Abstract
When treating trauma patients with severe hemorrhage, massive transfusions are often needed. Damage control resuscitation strategies can be used for such patients, but an adequate fresh frozen plasma: packed red blood cell (FFP:PRBC) administration ratio must be established. We retrospectively reviewed the medical records of 100 trauma patients treated with massive transfusions from March 2010 to October 2012. We divided the patients into 2 groups according to the FFP:PRBC ratio: a high-ratio (≥0.5) and a low-ratio group (<0.5). The patient demographics, fluid and transfusion quantities, laboratory values, complications, and outcomes were analyzed and compared. There were 68 patients in the high-ratio and 32 in the low-ratio group. There were statistically significant differences between groups in the quantities of FFP, FFP:PRBC, platelets, and crystalloids administered, as well as the initial diastolic blood pressure. Bloodstream infections were noted only in the high-ratio group, and the difference was statistically significant (P=0.028). Kaplan-Meier plots revealed that the 24-hr survival rate was significantly higher in the high-ratio group (71.9% vs. 97.1%, P<0.001). In severe hemorrhagic trauma, raising the FFP:PRBC ratio to 0.5 or higher may increase the chances of survival. Efforts to minimize bloodstream infections during the resuscitation must be increased.
Graphical Abstract
Keywords: Resuscitation, Transfusion, Blood Product Ratio, Survival, Trauma
INTRODUCTION
Hemorrhage is a preventable yet major cause of death among patients with severe trauma (1, 2, 3, 4, 5), as it can quickly cause acidosis, hypothermia, and coagulopathy, the so-called lethal triad, which is irreversible. Thus, to increase the chances of survival, quick hemostatic methods such as surgery or angioembolization, along with supportive care using fluids and blood transfusions, are crucial. However, there have been reports of adverse events arising from the conventional method of using crystalloid fluids in patients with major trauma who require massive blood transfusion. Thus, a new concept of damage control resuscitation (DCR) was developed based on recent military experiences and research (6, 7, 8).
DCR, now listed in surgery textbooks (9) as a method that involves permissive hypotension, limited use of crystalloid fluids, and early use of blood products, is now an essential concept that surgeons have to learn to be able to deal with patients in hypovolemic shock due to hemorrhage, even if they are not patients with trauma (10). However, there are various views on the validity of such a management technique. Further, the correct ratio of fresh frozen plasma (FFP) to packed red blood cells (PRBC) that should be used remains unclear. Recent studies have suggested that it is more beneficial to use more amount of FFP, to the extent that its ratio to PRBC is close to 1:1 (11, 12). However, the excessive use of FFP may cause infection, transfusion-related acute lung injury (TRALI), and acute respiratory distress syndrome (ARDS) (13, 14, 15). Conversely, other studies have suggested that patients receiving a large amount of FFP have a low mortality rate because they have higher chances of survival, which makes it possible for them to receive a large amount of FFP. Thus, this suggests a possible selection bias and the need for a prospective trial (16). In view of this, we had previously tried to estimate the correct ratio of PRBC to FFP for treating severe trauma patients. Prior to 2007, we administered FFP:PRBC according to the transfusion protocol at a ratio of <0.5, but we then increased the ratio because another study demonstrated better results with the use of a higher amount of FFP (8, 11, 17, 18). Furthermore, a more stable course was noted when less crystalloids and more FFP were used at an early stage of patient management; hence, we further increased the ratio 2 yr ago in an attempt to increase the FFP:PRBC ratio to 1:1. Thus, we felt the need to thoroughly analyze the recent data on patients with severe trauma who had received massive transfusions to establish an effective PRBC:FFP administration ratio. We plan to use the results of this study at our institution when treating patients with severe trauma who are expected to undergo surgery and may require massive transfusion.
MATERIALS AND METHODS
Medical records of 100 patients who were hospitalized at the Ajou University Hospital due to severe trauma and who received massive transfusion (≥10 units of RBCs in 24 hr) from March 2010 to October 2012 were retrospectively analyzed. Ajou University Hospital is a leading tertiary hospital in Korea, with 16,000 trauma patients visiting its emergency room (ER) every year, several of whom have injury severity scores (ISS) higher than 15.
