Abstract
Ketamine has anti-inflammatory, analgesic and antihyperalgesic effect and prevents pain associated with wind-up. We investigated whether low doses of ketamine infusion during general anesthesia combined with single-shot interscalene nerve block (SSISB) would potentiate analgesic effect of SSISB. Forty adult patients scheduled for elective arthroscopic shoulder surgery were enrolled and randomized to either the control group or the ketamine group. All patients underwent SSISB and followed by general anesthesia. During an operation, intravenous ketamine was infused to the patients of ketamine group continuously. In control group, patients received normal saline in volumes equivalent to ketamine infusions. Pain score by numeric rating scale was similar between groups at 1, 6, 12, 24, 36, and 48 hr following surgery, which was maintained lower than 3 in both groups. The time to first analgesic request after admission on post-anesthesia care unit was also not significantly different between groups. Intraoperative low dose ketamine did not decrease acute postoperative pain after arthroscopic shoulder surgery with a preincisional ultrasound guided SSISB. The preventive analgesic effect of ketamine could be mitigated by SSISB, which remains one of the most effective methods of pain relief after arthroscopic shoulder surgery.
Graphical Abstract
Keywords: Ketamine; Interscalene Nerve Block; Shoulder Surgery; Pain, Postoperative
INTRODUCTION
Arthroscopic shoulder surgery (ASS) is associated with moderate to severe postoperative pain, which is comparable to the pain following a gastrectomy or thoracotomy and can require intensive pain management for several days (1, 2, 3). Several modalities have been proven effective for postoperative analgesia, including a single-shot interscalene nerve block (SSISB), continuous interscalene nerve block (CISB), subacromial injection of local anesthetic, and suprascapular nerve block (4). The CISB is the gold standard for postoperative analgesia following ASS because it provides better analgesia than the other modalities (4). However, it remains a technically challenging procedure and needs more time and effort of anesthesiologists for patient care. The SSISB is the preferred technique, which might be attributable to its analgesic effectiveness and the relative ease of the procedure despite the limited duration of analgesia (4). Regardless of the type of nerve block, a single-injection nerve block is simple but has a limited duration. Many studies have attempted to prolong the analgesic effect of single-injection nerve blocks by adding adjuvants such as clonidine (5), opioids (6), dexamethasone (7, 8), and ketamine (9, 10, 11). An effective adjuvant that prolongs the duration of a single-injection nerve block may be a good alternative to a continuous nerve block.
Blockade of the N-methyl-D-aspartate (NMDA) receptor antagonist reduces the development of perioperative nociception-related neural sensitization, hypersensitivity, and opioid tolerance. The NMDA receptor-mediated spinal reflex is the pharmacological basis for the wind-up phenomenon. Ketamine, a non-competitive NMDA receptor antagonist, has analgesic, anti-inflammatory, and antihyperalgesic effects (12) and prevents the pain associated with wind-up (13). However, randomized studies of perioperative ketamine infusion have reported conflicting results. Studies conducted in total knee placement (10) and open thoracotomy (11) have shown better early postoperative pain relief, whereas studies conducted in abdominal hysterectomy (14) and open colorectal surgery (15) did not confirm such relief.
To our knowledge, no study has assessed the analgesic effects of ketamine when used as an intravenous adjuvant combined with SSISB on postoperative pain following ASS. Therefore, this double-blind, randomized, prospective study investigated the effects of low-dose ketamine infusions on postoperative pain relief. We hypothesized that pre-incisional and intraoperative ketamine infusion during general anesthesia combined with an SSISB would provide an additional benefit to the SSISB with a limited duration.
MATERIALS AND METHODS
Forty adult American Society of Anesthesiologists (ASA) physical status I-III patients scheduled for elective ASS were enrolled. Exclusion criteria were coagulopathy, pregnancy, the presence of infection at the site of the block, cerebrovascular disease, any neurological deficit in the surgical limb, uncontrolled hypertension, elevated intraocular pressure, severe lung disease, contralateral diaphragmatic paralysis, a known allergy to levobupivacaine and ketamine, neurological and psychiatric disorders and contraindications to nonsteroidal anti-inflammatory drugs.
