Abstract
The aim of this study was to determine the proportion of human papilloma virus (HPV)-positive cases in tonsillar carcinomas and investigate its development over the last decade. Further aim was to show the oncologic results in accord to HPV status and various treatment modalities. A retrospective study was conducted between 2000 and 2012 and included 275 patients treated for tonsillar carcinoma. P16 immunohistochemistry was used as a surrogate marker for HPV-associated carcinogenesis. A total of 101 (36.7%) patients proved to be p16 positive and 174 p16 negative. 80.2% of the p16-positive cases presented with T1-2 tumor. Of the early-stage patients, 79% of the p16-positive and 52.3% of the p16-negative presented with lymph node metastases. The percentage of p16-positive patients increased from 23.2% in the period 2005–2007 to 58.6% in the period 2010–2012 in the whole population and from 30.9% to 76.9% in T1-2 carcinomas. Early T-category p16-positive carcinomas had significantly better disease-specific survival (92.4% vs. 75.5%, P = 0.007) and overall survival (OS, 79.6% vs. 54.3%, P < 0.001) compared to p16-negative tumors. This study showed an increase in the percentage of p16-positive patients in tonsillar carcinoma from 23.2% in the years between 2005 and 2007 to 58.6% between 2010 and 2012. The majority (80.2%) of p16-positive patients presented with early T-category tumor but most of these (79.0%) had also lymph node metastases. Nevertheless, p16-positive patients had excellent oncologic results after surgery and adjuvant radiotherapy and could be considered for de-escalation of treatment.
Keywords: HPV, oropharyngeal cancer, p16 oncoprotein, surgery, survival, tonsillar carcinoma
Introduction
The role of human papilloma virus (HPV) in head and neck carcinoma and particularly in oropharyngeal carcinoma (OPC) has gained a great deal of attention in recent years. In contrast to other regions in head and neck oncology, the incidence of OPC has increased significantly in many countries 1. This continuous rise has been mainly attributed to HPV infection. Näsman et al. reported an increase in the proportion of HPV-related tonsillar and base of the tongue carcinomas in the Stockholm region, from approximately 23% in the 1970s to 93% in 2007 2. A recent systematic review and meta-analysis by Mehanna et al. showed that the proportion of HPV-related OPC has increased significantly worldwide from 40.5% in studies recruiting patients before 2000 to 72.2% in studies recruiting patients after 2005 3.
In addition to its causative role, HPV infection also proved to have a prognostic value in many studies. In a large, randomized, controlled study, Ang et al. showed that HPV status is an independent prognostic factor for survival in OPC 4. Other studies also demonstrated a significant survival benefit for HPV-positive OPC 5. Radiochemosensitivity of HPV-positive carcinomas has therefore been assumed and an alteration of treatment modality according to HPV status has been discussed 6. The aim of this study was to determine the proportion of HPV-positive cases in tonsillar carcinomas and to investigate its development over the last decade. A further aim was to show the oncologic results according to HPV status.
Methods
A total of 457 patients who referred to our academic tertiary referral center (Department of Otorhinolaryngology, Head and Neck Surgery, University of Erlangen-Nuremberg Medical School, Erlangen, Germany) between 2000 and 2012 with previously untreated squamous cell carcinomas (SCC) of the tonsillar region were considered for selection. Those patients were included about whom information about HPV status could be collected. HPV infection in tumor tissue was retrospectively determined using p16 immunohistochemistry as a highly sensitive and specific surrogate marker for HPV-associated carcinogenesis 7. P16 immunohistochemistry was performed using a primary antibody retrieved from Santa Cruz Biotechnology (clone JC8, dilution: 1:100). Tumors were considered positive for p16 when strong nuclear and cytoplasmic staining was present in >60% of cells 7. The status of p16 oncoprotein expression was successfully determined in 275 patients with available paraffin blocks. In 182 cases, tumor blocks were lost or damaged. Dependencies with regard to the two groups were controlled and rated using a chi-square test and phi coefficient.
