Abstract
Treatment of chronic lymphocytic leukemia (CLL) has dramatically changed over the last years, with significant improvement in overall survival (OS) and increased efficacy in genetically defined “high-risk” disease. Besides prospective clinical trials usually enrolling young and fit patients, retrospective studies were performed comparing the outcome of patients belonging to different age groups and showing longer survival in patients diagnosed in the most recent periods. In patients younger than 70 years the 10-year relative survival was 43–53% in the 1980s as compared with 59–63% in the 2000s. Likewise, the 10-year relative survival in patients >70 years was 22–42% in the 1980s and 46–55% in the 2000s. Improved outcome derived in part by the introduction of effective regimens in genetically defined “high-risk” disease (i.e., 17p−, 11q−, TP53, NOTCH1, SF3B1 mutations), especially in the younger and/or fit patients. The unfavorable prognostic significance of 11q− was overcome by chemoimmunotherapy. High-dose steroids with anti-CD52 appeared to improve the response rate in 17p-/TP53 mutated cases and allogeneic transplantation achieved prolonged disease control irrespective of high-risk disease. Further improvement is being generated by the new anti-CD20 obinutuzumab in the elderly and by mechanism-based treatment using kinase-targeting agents or anti-BCL2 molecules yielding high-response rate and impressive progression-free survival in the chemorefractory setting as well as in previously untreated patients.
Keywords: Chronic lymphocytic leukemia, chemoimmunotherapy, genetic lesions, tyrosine-targeted treatment, BCL2
Introduction
Treatment of chronic lymphocytic leukemia (CLL) has dramatically changed in several respects over the last years thanks to the convergence of basic research and well-conducted clinical studies leading to a clearer understanding of pathophysiology of the disease, to the identification of prognostic factors and to the design of effective treatment regimens 1–12.
Modern regimens produced high overall response rates (ORR), including complete remissions with negativity for minimal residual disease (MRD) and prolonged progression-free survival (PFS). The combination of rituximab fludarabine and cyclophosphamide (FCR) was shown to be superior to FC for all clinical endpoints including overall survival (OS), with the notable exception of the 17p− and the “normal FISH” subgoups 13. Meanwhile, evidence was provided that some disease subsets defined by molecular cytogenetic lesions represent “high-risk” disease with shorter PFS and survival with current treatment regimens 14–17. Novel agents interfering with unique biologic features, that is, B cell receptor (BCR) downstream signaling and BCL2, are being rapidly introduced in clinical practice, representing a new scenario of mechanism-driven treatment of CLL, producing rapid and durable responses in relapsed/refractory CLL 18,19.
With some exceptions 20, most clinical studies enrolled relatively young and fit patients and to address the issue of whether modern treatment produced a survival benefit in all age groups several retrospective studies were performed 21–23.
Modern treatment approaches will be reviewed here with reference to
their impact on OS in different age groups and
their activity in specific molecular cytogenetic subsets.
Impact of Treatment on Survival
Survival of the general population improved in the last decades in many western countries 24 and several factors may influence survival in historical series, including earlier diagnosis due to widespread use of automatic blood counters, more precise diagnosis allowing for the exclusion of lymphoma in leukemic phase in recent years, and improved supportive treatment. However, the following observations indicate that the overall outlook of CLL improved in the majority of age groups over the last decades.
Data from single centers and registries
Brenner and coworkers 21 assessed relative survival rates in CLL calculating the ratio of absolute survival of CLL patients divided by the expected survival of a group of well-matched persons in the general population. An improvement in survival in patients <80 years between 1980–1984 and 2000–2004 was documented in this analysis (Fig. 1A). In 2000–2004, patients <70 years reached a 10-year relative survival close to 65%, whereas a 55% 10-year relative survival was reached in the 70–79 age group.
Figure 1.
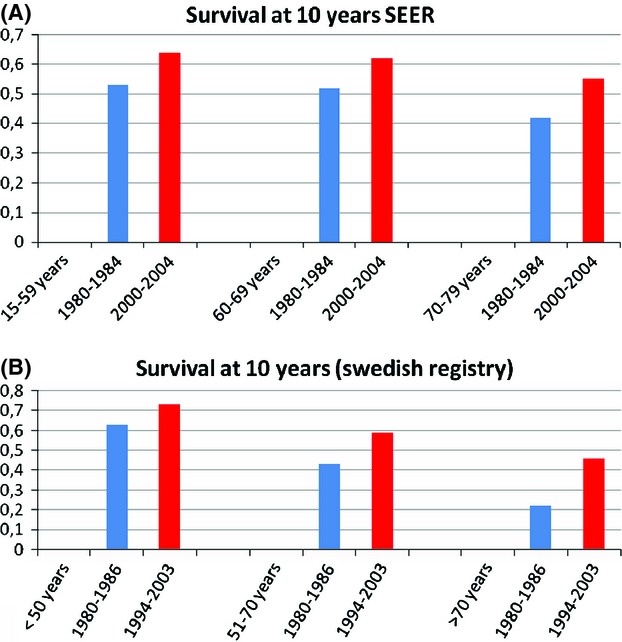
Improved 10-year survival in different age groups. Survival is expressed as ratio of absolute survival of CLL patients divided by the expected survival of a comparable group of persons in the general population. Data from (A) Brenner et al. 21 and (B) the Swedish registry 23. CLL, chronic lymphocytic leukemia.
