the treatment group contained red fluorescent (CM-DiI-positive) cells. Labeled cells were observed in the ciliary body (Fig. 3A), retinal outer nuclear layer (Fig. 3B), inner nuclear layer (Fig. 3C), ganglion cell layer (Fig. 3 D&E), optic papilla (Fig. 3 F & G), and within the optic nerve (Fig. 3 H), and all had intact nuclei as revealed by Hoechst staining. These results demonstrate that pre-induced adult hPBMCs survive for at least three months in vivo and migrate into all retinas layers of rds mice (Fig. 3 A-H). However, there was no obvious difference in the structure of retina between treatment and control groups.
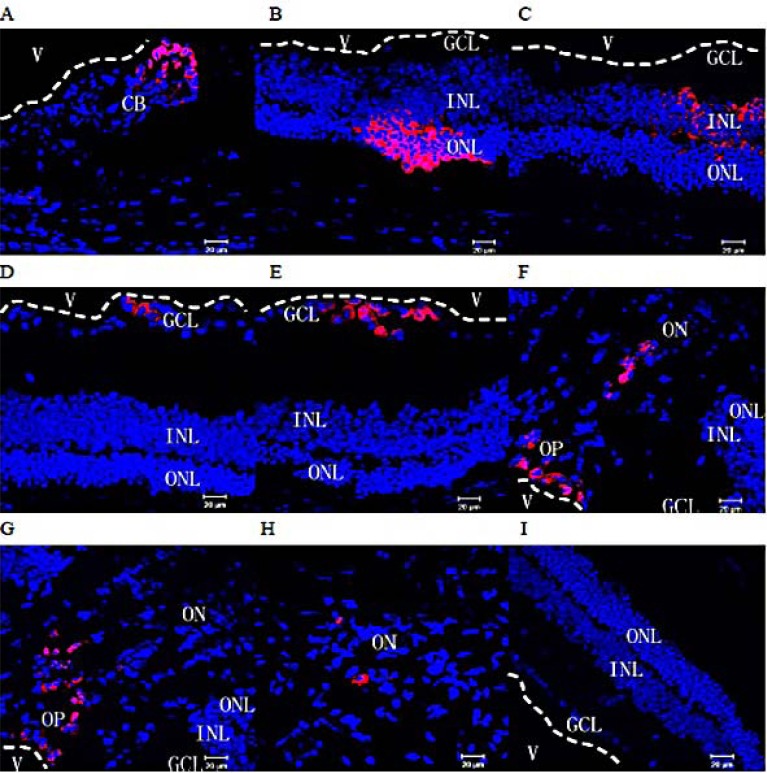
Fig. (3).
Migration within the eyeball of rds mice. Two and three months after subretinal transplantation into rds mice, the distribution of transplanted cells labeled with CM-Dil (red) was assessed in frozen retinal sections. Many transplanted cells were observed in aggregates in the ciliary body (A), the retinal outer nuclear layer (B), inner nuclear layer (C), ganglion cell layer (D&E), optic papilla (F&G), and within the optic nerve (H). (I) No CM-DiI-labeled cells were found in the retinas of rds mice from the control group (red: CM-Dil-labeled cells; Blue: cell nuclei counterstained with Hoechst 33342; V: vitreous cavity. The white dotted lines mark the boundaries of the vitreous cavity and the retina; CB: ciliary body; ONL: outer nuclear layer; INL: inner nuclear layer, GCL: ganglion cell layer, OP: optic papilla, ON: optic nerve). All scale bars represent 20 μm.
