Abstract
Previous epidemiologic studies have shown the clinical association between non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD). However, there is only limited information about the effect of NAFLD on the development of hypertension. Accordingly, we investigated the clinical association between NAFLD and prehypertension. A prospective cohort study was conducted on the 11,350 Korean men without prehypertension for 5 yr. The incidences of prehypertension were evaluated, and Cox proportional hazard model was used to measure the hazard ratios (HRs) for the development of prehypertension according to the degree of NAFLD (normal, mild, moderate to severe). The incidence of prehypertension increased according to NAFLD states (normal: 55.5%, mild: 63.7%, moderate to severe: 70.3%, P<0.001). Even after adjusting for multiple covariates, the HRs (95% confidence interval) for prehypertension were higher in the mild group (1.18; 1.07-1.31) and moderate to severe group (1.62; 1.21-2.17), compared to normal group, respectively (P for trend <0.001). The development of prehypertension is more potentially associated with the more progressive NAFLD than normal and milder state. These findings suggest the clinical significance of NAFLD as one of risk factors for prehypertension.
Keywords: Non-Alcoholic Fatty Liver Disease, Blood Pressure, Prehypertension
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is being recognized as not only most common liver disease but also metabolic disease (1, 2). Especially, as NAFLD was known to be deeply related to metabolic syndrome, there was increasing attention for the clinical association between NAFLD and cardiovascular disease (CVD). In practice, CVD was the second common cause of death next to cancer in 18 yr' follow up study for 132 NAFLD patients (3). In addition, diabetic patients with NAFLD had the higher prevalence of coronary arterial disease, cerebrovascular disease, and peripheral vascular disease than diabetic patients without NAFLD (4). As the pathophysiologic mechanisms of these associations, increased carotid artery intima-media thickness and decreased brachial artery endothelial flow-mediated vasodilation were suggested (5, 6, 7).
Nonetheless, there was only limited information about association between NAFLD and hypertension. Although Donati et al. (8) reported that prevalence of hypertension is high in the NAFLD patients with normal liver enzyme, their report has a limitation of the small scale with a few cases. Especially, considering the role of hypertension in the development of CVD, there is necessity for a study to investigate the association between NAFLD and hypertension. Accordingly, we conducted this study to investigate the effects of NAFLD on the development of prehypertension, identified as a predictor for developing hypertension.
MATERIALS AND METHODS
Study design
A prospective cohort study was conducted to examine the association between NAFLD and the development of prehypertension in Korean men participating in a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea. The background and purpose of the medical health check-up program is precisely described in the previous study of our group (9).
Study population
This study was based on the medical data derived from the health check-up program for Korean employees in 2005 yr. The study population was composed of Korean male workers, and the system of medical health check up was described in our previous study in detail (9).
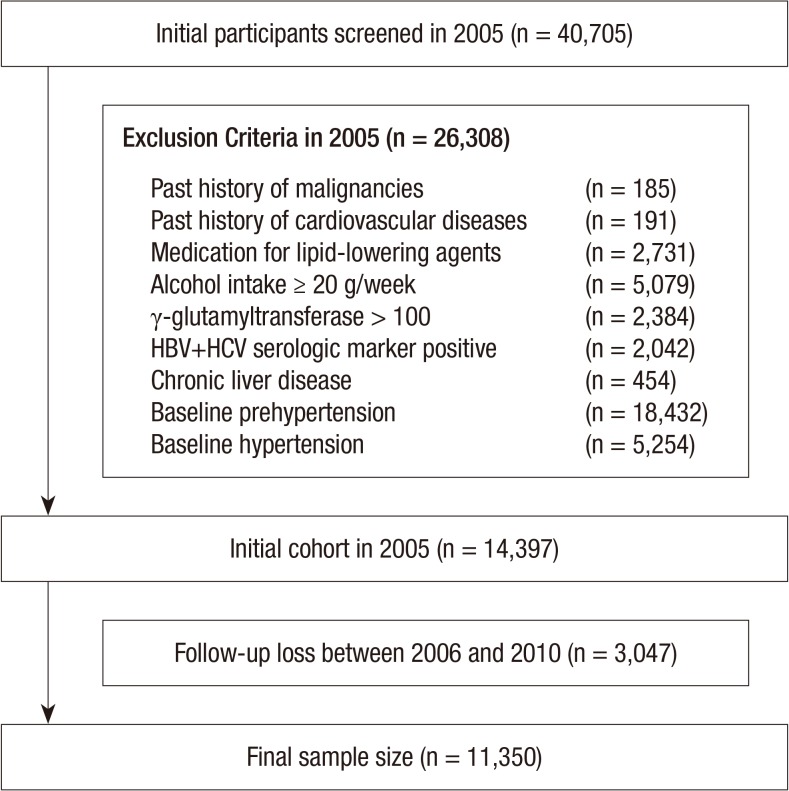
A total of 40,705 men, aged from 30 to 59 yr old, who underwent the abdominal ultrasonography (US) during a medical health check-up in 2005 participated in this study. Among the 40,705 participants, 26,308 men were excluded on the basis of the following exclusion criteria that might influence prehypertension or US findings: 185 had a past history of a malignancy; 191 had a past history of cardiovascular disease; 2,731 were receiving medication for lipid-lowering agents; 5,079 had an alcohol intake of ≥20 g/day; 2,384 had elevated γ-glutamyltransferase (GGT) levels (>100 U/L); 2,003 had a positive serologic marker for hepatitis B surface antigen (HBsAg); 39 had a positive serologic marker for hepatitis C virus antibody (HCVAb); 454 had abnormal US findings such as chronic liver disease, liver cirrhosis, and/or current or past history of clonorchiasis; 18,432 and 5,254 had a baseline prehypertension or hypertension, respectively at initial examinations.