Staging was reevaluated after reviewing pretherapeutic imaging, surgical, and pathology reports according to the 2010 American Joint Committee on Cancer (AJCC) and Union Internationale Contre le Cancer (UICC) classification 8. Standard diagnostics included clinical examination, ultrasonography, and computed tomography. Magnetic resonance imaging (MRI) was also used in a few cases. The appropriate treatment modality was decided by our interdisciplinary tumor board. Factors influencing the decision were the operability of the tumor, general health status, and personal preference of each patient.
The primary endpoint of the study was to examine the development of the proportion of p16-positive patients over the last decade. Further endpoints of the analysis were disease-specific survival (DSS), local control (LC), and regional control (RC). DSS was defined using the time from the date of diagnosis to death from the cancer or complications of treatment or last follow-up. Time to LC or RC was calculated from the date of initial diagnosis to the date of most recent clinical review when local or regional recurrence was confirmed or last follow-up. Local recurrence was defined as invasive carcinoma developing after completion of initial treatment at the anatomic site of the primary tumor. Calculations of 5-year overall DSS, LC, and RC were made using Kaplan–Meier estimates and compared with the log-rank test. A P value of less than 0.05 was considered significant. Considering the level of measurement, multivariate analysis was performed with an appropriate logistic regression. Resultant odds ratios were rated with corresponding Wald test and Nagelkerke's pseudo-R2. All statistical analyses were performed using SPSS Version 20 (SPSS In., Chicago, IL). Relevant approval was obtained from the institutional review board of the hospital (“Ethikkommission Universität”, Erlangen).
Results
The final study population included 275 patients who met the inclusion criteria. A total of 101 (36.7%) patients proved to be p16-positive and 174 were p16-negative. A proportion of 82.7% (225/275) of the patients presented with a primary tumor, 11.8% (32/275) with a second malignancy, and 5.5% (15/275) with multiple tumors. The median age at presentation was 57 years, ranging from 38 to 88 years (SD 9.95). The p16-positive patients had a median age of 56 years and the corresponding figure in p16-negative patients was 59 years. Fifty-three (19.3%) patients were women, with a men-to-women ratio of 4.2:1. Mean follow-up was 3.1 years (range 0.3–11.5 years). Detailed patient demographics according to p16 status are presented in Table 1.
Table 1.
Detailed description of demographics, treatment modalities, and histological differentiation according to p16 status
| Characteristics | p16-Positive (101) | p16-Negative (174) |
|---|---|---|
| Gender | Male: 74 (73.3%) | Male: 148 (85.1%) |
| Female: 27 (26.7%) | Female: 26 (14.9%) | |
| Age | Median: 56, range: 38–83 | Median: 59, range: 42–88 |
| Smoking | Smokers: 53 (52.5%), Ex-smokers: 15 (14.9%), Nonsmokers: 33 (32.7%) | Smokers: 121 (69.5%), Ex-smokers: 39 (22.4%), Nonsmokers: 14 (8.1%) |
| Primary treatment | Surgical: 86 (85.1) | Surgical: 118 (67.8%) |
| Nonsurgical: 15 (14.9%) | Nonsurgical: 56 (32.2%) | |
| Surgical technique | TLM: 11 (12.8%) | TLM: 13 (11.0%) |
| Electrocautery: 71 (82.6%) | Electrocautery: 89 (75.4%) | |
| Combined: 4 (4.6%) | Combined: 16 (13.6%) | |
| Adjuvant treatment | None: 15 (17.4%) | None: 35 (29.7%) |
| RT: 33 (38.4%) | RT: (39.0%) | |
| RCT: 38 (44.2%) | RCT: 37 (31.4%) | |
| Histological differentiation | Well-moderate (G1, G2): 51 (50.5%) | Well-moderate (G1,G2): 117 (67.2%) |
| Poor – undifferentiated (G3, G4): 50 (49.5%) | Poor – undifferentiated (G3,G4): 57 (32.8%) |
TLM, tranoral laser microsurgery; RT, radiotherapy; RCT, radiochemotherapy
Table 2 shows the distribution of patients according to T-category and p16 status. 80.2% of the p16-positive cases presented with early T-category. On the other hand, 50.6% of the p16-negative patients had early T-category and 47.7% had advanced T-category. Table 3 shows the distribution of patients according to N-category. Furthermore, we investigated the tendency for cervical metastases in early and advanced T-category separately, because most of the p16-positive patients presented with early T-category. Of the early-stage patients, 79% of the p16-positive, and 52.3% of the p16-negative patients presented with lymph node metastases (P < 0.001). Of 16 p16-positive cases that initially presented with cN0 status and underwent an elective neck dissection, 11 had at least one lymph node metastasis, giving an occult metastasis rate of 68.7% (11/16). By contrast, p16-negative patients had an occult metastasis rate of 18.2% (6/33).