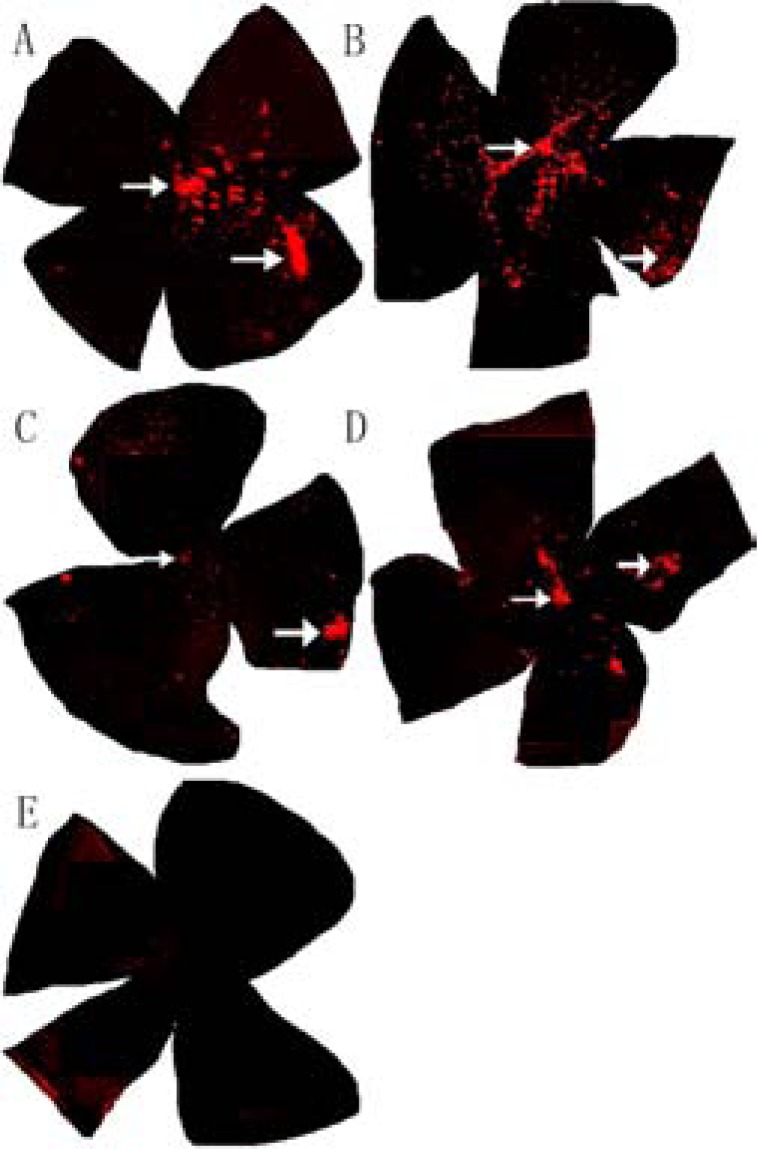
Fig. (4).
The extent of radial migration within retinas of rds mice. Two and three months after subretinal transplantation, the distribution of CM-DiI-labeled transplanted cells (red) was assessed in whole-mount retinas from the treated group (× 50 Magnification). Most of the transplanted cells remained near the injection site (white arrow in the right section of each specimen) but aggregates were also found in the optic papilla (white arrows at the center). CM-DiI staining was more intense and widespread after 2 months (A&B) than after 3 months (C& D). (E) There were no CM-DiI-labeled cells in whole-mount retinas from control group rds mice.
To assess the extent of radial migration, CM-DiI labeling was examined in whole-mount retinas. After two months (Fig. 4 A & B) or three months (Fig. 4C & D), red fluorescent cells were widely distributed throughout the retina (Fig. 4 A-D; white arrows point to the injection site and optic nerve), while no fluorescence was observed in whole-mount retinas from sham-injected mice (Fig. 4E). The CM-DiI fluorescence at two months (Fig. 4 A & B) was stronger and more widely distributed than after three months (Fig. 4C & D). The mean distance from the injection site to the point of farthest migration was 4021.66 ±373.88 µm.