Because some participants had more than one exclusion criteria, the total number of men eligible for the study was 14,397. We further excluded 3,047 participants who lost to follow-up between 2006 and 2010. The lost to follow-up was developed for the reasons such as retirement, unexpected disease, accidents or changing hospital for their will. Accordingly, 11,350 participants were included in the final analysis (Fig. 1). The total follow-up period was 32,180.1 person year and average follow-up period was 2.84 (standard deviation [SD], 1.44) person year.
Fig. 1.
Flow chart of the enrolled study population.
Clinical and laboratory measurements
Study data included a medical history, a physical examination, information provided by a questionnaire, anthropometric measurements and laboratory measurements. The medical history and the history of drug prescription were assessed by the examining physicians. All the participants were asked to respond to a questionnaire on health-related behavior. Questions about alcohol intake included the frequency of alcohol consumption on a weekly basis and the usual amount that was consumed on a daily basis (≥20 g/day). Persons smoking at that time were considered to be current smokers. In addition, the participants were asked about their weekly frequency of physical activity, such as jogging, bicycling, and swimming that lasted long enough to produce perspiration (≥1 time/week).
Anthropometric measurements and procedures for obtaining and examining the blood samples were described in detail elsewhere (9). Systolic and diastolic blood pressure (BP) was measured by a standardized mercury sphygmomanometer after at least 5 min of seated rest using the Hypertension Detection Protocol (10). According to the JNC-7 guidelines, normal BP was defined as a systolic BP less than 120 mmHg and a diastolic BP less than 80 mmHg, prehypertension as a systolic BP of 120-139 mmHg or a diastolic BP of 80-89 mmHg, hypertension as a systolic BP of at least 140 mmHg or a diastolic BP at least 90 mmHg, or current use of antihypertensive agents (11). Diabetes mellitus was defined as fasting serum glucose ≥126 mg/dL, or current use of diabetic medications.
The clinical laboratory has been accredited and participated annually in inspections and surveys by the Korean Association of Quality Assurance for Clinical Laboratories.
The diagnosis of fatty liver and the evaluation of its degree were based on the results of abdominal US with a 3.5-MHz transducer (Logic Q700 MR, GE, Milwaukee, WI, USA). Abdominal US were carried out by experienced radiologists who were unaware of the aims of the study and blind to the laboratory values. Images were captured in a standard fashion, with the patient in the supine position, with the right arm raised above the head. The fatty liver disease was diagnosed according to the standard criteria described by previous studies, including parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, and bright vessel walls (12, 13, 14). To assess the intra- and inter-observer reliability of ultrasound diagnosis of fatty liver, random samples of 200 stored ultrasonographic images were re-read at least two weeks apart by the eleven radiologists. All radiologists were blinded to clinical information. The inter-observer reliability and intra-observer reliability for fatty liver diagnosis were substantial (kappa static of 0.74) and excellent (kappa static of 0.94), respectively.
The presence of metabolic syndrome (MetS) was evaluated according to the joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention (15).
Statistical analysis
Data were expressed as means±(SD) or medians (interquartile range) for continuous variables and percentages of the number for categorical variables.