Table 2.
Number of cases according to T-category and p16 status
| T-category | T1 | T2 | T3 | T4a | T4b | Tx | All |
|---|---|---|---|---|---|---|---|
| p16 Positive | 32 (31.7%) | 49 (48.5%) | 11 (10.9%) | 7 (6.9%) | 2 (2.0%) | 0 | 101 |
| p16 Negative | 40 (23.0%) | 48 (27.6%) | 36 (20.7%) | 42 (24.1%) | 5 (2.9%) | 3 (1.7%) | 174 |
| All | 72 (26.2%) | 97 (35.3%) | 47 (17.1%) | 49 (17.8%) | 7 (2.5%) | 3 (1.1%) | 275 |
Table 3.
Number of cases according to N-category, p16 status, and T subcategory
| N-category | N0 | N1 | N2 | N3 | All | |
|---|---|---|---|---|---|---|
| p16 Positive | T1-2 | 17 (21.0%) | 17 (21.0%) | 46 (56.8%) | 1 (1.2%) | 81 |
| T3-4 | 7 (35.0%) | 0 | 12 (60.0%) | 1 (5.0%) | 20 | |
| All | 24 (23.8%) | 17 (16.8%) | 58 (57.4%) | 2 (2.0%) | 101 | |
| p16 Negative | T1-2 | 42 (47.7%) | 12 (13.6%) | 30 (34.1%) | 4 (4.6%) | 88 |
| T3-4 | 15 (17.4%) | 3 (3.5%) | 60 (69.8%) | 8 (9.3%) | 86 | |
| All | 57 (32.8%) | 15 (8.6%) | 90 (51.7%) | 12 (6.9%) | 174 | |
| All | 81 | 32 | 148 | 14 | 275 |
The development of incidence of p16-positive tonsillar carcinoma as a percentage of the whole population over the years 2000–2012 is shown in Figure 1. A sudden increase in the percentage of p16-positive tonsillar carcinomas can be noted from 2010 onwards. In order to improve statistical comparability of the development of incidence over the years, we divided the study period into intervals with comparable patient numbers: 2000–2004, 2005–2007, 2007–2009, and 2010–2012. The results can be seen in Table 4. The first group (2000–2004) is biased by a higher percentage of p16-positive male patients and a higher proportion of T2-carcinomas. Therefore, interval 2005–2007 was set as the reference category for further analysis. The years 2005–2007 and 2008–2009 show no significant dependence on p16 (P = 0.887). On the other hand, patients from the period 2010–2012 had a statistically significant higher chance of being p16-positive compared to the period 2005–2007. The increase was from 23.2% to 58.6% with Nagelkerke's pseudo-R2 of 0.099 (P < 0.001, OR = 4.693, Phi = 0.361, 95% CI: 2.18–10.09). If early tonsillar carcinomas (T1-2) are considered alone, then the percentage of p16-positive carcinomas also increases suddenly from 2010 onwards. The comparison of the intervals 2005–2007 and 2010–2012 revealed an increase from 30.9% to 76.9% with Nagelkerke's pseudo-R2 of 0.192 (P < 0.001, OR = 7.44, Phi = 0.46, 95% CI: 2.76–20.04).
Figure 1.