Patients were randomized using a computer-generated randomization table to either the normal saline (N) or ketamine (K) group. An anesthesiologist blinded to group assignment prepared all of the study drugs. Patients and the attending anesthesiologists participating in the study were unaware of the group assignment. Investigators who evaluated postoperative pain were blinded to the study groups. On arrival in the operating room, standard ASA monitors were used throughout the surgery. All patients were given a standard SSISB under ultrasound guidance (SonoSite®, M-Turbo™, SonoSite; Bothell, WA, USA). The blocks were performed by one experienced anesthesiologist and were facilitated by sedation with intravenous (IV) midazolam 1-3 mg and fentanyl 25-50 µg. The patient was placed in a supine position with the head turned away from the side to be blocked. Using a 5-12 MHz linear probe, hypoechoic nerve roots or the superior trunk located between the anterior and middle scalene muscles were identified by their round to oval appearance in the short-axis view. A 50-mm, 22-gauge insulated needle (Stimuplex® A, B-Braun; Melsungen, Germany) attached to a nerve stimulator (Stimuplex® Dig RC, B-Braun) was used. After sterile skin preparation, the point where the C5, C6, and C7 roots or superior trunk were most visible was selected. The needle tip was advanced to between the C5 and C6 roots or superior trunk within the sheath using the in-plane method. The needle placement was considered adequate if the deltoid, pectoralis major, triceps, or biceps motor response was still present at less than 0.5 mA of electrical stimulation. After localization of the brachial plexus and negative aspiration, 10 mL of 0.5% levobupivacaine (Chirocaine®, Abbott Scandinavia; Solna, Sweden) mixed with dexamethasone 5 mg was injected in increments.
After the procedure, general anesthesia was induced with intravenous propofol and remifentanil, which were also used for intraoperative maintenance as a continuous infusion using a TCI device (Orchestra Base Primea®, Fresenius Kabi; France) with a 50% oxygen-air mixture. Tracheal intubation was facilitated with 0.6 mg/kg of rocuronium. The patients in group K (n =20) received an intravenous bolus of 0.3 mg/kg ketamine (Ketomin®, Dai Han Pharm, Seoul, Korea) after anesthesia induction and before the surgical incision and a continuous intraoperative infusion of ketamine 0.15 mg/kg/h, which was stopped 5 min before the end of surgery. In group N (n=20), the patients received a preincisional bolus and continuous intraoperative normal saline in volumes equivalent to the ketamine infusions until 5 min before the end of surgery. Propofol was targeted to achieve a bispectral index of 40-60. The systolic arterial pressure and heart rate were kept within 20% of baseline values. Patients were then placed in the lateral decubitus position, and all surgeries were performed by one surgeon. Propofol and remifentanil were discontinued when suturing the skin. In all patients, ramosetron 0.3 mg IV (Nasea®, Astellas Pharma Korea, Tokyo, Japan) was administered for antiemetic prophylaxis. Patients were extubated in the operating room after reversing the residual muscle relaxation.
In the recovery room, an intravenous patient-controlled analgesia (IV-PCA) pump, which contained a mixture of 1,500 µg fentanyl and 150 mg ketorolac diluted with saline solution to a total volume of 100 mL, was attached to all patients. A 2-mL bolus was administered on demand, with a 20-min lockout time. A doctor blinded to the groups visited the patients and evaluated pain using a numerical rating scale (NRS: 0=no pain, 10= most severe pain imaginable) 0, 1, 6, 12, 24, 36, and 48 hr after an arrival at the recovery room. Patients were educated to press the button when the NRS was >3 or the patient requested analgesic. All patients had a plain chest radiograph taken to rule out phrenic nerve palsy. Patients were assessed for possible complications such as arm weakness, arm numbness, nausea, vomiting, dizziness, respiratory difficulties, and Horner syndrome. The number of total attempts and the amount of PCA medication used for 48 hr was printed automatically by the PCA machine (Baxter PCA II, Baxter Corporation; Mississauga, Ontario, Canada).