To reduce bias, the patients who died upon arrival at the hospital or within an hour of their arrival were excluded. The demographic, transfusion, laboratory evaluation, time-to-death after ER admission, and outcome data of the 100 patients were analyzed retrospectively using their medical records. All time-to-death data were calculated based on the time of ER admission of the patients. The PRBC and FFP units were adjusted to standard units and totaled. The crystalloid and colloid amounts were adjusted to the infused volume (L) and similarly recorded.
Patients were categorized according to their FFP:PRBC ratio. Those with ratios of 0.5 or greater were assigned to the "high-ratio" group, and those with ratios of less than 0.5 were assigned to the "low-ratio" group; the demographic, laboratory, and clinical characteristics of the 2 groups were compared. The continuous variables were compared using a Student's t-test, and the categorical variables were tested using chi-square analysis or a Fisher's exact test, as appropriate. The relationship between the survival time and the FFP:PRBC ratio was assessed with Kaplan-Meier methods. The 24-hr and 30-day survival rates of the 2 groups were compared using the Kaplan-Meier plots. In addition, the complications, such as infection (pneumonia, bloodstream infection, catheter-related infection, urinary tract infection, and wound infection), as well as the incidence rates of TRALI and ARDS, in the 2 groups were compared and analyzed.
Ethics statement
This study was approved by the institutional review board of Ajou University Hospital (IRB No. MED-MDB-13-114). Informed consent was waived by the bvoard because of the observational nature of the study.
RESULTS
During the 32-month study period, there were 1,111 (approximately 416.5 per years and 34.8 per remaining month) trauma patients in our hospital with an ISS score higher than 15. Of these, the complete and available medical records of 100 patients who met the criteria for this study were analyzed. Patients had a mean age of 47 (13-87) yr, 83% of them were male patients, and most (93%) of them had experienced blunt traumas (Table 1). Up to 2,723 units of PRBC and 1,755 units of FFP were transfused during the 24-hr resuscitation period. The first PRBC unit were given at a median time of 34 min (range: 12-410 min), and the first FFP unit was given at a median time of 146 min (range: 12-1,430 min).