The CLL-attributable mortality for patients diagnosed in 1995–2004 and 1980–1994 was calculated in the Barcelona series as deaths per 1000 patient-year, with an incidence rate ratio of 0.46 and 0.65 at 5-years and 10 years, respectively 22. Improved 5- and 10-year relative survival in the 1995–2004 period as compared with the 1980–1994 period was more pronounced in stage B/C patients <70 years. These data suggest that more effective treatment produced longer survival in young patients with intermediate-advanced stage, whereas no obvious improvement was noted in this analysis in limited-stage disease and in the elderly.
Using population-based data in an efficient Swedish registry, Kristinsson and coworkers 23 assessed variations in survival among CLL patients and found significantly improved 5-, 10-year relative survival ratio for the entire cohort during the study period for the majority of the age groups (Fig. 1B). Stable age-adjusted incidence and stable mean age at CLL diagnosis over the study period seem to outrule lead time bias due to early CLL detection as confounding factor in this analysis; however, improved survival in the general population, including CLL patients, might have played a role here. An unexplained observation in this study was that the 5-year relative survival ratio improved only in the 1973–1980 period and was stable thereafter in the youngest CLL population. Interestingly, adverse prognostic markers were found to be more common among young patients 25 and this might have influenced the outcome in this age subset in an era preceding the widespread use of biologic agents.
Comparisons with historical controls
Chemoimmunotherapy upfront was found to prolong survival at 6 years (77%) with respect to previous trials using fludarabine-based treatment (54–59%) 12. More recently, PFS and OS were retrospectively assessed in four successive frontline CALGB trials 26. With a median follow-up across studies of 92 months, OS was improved with fludarabine over chlorambucil (31% reduction of risk of death) among patients <70 years, but not in older adults. Importantly, a 35% reduction of risk of death was observed with the adjunct of rituximab to fludarabine, irrespective of age.
Randomized trials
At an extended follow-up analysis with a median observation of 5.9 years in the CLL8 trial, 69.4% of the patients were alive in the FCR group versus 62.3% in the FC group 27. Inclusion criteria in this protocol precluded enrollment of many elderly patients and when restricting outcome analysis in the 30% study population ≥65 years, improved complete remission (CR) rate and PFS were maintained in the chemoimmunotherapy arm, whereas no significant advantage in survival was noted in this age subset.
Although no difference in survival was observed in a trial comparing fludarabine versus chlorambucil in the elderly 28, a planned interim analysis of the CLL11 trial designed for unfit patients and comparing chlorambucil versus chlorambucil plus rituximab or the novel anti-CD20 monoclonal antibody obinutuzumab, found survival advantage in the chlorambucil plus obinutuzumab arm as compared with chlorambucil 20. Notably improved PFS was recorded in the obinutuzumab arm as compared with the rituximab 20. These data overall indicate that true improvement in survival is nowadays achievable in the majority of age groups, especially for those patients eligible to chemoimmunotherapy.
Efficacy of Treatment in Specific Molecular Cytogenetic Subsets of CLL
There is evidence deriving from single-center studies and from prospective multicenter trials that specific molecular-cytogenetic lesions, that is, 17p− 11q−, TP53, NOTCH1, and SF3B1 mutations occur in all age groups and may predict for chemorefractoriness and worse prognosis 29–36. Improved outcome in CLL derived in part by the introduction of novel regimens which proved to be effective in all risk categories, including genetically defined “high-risk” disease (i.e., 17p−, 11q−, TP53, NOTCH1, SF3B1 mutations). These regimens were tested preferentially in younger and/or fit patients. Efficacy data of chemoimmunotherapy in the frontline setting in distinct cytogenetic subsets are presented in Table 1.
Table 1.
Efficacy of the main frontline treatment regimens in different cytogenetic subsets of CLL
| Reference | Regimen | Response rate expressed as %ORR/%CR | Survival expressed as PFS/OS (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All groups | 13q− | +12 | 11q− | 17p− | All groups | 13q− | +12 | 11q− | 17p− | ||
| Hillmen et al. 80 | Chlorambucil | 55.4/NR | 62/NR | 80/NR | 29/NR | 20/NR | 11.7/NR | 13/NR | 12.9/NR | 8.5/NR | 2.2/NR |
| Hillmen et al. 80 | Alemtuzumab | 83.2/NR | 91/NR | 83/NR | 87/NR | 64/NR | 14.6/NR | 24.4/NR | 18.3/NR | 8.5/NR | 10.7/NR |
| Hallek et al. 13 | Fludarabine cyclophosphamide | 80/22 | 80/23 | 84/19 | 87/15 | 34/0 | 45%/83%* | 52%/89%* | 48%/86%* | 32%/83%* | 0%/37% * |
| Hallek et al. 13 | Fludarabine cyclophosphamide rituximab | 90/44 | 96/48 | 100/71 | 93/51 | 68/5 | 65%/87% * | 76%/95%* | 83%/96%* | 64%/94%* | 18%/38%* |
| Bosch et al. 81 | Rituximab fludarabine cyclophosphamide mitoxantrone | 82/11 | NR/82 | NR/100 | NR/87 | NR/25 | NR | NR | NR | NR | NR |
| Parikh et al. 82 | Cyclophosphamide fludarabine alemtuzumab rituximab | 92/70 | 100/64 | 100/93 | 90/80 | 78/57 | 38/NR | 42**/NR | 42**/NR | 27/NR | 15/NR |
| Fisher et al. 69 | Rituximab bendamustine | 88/23 | 93.3/13.3 | 94.7/21 | 90/40 | 37.5/0 | 33.8/NR | 34.4/NR | Not reached/NR | 29.7/NR | 7.9/NR |
| Pettitt et al. 39 | Alemtuzumab methylprednisolone | NA | NR | NR | NR | 88/65 | NA | NR | NR | NR | 18.3/39 |
CLL, chronic lymphocytic leukemia; ORR, overall response rate; CR, complete remission; PFS, progression-free survival; OS, overall survival; NR, not reported; NA, not applicable.