Statistical analysis
SPSS (ver. 18.0, Chicago, IL, USA) was used for the statistical analysis. The sample size was based on postoperative pain. The primary endpoint is NRS 24 hr postoperatively. Data from a preliminary study in our institution, detection of a mean shift of 2 points (an SD of 2.0 points) in NRS after dropouts would require 20 subjects in each group (unpaired t test, α=0.05, 2-tailed, power=80%). Student's t-tests were done to compare continuous variables between groups. Discrete variables were analyzed using chi-squared tests. Pain score during postoperative 48 hr, cumulative number of PCA attempts and total consumption of PCA medication were analyzed using repeated measured ANOVA. The time to first analgesic request was analyzed using Mann-Whitney U test. P values of less than 0.05 were considered statistically significant. Data are presented as the mean±standard deviation unless noted otherwise.
Ethics statement
This study was approved by the institutional review board (IRB) of Ewha Womans Hospital (IRB No. ECT 12-29-04). Written informed consent was obtained from all participating subjects.
RESULTS
Forty patients scheduled to undergo ASS were enrolled. Demographic data were similar in both groups (Table 1). The preoperative NRS pain score, durations of anesthesia and surgery, type of surgery, and amounts of propofol and remifentanil used during anesthesia did not differ significantly between the groups (Table 2).
Table 1.
Demographic characteristics by groups
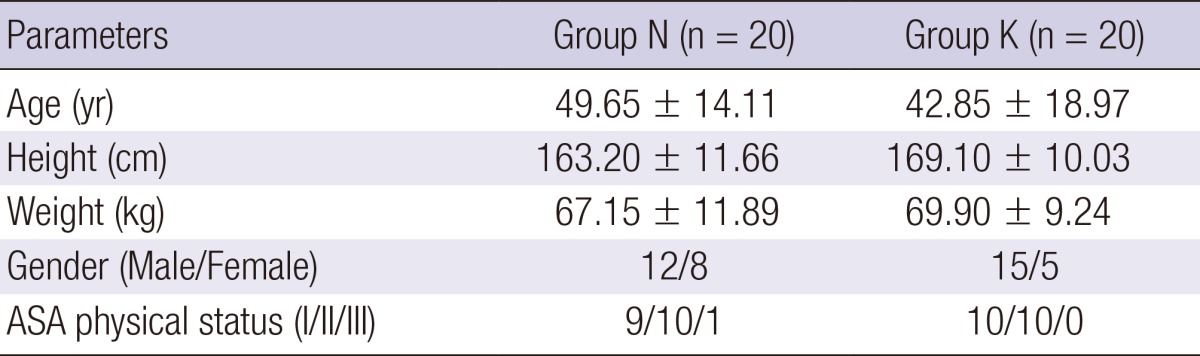
Values are mean±SD or numbers. There were no significant differences between groups. Group N, the control group; Group K, the ketamine group; ASA, American Society of Anesthesiologists.
Table 2.