Table 1.
Comparison of baseline characteristics of massive transfusion patients in the 2 groups
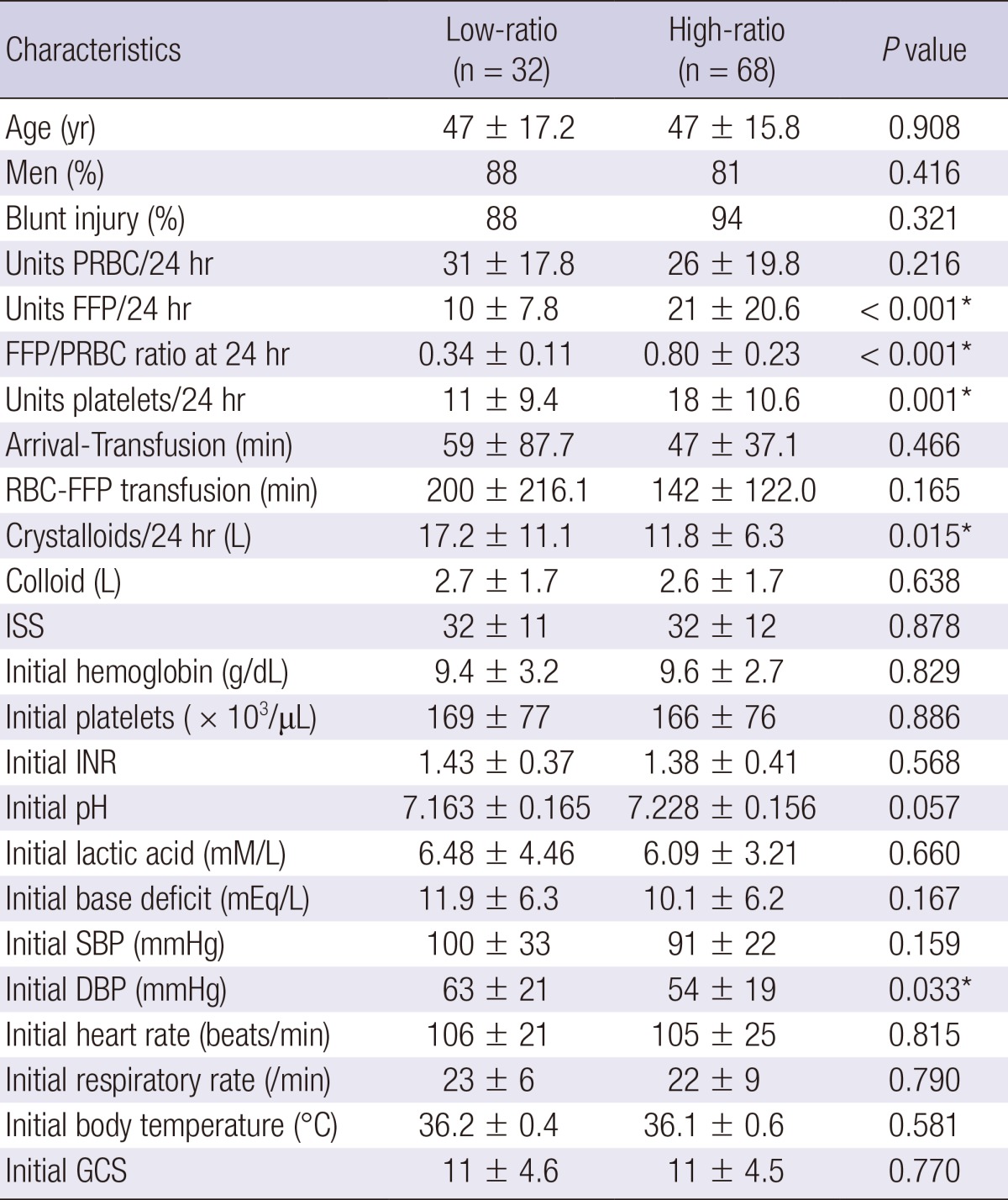
*Statistically significant. PRBC, packed red blood cell; FFP, fresh frozen plasma; ISS, injury severity score; INR, international normalized ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; GCS, glasgow coma scale.
The overall in-hospital mortality was 36% (36 deaths) (Table 2), and 11% of these deaths occurred within 24 hr of admission. The causes of death in these 36 patients were exsanguinating hemorrhage (ongoing bleeding) in 14 patients (38.9%), multisystem organ failure in 15 patients (41.7%), traumatic brain injury in 6 patients (16.7%), and another cause in 1 patient (2.8%). All 11 patients who died within 24 hr died due to exsanguinating hemorrhage. The 14 patients who died due to exsanguinating hemorrhage died after an average of 17.1 hr upon their arrival at the hospital, with a median time of death of 8.0 hr. Fifteen patients died because of multi-organ failure in a mean time of 718.4 hr (29.9 days) and a median value of 432 hr (18 days) after they arrived at the hospital. Six patients who initially had brain injury that either progressed or did not subside despite bleeding control and who had no organ failure were classified as having died due to traumatic brain injury.
Table 2.
Comparison of mortality and complication rates between patients who received a high FFP:PRBC and a low FFP:PRBC transfusion ratio
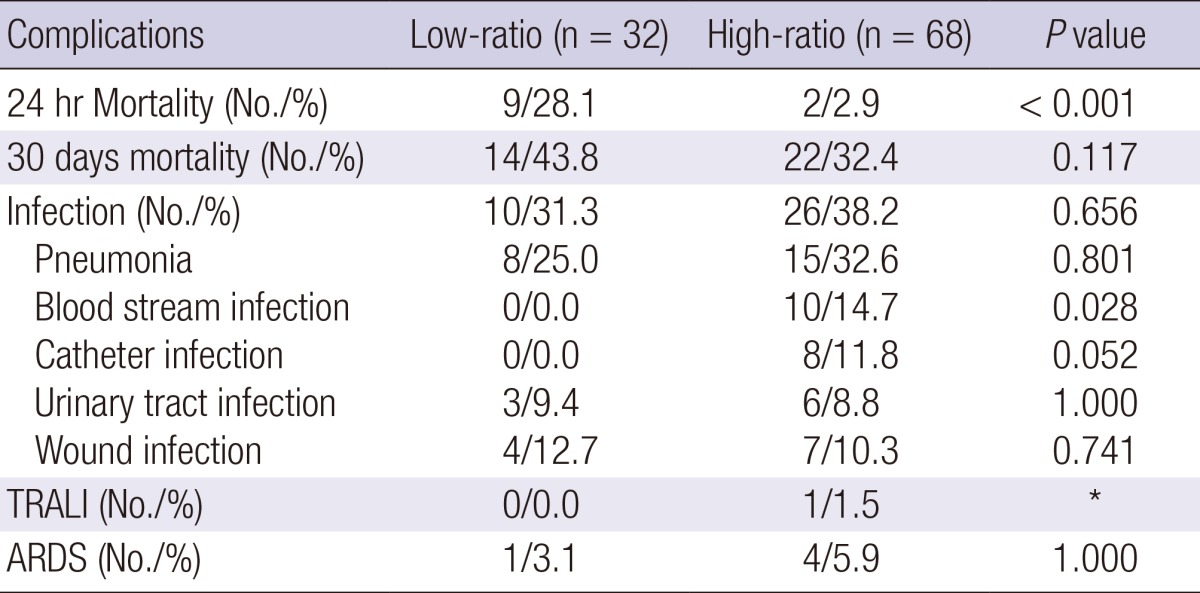
*Events reported were scarce. TRALI, transfusion-related acute lung injury; ARDS, acute respiratory distress syndrome.
There were 68 patients in the high-ratio (FFP:PRBC ratio≥0.5) group and 32 in the low-ratio (FFP:PRBC ratio<0.5) group. The clinical and laboratory characteristics of the 2 groups are shown in Table 1. There were no statistical differences between the 2 groups with respect to sex, age, type of accidents, ISS values, initial blood test results, and systolic blood pressure. However, FFP (P<0.001), FFP/PRBC (P<0.001), platelets (P=0.001), and crystalloid (P=0.015) administration as well as the initial diastolic blood pressure (DBP) (P=0.033) differed significantly. Thus, more amount of FFP and platelets was administered in the high-ratio group than in the low-ratio group, and the high-ratio group presented with lower initial DBPs upon their arrival at the hospital.
