Abstract
The aim of this study was to evaluate the relation between neutrophil-to-lymphocyte ratio (NLR) and plaque components assessed by virtual histology-intravascular ultrasound in 399 coronary artery disease (CAD) patients with 471 coronary lesions. We classified the lesions into two groups according to the NLR on admission {low NLR group (NLR≤2.73 [n=370]) vs. high NLR group (NLR>2.73 [n=101])}. By volumetric analysis, total atheroma and the absolute necrotic core (NC) volumes were significantly greater in high NLR group (249.9±149.7 µL vs. 192.5±127.7 µL, P=0.001, and 32.7±26.8 µL vs. 22.8±19.4 µL, P=0.001, respectively) and thin-cap fibroatheroma (TCFA) was observed more frequently in high NLR group (33% vs. 18%, P=0.001). ST segment elevation myocardial infarction (odds ratio [OR], 2.159; 95% CI, 1.000-4.660, P=0.050) and NLR>2.73 (OR, 1.848; 95% CI, 1.016-3.360, P=0.044) and total atheroma volume (OR, 1.003; 95% CI, 1.001-1.004, P=0.004) were the independent predictors of TCFA. CAD patients with high NLR had more vulnerable plaque components (greater NC-containing plaques) than those with low NLR.
Keywords: Coronary Disease, Neutrophils, Lymphocytes, Plaque, Ultrasonography, Interventional
INTRODUCTION
Inflammatory processes promote initiation and evolution of atheroma and contribute decisively to acute thrombotic complications of atheroma (1). White blood cell count is known to be an independent predictor of cardiovascular events and all-cause mortality (2). White blood cell subtypes, especially the neutrophil-to-lymphocyte ratio (NLR), has been proposed as a prognostic marker and seemed to be related to a proinflammatory state imposing worse clinical outcomes in patients with cardiovascular disease (3). Also NLR provides a simple and inexpensive method for assessment of inflammatory status in patients with acute coronary syndrome (4). Several studies have demonstrated a relation between the NLR and the severity of atherosclerosis and clinical outcome (5, 6, 7, 8, 9, 10, 11). However, the virtual histology-intravascular ultrasound (VH-IVUS) findings according to NLR were not well known.
Therefore, the aim of the present study was to evaluate the relation between NLR and plaque components assessed by VH-IVUS in patients with coronary artery disease (CAD).
MATERIALS AND METHODS
Patient population
This study was a retrospective, single-center study. From March 2006 to March 2010, a total of 399 CAD patients with 471 coronary lesions who underwent pre-intervention VH-IVUS at Chonnam National University Hospital were enrolled in this study. The presence of stable angina was determined by typical effort-induced chest pain which was relieved by resting. The presence of unstable angina was determined by chest pain within the preceding 72 hr with or without ST-T wave changes of positive cardiac biochemical markers. The presence of ST-segment elevation myocardial infarction was determined by >30 min of continuous chest pain, a new ST-segment elevation ≥2 mm on at least two contiguous electrocardiographic leads, and creatine kinase-myocardial band (MB) >3 times normal. The presence of non-ST-segment elevation myocardial infarction was diagnosed by chest pain and a positive cardiac biochemical marker without new ST-segment elevation. We excluded patients with subacute or late stent thrombosis, restenosis after stenting, coronary artery bypass graft failure, factors associated with increased risk of bleeding, severe heart failure or cardiogenic shock, important systemic disease, or creatinine ≥2.5 mg/dL, and patients in whom adequate IVUS images could not be obtained. The NLR was calculated as the ratio of neutrophil count to lymphocyte count. Based on the previously published article (11), we decided the cut-off value of NLR as 2.73, and we classified the patients into two groups according to the NLR on admission {low NLR group (NLR≤2.73 [370 lesions in 315 patients]) vs. high NLR group (NLR>2.73 [101 lesions in 84 patients])}.
Laboratory analysis
The blood samples were centrifuged, and serum was collected and stored at -70℃ until the assay was performed. Absolute creatine kinase-myocardial band levels were determined by radioimmunoassay (Dade Behring Inc., Miami, FL, USA). Cardiac-specific troponin I levels were measured by a paramagnetic particle, chemiluminescent immunoenzymatic assay (Beckman, Coulter Inc., Fullerton, CA, USA). Serum levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were measured by standard enzymatic methods. High-sensitivity C-reactive protein was analyzed turbidimetrically with sheep antibodies against human C-reactive protein; this has been validated against the Dade-Behring method (12).