The perioperative data by groups
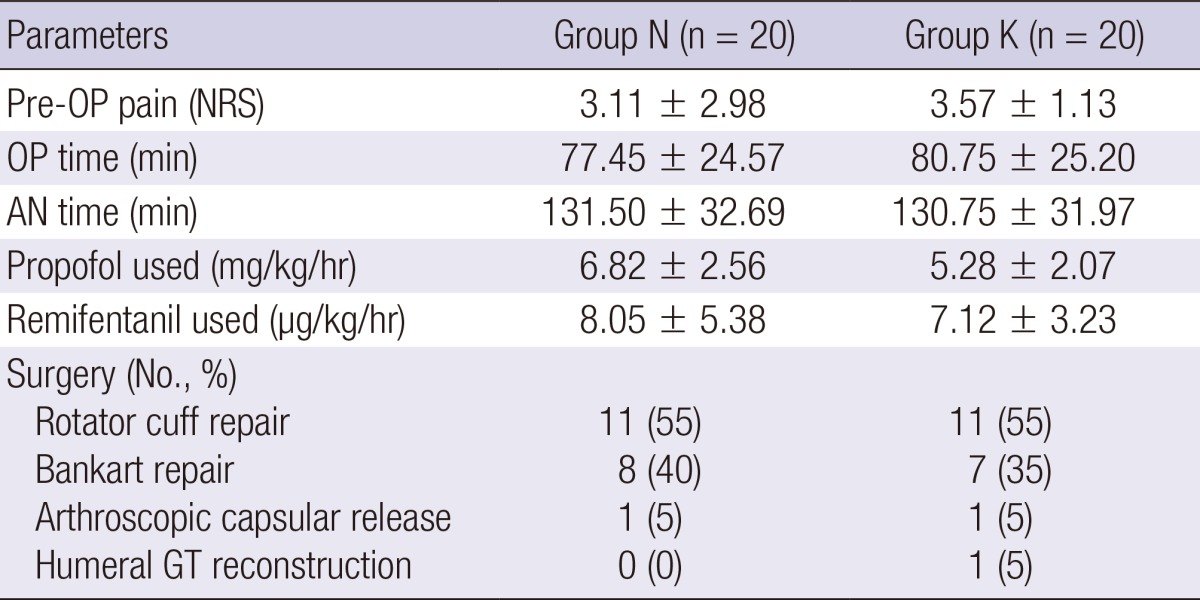
Values are mean±SD or number (percentage). There were no significant differences between groups. Group N, the control group; Group K, the ketamine group; OP, operation; AN, anesthesia; NRS, numeric rating scale; GT, greater tuberosity.
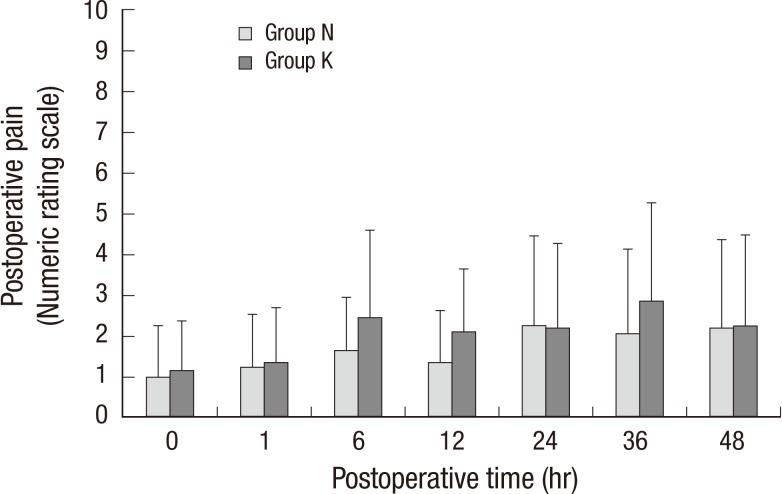
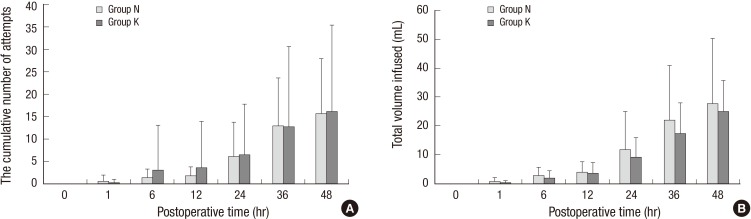
The NRS pain score was similar in both groups at 1, 6, 12, 24, 36, and 48 hr postoperatively (Fig. 1). The NRS was maintained at <3 in both groups during the 48 postoperative hours. There was no appreciable difference between groups in the cumulative number of attempts at PCA (Fig. 2A) and total consumption of PCA medication during the 48 hr (Fig. 2B). The median time (inter-quartile range) to first analgesic request after admission to the post-anesthesia care unit was 520.00 min (37.75-1,272.25 min) in group N and 670.50 min (57.75-1,263.75 min) in group K (P=0.925).