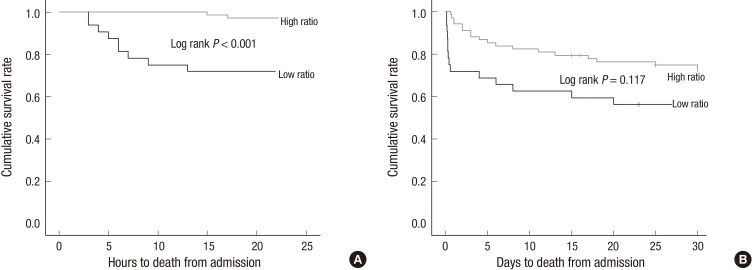
The comparison of the 24-hr survival rates indicated that the high-ratio group had a significantly higher survival rate than the low-ratio group (97.1% vs. 71.9%, respectively [P<0.001]) (Fig. 1A and Table 2). The 30-day survival rate of the high-ratio group was only slightly higher than that of the low-ratio group, but the difference was not statistically significant (67.6% and 56.2%, respectively [P=0.117]) (Fig. 1B and Table 2).
Fig. 1.
Kaplan-Meier survival plots for the 2 groups. (A) First 24 hr, (B) First 30 days after admission.
Table 2 shows the complications of massive transfusions noted in our study patients. Although infection, TRALI, and ARDS were investigated, but there was only 1 case of TRALI in the high-ratio group out of the 100 patients; hence, we excluded it from Table 2 because its incidence was considered insignificant. There was no significant difference in the overall infection incidence rates between the 2 groups. However, 10 cases of bloodstream infection were reported in the high-ratio group, with the difference being statistically significant (P=0.028). Eight cases of catheter-related infection were reported in the high-ratio group (P=0.052). There was 1 case of ARDS in the low-ratio group and 4 in the high-ratio group, but the difference between the 2 groups was not significant.
DISCUSSION
The ability to control the bleeding successfully is the most important factor while treating severe trauma patients with hemorrhage. Trauma surgeons can now control bleeding more efficiently owing to the remarkable improvements in damage control surgery and radiologic intervention techniques. Further, studies (6, 7, 8) have suggested that changes in resuscitation before and after surgery, along with surgery and radiologic intervention, which is performed in DCR or hemostatic resuscitation, can also alter patient prognoses. This method is currently being applied through several approaches on civilians with good results.