At 3 years;
median not reached.
Over the last few years, however, the introduction of novel treatment regimens and the recent development of molecules interfering with specific biologic mechanisms changed the treatment paradigm in CLL 37, especially in high-risk disease and chemorefractory disease. The efficacy and safety of novel regimens in the relapsed-refractory setting including the “unfavorable” genetic subsets of CLL is illustrated below and summarized in Table 2.
Table 2.
Efficacy and safety of some classical and novel treatment options in relapsed refractory CLL
| Regimen (reference) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Various regimens 83 | FCR 84,85 | Ofatumumab 86 | Lenalidomide + R 62 | Ibrutinib 9 | Idelalisib + R/B/RB 51 | ABT-199 57 | B + R 72 | R-BAC 41 | Flavopiridol 42 | ||
| Number of patients | 99 | 276/284 | 138 | 59 | 85 | 51 | 56 | 78 | 13 | 40 | |
| Number previous regimens (median) | NA (fludarabine refractory) | 1/2 | 4 (fludarabine refractory) | 2 | 4 | 1–10 (range) | 4 | 2 | 3 | 4 | |
| Response | |||||||||||
| CR | 0% | 24/30% | 0–1% | 12% | 2% | 78–87% (ORR) | 21% | 9% | 38% | 46% (ORR) | |
| PR | 23% | 45/44% | 47–58% | 54% | 69%(iii) | 63% | 50% | 46% | |||
| Follow-up (i) | NA | 25/43 | NA | 33 | 26 | >40 weeks | NA | 24 | 17 | NA | |
| PFS | 2–3 | 30/21 | 5.7–5.9 | 17,4 (ii) | 75%(iv) | 74–87%(iv) | 15.2 | 16 | 10.4 | ||
| Survival | 9 | NR/47 | 13.7–15.4 | 71% | 83% | NA | 33.9 | NR | 19.8 | ||
| Grade 3/4 | |||||||||||
| infections | 54% | 18/16% | 8–12% | 24% | 17% | 0–29% (v) | 7% (vi) | 0–3.4% | 8%(vi) | NA | |
| neutropenia | NA | 89/81% | 6–14% | 73% | 15% | 32–67% | 41% | 4.8–5.4% | 84% | NA | |
PFS, progression-free survival; CLL, chronic lymphocytic leukemia; NA, not available; NR, not reached; ORR, overall response rate; R, rituximab; B, bendamustine; (i) months, median value; (ii) time to treatment failure; (iii) an additional 18% patients had PR with lymphocytosis; (iv) % at 26 months for ibrurinib and % at 1 year for idelalisib; (v) pneumonia; (vi) febrile neutropenia.
17p−/TP53 Mutations
This subset of CLL is mostly refractory to fludarabine and alkylating agents and shows, with few exceptions 38, a poor prognosis with expected median survival of few years even with intensive regimens. Because the anti-CD52 monoclonal antibody alemtuzumab and high-dose steroids kill CLL cells through a p53 independent mechanism the efficacy of these drugs in combination was assessed 39, producing a 65% CR rate, with 36% MRD-disease and PFS median of 18.3 months in untreated patients. Despite representing a progress with respect to other regimens, virtually all patients are expected to relapse.
Allogeneic transplantation is an option for these patients. Interestingly, 6-year OS and event-free survival were 58% and 38%, respectively, in a study of 90 allografted high-risk patients, 49% of whom were fludarabine resistant. The efficacy results of this procedure were independent of the presence of unfavorable genetic features, including 17p− 40.
The combination of rituximab, bendamustine, and cytarabine in nine heavily pretreated patients with 17p− achieved CR in three cases and PR in four, with an ORR of 78% and a median PFS of 16 months in the entire series including four additional patients with 11q− 41. Flavopiridol as single agent attained a 48% ORR in 40 pretreated patients with 17p− with median PFS of 10.4 months; these data were not significantly different among the cytogenetic groups included in the study 42.
Novel agents showed promising efficacy in this cytogenetic subsets of CLL as summarized below.