Coronary angiographic analysis
Coronary angiogram was analyzed with validated QCA system (Phillips H5000 or Allura DCI program, Philips Medical Systems, Eindhoven, the Netherlands) (13). With the outer diameter of the contrast-filled catheter as the calibration standard, the minimal lumen diameter and reference diameter were measured in diastolic frames from orthogonal projections.
IVUS imaging and analysis
All IVUS examinations were performed a 20-MHz, 2.9F IVUS imaging catheter (Eagle Eye, Volcano Corp, Rancho Cordova, CA, USA) was advanced >10 mm beyond the lesion; and automated pullback was performed to a point >10 mm proximal to the lesion at a speed of 0.5 mm/sec.
Grey-scale IVUS and VH-IVUS data were analyzed by 2 independent observers. The levels of reproducibility for external elastic membrane, lumen, and plaque plus media cross-sectional areas using the Spearman rank-order correlation coefficients were 0.95, 0.97, and 0.97, respectively. Similarly, for plaque components by VH-IVUS, reproducibility for the fibrous, fibro-fatty, dense calcium, and necrotic core volume measurements using the Spearman rank-order correlation coefficients were 0.95, 0.92, 0.93, and 0.93, respectively.
Quantitative volumetric grey-scale and VH-IVUS analyses were performed across the entire lesion segment, and cross-sectional analysis was performed at the minimum lumen area sites and at the largest nectoric core sites. Conventional quantitative volumetric grey-scale IVUS analysis was performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (14). Measurements were made by every 1-mm interval for the region of interest, which was defined as the segment between distal to proximal reference sites that were the most normal looking within 5 mm proximal and distal to the lesion. Volumetric data were generated by the software using Simpson's method. External elastic membrane and lumen cross-sectional areas were measured. Plaque plus media cross-sectional area was calculated as external elastic membrane minus lumen cross-sectional area; and plaque burden was calculated as plaque plus media divided by external elastic membrane minus lumen cross-sectional area. Total atheroma volume (TAV) was calculated by summation of atheroma area from each measured image as: TAV=Σ(external elastic membrane area-lumen area). The percent atheroma volume (PAV) was determined using the formula: PAV=100×(Σ[external elastic membrane area-lumen area]/Σ[external elastic membrane area]). VH-IVUS analysis classified the color-coded tissue into four major components: green (fibrous), yellow-green (fibro-fatty), white (dense calcium), and red (necrotic core) (15). VH-IVUS analysis was reported in absolute amounts and as a percentage of plaque area or volume. We defined thin-cap fibroatheroma (TCFA) as necrotic core ≥10% of plaque area in at least 3 consecutive frames without overlying fibrous tissue in the presence of ≥40% least 3 plaque burden (16).
Statistical analysis
The statistical Package for Social Sciences (SPSS) for Windows, version 19.0 (Chicago, IL, USA) was used for all analyses. Continuous variables were presented as the mean value±1SD; comparisons were conducted by Student's t-test, Discrete variables were presented as percentages and frequencies; comparisons were conducted by chi-square test, where appropriate. Multivariate analysis was performed to determine the independent predictor of TCFA. A P value<0.05 was considered statistically significant.
Ethics statement
This study protocol was reviewed and approved by the institutional review board of Chonnam National University Hospital, Gwangju, Korea (CNUH-2013-054). Informed consent was waived by the board.
RESULTS
Baseline characteristics
The baseline characteristics are summarized in Table 1. High NLR group had more acute coronary syndrome (ACS) compared with low NLR group. High NLR group had higher white blood cell counts and high-sensitivity C-reactive protein, and lower ejection fraction compared with low NLR group. The creatine kinase-myocardial band and troponin-I, and N-terminal pro-B type natriuretic peptide level were significantly higher in high NLR group.
Table 1.