The components of DCR can be summarized as permissive hypotension, the use of blood products over isotonic fluid for volume replacement, and the fast and early correction of coagulopathy with component therapy (9). However, there have been numerous arguments in applying the principles of permissive hypotension for severe traumatic hemorrhagic patients. In addition, the limited use of fluids during resuscitation efforts is in direct opposition to guidelines put forth by the American College of Surgeons and the ATLS protocol (10). Although the SBP in the group of high ratio group is lower than in the group of low ratio in our results, the difference was not significant enough to show the effects of permissive hypotension. Therefore, the main concept of DCR that we wanted to focus on is using less crystalloids and more blood products as early as possible to correct the hypovolemic state. The FFP:PRBC ratio has attracted a great deal of attention and has been recently the subject of numerous studies. Ever since Hewson et al. (19) suggested the use of an FFP:PRBC ratio of 1:1 in 1986, it has been largely ignored until consensus on this topic started building up again recently (11, 17), to the point where it is now included in the chapter (9) on shock in a surgery textbook. There are still arguments that using more FFP to produce better results is based on bias (16), and that using more blood products (especially FFPs, platelets, and plasma-based solutions, compared to PRBCs) causes more infections and leads to TRALI and ARDS (13, 14, 15, 20).
In our institution, during the past 3 yr, the amount of crystalloids used for hypovolemic state correction has been reduced and more blood products have been used to treat severe trauma patients. We have been trying to increase the FFP:PRBC ratio to 1:1 and are preparing a massive transfusion protocol based on the results of other studies. In the past 32 months, we administered 2,723 units of PRBC, 1,755 units of FFP, and 1,544 units of platelets to 100 severe trauma patients who received massive transfusions. There were significant differences in the DBPs at admission and the amounts of FFP, platelets, and crystalloids received by patients in the high- and low-ratio groups of this study. Of note, patients who presented with a lower DBP upon their hospital admission received more FFPs and platelets than crystalloids and these factors were considered while determining the 24-hr survival rates. Further, patients in the high-ratio group who had a lower DBP and a worse shock state showed a higher survival rate when they received less crystalloids and more FFPs and platelets. Although the 30-day survival rates of the 2 groups did not differ significantly, initial management with fluid and blood transfusion seems to have been a deciding factor of their short-term survival rates.
The present study results indicate that the use of platelets in the 2 groups also differed, but such analyses were not the focus of this study, as fewer platelet transfusions were used than the amount recommended by the 1:1:1 ratio stated in previous studies (17, 21, 22). Additionally, considering the limited blood product supply in our country, as in most countries, scarce supply of platelets hindered the surgeons from administering sufficient amounts at the required times. It is true that we were cautious about the quantities and timing of platelet transfusions administered to patients with varied conditions and still do not include the use of platelets in our massive transfusion protocol. However, we felt the need to arrive first on the correct FFP:PRBC ratio; hence, this was the focus of our data analysis. However, we are planning further studies on a comprehensive massive transfusion protocol that includes the use of platelets.
A total of 100 patients were administered FFP:PRBC at an average ratio of 0.65, and these patients had a survival rate of 64%. Considering the 41%-74% survival rate reported by a multicenter study (23) conducted in 16 American Level I trauma centers, the results of the present study can be considered as acceptable. Eleven of the 36 deaths took place within 24 hr and were due to exsanguinating hemorrhage. There was a statistically significant difference between groups when comparing the 9 deaths in the low-ratio group and the 2 deaths in the high-ratio group (71.9% vs. 97.1%, respectively [P<0.001]). However, this significant difference could be biased by the fact that those who survived allowed more time for the medical staff to administer larger quantities of FFP. To clarify this issue, perhaps a new prospective study should be conducted with a larger sample of patients.