Fig. 1.
Numeric rating scale of pain during postoperative 48 hr. There was no significant difference between groups. Group N, the control group; Group K, the ketamine group. Numeric rating scale; 0, no pain; 10, worst pain imaginable.
Fig. 2.
The cumulative number of PCA attempts (A) and total consumption of PCA medication (B) for postoperative 48 hr. There was no significant difference between groups. Group N, the control group; Group K, the ketamine group; PCA, patient-controlled analgesia.
Adverse effects observed during the study period are summarized in Table 3. There were no significant differences in complications, including arm weakness, numbness, nausea, vomiting, dizziness, phrenic nerve palsy (ipsilateral diaphragm elevation), and dyspnea. Arm numbness for the second 24 postoperative hours was noted in five patients in group N and one in group K; all cases resolved by the following day. Complications related to ketamine such as hallucinations or drowsiness were not observed.
Table 3.
Adverse effects by groups
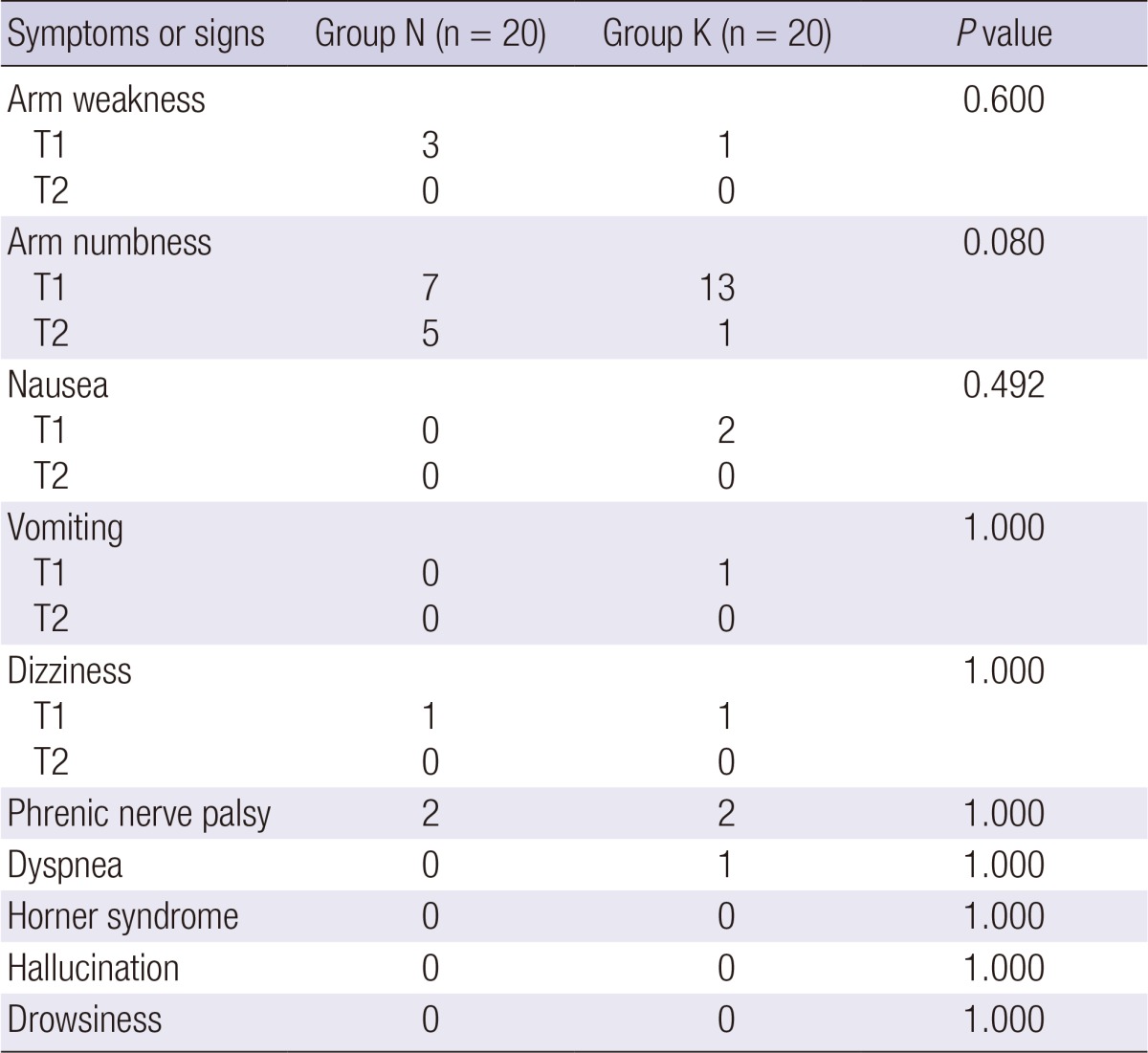
Values are numbers. Group N, the control group; Group K, the ketamine group; T1, 0-24 hr postoperatively; T2, 24-48 hr postoperatively.