Baseline characteristics
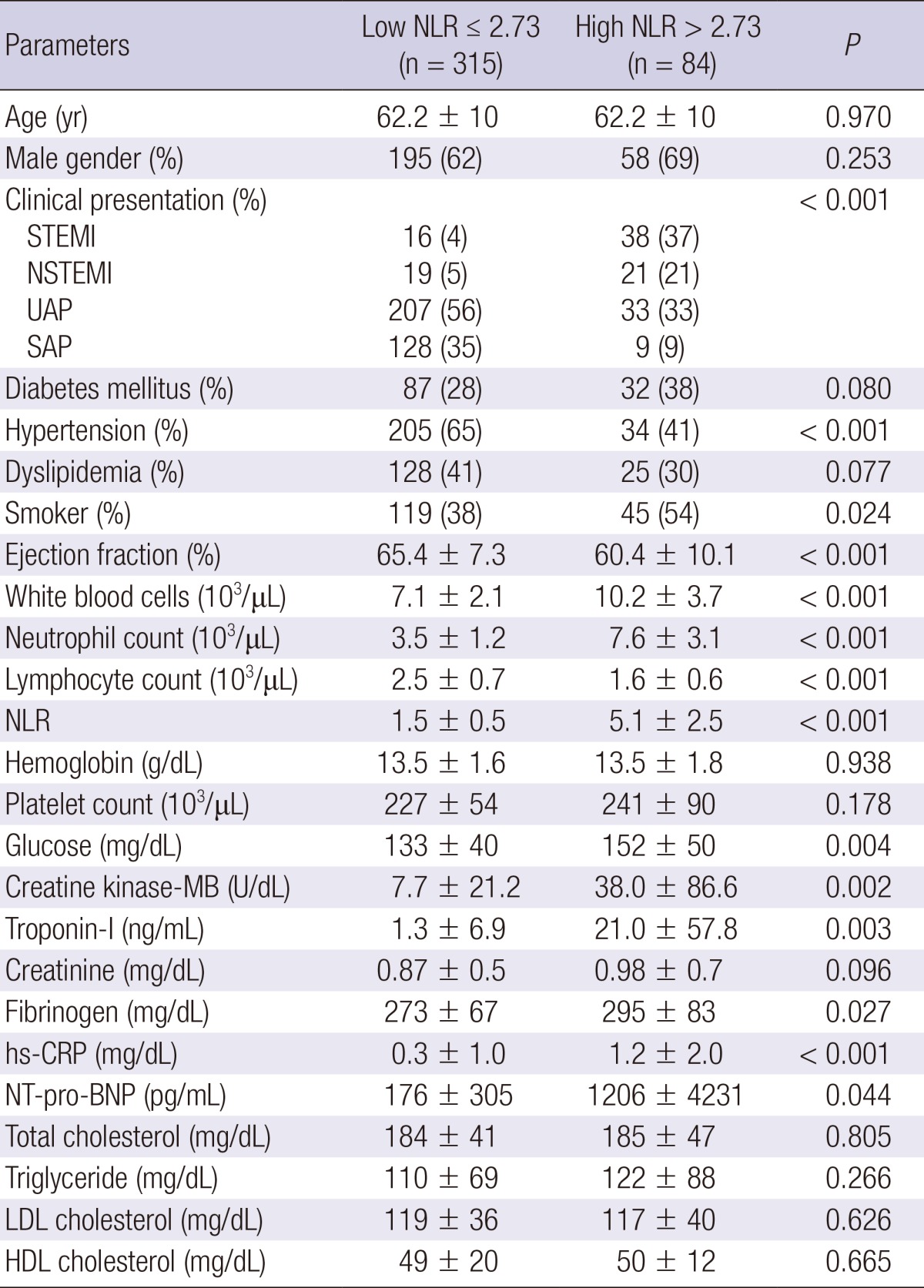
Data are presented as the No. (%) of patients or mean±SD. NLR, Neutrophil-to-Lymphocyte ratio; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; UAP, unstable angina pectoris; SAP, stable angina pectoris; hs-CRP, high-sensitivity C-reactive protein; NT-pro-BNP, N-terminal pro-B type natriuretic peptide; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Coronary angiographic findings
Coronary angiographic findings are summarized in Table 2. The minimal luminal diameter was significantly smaller and percent diameter stenosis was significantly greater in high NLR group.
Table 2.