BCR-Targeted Therapy
Ibrutinib
The Bruton tyrosine kinase (BTK) is a cytoplasmic tyrosine kinase that is essential for BCR signaling, inducing cell proliferation, and activation of the NF-κB pathway. Ibrutinib is an oral agent which binds covalently to Cys-481 of BTK, causing its inhibition.
The publication by Byrd and coworkers 9 of a phase Ib-2 multicenter study to assess the safety and efficacy of ibrutinib in 85 relapsed-refractory CLL who had received a median of four previous lines of treatment was welcomed as the first mechanism-driven treatment for CLL 18.
The drug induced rapid shrinkage of lymph nodes with increase in the absolute lymphocyte count, reflecting a compartment shift. Over time, this lymphocytosis gradually resolved in the majority of the cases.
Toxicity was modest (Table 2), with grade 1–2 diarrhea, fatigue, and upper respiratory tract infection being the most common events.
Responses were independent of stage, number of previous therapies, and 17p−. At 26 months an impressive 75% PFS and 83% OS were observed. In this and in another phase II trial 43, there was no apparent difference in the incidence of response between patients with and without 17p−. However, disease progression occurred in 11 patients in the trial by Byrd and coworkers 9, 10 of whom had 17p− or 11q−. Interestingly, whole exome sequencing at baseline and after disease progression showed single nucleotide variations in three patients in the relapse sample 44. Two patients had distinct mutations that encoded a cysteine-to-serine substitution at position 481 of BTK (C481S) and the third patient acquired a potential gain-of-function mutation encoding a R665W substitution in PLCg2, a substrate of BTK, consistent with constitutive PLCg2 activation. Although rare, the acquisition of C481S BTK and R665W PLCg2 mutations in the setting of resistance suggests mechanisms of ibrutinib resistance. In another study 45, resistance to ibrutinib was observed in patients showing clonal evolution with the appearance of driver SF3B1 mutations or 8p deletion arising from a background of preexisting 17p− or 11q−.
The favorable therapeutic index, along with its tolerability and efficacy in the first-line setting 46 may facilitate the use of ibrutinib in combination with other agents to limit the increase in peripheral lymphocytosis and to further improve its efficacy 47,48.
Idelalisib (GS1101–CAL101)
CAL-101 inhibits PI3K-Δ, causing apoptosis in CLL cells, sparing T-cells or NK cells. In vitro, CAL-101 was able to sensitize CLL cells to the effects of cytotoxic drugs and steroids and to interact with BCR signaling, possibly reflecting a dual mechanism of action 49.
A clear benefit of idelalisib and rituximab over rituximab alone was documented independent of the presence or absence of 17p− in heavily pretreated patients who were not able to receive chemotherapy due to cytopenias or comorbidities 50. Furthermore, Coutre and coworkers 51 demonstrated durable responses in the majority of patients using idelalisib in combination with rituximab and/or bendamustine. As with ibrutinib, nodal response was associated with lymphocytosis; this effect was limited by adding ofatumumab in one study of 15 patients producing a 94% ORR 52. The favorable safety profile of idelalisib allowed the administration of this oral PI3K-Δ inhibitor at the full single dosage with concomitant chemoimmunotherapy and provided the basis for the initiation of studies evaluating its efficacy in combination with rituximab or bendamustine ± rituximab, with fludarabine or chlorambucil. Idelalisib showed robust activity independent of the presence of 17p− both in pretreated and in untreated patients 53,54.
BCL2 Antagonists
BCL2 is overexpressed by CLL and plays an antiapoptotic role 55. The BCL2 gene antisense nucleotide oblimersen did not produce significant survival advantage in an intent to treat analysis 56. More recently, the Bcl-2 antagonist ABT-263 showed activity in CLL with dose-limiting thrombocytopenia due to concomitant Bcl-xL inhibition. A single dose of the compound ABT-199 targeting more specifically Bcl-2 resulted in potent tumor lysis without significant effect on platelet count in three patients 19. Fifty-six previously treated patients were enrolled in a phase-I study of ABT-199 57 producing a 21% CR rate with few grade 3/4 adverse events. Interestingly responses were independent of the presence of 17p− and of fludarabine-refractory disease. Consistent with these results a greater that 87.5% ORR was reported in relapsed/refractory CLL with 17p− and/or TP53 mutation 58.
Lenalidomide
Treatment interfering with the interactions of CLL lymphocytes in the microenvironment and the immune system using lenalidomide is under investigation 59,60.
Shanafelt and coworkers 61 reported on a trial of pentostatine, cyclophosphamide, and rituximab as induction regimen followed by lenalidomide consolidation in untreated CLL, showing improvement in the quality of response in 24%.
The association of lenalidomide with rituximab was effective in relapsed/refractory CLL (Table 2), including the 17p− subset, where a 53% ORR was observed 62. Preliminary data showed that this combination was effective in untreated CLL 63.
11q−
11q deletion occurs in 10–15% of the cases and involves ATM, a principal DNA damage response gene 64–66.
11q− was associated with an inferior prognosis 34,67, however, the combination of fludarabine and alkylating agents improved the outcome in this cytogenetic subset of CLL 68. A 40% CR rate in previously untreated 11q− patients was reported by using bendamustine and rituximab 69 and evidence was provided that the combination of purine analogs with cyclophosphamide and rituximab may overcome the negative prognostic impact of this chromosome deletion 70. An analysis of prognostic factors in the CLL8 trial did not identify 11q− as predictor of a shorter PFS in multivariable analysis 71.