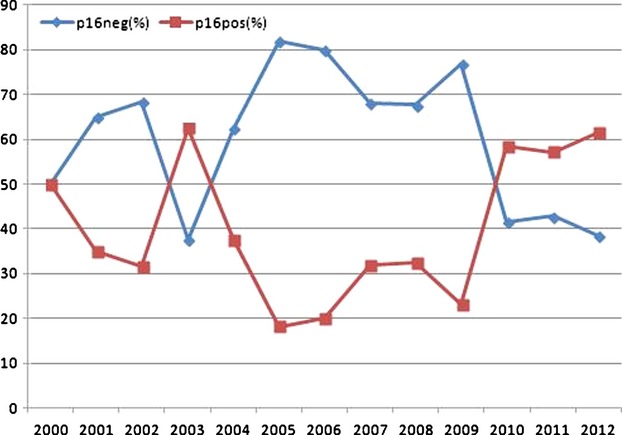
Incidence of p16-positive tonsillar carcinoma as a percentage of the whole population over the years 2000–2012
Table 4.
Development of p16-positive cases in comparison with p16-negative cases from 2000 to 2012 in accordance with T-category
| Year grouped | 2000–2004 | 2005–2007 | 2008–2009 | 2010–2012 | |
|---|---|---|---|---|---|
| T1-2 | p16 Positive | 25 | 13 | 13 | 30 |
| p16 Negative | 19 | 29 | 21 | 9 | |
| All T | p16 Positive | 31 | 16 | 20 | 34 |
| p16 Negative | 44 | 53 | 53 | 24 | |
| All | 275 | 75 | 69 | 73 | 58 |
The second aim of the study was to compare the oncologic results of patients with tonsillar carcinomas according to p16 status. Patients with p16-positive carcinomas (101 cases) had significantly better DSS (89.1% vs. 64.9%, P < 0.001) and OS (76.5% vs. 46.1%, P < 0.001) compared to p16-negative patients (174 cases). Due to the fact that over 80% of patients with p16-positive tumors presented with a locally early tumor (T1-2), comparison of the whole patient group is accompanied by a strong bias and therefore not acceptable. On the other hand, early T-category patients had an almost identical distribution between p16-positive (81 patients) and p16-negative (88 patients) patients. Further oncologic analysis was therefore performed in 169 patients with T1-2 tonsillar carcinoma. Primary surgical therapy was performed in 147 cases and primary nonsurgical therapy in 22 cases. Of the patients who underwent surgery, 42 had monotherapy and 113 also had adjuvant therapy. Table 5 shows the oncologic results according to p16 status in early carcinoma. Patients with p16-positive carcinomas had significantly better DSS (92.4% vs. 75.5%, P = 0.007) (Fig. 2) and OS (79.6% vs. 54.3%, P < 0.001). The results of LC and RC were flawed because of the low number of events. Although most p16-positive patients presented with lymph node metastases (64/81), they showed excellent OS (81.1%) and DSS (91.7%), as shown in Table 6. A statistical comparison was not possible because of the low number of N0 patients. On the other hand, in p16-negative patients, presence of cervical lymph node metastases had a statistically significant impact on OS (60.8% vs. 48.4%, P = 0.003) and DSS (82.2 vs. 69.9%, P = 0.041).
Table 5.
Oncologic results according to p16 status in T1-2 tonsillar carcinoma
| 5-year KM estimate (%) (Total number of events) (95% CI) | |||||
|---|---|---|---|---|---|
| No. of patients | OS (P < 0.001) | DSS (P = 0.007) | LC | RC | |
| p16 Positive | 81 | 79.6 (13) (68–91) | 92.4 (5) (86–99) | 97.8 (1) (93–100)* | 98.6 (1) (96–100)* |
| p16 Negative | 88 | 54.3 (35) (42–66) | 75.5 (17) (65–86) | 86.6 (9) (78–95)* | 87.7 (7) (79–97)* |
| All | 169 | 66.0 (48) (57–75) | 83.9 (22) (77–90) | 92.2 (10) (87–97) | 93.5 (8) (89–98)* |
No meaningful interpretation possible, due to insufficient number of events.
Figure 2.