DISCUSSION
This study found no beneficial effects of adding intravenous low-dose ketamine during the operation to SSISB for patients undergoing ASS under total IV anesthesia with propofol and remifentanil. Low-dose ketamine did not reduce the early postoperative pain score or prolong the time to first analgesic request.
Whether intraoperative ketamine infusion reduces postoperative pain remains controversial. Previous studies of ketamine infusion can be divided into two types according to whether ketamine was also infused during the postoperative period (16). Peripheral tissue injury during an operation induces peripheral and central sensitization, which contribute to postoperative pain. The inflammatory response to the damaged tissue also leads to postoperative nociceptive input, which can sustain the hypersensitive state (17). Therefore, limiting central sensitization during the preoperative and intraoperative periods may be insufficient. In this study, an IV bolus of ketamine was given after inducing anesthesia and before the surgical incision, and the ketamine was infused continuously until 5 min before the end of surgery. Our design might be criticized for the lack of postoperative ketamine infusion. In clinical practice, ASS is commonly performed as outpatients surgery or it requires only a short hospital stay so that continuous ketamine infusion in a postoperative period would not be available to the general surgical population. Interestingly, ketamine also inhibits the early postoperative inflammatory response, which contributes to sustained nociceptive input and the hypersensitive state (12, 18). This study sought to evaluate whether intraoperative ketamine infusion itself potentiated the analgesia of SSISB and extended its duration.
We postulated that intravenous ketamine did not confer any benefit on the analgesic effect of SSISB for two reasons. First, we used a dexamethasone as an adjuvant to local anesthetics for the SSISB. Many studies have shown that dexamethasone prolongs the sensory block of SSISB significantly (7, 8, 19). In our institution, we routinely add 5 mg of dexamethasone to the local anesthetics for SSISB. It is possible that the potent anti-inflammatory effect of dexamethasone may have limited the impact of any additional anti-inflammatory effect of ketamine on the postoperative inflammatory response. Second, SSISB is commonly used for anesthesia for shoulder surgery, as well as for postoperative analgesia. The pain score was zero in most patients when they recovered from the general anesthesia. This might involve a limited effect of ketamine, which prevents an activity-dependent increase in the excitability of spinal neurons. Mathisen et al. (20) stated that intravenous ketamine might not provide any additional benefit when adequate analgesia is obtained with a conventional multimodal approach.
Previous studies tried various doses of ketamine (0.12-1 mg/kg bolus before the surgical incision and 0.12-1 mg/kg/hr infusion until skin closure) for abdominal, gynecological, thoracic, and orthopedic surgery (16). The plasma ketamine concentrations for analgesia and anesthesia are 100-200 ng/mL and 700 ng/mL, respectively, and the dose of ketamine for analgesia is 0.2-0.4 mg/kg (21). We chose 0.3 mg/kg pre-incisional and 0.15 mg/kg/hr intraoperative ketamine as the dosing regimen based on our previous study with positive results (22). A quantitative meta-analysis of 37 trials showed that there was no morphine-sparing effect from increasing the ketamine dose above 30 mg/24 hr (16). The mean ketamine doses infused during the operation in our series was 43.05 mg, as calculated using the weight and anesthesia time for our patients.