In the relapsed/refractory setting a 92% and 57% ORR were achieved by the combination bendamustine and rituximab 72 and by flavopiridol as single agent, respectively 42.
BTK inhibitor ibrutinib proved effective in this cytogenetic subset, however it is worth noting that, possibly due to clonal evolution, those patients with 11q− or 17p− may progress under ibrutinib more frequently than patients without 11q−/17p− 9. Likewise treatment by idelalisib and ABT-199 proved effective irrespective of the presence of 11q− 53,58.
SF3B1 and NOTCH1 Mutations
Lesions of SF3B1, NOTCH1, and BIRC3 were clearly associated with relapsed/refractory disease 17,73.
The significance of SF3B1 and NOTCH1 mutations were studied in 494 patients enrolled in the UK LRFCLL4 trial, randomizing patients to receive chlorambucil, fludarabine, or fludarabine and cyclophosphamide 36. While no difference in terms of ORR was noted in each trial arm for these lesions, NOTCH1 and SF3B1 mutations were associated with shorter OS and SF3B1 gene was associated with reduced PFS in FC-treated patients. Likewise, SF3B1 mutations showed independent negative prognostic value for PFS in patients receiving first-line FC and FCR treatment in the CLL8 trial 74. Interestingly, NOTCH1 mutations appeared to identify a subset of CLL patients that did not benefit from the addition of rituximab to FC 74. In line with these findings the adjunct of the anti-CD20 antibody ofatumumab to chlorambucil prolonged significantly PFS in a phase III trial, but this benefit was not observed in those patients with NOTCH1 mutations 75. In another analysis, 18 NOTCH1-mutated patients showed an inferior CR rate under fludarabine associated with alemtuzumab or cyclophosphamide as compared with patients without this aberration 76.
To the contrary, idelalisib, alone in combination with rituximab or chemoimmunotherpy combinations proved equally effective irrespective of the presence of NOTCH1 mutations in a study including 232 patients 53.
Conclusion
Modern CLL treatment is a remarkable example of how biologic studies and clinical expertise may converge, providing a rationale basis for the development of effective treatments, resulting in a significant improvement of the number and quality of responses, quality of life, and survival 77 in the majority of age groups, including the elderly population 20,26.
In genetically defined high-risk disease effective first-line chemoimmunotherapy combinations improved the quality and duration of response in 11q− and 17p-/TP53 mutated cases; allogeneic bone marrow transplantation overcame the unfavorable prognostic significance of 11q−, 17p−/TP53 mutations, NOTCH1, SF3B1 mutations and very effective compounds targeting the BCR signaling or BCL2, provided excellent and durable results in the setting of chemorefractory disease and in untreated patients 46.
The cost of hemopoietic neoplasms is an issue in high-income countries 78 and the development on novel treatment in CLL is likely to become soon a real challenge for the national health systems 79.
However, it is worth noting that the pharmacoeconomic analysis performed by the National Institute of Health and Clinical Excellence in the United Kingdom recognized that FCR, a regimen improving survival in a direct comparison with the best chemotherapy combination was a cost-effective use of NHS resources. The predicted efficacy of very potent, targeted, and nonchemotherapeutic drugs in CLL along with the development of sensitive predictors of response offer a unique opportunity to intensify coordinated research programmes aimed at providing compelling evidence of the positive cost/efficacy ratio of these novel agents.
Conflict of Interest
None declared.
References
- 1.Hillmen P. Using the biology of chronic lymphocytic leukemia to choose treatment. Hematol. Am. Soc. Hematol. Educ. Prog. 2011;2011:104–109. doi: 10.1182/asheducation-2011.1.104. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N. Development and evolution of chronic lymphocytic leukemia. Hematol. Educ. 2012;6:73–92. [Google Scholar]
- 3.Rigolin GM, Saccenti E, Rizzotto L, Ferracin M, Martinelli S, Formigaro L, et al. Genetic subclonal complexity and miR125a-5p down-regulation identify a subset of patients with inferior outcome in low-risk CLL patients. Oncotarget. 2014;5:140–149. doi: 10.18632/oncotarget.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Rabe KG, Kay NE, Zent CS, Jelinek DF, Reinalda MS, et al. Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer. 2010;116:4777–4787. doi: 10.1002/cncr.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccone M, Agostinelli C, Rigolin GM, Piccaluga PP, Cavazzini F, Righi S, et al. Proliferation centers in chronic lymphocytic leukemia: correlation with cytogenetic and clinicobiological features in consecutive patients analyzed on tissue microarrays. Leukemia. 2012;26:499–508. doi: 10.1038/leu.2011.247. [DOI] [PubMed] [Google Scholar]