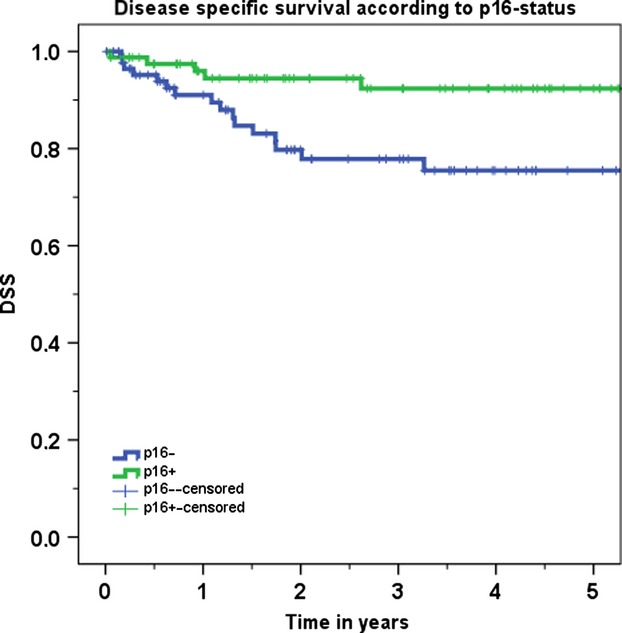
Disease-specific survival according to p16 status for T1-2 tonsillar carcinomas
Table 6.
Oncologic results according to p16 status and N-category in T1-2 tonsillar carcinoma
| 5-year KM estimate (%) (Total number of events) (95% CI) | ||||
|---|---|---|---|---|
| N0 versus N+ | OS | DSS | RC | |
| p16 Positive (81) | N0 (17) | 73.2 (4) (46–100)* | – (1) (–;–)* | – (0) (–;–)* |
| N+ (64) | 81.1 (9) (68–94) | 91.7 (4) (84–100)* | – (1) (–;–)* | |
| p16 Negative (88) | N0 (42) | 60.8 (15) (44–77) | 82.2 (7) (69–95)* | 84.3(4) (70–99)* |
| N+ (46) | 48.4 (20) (31–66) | 69.9 (10) (54–86) | 91.9 (3) (83–100)* | |
| All | 169 | 66.0 (48) (57–75) | 83.9 (22) (77–90) | 93.5 (8) (89–98)* |
No meaningful interpretation possible, due to insufficient number of events.
Discussion
The causative and prognostic association of HPV in head and neck carcinomas and particularly OPC has focused a great deal of attention on this infection 9. In older studies, there was a clear regional difference in incidence of HPV-related OPC, but recent studies show that differences in incidence between various countries, especially the United States and Europe, seem to have disappeared 3. In many countries, including the United Kingdom, the incidence of HPV-related OPC has doubled in the last 10 years. Data from Germany are very sparse and suggest an incidence of HPV-related OPC between 20 and 40% 10,11. Even within OPC, however, tonsillar and base of the tongue carcinomas seem to represent subsites of special focus. This study, therefore, also concentrates solely on tonsillar carcinoma 11–13. The weakness of this study is its retrospective nature and the use of p16 immunohistochemistry as an indirect marker of HPV-positive tonsillar carcinoma. The combination of p16 immunohistochemistry and HPV PCR testing could lead to improved validation of HPV positivity.
This study showed an overall incidence of 36.7% for HPV-related tonsillar carcinomas. As the majority of p16-positive patients presented with early local tumor, these carcinomas represent 47.9% (81/169) of T1 and T2 tumors in this study. One possible explanation for this phenomenon could be that p16-positive patients are present at a younger age and are more differentiated, therefore seeking medical care before the local tumor reached an advanced category. Another explanation could be that the presence of cervical metastases, which occurs earlier in p16-positive patients, leads them to seek medical advice earlier and also receive a more aggressive treatment. The development of the incidence of p16-positive patients over time in the last decade supplies important information about the demographics of the disease and future expectations. In this study, the percentage of p16-positive patients varied between 23.2% in the period 2005–2007 and 58.6% in the period 2010–2012. We could not identify any graduated increase in p16-positive patients over the years, but we did observe a sudden increase during the last 3 years of the study period. The same was true when only patients with T1-2 carcinomas were considered; there was an increase from 30.9% in the period 2005–2007 to 76.9% between 2010 and 2012. The reasons for this sudden increase remain unclear and although the results are statistically significant, they have to be interpreted with caution and more study years are needed before this change can be considered definitive. Nevertheless, this high incidence of HPV-related tonsillar carcinoma, particularly in early T-category, shows that preventive and therapeutic modalities should be developed to better fit the demands of this patient group.