Ketamine reduces postoperative nausea and vomiting (16). The incidence of nausea and vomiting was similarly low in both groups of our study. This likely resulted from the reduced opioid consumption due to the low pain scores, routine use of preventive antiemetics, and antiemetic effect of dexamethasone in both groups (23). This concurs with previous articles that described the adverse effects as mild or absent (16).
There are the concerns about the 'off-label' use of perineural dexamethasone. Although no previous clinical trials using perineural dexamethasone have reported neurotoxicity related with dexamethasone, it requires enormous sample sizes to conclude safety concerns with rare adverse events. Though, several animal experiments (24, 25) demonstrated no significant long-term adverse effects. In addition, epidural injections of corticosteroids have been used to treat radiculopathy for a long time, and the neurologic risk of single use of small amounts (less than 8 mg) of dexamethasone, or use within 24 hr is considered to be small for adults (7, 26). Neural damage is also related to direct trauma by the needle. In our study, SSISB was conducted within the sheath of the interscalene brachial plexus. Using the in-plane approach, direct damage to nerves and surrounding tissue from needle insertion was prevented by checking ultrasound images, and appropriate spread of local anesthetics within the interscalene brachial plexus sheath was ascertained during administration. This allowed local anesthetics to spread into the appropriate areas for sufficient perfusion through the nerve trunk and roots so successful nerve block was properly achieved in all patients without serious neurologic complications.
There are several limitations to this study. First, we focused on acute postoperative pain and did not evaluate the postoperative shoulder function or rehabilitation outcomes. Secondly, this study included a relatively small number of patients. Further studies with prolonged use of ketamine until the postoperative period or pre-incisional SSISB without the use of dexamethasone in a larger patient population might find different results.
In conclusion, intraoperative low-dose ketamine does not decrease acute postoperative pain following ASS. The preventive analgesic effect of ketamine found in other studies could be mitigated by SSISB, which remains one of the most effective methods of pain relief after ASS.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Borgeat A, Schäppi B, Biasca N, Gerber C. Patient-controlled analgesia after major shoulder surgery: patient-controlled interscalene analgesia versus patient-controlled analgesia. Anesthesiology. 1997;87:1343–1347. doi: 10.1097/00000542-199712000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AT, Nicholson E, Burton L, Wild C. Analgesia for day-case shoulder surgery. Br J Anaesth. 2004;92:414–415. doi: 10.1093/bja/aeh071. [DOI] [PubMed] [Google Scholar]
- 3.Tuominen M, Pitkänen M, Rosenberg PH. Postoperative pain relief and bupivacaine plasma levels during continuous interscalene brachial plexus block. Acta Anaesthesiol Scand. 1987;31:276–278. doi: 10.1111/j.1399-6576.1987.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 4.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–624. doi: 10.1111/j.1365-2044.2009.06231.x. [DOI] [PubMed] [Google Scholar]
- 5.Hutschala D, Mascher H, Schmetterer L, Klimscha W, Fleck T, Eichler HG, Tschernko EM. Clonidine added to bupivacaine enhances and prolongs analgesia after brachial plexus block via a local mechanism in healthy volunteers. Eur J Anaesthesiol. 2004;21:198–204. doi: 10.1017/s0265021504003060. [DOI] [PubMed] [Google Scholar]
- 6.Karakaya D, Büyükgöz F, Bariş S, Güldoğuş F, Tür A. Addition of fentanyl to bupivacaine prolongs anesthesia and analgesia in axillary brachial plexus block. Reg Anesth Pain Med. 2001;26:434–438. doi: 10.1053/rapm.2001.24675. [DOI] [PubMed] [Google Scholar]