Coronary angiographic findings
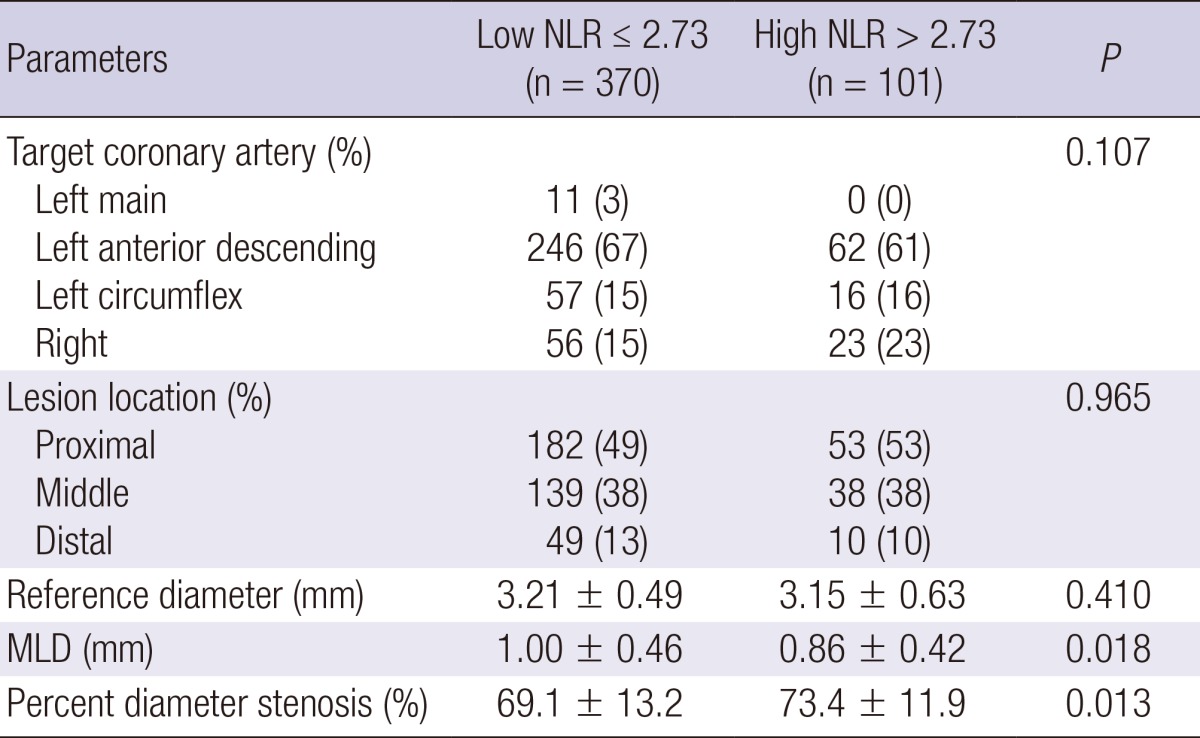
Data are presented as the No. (%) of patients or mean±SD. NLR, Neutrophil-to Lymphocyte ratio; MLD, minimal luminal diameter.
Grey-scale and VH-IVUS findings
Grey-scale IVUS findings are summarized in Table 3. At the proximal reference, lumen cross-sectional area was significantly greater in high NLR group. At the minimum lumen area site, external elastic membrane cross-sectional area and plaque plus media cross-sectional area and plaque burden were significantly greater, and IVUS lesion was significantly longer in high NLR group. At the largest necrotic core site external elastic membrane cross-sectional area and plaque plus media cross-sectional area and plaque burden were significantly greater in high NLR group. By volumetric analysis, external elastic membrane volume and lumen volume and total atheroma volume were significantly greater in high NLR group.
Table 3.
Grey-scale intravascular ultrasound findings
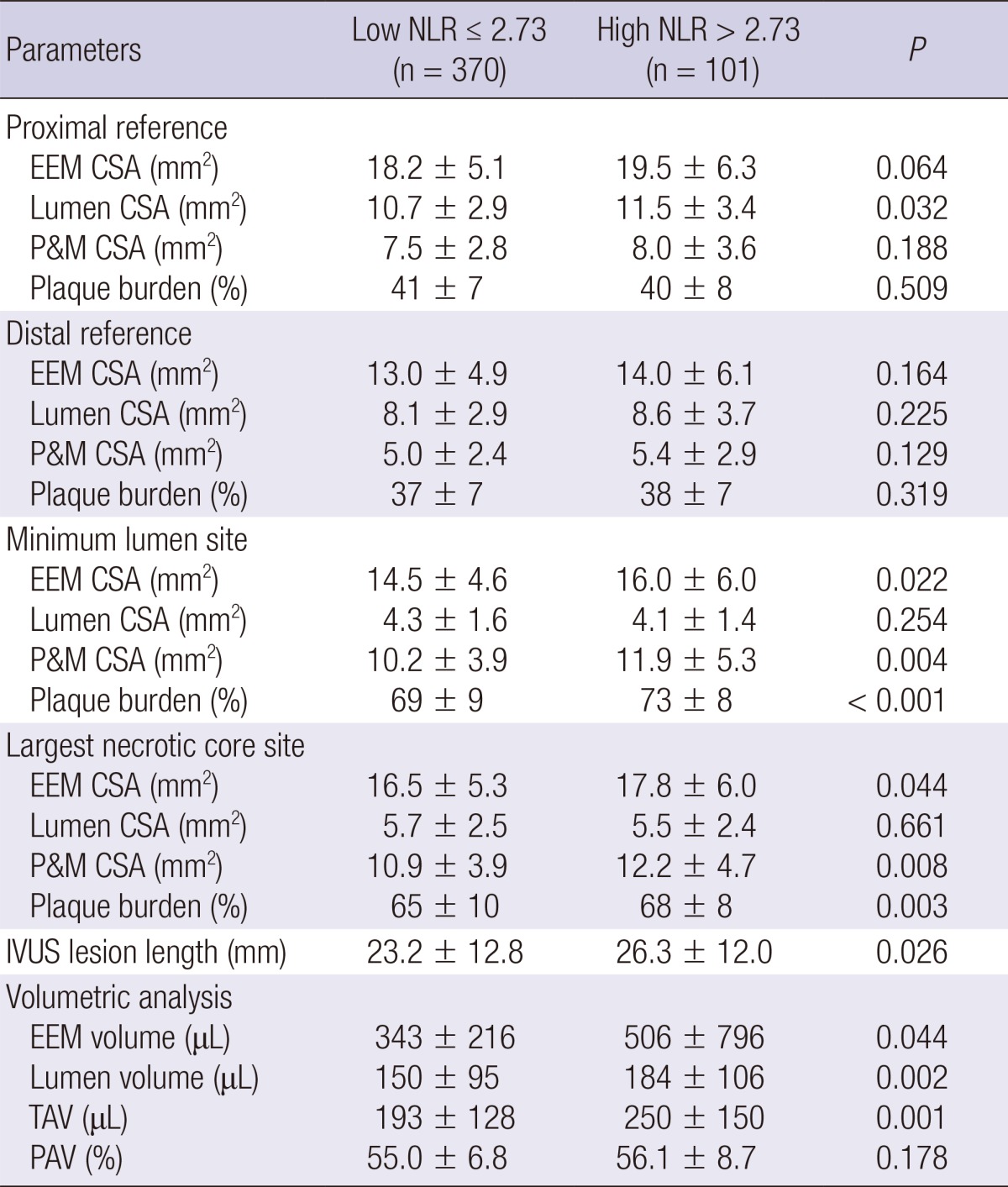
Data are presented as the No. (%) of patients or mean±SD. NLR, Neutrophil-to-Lymphocyte ratio; EEM, external elastic membrane; CSA, cross-sectional area; P&M, plaque plus media; IVUS, intravascular ultrasound; TAV, total atheroma volume; PAV, percent atheroma volume.
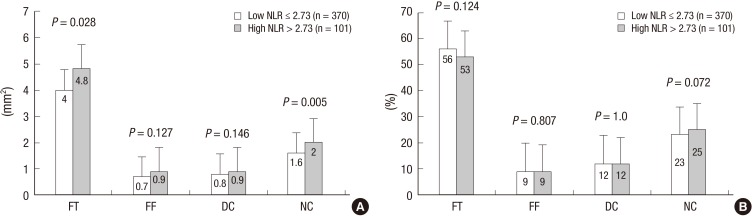
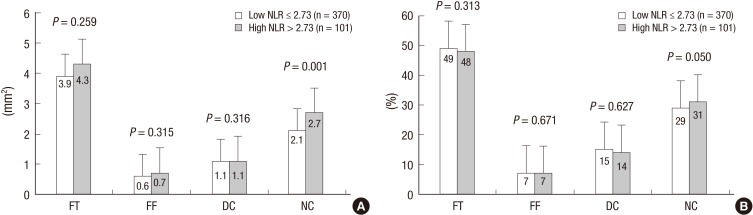
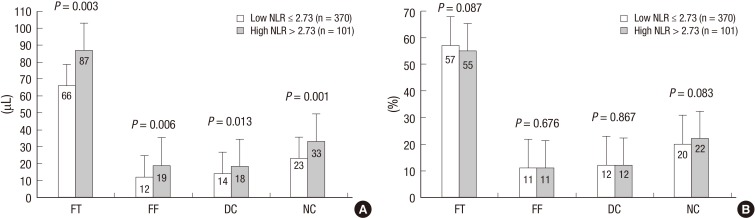
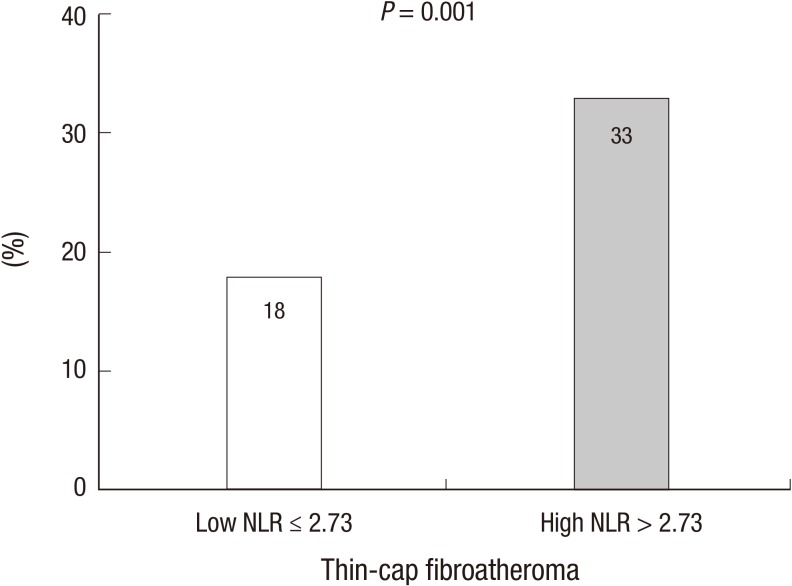