The difference in bloodstream infection between groups was statistically significant (low ratio 0 vs. high ratio 10, [P=0.028]); further, the incidence of catheter-related infection differed (low ratio 0 vs. high ratio 8, [P=0.052]), but not significantly. As catheter-related and bloodstream infections were more frequent in the high-ratio group, unlike other infections, we can conclude that the increase in FFP transfusions led to an increased rate of infection, as previously considered. Unlike other studies (20, 24, 25) that showed an incidence rate higher than 30% for ARDS, we observed a lower incidence (5%) of ARDS. We had only a few cases of ARDS, and as for TRALI, the comparison between both groups seemed insignificant. Although we could not explain clearly the reason why there were fewer cases of ARDS and TRALI than that reported in other studies, it was obvious that complications such as ARDS and TRALI did not create major issues while applying damage control resuscitation strategies to the patients in need of massive transfusions.
This study was limited by the fact that it was a retrospective study with a small sample of patients. However, the 2 groups had remarkable differences in the amounts of their FFP and platelet transfusions received, FFP:PRBC ratio targeted, and crystalloids administered, despite the absence of clinical differences on blood tests and in their hemodynamic states (excluding DBP), which may have contributed to the difference in their survival rates.
To conclude, when treating severe trauma patients with hemorrhage, increasing the FFP:PRBC ratio to greater than 0.5 can result in a higher survival rate, especially the 24-hr survival rate. However, to decrease the risk of complications such as catheter-related and bloodstream infections, which were more frequent in the high-ratio group, careful and aseptic techniques must be used when administering such regimens. A larger sample size for our future prospective study is expected to contribute significantly to the establishment of a massive transfusion protocol.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A2A2A04013317, 2010-0028631).
The authors have no conflicts of interests to disclosure.
References
- 1.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. Causes of death in US. Special Operations Forces in the global war on terrorism: 2001-2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. J Trauma. 2008;64:S21–S26. doi: 10.1097/TA.0b013e318160b9fb. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira PG, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, Browder T, Noguchi TT, Demetriades D. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63:1338–1346. doi: 10.1097/TA.0b013e31815078ae. [DOI] [PubMed] [Google Scholar]
- 4.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62:142–146. doi: 10.1097/01.ta.0000251558.38388.47. [DOI] [PubMed] [Google Scholar]
- 5.Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–380. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, Wade CE, Holcomb JB. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64:S69–S77. doi: 10.1097/TA.0b013e318160ba2f. [DOI] [PubMed] [Google Scholar]
- 7.Fox CJ, Gillespie DL, Cox ED, Kragh JF, Jr, Mehta SG, Salinas J, Holcomb JB. Damage control resuscitation for vascular surgery in a combat support hospital. J Trauma. 2008;65:1–9. doi: 10.1097/TA.0b013e318176c533. [DOI] [PubMed] [Google Scholar]
- 8.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 9.Rhee P. Shock, electrolytes, and fluid. In: Sabiston DC, Townsend CM, editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. 19th ed. Philadelphia: Elsevier Saunders; 2012. pp. 66–119. [Google Scholar]
- 10.Duchesne JC, McSwain NE, Jr, Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, Marr AB, Gonzalez EA, Phelan HA, Bilski T, et al. Damage control resuscitation: the new face of damage control. J Trauma. 2010;69:976–990. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 11.Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, Wright MJ, McSwain NE., Jr Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 12.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer. J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 13.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med. 2008;36:3080–3084. doi: 10.1097/CCM.0b013e31818c3801. [DOI] [PubMed] [Google Scholar]
- 15.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–572. [PubMed] [Google Scholar]
- 16.Snyder CW, Weinberg JA, McGwin G, Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW, 3rd, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66:358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 17.Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MJ, Jr, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 18.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60:S51–S58. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 19.Hewson JR, Neame PB, Kumar N, Ayrton A, Gregor P, Davis C, Shragge BW. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med. 1985;13:387–391. doi: 10.1097/00003246-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, et al. An FFP:PRBC transfusion ratio >/= 1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 21.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 24.Silverboard H, Aisiku I, Martin GS, Adams M, Rozycki G, Moss M. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717–723. [PubMed] [Google Scholar]
- 25.Sharpe JP, Weinberg JA, Magnotti LJ, Fabian TC, Croce MA. Does plasma transfusion portend pulmonary dysfunction? a tale of two ratios. J Trauma Acute Care Surg. 2013;75:32–36. doi: 10.1097/TA.0b013e318294672d. [DOI] [PubMed] [Google Scholar]