There are a number of studies in the recent literature that identify HPV infection as an important prognostic factor leading to favorable oncologic outcomes in OPC 4,13–15. Most of these studies focus on locally advanced OPC and use radiochemotherapy as the primary treatment option. The well-known study by Ang et al., for example, does not include T1 tumors and all patients had either stage III or IV disease 4. There are very few studies that investigate local early carcinomas and the use of surgery as treatment modality 10,16. This study is the largest to date that compares p16-positive with p16-negative patients only in cases of locally early tumor extension. A statistically highly significant difference in favor of p16-positive cases was found in both OS and DSS.
Another very interesting result of our analysis is that, in most cases, p16-positive patients presented with a locally early T-category but with lymph node metastases. Therefore, although 80.2% of p16-positive patients had an early T-category, only 21.0% (17/81) of these patients also had an early stage of disease. Perhaps this also explains the highest rate of p16-positive lymph node metastases in patients with carcinoma of unknown primary. A recent study suggests that a downregulation of E-cadherin, a cell adhesion molecule, at the primary tumor site of HPV-related tonsillar carcinomas could be related to the increased tendency toward regional metastases 17. Nevertheless, p16-positive patients had excellent oncologic results even with the combination of early T-category and the presence of lymph node metastases. Most of these cases were treated with primary surgery and postoperative radiotherapy. These results contrast with recently published data on surgically treated early OPC 10. In that study, 31 p16-positive patients with T1-2 N0-1 OPC had similar OS (80.8% vs. 79.5%, P = 0.59) and DSS (95.2% vs. 91.9%, P = 0.44) to 52 p16-negative patients. Perhaps the reason for this discrepancy was that this study excluded patients with advanced N-category (N2-3), therefore leading to excellent oncologic results even in p16-negative patients. The results of this study strengthen this hypothesis, as the presence of cervical metastases had a significant impact on survival of p16-negative (DSS 82.2 vs. 69.9%, P = 0.037) but not p16-positive patients. Future studies should clarify why HPV-related carcinomas so frequently lead to regional metastases and why the impact on survival in these cases is not as devastating as with lymph node metastases in all other head and neck carcinomas.
This study is the first to show that p16-positive carcinomas not only frequently present with lymph node metastases but that they also have a very high rate of occult cervical metastases (68.7%), in cases with cN0 status. This rate is not only much higher compared to p16-negative patients but also compared to advanced carcinomas in other head and neck regions 18. The logical consequence would be to perform an elective neck dissection in all patients with p16-positive tonsillar carcinomas, but as many studies failed to show a survival benefit after elective neck dissection in head and neck carcinomas 19–21, future studies should answer this question specifically for HPV-positive cases. Furthermore, a possible indication for a contralateral neck dissection should be investigated in HPV-positive patients. Although previous studies showed a very low incidence of contralateral lymph node metastases in tonsillar carcinoma, the situation might be different if only HPV-positive cases are investigated 22. The higher rate of lymph node metastases could also explain the higher risk of distant metastases and poor survival found in a recent study with advanced HPV-related OPC 23.
There has been a great deal of discussion on de-escalation of treatment in HPV-positive patients 24. The good oncologic results obtained in this patient group and the younger age at presentation make the long-term toxicity of the therapy and functional problems even more devastating for these patients. Most studies currently being performed concentrate on reducing toxicity by using induction therapy and a reduced radiation therapy 25,26. Nevertheless, local early OPC would present ideal candidates for primary surgical treatment. A transoral resection of the tumor is possible in most cases. The development of laser microsurgery and transoral robotic surgery improves the efficacy of surgical treatment 27–30. Many studies have confirmed that, with primary surgical treatment, excellent oncologic results with acceptable functional results can be achieved 31. A recent study showed that 266 patients with T1-2N0-1 OPC treated with surgery ± adjuvant therapy had DSS of 88.7% 10. In their study that included 223 patients with primary surgically treated T1 OPC, Karatzanis et al. showed that no fatal complications occurred 32. The overall complication rate was 6.2%, permanent tracheotomies were necessary in 3.1% of cases and no permanent gastrostomies were necessary. This study shows that p16-positive patients with early local tumor and presence of lymph node metastases would be ideal cases for de-escalation of treatment. Future trials should investigate whether primary transoral surgery with neck dissection as monotherapy could achieve similar oncologic results compared to those seen in patients after use of adjuvant radiotherapy. Alternatively, the effectiveness of a reduced dose of adjuvant radiotherapy could also be investigated.