The one-way ANOVA and chi-square-test were used to analyze the statistical differences among the characteristics of the study participants at the time of enrollment in relation to the NAFLD categories. Categories of the NAFLD comprised the following: normal, mild, moderate and severe. Moderate (n=382) and severe NAFLD (n=9) was combined into moderate to severe NAFLD category for analyses, owing to the small number of severe NAFLD. The distributions of continuous variables were evaluated, and log transformations were used in the analysis as required. For incident prehypertension cases, the time of prehypertension development was assumed to be the midpoint between the time of visit when prehypertension was first diagnosed and the time of baseline visit in 2005. The person years were calculated as the sum of follow-up times from the baseline to an assumed time of prehypertension development or to the final examination of each individual. Cox proportional hazards model was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for incident prehypertension comparing the mild and moderate to severe NAFLD categories vs. the normal group. The data were adjusted, first for age alone, then for the multiple covariates. In the multivariate models, we included variables that might confound the relationship between NAFLD and prehypertension, which include: age, log (hsCRP), HDL-cholesterol, serum creatinine, recent smoking status, regular exercise, MetS and diabetes mellitus. For the linear trends of risk, the number of NAFLD categories was used as a continuous variable and tested on each model. To use the Cox proportional hazards models, we checked the validity of the proportional hazards assumption. Two approaches were used to assess the validity of the proportional hazards assumption. First, the assumption was assessed by log-minus-log-survival function and found to graphically hold. Second, to confirm the validity of the proportional hazards assumption, time-dependent covariate analysis was used. The time-dependent covariate was not statistically significant, suggesting that the proportional hazards assumption is not violated (P=0.441). P values <0.05 were considered to be statistically significant. Statistical analyses were performed PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Ethics statement
Ethics approvals for the study protocol and analysis of the data were obtained from the institutional review board of Kangbuk Samsung Hospital (IRB number: KBC12060). The informed consent requirement was exempted by the board because researchers only accessed retrospectively a de-identified database for analysis purposes.
RESULTS
During 32,180.1 person-years of follow-up, 6,602 (58.2%) incident cases of prehypertension developed between 2006 and 2010. Compared with analytic cohort (n=11,350), 3,047 participants not included in analytic cohort were 0.7 yr older (42.1 vs. 41.4) and had a less favorable baseline metabolic profiles in age, diastolic BP, fasting serum glucose, smoking status and diabetes mellitus (Table 1).
Table 1.
Comparison between exclusion from analysis and inclusion in analysis
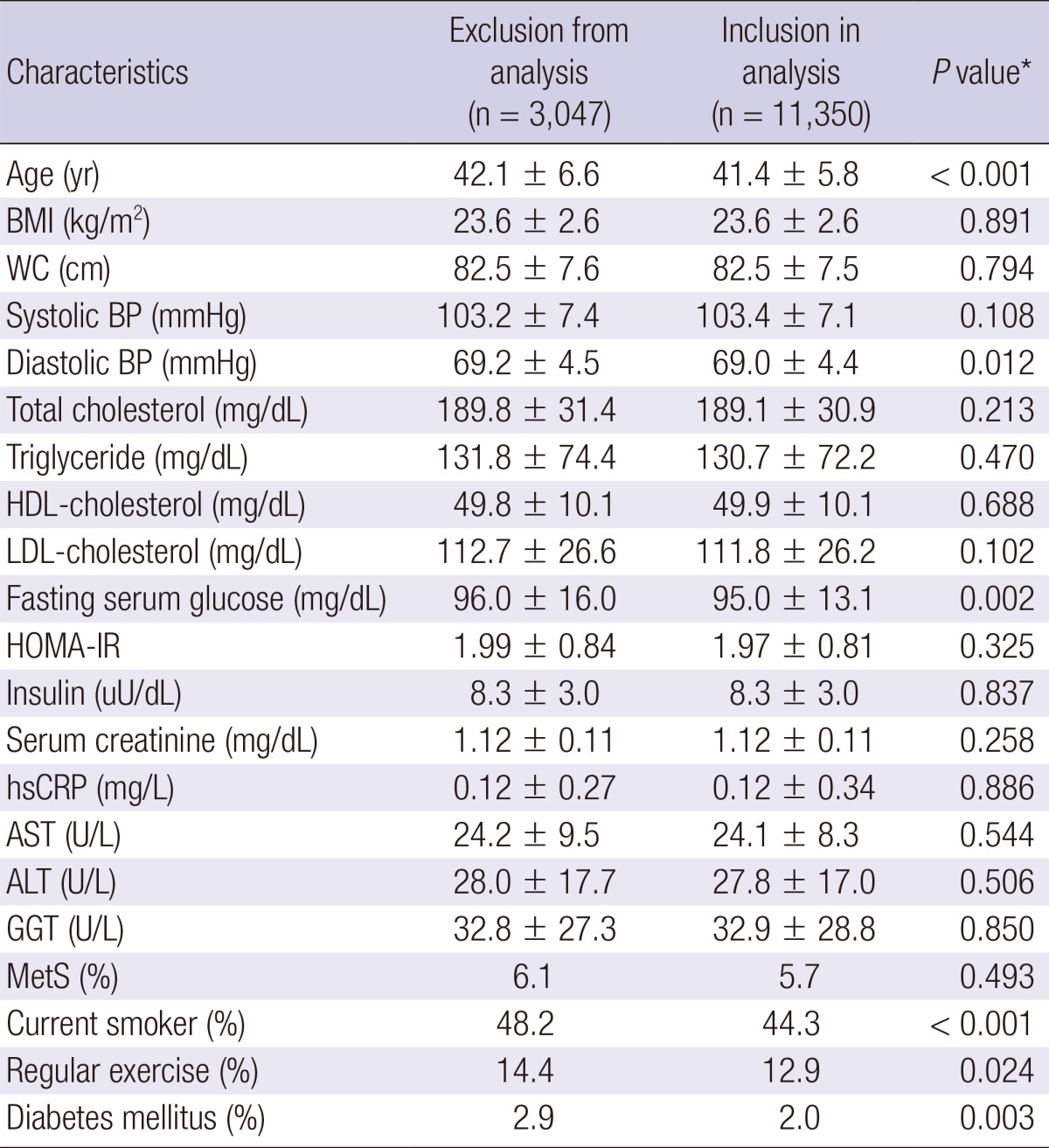
Data are expressed as means±standard deviation or percentages. *P value by t-test for continuous variables and chi square test for categorical variables. BMI, body mass index; WC, waist circumference; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity c-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; MetS, metabolic syndrome.