- 7.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–288. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 8.Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth. 2011;25:704–709. doi: 10.1007/s00540-011-1180-x. [DOI] [PubMed] [Google Scholar]
- 9.Joseph C, Gaillat F, Duponq R, Lieven R, Baumstarck K, Thomas P, Penot-Ragon C, Kerbaul F. Is there any benefit to adding intravenous ketamine to patient-controlled epidural analgesia after thoracic surgery? a randomized double-blind study. Eur J Cardiothorac Surg. 2012;42:e58–e65. doi: 10.1093/ejcts/ezs398. [DOI] [PubMed] [Google Scholar]
- 10.Adam F, Chauvin M, Du Manoir B, Langlois M, Sessler DI, Fletcher D. Small-dose ketamine infusion improves postoperative analgesia and rehabilitation after total knee arthroplasty. Anesth Analg. 2005;100:475–480. doi: 10.1213/01.ANE.0000142117.82241.DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Haraguti S, Sugimoto K, Kikutani T, Shimada Y, Sakamoto A. Low-dose intravenous ketamine potentiates epidural analgesia after thoracotomy. Anesthesiology. 2006;105:111–119. doi: 10.1097/00000542-200607000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? a systematic review and meta-analysis. Anesth Analg. 2012;115:934–943. doi: 10.1213/ANE.0b013e3182662e30. [DOI] [PubMed] [Google Scholar]
- 13.Hughes AM, Rhodes J, Fisher G, Sellers M, Growcott JW. Assessment of the effect of dextromethorphan and ketamine on the acute nociceptive threshold and wind-up of the second pain response in healthy male volunteers. Br J Clin Pharmacol. 2002;53:604–612. doi: 10.1046/j.1365-2125.2002.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grady MV, Mascha E, Sessler DI, Kurz A. The effect of perioperative intravenous lidocaine and ketamine on recovery after abdominal hysterectomy. Anesth Analg. 2012;115:1078–1084. doi: 10.1213/ANE.0b013e3182662e01. [DOI] [PubMed] [Google Scholar]
- 15.Guignard B, Coste C, Costes H, Sessler DI, Lebrault C, Morris W, Simonnet G, Chauvin M. Supplementing desflurane-remifentanil anesthesia with small-dose ketamine reduces perioperative opioid analgesic requirements. Anesth Analg. 2002;95:103–108. doi: 10.1097/00000539-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Bell RF, Dahl JB, Moore RA, Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review) Acta Anaesthesiol Scand. 2005;49:1405–1428. doi: 10.1111/j.1399-6576.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 17.Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 18.Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anaesth. 2011;107:123–126. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- 19.Cummings KC, 3rd, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, Sessler DI. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107:446–453. doi: 10.1093/bja/aer159. [DOI] [PubMed] [Google Scholar]
- 20.Mathisen LC, Aasbø V, Raeder J. Lack of pre-emptive analgesic effect of (R)-ketamine in laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1999;43:220–224. doi: 10.1034/j.1399-6576.1999.430218.x. [DOI] [PubMed] [Google Scholar]
- 21.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53:27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Baik HJ, Kim JH. The effects of the intravenous continuous infusion of low-dose ketamine on postoperative pain after total intravenous anesthesia. Korean J Anesthesiol. 2005;48:163–170. [Google Scholar]
- 23.Choi DH, Ko JS, Ahn HJ, Kim JA. A Korean predictive model for postoperative nausea and vomiting. J Korean Med Sci. 2005;20:811–815. doi: 10.3346/jkms.2005.20.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams BA, Hough KA, Tsui BY, Ibinson JW, Gold MS, Gebhart GF. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011;36:225–230. doi: 10.1097/AAP.0b013e3182176f70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, Liu L, Ding Z. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. 2010;167:329–342. doi: 10.1016/j.neuroscience.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 26.Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg Am. 1992;17:645–647. doi: 10.1016/0363-5023(92)90309-d. [DOI] [PubMed] [Google Scholar]