- 6.Gaidano G, Foà R, Dalla-Favera R. Molecular pathogenesis of chronic lymphocytic leukemia. J. Clin. Invest. 2012;122:3432–3438. doi: 10.1172/JCI64101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigolin GM, Maffei R, Rizzotto L, Ciccone M, Sofritti O, Daghia G, et al. Circulating endothelial cells in patients with chronic lymphocytic leukemia: clinical-prognostic and biologic significance. Cancer. 2010;116:1926–1937. doi: 10.1002/cncr.24961. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2013;88:803–816. doi: 10.1002/ajh.23491. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, Murphy T, Howard RS, Lucas MS, Goodrich A, Park K, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J. Clin. Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 12.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 14.Cuneo A, Rigolin GM, Bigoni R, De Angeli C, Veronese A, Cavazzini F, et al. Chronic lymphocytic leukemia with 6q− shows distinct hematological features and intermediate prognosis. Leukemia. 2004;18:476–483. doi: 10.1038/sj.leu.2403242. [DOI] [PubMed] [Google Scholar]
- 15.Cavazzini F, Hernandez JA, Gozzetti A, Russo Rossi A, De Angeli C, Tiseo R, et al. Chromosome 14q32 translocations involving the immunoglobulin heavy chain locus in chronic lymphocytic leukaemia identify a disease subset with poor prognosis. Br. J. Haematol. 2008;142:529–537. doi: 10.1111/j.1365-2141.2008.07227.x. [DOI] [PubMed] [Google Scholar]
- 16.Zenz T, Fröhling S, Mertens D, Döhner H, Stilgenbauer S. Movingfromprognostic to predictivefactors in chronic lymphocytic leukaemia (CLL) Best Pract. Res. Clin. Haematol. 2010;23:71–84. doi: 10.1016/j.beha.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Foà R, Del Giudice I, Guarini A, Rossi D, Gaidano G. Clinical implications of the molecular genetics of chronic lymphocytic leukemia. Haematologica. 2013;98:675–685. doi: 10.3324/haematol.2012.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foà R, Guarini A. A mechanism-driven treatment for chronic lymphocytic leukemia? N. Engl. J. Med. 2013;369:85–87. doi: 10.1056/NEJMe1303054. [DOI] [PubMed] [Google Scholar]
- 19.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 20.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1313984. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111:4916–4921. doi: 10.1182/blood-2007-12-129379. [DOI] [PubMed] [Google Scholar]
- 22.Abrisqueta P, Pereira A, Rozman C, Aymerich M, Giné E, Moreno C, et al. Improving survival in patients with chronic lymphocytic leukemia (1980–2008): the Hospital Clinic of Barcelona experience. Blood. 2009;114:2044–2050. doi: 10.1182/blood-2009-04-214346. [DOI] [PubMed] [Google Scholar]
- 23.Kristinsson SY, Dickman PW, Wilson WH, Caporaso N, Björkholm M, Landgren O. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973-2003 in Sweden. Haematologica. 2009;94:1259–1265. doi: 10.3324/haematol.2009.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenbach JP, Karanikolos M, McKee M. The unequal health of Europeans: successes and failures of policies. Lancet. 2013;381:1125–1134. doi: 10.1016/S0140-6736(12)62082-0. [DOI] [PubMed] [Google Scholar]
- 25.Parikh SA, Rabe KG, Kay NE, Call TG, Ding W, Schwager SM, et al. Chronic lymphocytic leukemia in young (less than 55 years) patients: a comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99:140–147. doi: 10.3324/haematol.2013.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J, et al. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential cancer and leukemia group B studies. J. Clin. Oncol. 2012;31:440–447. doi: 10.1200/JCO.2011.41.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer K, Bahlo J, Fink AM, Busch R, Böttcher S, Mayer J, et al. Chemoimmunotherapy based treatment for CLL: extended follow up of the CLL8 protocol, a randomized phase-III trial of the German CLL Study Group (GCLLSG) comparing fludarabine and cyclophosphamide (FC) to FC plus rituximab (FCR) for previously untreated patients with chronic lymphocytic leukemia (CLL): results on survival, progression-free survival, delayed neutropenias and secondary malignancies confirm superiority of the FCR regimen. Blood. 2012;120 (ASH Annual Meeting Abstracts) abstract 435. [Google Scholar]
- 28.Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 29.Shanafelt TD. Predicting clinical outcome in CLL: how and why. Hematol. Am. Soc. Hematol. Educ. Prog. 2009;2009:421–429. doi: 10.1182/asheducation-2009.1.421. [DOI] [PubMed] [Google Scholar]
- 30.Cavazzini F, Ciccone M, Negrini M, Rigolin GM, Cuneo A. Clinicobiologic importance of cytogenetic lesions in chronic lymphocytic leukemia. Expert Rev. Hematol. 2009;2:305–314. doi: 10.1586/ehm.09.22. [DOI] [PubMed] [Google Scholar]