VH-IVUS findings are summarized in figures. At the minimum lumen area site, absolute fibrous and necrotic core areas were significantly greater in high NLR group (Fig. 1A). At the largest necrotic core site, absolute and relative necrotic core areas were significantly greater in high NLR group (Fig. 2). By volumetric analysis, absolute fibrous and fibro-fatty and dense calcium and necrotic core volumes were significantly greater in high NLR group (Fig. 3A). Also TCFA was observed more frequently in high NLR group compared with low NLR group (Fig. 4).
Fig. 1.
Absolute (A) and relative (B) plaque components at the minimum lumen area sites. Absolute fibrous and necrotic core areas are significantly greater in high NLR group. FT, fibrous; FF, fibro-fatty; DC, dense calcium; NC, necrotic core.
Fig. 2.
Absolute (A) and relative (B) plaque components at the largest necrotic core sites. Absolute necrotic core and relative necrotic core (%) areas are significantly greater in high NLR group.
Fig. 3.
Absolute (A) and relative (B) plaque components by volumetric analysis. Absolute fibrous and fibro-fatty and dense calcium and necrotic core volumes are significantly greater in high NLR group (A).
Fig. 4.
The incidence of thin-cap fibroatheroma. Thin-cap fibroatheroma is observed more frequently in high NLR group compared with low NLR group.
We performed separate analysis in the stable angina and ACS patients. In a subgroup analysis, ACS patients had greater total atheroma volume and absolute necrotic core volume compared with stable angina patients (216.2±141.9 µL vs. 177.2±110.7 µL, P=0.004, and 26.9±23.2 µL vs. 20.0±15.8 µL, P<0.001, respectively), and TCFA was observed more frequently in ACS patients compared with stable angina patients (24% vs. 13%, P=0.009).