Although most studies concentrate on HPV-related OPC, HPV-negative OPC should not be neglected, as they still account for the majority of patients treated 6. Most studies in the literature show poor results after combinations of radiation and chemotherapy 4. This study shows that the use of primary surgery (mostly with adjuvant radiotherapy) can lead to acceptable oncologic results in this patient group and can be used as a basis for future trials.
Conclusion
This study showed an increase in the percentage of p16-positive patients in tonsillar carcinoma from 23.2% in the years between 2005 and 2007 to 58.6% between 2010 and 2012. The majority (80.2%) of p16-positive patients presented with early T-category tumor but most of these (79.0%) had also lymph node metastases. The p16-positive cases also had a very high rate of occult cervical metastases (68.7%). Nevertheless, p16-positive patients had excellent oncologic results and could be considered for de-escalation of treatment.
Acknowledgments
The authors would like to thank Philipp Grundtner for statistical analysis in this study.
Conflict of Interest
None declared.
References
- 1.Licitra L, Zigon G, Gatta G, Sanchez MJ, Berrino F, Group EW. Human papillomavirus in HNSCC: a European epidemiologic perspective. Hematol. Oncol. Clin. North Am. 2008;22:1143–1153. doi: 10.1016/j.hoc.2008.10.002. vii–viii. [DOI] [PubMed] [Google Scholar]
- 2.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int. J. Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 3.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Mehanna H, Olaleye O, Licitra L. Oropharyngeal cancer – is it time to change management according to human papilloma virus status? Curr. Opin. Otolaryngol. Head Neck Surg. 2012;20:120–124. doi: 10.1097/MOO.0b013e3283509735. [DOI] [PubMed] [Google Scholar]
- 7.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS, Jr, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am. J. Surg. Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 9.Sudhoff HH, Schwarze HP, Winder D, Steinstraesser L, Gorner M, Stanley M, et al. Evidence for a causal association for HPV in head and neck cancers. Eur. Arch. Otorhinolaryngol. 2011;268:1541–1547. doi: 10.1007/s00405-011-1714-8. [DOI] [PubMed] [Google Scholar]
- 10.Psychogios G, Mantsopoulos K, Agaimy A, Koch M, Zenk J, Waldfahrer F, et al. Prognostic factors in limited (T1-2, N0-1) oropharyngeal carcinoma treated with surgery +/- adjuvant therapy. Head Neck. 2013;35:1752–1758. doi: 10.1002/hed.23229. [DOI] [PubMed] [Google Scholar]
- 11.Wittekindt C, Gultekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP, et al. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv. Otorhinolaryngol. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- 12.Marklund L, Nasman A, Ramqvist T, Dalianis T, Munck-Wikland E, Hammarstedt L, et al. Prevalence of human papillomavirus and survival in oropharyngeal cancer other than tonsil or base of tongue cancer. Cancer Med. 2012;1:82–88. doi: 10.1002/cam4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist D, Romanitan M, Hammarstedt L, Nasman A, Dahlstrand H, Lindholm J, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol. Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 15.Lowy DR, Munger K. Prognostic implications of HPV in oropharyngeal cancer. N. Engl. J. Med. 2010;363:82–84. doi: 10.1056/NEJMe1003607. [DOI] [PubMed] [Google Scholar]
- 16.Olsen SM, Moore EJ, Laborde RR, Garcia JJ, Janus JR, Price DL, et al. Transoral surgery alone for human-papillomavirus-associated oropharyngeal squamous cell carcinoma. Ear Nose Throat J. 2013;92:76–83. [PubMed] [Google Scholar]