- 31.Rigolin GM, Cibien F, Martinelli S, Formigaro L, Rizzotto L, Tammiso E, et al. Chromosome aberrations detected by conventional karyotyping using novel mitogens in chronic lymphocytic leukemia with “normal” FISH: correlations with clinicobiologic parameters. Blood. 2012;119:2310–2313. doi: 10.1182/blood-2011-11-395269. [DOI] [PubMed] [Google Scholar]
- 32.Rossi D, Rasi S, Spina V, Bruscaggin A, Monti S, Ciardullo C, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121:1403–1412. doi: 10.1182/blood-2012-09-458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 34.Grever MR, Lucas DM, Dewald GW, Neuberg DS, Reed JC, Kitada S, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III Trial E2997. J. Clin. Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 35.Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S, et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95:1705–1712. doi: 10.3324/haematol.2010.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oscier DG, Rose-Zerilli MJ, Winkelmann N, Gonzalez de Castro D, Gomez B, Forster J, et al. The clinical significance of NOTCH1 and SF3B1 mutations in the UK LRF CLL4 trial. Blood. 2013;121:468–475. doi: 10.1182/blood-2012-05-429282. [DOI] [PubMed] [Google Scholar]
- 37.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Prog. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Tam CS, Shanafelt TD, Wierda WG, Abruzzo LV, Van Dyke DL, O'Brien S, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–964. doi: 10.1182/blood-2009-03-210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettitt AR, Jackson R, Carruthers S, Dodd J, Dodd S, Oates M, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J. Clin. Oncol. 2012;30:1647–1655. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 40.Dreger P, Schnaiter A, Zenz T, Böttcher S, Rossi M, Paschka P, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121:3284–3288. doi: 10.1182/blood-2012-11-469627. [DOI] [PubMed] [Google Scholar]
- 41.Visco C, Finotto S, Pomponi F, Sartori R, Laveder F, Trentin L, et al. The combination of rituximab, bendamustine, and cytarabine for heavily pretreated relapsed/refractory cytogenetically high-risk patients with chronic lymphocytic leukemia. Am. J. Hematol. 2013;88:289–293. doi: 10.1002/ajh.23391. [DOI] [PubMed] [Google Scholar]
- 42.Woyach JA, Lozanski G, Ruppert AS, Lozanski A, Blum KA, Jones JA, et al. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia. 2012;26:1442–1444. doi: 10.1038/leu.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farooqui M, Aue G, Valdez J, Martyr S, Jones J, Soto S, et al. Single agent ibrutinib (PCI-32765) achieves equally good and durable responses in chronic lymphocytic leukemia (CLL) patients with and without deletion 17p. Blood. 2013 (ASH Annual Meeting Abstracts), abs no. 673. [Google Scholar]
- 44.Chang B. Y RRFurman, Zapatka M, Barrientos JC, Li D, Steggerda S, et al. Use of tumor genomic profiling to reveal mechanisms of resistance to the BTK inhibitor ibrutinib in chronic lymphocytic leukemia (CLL) J. Clin. Oncol. 2013;31 (Suppl. abs no. 7014) [Google Scholar]
- 45.Burger I, Landau D, Hoellenriegel J, Sougnez C, Schlesner M, Ishaque N, et al. Clonal evolution in patients with chronic lymphocytic leukemia (CLL) developing resistance to BTK inhibition. Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 866. [Google Scholar]
- 46.O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown JR, Barrientos JC, PM Barr, Flinn I, Burger J, Salman Z, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: final results of a phase 1b study. Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 525. [Google Scholar]
- 48.Burger JA KeatingMJ, WG Wierda, Hoellenriegel J, Jeyakumar G, Ferrajoli A, et al. Ibrutinib in combination with rituximab (iR) is well tolerated and induces a high rate of durable remissions in patients with high-risk chronic lymphocytic leukemia (CLL): new, updated results of a phase II trial In 40 patients. Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 675. [Google Scholar]
- 49.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1315226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coutre SE, Leonard JP, Furman RR, Barrientos JC, de Vos S, Flinn IW, et al. Combinations of the selective phosphatidylinositol 3-kinase-delta (PI3Kdelta) inhibitor GS–1101 (CAL-101) with rituximab and/or bendamustine are tolerable and highly active in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): results from a phase I study. Blood. 2012;120 (ASH Annual Meeting Abstracts), abs no. 191. [Google Scholar]
- 52.Furman RR, Barrientos JC, Sharman JP, De Vos S, Leonard J, Coutre SE, et al. 2012. A phase I/II study of the selective phosphatidylinositol 3-kinase-delta (PI3K{delta}) inhibitor, GS-1101 (CAL-101), with ofatumumab in patients with previously treated chronic lymphocytic leukemia (CLL). ASCO Annual Meeting Abstract 6518.