Transplanted Cells in rds Mice Retinas Expressed Human-specific Neuronal and Photoreceptors Protein Markers
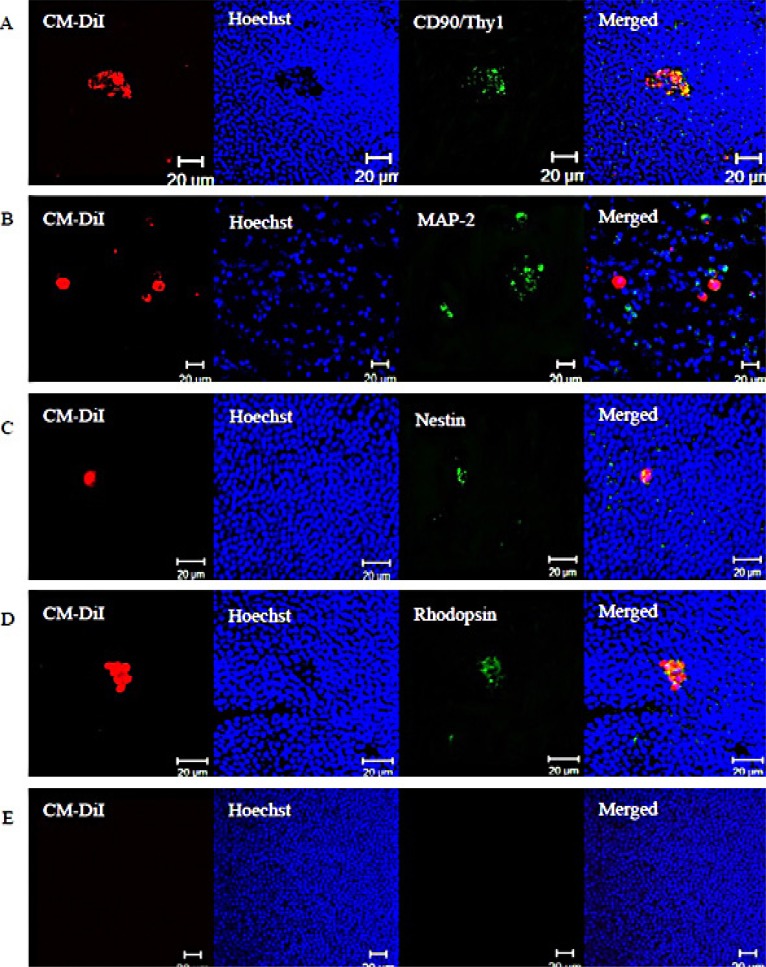
The protein expression phenotypes of hPBMCs three months after injection were assessed by immunostaining whole-mount retinas for markers of mature neurons, neural progenitors, and photoreceptors. Subpopulations of CM-DiI-positive cells expressed CD90/Thy1 (Fig. 5A), MAP-2 (Fig. 5B), nestin (Fig. 5C), or rhodopsin (Fig. 5D), but none expressed β-tubulin III (data not show). Some cells expressing human protein markers were clustered in aggregates, while others were dispersed as single isolated cells. However, none exhibited obvious neural processes. The retinas of mice injected with serum-free culture medium did not express any of these human markers (Fig. 5E).
Fig. (5).
The expression of human retina-specific protein markers. Three months after subretinal transplantation, subpopulations of transplanted cells expressed (A) CD90/Thy1, (B) MAP-2, (C) nestin, or (D) rhodopsin. (E) Retinas from the control group exhibited no red- or green-labeled cells. Red cells are CM-Dil labeled. Human specific proteins are immunolabeled using a green fluorophore. Nuclei were stained blue with Hoechst 33342. All scale bars represent 20 μm.
Injected hPBMCs survived in the retina for three months after subretinal transplantation. Moreover, many expressed human protein markers, although others remained antibody negative. Additional studies are required to determine if expression profile are consistent with specific cell types.