We performed analysis in patients with ACS and stable angina using reference value of NLR (NLR: 2.7), respectively. We classified the lesions into two groups according to the NLR on admission in patients with ACS {low NLR group (NLR<2.7 [n=242]) vs. high NLR group (NLR≥2.7 [n=92]) and stable angina {low NLR group (NLR<2.7 [n=126]) vs. high NLR group (NLR≥2.7 [n=11]), respectively}. In ACS patients, at the minimum lumen area site and at the largest necrotic core site, high NLR group had greater absolute nectoric core area compared with low NLR group (1.58±1.04 mm2 vs. 2.02±1.38 mm2, P=0.002, and 2.16±1.11 mm2 vs. 2.72±1.50 mm2, P<0.001, respectively), and high NLR group had greater total atheroma volume and absolute necrotic core volume compared with low NLR group (201.7±134.6 µL vs. 254.3±153.8 µL, P=0.002, and 24.3±20.8 µL vs. 33.7±27.7 µL, P=0.001, respectively), and TCFA was observed more frequently in high NLR group compared with low NLR group (35% vs. 20%, P=0.007). However, there were no significant differences in VH-IVUS parameters between high NLR group and low NLR group in stable angina patients using reference value (NLR: 2.7).
Independent predictors of TCFA
Independent predictors of TCFA are summarized in Table 4. The following variables were tested to determine the independent predictors of TCFA (variables with P<0.2 in univariate analysis): ST segment elevation myocardial infarction, NLR>2.73, total atheroma volume, high-sensitivity C-reactive protein, plaque burden at the minimum lumen area site, plaque burden at the largest necrotic core site, hypertension, creatine kinase-myocardial band, ejection fraction. ST segment elevation myocardial infarction (odds ratio [OR], 2.159; 95% CI, 1.000-4.660, P=0.050) and NLR>2.73 (OR, 1.848; 95% CI, 1.016-3.360, P=0.044) and total atheroma volume (OR, 1.003; 95% CI, 1.001-1.004, P=0.004) were independent predictors of TCFA.