- 53.Coutre SE, Leonard JP, Barrientos JC, De Vos S, Flinn I, Furman RR, et al. Clinical activity of idelalisib (GS-1101), a selective inhibitor of pi3kδ, in phase 1 and 2 trials in chronic lymphocytic leukemia (CLL): effect of Del(17p)/TP53 mutation, Del(11q), IGHV mutation, and NOTCH1 mutation. Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 1632. [Google Scholar]
- 54.De Vos S, Furman RR, Barrientos JC, Wagner-Johnston ND, Flinn I, Sharman JP, et al. Idelalisib, a selective inhibitor Of PI3Kδ, in combination with bendamustine, fludarabine or chlorambucil in patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 2878. [Google Scholar]
- 55.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82:1820–1828. [PubMed] [Google Scholar]
- 56.O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J. Clin. Oncol. 2009;27:5208–5212. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seymour JF, Davids MS, Pagel JM, Kahl BS, Wierda WG, Puvvada S, et al. Bcl-2 inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 872. [Google Scholar]
- 58.Anderson MA, Tam CS, Seymour JF, Bell A, Westerman DA, Juneja S, et al. Selective Bcl-2 inhibition with ABT-199 is highly active against chronic lymphocytic leukemia (CLL) irrespective of TP53 mutation or dysfunction. Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 1304. [Google Scholar]
- 59.Lapalombella R, Andritsos L, Liu Q, May SE, Browning R, Pham LV, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–2629. doi: 10.1182/blood-2009-09-242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL) Blood. 2013;121:4137–4141. doi: 10.1182/blood-2012-12-470005. [DOI] [PubMed] [Google Scholar]
- 62.Badoux XC, Keating MJ, Wen S, Wierda WG, O'Brien SM, Faderl S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2013;31:584–591. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.James DF, Brown JR, Werner L, Wierda WG, Barrientos JC, Castro J, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia (CLL) a multicenter study of the CLL research consortium. Blood. 2011;118 (ASH Annual Meeting Abstracts), abstract 291. [Google Scholar]
- 64.Skowronska A, Parker A, Ahmed G, Oldreive C, Davis Z, Richards S, et al. Biallelic ATM inactivation significantly reduces survival in patients treated on the United Kingdom Leukemia Research Fund Chronic Lymphocytic Leukemia 4 trial. J. Clin. Oncol. 2012;30:4524–4532. doi: 10.1200/JCO.2011.41.0852. [DOI] [PubMed] [Google Scholar]
- 65.Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia. 2007;21:2442–2451. doi: 10.1038/sj.leu.2404935. [DOI] [PubMed] [Google Scholar]
- 66.Zenz T, Mohr J, Eldering E, Kater AP, Bühler A, Kienle D, et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–3808. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 67.Döhner H, Stilgenbauer S, James MR, Benner A, Weilguni T, Bentz M, et al. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89:2516–2522. [PubMed] [Google Scholar]
- 68.Ding W, Ferrajoli A. Evidence-based mini-review: the role of alkylating agents in the initial treatment of chronic lymphocytic leukemia patients with the 11q deletion. Hematol. Am. Soc. Hematol. Educ. Prog. 2010;2010:90–92. doi: 10.1182/asheducation-2010.1.90. [DOI] [PubMed] [Google Scholar]
- 69.Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 70.Tsimberidou AM, Tam C, Abruzzo LV, O'Brien S, Wierda WG, Lerner S, et al. Chemoimmunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer. 2009;115:373–380. doi: 10.1002/cncr.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fink AM, Böttcher S, Ritgen M, Fischer K, Pflug N, Eichhorst B, et al. Prediction of poor outcome in CLL patients following first-line treatment with fludarabine, cyclophosphamide and rituximab. Leukemia. 2013;27:1949–1952. doi: 10.1038/leu.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 2011;29:3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 73.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stilgenbauer S, Busch R, Schnaiter A, Paschka P, Rossi M, Döhner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2012;120:433. doi: 10.1182/blood-2014-01-546150. (ASH Annual Meeting Abstracts) [DOI] [PubMed] [Google Scholar]
- 75.Tausch E, Beck P, Schlenk RF, Kless S, Galler C, Hillmen P, et al. NOTCH1 mutation and treatment outcome in CLL patients treated with chlorambucil (Chl) or ofatumumab-Chl (O-Chl): results from the phase III study complement 1 (OMB110911) Blood. 2013;122 (ASH Annual Meeting Abstracts), abs no. 527. [Google Scholar]
- 76.Chiaretti S, Marinelli M, Del Giudice I, Bonina S, Gabrielli S, Piciocchi A, et al. NOTCH1, SF3B1 and BIRC3 mutations in chronic lymphocytic leukemia (CLL) patients requiring first-LINE treatment: correlation with biological parameters and response to treatment. Blood. 2012;120 doi: 10.3109/10428194.2014.898760. (ASH Annual Meeting Abstracts), abs no. 1784. [DOI] [PubMed] [Google Scholar]
- 77.Hallek M. Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies. Blood. 2013;122:3723–3734. doi: 10.1182/blood-2013-05-498287. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 79.Blankart CR, Koch T, Linder R, Verheyen F, Schreyögg J, Stargardt T. Cost of illness and economic burden of chronic lymphocytic leukemia. Orphanet J. Rare Dis. 2013;8:32. doi: 10.1186/1750-1172-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 81.Bosch F, Abrisqueta P, Villamor N, Terol MJ, González-Barca E, Ferra C, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J. Clin. Oncol. 2009;27:4578–4584. doi: 10.1200/JCO.2009.22.0442. [DOI] [PubMed] [Google Scholar]
- 82.Parikh SA, Keating MJ, O'Brien S, Wang X, Ferrajoli A, Faderl S, et al. Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab, and rituximab for high-risk chronic lymphocytic leukemia. Blood. 2011;118:2062–2068. doi: 10.1182/blood-2011-01-329177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tam CS, O'Brien S, Lerner S, Khouri I, Ferrajoli A, Faderl S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk. Lymphoma. 2007;48:1931–1939. doi: 10.1080/10428190701573257. [DOI] [PubMed] [Google Scholar]
- 84.Robak T, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 85.Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]