DISCUSSION
Adult hPBMCs pre-induced in vitro by co-culture with neonatal rat retinal explants can survive for at least 3 months in the eyes of rds mice. Transplanted cells were found well beyond the injection site, extending to the inner nuclear layer, outer nuclear layer, ganglion cell layer, ciliary body, optic papilla, and optic nerve, as well as radially toward the temporal/nasal edge of the retina. Rhodopsin, MAP-2, CD90/THY1, and nestin were expressed by subpopulations of transplanted cells three months after subretinal transplantation, suggesting that hPBMCs have the potential to replace retinal cells lost to degenerative diseases.
We previously demonstrated that subretinal injection greatly enhances the in vivo survival and differentiation potential of adult hPBMCs compared to intravitreal injection. In this study, we attempted to further enhance survival and (or) differentiation capacity by pre-induction. The molecular changes induced by pre-induction are unknown. After 4 days in neural stem cell medium conditioned by explant neonatal retinal tissue, there were no observable changes in cell morphology, possibly due to the short induction period [33-35]. Nonetheless, pre-induction was sufficient to expand the stem/progenitor cell population and reduce the immunocyte population as indicated by flow cytometry. Enrichment of stem/progenitors may have enhanced in vivo survival, although further studies are needed to confirm this proposal.
To track transplanted cells in rds mice, we labeled preconditioned hPBMCs with CM-DiI, a lipophilic red fluorochrome that is weakly fluorescent in cytoplasm but highly fluorescent and photostable when incorporated into membranes. This dye allowed for effective tracking of hPBMCs [36] due both to the highly efficient labeling 92.8%±5.3% and the retention of dye by at least a fraction of injected cells for 2 to 3 months after transplantation [37]. However, more fluorescent cells were observed at 2 months, so some CM-DiI fluorescence may have been lost over time or diluted during cell division [38]. Studies with additional time points are necessary to assess cell fate and the stability of the tracer in more detail. Moreover, there are reports of phagocytic dye staining by host cells [39], so it is possible that some CM-DiI cells were not transplanted. In order to confirm that the observed red-stained cells were indeed those transplanted, we demonstrated immunoreactivity to human antibodies that do not cross-react with mouse retinal tissue.
Liu and colleagues [31] showed that differentiated adult hPBMCs could migrate and integrate into the retina 4 weeks after intravitreal injection. In our study, we used the trans-scleral approach for subretinal transplantation instead because this delivery method places the transplanted cells physically nearer to the photoreceptors and adjacent to a rich blood supply. Hambright et al. [39] showed that immuno-suppression was not mandatory for xenogenic graft survival in the retina so long as the blood-retinal barrier was not breached by the transplantation process. They also indicated that the subretinal space, but not the vitreous space, can promote maturation of transplanted cells into photoreceptors. Our results are consistent with these previous studies. Indeed, two and three months after subretinal injection, some red fluorescent (CM-DiI-positive) cells expressed rhodopsin.
Adult hPBMCs were induced in the presence of 1-day-old SD rat retinas, which still contain numerous neuronal stem/progenitor cells [40-42]. Moreover, retinal tissue at this age is at peak photoreceptor genesis [42]. Thus, these retinas likely secrete a variety of trophic factors that contribute to lineage specification. In addition, the culture medium contained hbFGF, hEGF, hSCF, and B27 supplement used for nerve cell culture [31, 43]. It was reported that a culture medium containing these factors could induce hPBMCs to differentiate into neural-like cells [27, 31, 44]. Both EGF and FGF are mitogenic factors that stimulate proliferation of neural stem cells [45, 46], and bFGF is essential for development of the neural retina [47-50]. The presence of these factors may explain the relatively higher percentage of neuron-like cells found in the ganglion cell layer of the host.