Table 4.
Multivariate analysis for thin-cap fibroatheroma
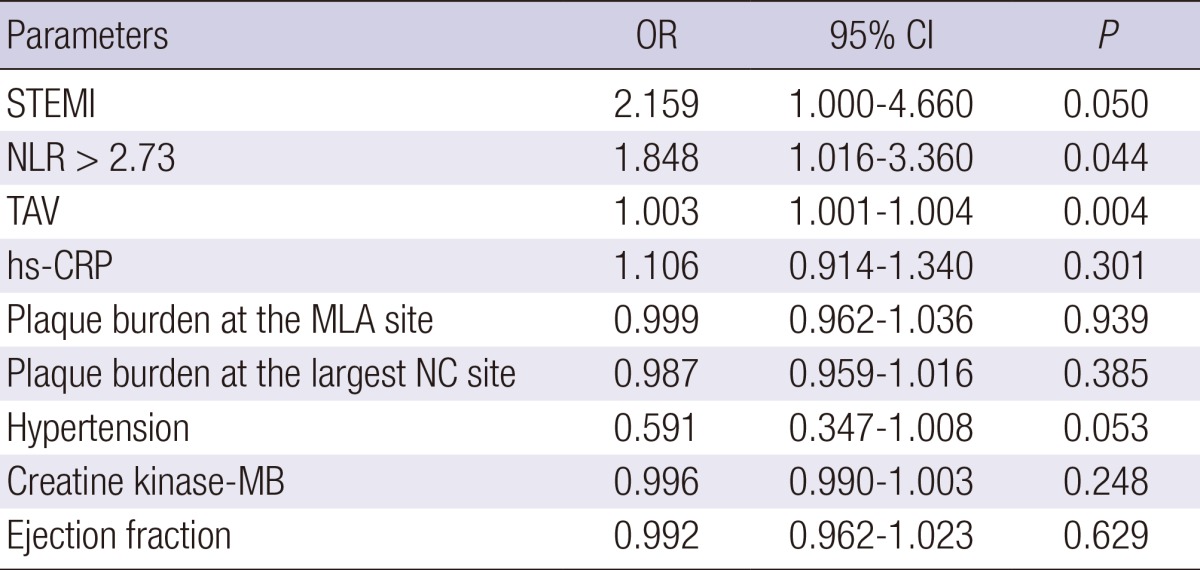
STEMI, ST segment elevation myocardial infarction; NLR, Neutrophil to Lymphocyte ratio; TAV, total atheroma volume; hs-CRP, high-sensitivity C-reactive protein; MLA, minimum lumen area; NC, necrotic core.
DISCUSSION
The present VH-IVUS study demonstrated that 1) patients with high NLR had more acute coronary syndrome; 2) necrotic core components were significantly greater in high NLR group compared with low NLR group; 3) the TCFA was observed more frequently in high NLR group compared with low NLR group; and 4) ST segment elevation myocardial infarction, NLR>2.73 and total atheroma volume were independent predictors of TCFA.
Atherosclerosis is known to be an inflammatory process and NLR is associated with enhanced inflammatory response (17). Increased inflammatory response can cause atherosclerosis to destabilize and become clinical cardiovascular disease (18, 19, 20, 21). Muhmmed Suliman et al. (4) reported that high NLR increased mortality, thus providing an additional level of risk stratification in patients with acute coronary syndrome. In the present study, patients with high NLR had more incidence of acute coronary syndrome and had higher white blood cell counts and high-sensitivity C-reactive protein levels compared with those with low NLR.
Previous studies have reported that NLR was related to the development and progression of CAD (5, 22). In the present study, the percent diameter stenosis was significantly greater and total atheroma volume was significantly greater in patients with high NLR compared with those with low NLR. These results suggest that inflammatory process indicated by high NLR are associated with the progression of atherosclerosis.
Vulnerable plaque in coronary artery can progress to plaque rupture and thrombosis, and have a strong potential to induce ACS (23, 24). Also inflammation and necrotic core size play a greater role in the progression of atherosclerosis in diabetic subjects who experienced sudden coronary death (25). In the present study, the necrotic core component was greater and TCFA within culprit lesions was observed more frequently in patients with high NLR compared with those with low NLR. The results of the present study suggest that CAD patients with high NLR have a greater possibility having vulnerable plaque and higher inflammatory status, which can lead to acute coronary events. Therefore NLR can be used as a useful tool to detect not only significant atherosclerosis but also plaque vulnerability in patients with CAD.