- 17.Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJ, et al. Nuclear translocation of beta-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: an early event in human papillomavirus-related tumour progression? Histopathology. 2011;58:1117–1126. doi: 10.1111/j.1365-2559.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 18.Psychogios G, Mantsopoulos K, Bohr C, Koch M, Zenk J, Iro H. Incidence of occult cervical metastasis in head and neck carcinomas: development over time. J. Surg. Oncol. 2013;107:384–387. doi: 10.1002/jso.23221. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigo JP, Shah JP, Silver CE, Medina JE, Takes RP, Robbins KT, et al. Management of the clinically negative neck in early-stage head and neck cancers after transoral resection. Head Neck. 2011;33:1210–1219. doi: 10.1002/hed.21505. [DOI] [PubMed] [Google Scholar]
- 20.Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011;47:320–324. doi: 10.1016/j.oraloncology.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Psychogios G, Mantsopoulos K, Koch M, Klintworth N, Kapsreiter M, Zenk J, et al. Elective neck dissection vs observation in transorally treated early head and neck carcinomas with cN0 neck. Acta Otolaryngol. 2013;133:313–317. doi: 10.3109/00016489.2012.743032. [DOI] [PubMed] [Google Scholar]
- 22.Mantsopoulos K, Psychogios G, Waldfahrer F, Zenk J, Iro H. Surgical treatment of locally limited tonsillar cancer. Surg. Oncol. 2012;21:e13–e16. doi: 10.1016/j.suronc.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, Chen TC, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS ONE. 2012;7:e40767. doi: 10.1371/journal.pone.0040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin. Radiat. Oncol. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra B, Schwartz DL, Frank D. Saltman B, Roy R, Lebowicz YZ, et al. Phase II trial of neoadjuvant chemotherapy for HPV-associated squamous cell carcinoma of the oropharynx followed by reduced-dose radiotherapy/chemoradiotherapy for responders or standard dose chemoradiotherapy for nonresponders. J. Clin. Oncol. 2012;30(Suppl) ASCO Meeting Abstracts abstr TPS5601. [Google Scholar]
- 26.Loewenthal M, Vitez E, Laban S, Munscher A, Guldenzoph B, Knecht R, et al. New aspects of current therapeutic strategies in oropharyngeal carcinoma: highlights of the 2012 ASCO meeting. HNO. 2012;60:951–956. doi: 10.1007/s00106-012-2597-8. [DOI] [PubMed] [Google Scholar]
- 27.Iro H, Mantsopoulos K, Zenk J, Waldfahrer F, Psychogios G. Results of transoral laser resection in T1-2 oropharyngeal, hypopharyngeal and laryngeal carcinomas. Laryngorhinootologie. 2011;90:481–485. doi: 10.1055/s-0031-1283154. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein GS, Quon H, Newman HJ, Chalian JA, Malloy K, Lin A, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch. Otolaryngol. Head Neck Surg. 2012;138:628–634. doi: 10.1001/archoto.2012.1166. [DOI] [PubMed] [Google Scholar]
- 29.Psychogios G, Mantsopoulos K, Kuenzel J, Koch M, Zenk J, Harreus U, et al. Primary surgical treatment of T2 oropharyngeal carcinoma. J. Surg. Oncol. 2012;105:719–723. doi: 10.1002/jso.23026. [DOI] [PubMed] [Google Scholar]
- 30.Quon H, Cohen MA, Montone KT, Ziober AF, Wang LP, Weinstein GS, et al. Transoral robotic surgery and adjuvant therapy for oropharyngeal carcinomas and the influence of p16(INK4a) on treatment outcomes. Laryngoscope. 2013;123:635–640. doi: 10.1002/lary.22172. [DOI] [PubMed] [Google Scholar]
- 31.Kunzel J, Iro H, Psychogios G, Zenk J, Koch M. Closure of defects after resection of tumors of the oral cavity and the pharynx: medium- to long-term oncologic and functional results with the myocutaneous platysma flap. Eur. Arch. Otorhinolaryngol. 2013;270:2537–2545. doi: 10.1007/s00405-013-2389-0. [DOI] [PubMed] [Google Scholar]
- 32.Karatzanis AD, Psychogios G, Waldfahrer F, Zenk J, Velegrakis GA, Iro H. Surgical management of T1 oropharyngeal carcinoma. Head Neck. 2012;34:1277–1282. doi: 10.1002/hed.21916. [DOI] [PubMed] [Google Scholar]