Rhodopsin, MAP-2, CD90/THY1, and nestin were expressed by different CM-DiI-labeled cells, while the neural marker β-tubulin III was not expressed. Antibody-positive cells did not show nerve cell processes, possibly due to insufficient time in the host for full differentiation. Therefore, future studies must examine longer time points after transplantation. Moreover, many CM-DiI-positive cells were not immunoreactive for the human antibodies tested. One possible reason for expression failure is that some transplanted cells may have already terminally differentiated under the guidance of cues in vitro (from rat retinal explants) and so were not responsive to the host microenvironment. Therefore, further purification of pre-induced cells may be required prior to transplantation. Second, the cells were injected prior to significant retinal degeneration. At the time of injection, rds mice were one and a half months old and by the end of the experiment, the oldest were four and a half months old. In rds mice, slow loss of visual cells begins at around two months, but the outer nuclear layer is not reduced to half its normal thickness until 18 months [51]. Thus, the exact timing of transplantation relative to pathological progression may influence differentiation. Indeed, there was no significant difference in retinal structure between control and treated mice by the end of the study. Future experiments must also test the effects of transplant in older rds mice. Moreover, the temporal course of retinal degeneration in untreated rds mice should be re-evaluated.
CONCLUSIONS
Pre-induced adult hPBMCs survived for at least 3 months in rds mouse retina after subretinal transplantation, migrated to all retinal layers, and expressed neuronal and photoreceptor markers, suggesting that hPBMCs have potential applications in cell replacement therapy to treated retinal degenerative disease. Whether pre-induced adult hPBMCs can live for longer periods and eventually replace functional cells lost to degeneration requires further study.
ACKNOWLEDGEMENTS
We thank the following for technical assistance: Jianfa Huang assisted in the use of laser confocal microscopy and Ting Luo assisted in flow cytometry. Hebi Liu assisted in the use of the cryostat microtome. We are grateful to members of the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, for discussion and advice.
LIST OF ABBREVIATIONS
- hPBMCs =
Human peripheral blood mononuclear cells
- RP =
Retinitis pigmentosa
- HuCB-MNCs =
Human umbilical cord blood mononuclear cells
- NSCs =
Neural stem cells
- RPE =
Retinal pigment epithelium
- iPSC =
Induced pluripotent stem cells
- BMSCs =
Bone mesenchymal stem cells
- rds =
Retinal degeneration slow
- SD =
Sprague-Dawley
- hbFGF =
Human basic fibroblast growth factor
- hSCF =
Human stem cell factor
- hEGF =
Human epidermal growth factor
- DMEM =
Dulbecco’s modified Eagles medium
- MAP-2 =
Microtubule-associated protein type 2
CONFLICT OF INTEREST
The authors declare that they have no competing interests. This work was supported by the Science and Technology Projects of Guangdong Province, China (NO.2012B040304009, NO.2010B060200008).
REFERENCES
- 1.Silva GA, Silva NF, Fortunato TM. Stem cell and tissue engineering therapies for ocular regeneration. Curr Stem Cell Res Ther. 2011;6(3):255–72. doi: 10.2174/157488811796575369. [DOI] [PubMed] [Google Scholar]
- 2.Wallace VA. Stem cells a source for neuron repair in retinal disease. Can J Ophthalmol. 2007;42(3):442–6. [PubMed] [Google Scholar]
- 3.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24(2):274–83. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLaren RE, Pearson RA, MacNeil A etal. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 5.Melville H, Carpiniello M, Hollis K, Staffaroni A, Golestaneh N. Stem cells a new paradigm for disease modeling and developing therapies for age-related macular degeneration. J Transl Med. 2013;11:53. doi: 10.1186/1479-5876-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Atmaca-Sonmez P, Schanie CL, Ildstad ST, Kaplan HJ, Enzmann V. Endogenous bone marrow derived cells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage. Invest Ophthalmol Vis Sci. 2007;48(9):4321–7. doi: 10.1167/iovs.06-1015. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Lewallen M, Chen S, Yu W, Zhang N, Xie T. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013;23(6):788–802. doi: 10.1038/cr.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. 2013;36(7):385–95. doi: 10.1016/j.tins.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Carr AJ, Vugler AA, Hikita ST , et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLOS One. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwart I, Hill AJ, Al-Allaf F etal. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216(2):439–48. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 11.MacNeil A, Pearson RA, MacLaren RE, Smith AJ, Sowden JC, Ali RR. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007;25(10):2430–8. doi: 10.1634/stemcells.2007-0035. [DOI] [PubMed] [Google Scholar]
- 12.Chacko DM, Rogers JA, Turner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun. 2000;268(3):842–6. doi: 10.1006/bbrc.2000.2153. [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Chen G, Hu B. Induced pluripotency for translational research. Genomics Proteomics Bioinformatics. 2013;11(5):288–93. doi: 10.1016/j.gpb.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu SH, Kuo WC, Chen YT etal. New nerve regeneration strategy combining laminin-coated chitosan conduits and stem cell therapy. Acta Biomater. 2013;9(5):6606–15. doi: 10.1016/j.actbio.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Iriyama A, Ueno S etal. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85(2):234–41. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro-Resende VT, Pimentel-Coelho PM etal. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009;159(2):540–9. doi: 10.1016/j.neuroscience.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 17.Milwid JM, Ichimura T, Li M etal. Secreted factors from bone marrow stromal cells upregulate IL-10 and reverse acute kidney injury. Stem Cells Int. 2012;2012:e392050. doi: 10.1155/2012/392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 19.Marmont AM, Gualandi F, Piaggio G etal. Allogeneic bone marrow transplantation (BMT) for refractory Behcet's disease with severe CNS involvement. Bone Marrow Transplant. 2006;37(11):1061–3. doi: 10.1038/sj.bmt.1705372. [DOI] [PubMed] [Google Scholar]
- 20.Lisianyi M I. Mesenchymal stem cells and their immunological properties. Fiziol Zh. 2013;59(3):126–34. [PubMed] [Google Scholar]
- 21.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A etal. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574–91. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane RJ, Graham SM, Davies PS etal. Anti-inflammatory role and immunomodulation of mesenchymal stem cells in systemic joint diseases potential for treatment. Expert Opin Ther Targets. 2013;17(3):243–54. doi: 10.1517/14728222.2013.746954. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Li XR, Yuan JQ. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):503–14. doi: 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Huang B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res Ther. 2012;3(6):e48. doi: 10.1186/scrt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama H, Inoue T, Watanabe R etal. Neurogenic potential of progenitors derived from human circulating CD14+monocytes. Immunol Cell Biol. 2006;84(2):209–17. doi: 10.1111/j.1440-1711.2006.01424.x. [DOI] [PubMed] [Google Scholar]
- 26.Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio J Med. 2007;56(2):41–7. doi: 10.2302/kjm.56.41. [DOI] [PubMed] [Google Scholar]
- 27.Horschitz S, Meyer-Lindenberg A, Schloss P. Generation of neuronal cells from human peripheral blood mononuclear cells. Neuroreport. 2010;21(3):185–90. doi: 10.1097/WNR.0b013e328334be4e. [DOI] [PubMed] [Google Scholar]
- 28.Kijima Y, Ishikawa M, Sunagawa T etal. Regeneration of peripheral nerve after transplantation of CD133+cells derived from human peripheral blood. J Neurosurg. 2009;110(4):758–67. doi: 10.3171/2008.3.17571. [DOI] [PubMed] [Google Scholar]