There are several limitations to be mentioned. First, the present study is retrospective single center study, so is subject to limitations inherent in this type of clinical investigation. Second, IVUS and VH-IVUS imaging were performed at the discretion of the individual operators leading to potential selection bias. Third, there is limitation using 20 MHz IVUS because this low frequency IVUS has a limitation to detect the plaque in detail, especially in the near field. Fourth, heavily calcified plaques may induce an artifact regarding the codification of plaques by VH-IVUS resulting in an increase in necrotic core content. Fifth, serial follow-up of serum neutrophil and lymphocyte levels were not performed. Therefore, we did not demonstrate the impact of sequential change of NLR levels on plaque components.
In conclusion, the present study demonstrates that CAD patients with high NLR had more vulnerable plaque components (greater necrotic core containing plaques) than those with low NLR. Our results of the present study suggest that CAD patients with high NLR have a greater possibility having vulnerable plaque and higher inflammatory status, which can lead to acute coronary events. Therefore NLR can be used as a useful tool to detect not only significant atherosclerosis but also plaque vulnerability in patients with CAD.
Footnotes
Funding: This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0163), and a grant of The Korean Society of Cardiology, The Korea Centers for Disease Control and Prevention (2013-E63005-00), and The Korean Health Technology R&D Project (HI13C1527), Ministry of Health & Welfare, and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2012M3A9C6049744), and the National Research Foundation of Korea Grant funded by the Korean Government (2011-0008875), and the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs (HI12C0275), and Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 11080-21), Republic of Korea.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 3.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Muhmmed Suliman MA, Bahnacy Juma AA, Ali Almadhani AA, Pathare AV, Alkindi SS, Uwe Werner F. Predictive value of neutrophil to lymphocyte ratio in outcomes of patients with acute coronary syndrome. Arch Med Res. 2010;41:618–622. doi: 10.1016/j.arcmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 7.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, Santas E, Merlos P, Rumiz E, Darmofal H, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Cho KH, Jeong MH, Ahmed K, Hachinohe D, Choi HS, Chang SY, Kim MC, Hwang SH, Park KH, Lee MG, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107:849–856. doi: 10.1016/j.amjcard.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 10.Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, Youn TJ, Cho GY, Chae IH, Choi DJ. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Turak O, Ozcan F, Isleyen A, Tok D, Sokmen E, Buyukkaya E, Aydogdu S, Akpek M, Kaya MG. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am J Cardiol. 2012;110:1405–1410. doi: 10.1016/j.amjcard.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications: part 2. Clin Chem. 2001;47:418–425. [PubMed] [Google Scholar]
- 13.Reiber JH, van der Zwet PM, Koning G, von Land CD, van Meurs B, Gerbrands JJ, Buis B, van Voorthuisen AE. Accuracy and precision of quantitative digital coronary arteriography: observer-, short-, and medium-term variabilities. Cathet Cardiovasc Diagn. 1993;28:187–198. doi: 10.1002/ccd.1810280301. [DOI] [PubMed] [Google Scholar]
- 14.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS): a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 15.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Granillo GA, García-García HM, Mc Fadden EP, Valgimigli M, Aoki J, de Feyter P, Serruys PW. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038–2042. doi: 10.1016/j.jacc.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haim M, Boyko V, Goldbourt U, Battler A, Behar S. Predictive value of elevated white blood cell count in patients with preexisting coronary heart disease: the Bezafibrate Infarction Prevention Study. Arch Intern Med. 2004;164:433–439. doi: 10.1001/archinte.164.4.433. [DOI] [PubMed] [Google Scholar]
- 19.Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J. 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakachi T, Kosuge M, Hibi K, Ebina T, Hashiba K, Mitsuhashi T, Endo M, Umemura S, Kimura K. C-reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non-ST-segment elevation acute coronary syndrome. Circ J. 2008;72:1953–1959. doi: 10.1253/circj.cj-08-0185. [DOI] [PubMed] [Google Scholar]
- 21.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 22.Fowler AJ, Agha RA. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography: the growing versatility of NLR. Atherosclerosis. 2013;228:44–45. doi: 10.1016/j.atherosclerosis.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 24.Virmani R, Burke AP, Kolodgie FD, Farb A. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol. 2003;16:267–272. doi: 10.1034/j.1600-0854.2003.8042.x. [DOI] [PubMed] [Google Scholar]
- 25.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]