- 29.Fujioka Y, Tanaka N, Nakanishi K etal. Magnetic field-based delivery of human CD133 (+) cells promotes functional recovery after rat spinal cord injury. Spine. 2012;37(13):e768–77. doi: 10.1097/BRS.0b013e318246d59c. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Huang B. Peripheral blood stem cells: phenotypic diversity and potential clinical applications. Stem Cell Rev. 2012;8(3):917–25. doi: 10.1007/s12015-012-9361-z. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Guan L, Huang B etal. Adult peripheral blood mononuclear cells transdifferentiate in vitro and integrate into the retina in vivo. Cell Biol Int. 2011;35(6):631–8. doi: 10.1042/CBI20100146. [DOI] [PubMed] [Google Scholar]
- 32.Yichi Zhang, Yan Luo, Kaijing Li etal. Pre-induced adult human peripheral blood mononuclear cells migrate widely into the degenerative retinas of rd1 mice. Cytotherapy. 2013;15:1416–25. doi: 10.1016/j.jcyt.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Bibel M, Richter J, Lacroix E, Barde YA. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2(5):1034–43. doi: 10.1038/nprot.2007.147. [DOI] [PubMed] [Google Scholar]
- 34.Yoshino H, Watanabe N, Takahashi K etal. The potential of patients' peripheral blood mononuclear cells to differentiate into dendritic cells after hematopoietic stem cell transplantation. Life Sci. 2011;89(25-26):946–55. doi: 10.1016/j.lfs.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Li DY, Gu C, Min J, Chu ZH, Ou QJ. Maturation induction of human peripheral blood mononuclear cell-derived dendritic cells. Exp Ther Med. 2012;4(1):131–4. doi: 10.3892/etm.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrade W, Seabrook TJ, Johnston MG, Hay JB. The use of the lipophilic fluorochrome CM-DiI for tracking the migration of lymphocytes. J Immunol Methods. 1996;194(2):181–9. doi: 10.1016/0022-1759(96)00083-x. [DOI] [PubMed] [Google Scholar]
- 37.Schormann W, Hammersen FJ, Brulport M etal. Tracking of human cells in mice. Histochem Cell Biol. 2008;130(2):329–38. doi: 10.1007/s00418-008-0428-5. [DOI] [PubMed] [Google Scholar]
- 38.Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999;77(6):499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- 39.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–36. [PMC free article] [PubMed] [Google Scholar]
- 40.Ohta K, Ito A, Tanaka H. Neuronal stem/progenitor cells in the vertebrate eye. Dev Growth Differ. 2008;50(4):253–9. doi: 10.1111/j.1440-169X.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 41.Wohl SG, Schmeer CW, Isenmann S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog Retin Eye Res. 2012;31(3):213–42. doi: 10.1016/j.preteyeres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212(2):199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 43.Yuan T, Liao W, Feng NH , et al. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurological function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther. 2013;4(3):e73. doi: 10.1186/scrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, Honmou O, Kato K etal. Neural differentiation potential of peripheral blood-and bone-marrow-derived precursor cells. Brain Res. 2006;1123(1):27–33. doi: 10.1016/j.brainres.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson BR, Hartman BH, Georgi SA, Lan MS, Reh TA. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev Biol. 2007;304(2):479–98. doi: 10.1016/j.ydbio.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieto-Estevez V, Pignatelli J, Arauzo-Bravo MJ, Hurtado-Chong A, Vicario-Abejon C. A global transcriptome analysis reveals molecular hallmarks of neural stem cell death, survival, and differentiation in response to partial FGF-2 and EGF deprivation. PLOS One. 2013;8(1):e53594. doi: 10.1371/journal.pone.0053594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinothkumar S, Rastegar S, Takamiya M, Ertzer R, Strähle U. Sequential and cooperative action of Fgfs and Shh in the zebrafish retina. Dev Biol. 2008;314(1):200–14. doi: 10.1016/j.ydbio.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124(4):805–16. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- 49.Kodama H, Inoue T, Watanabe R , et al. Neurogenic potential of progenitors derived from human circulating CD14+ monocytes. Immunol Cell Biol. 2006;84(2):209–17. doi: 10.1111/j.1440-1711.2006.01424.x. [DOI] [PubMed] [Google Scholar]
- 50.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347(6288):83–6. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 51.Hawkins RK, Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41(6):701–20. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]