The baseline characteristics of the study participants in relation to the NAFLD categories are presented in Table 2. At baseline, the mean (SD) age and BMI of study participants were 41.4 (5.9) yr and 23.6 (2.6) kg/m2, respectively. There were clear dose response relationships between all of the listed variables and NAFLD categories.
Table 2.
Baseline characteristics of participants according to NAFLD categories (n=11,350)
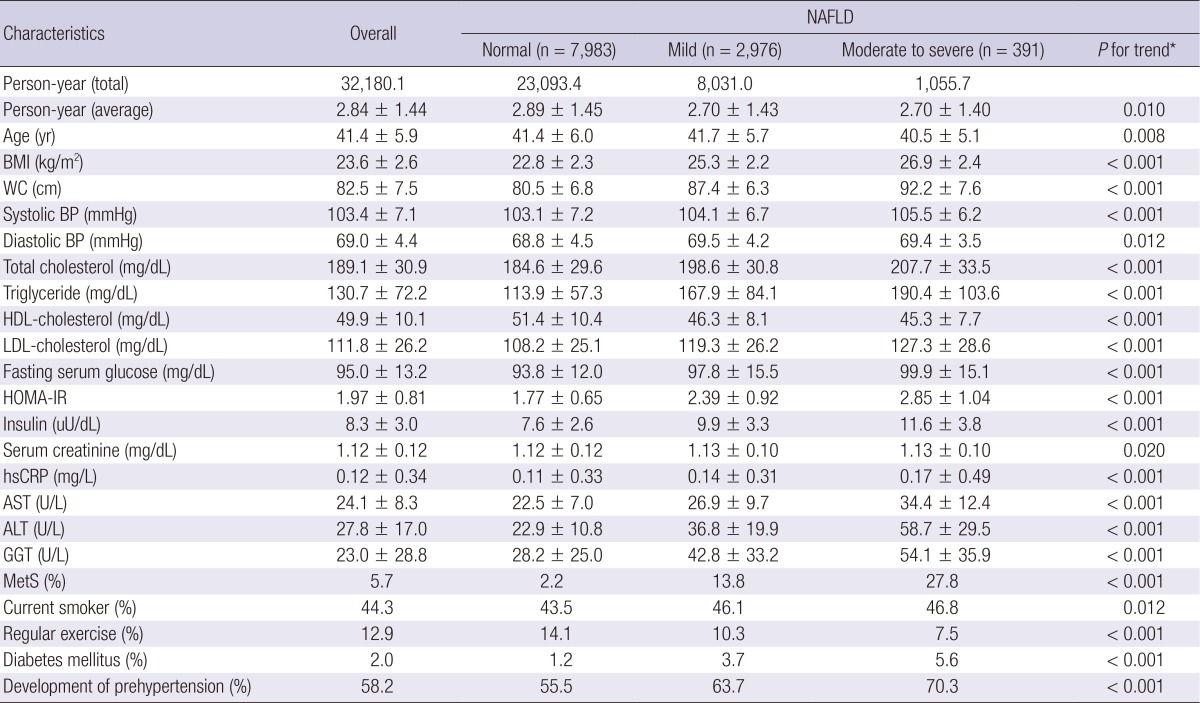
Data are means±standard deviation, medians or percentages. *P value by ANOVA-test for continuous variables and chi square test for categorical variables. BMI, body mass index; WC, waist circumference; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity c-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; MetS, metabolic syndrome.
In contrast to participants without development of prehypertension, those with development of prehypertension were slightly older (42.0 vs. 40.6) and more likely to have the diabetes mellitus and NAFLD. As expected, all clinical variables showed statistically significant differences between two groups except for smoking status (Table 3).
Table 3.
Comparison between participants with and without incident prehypertension
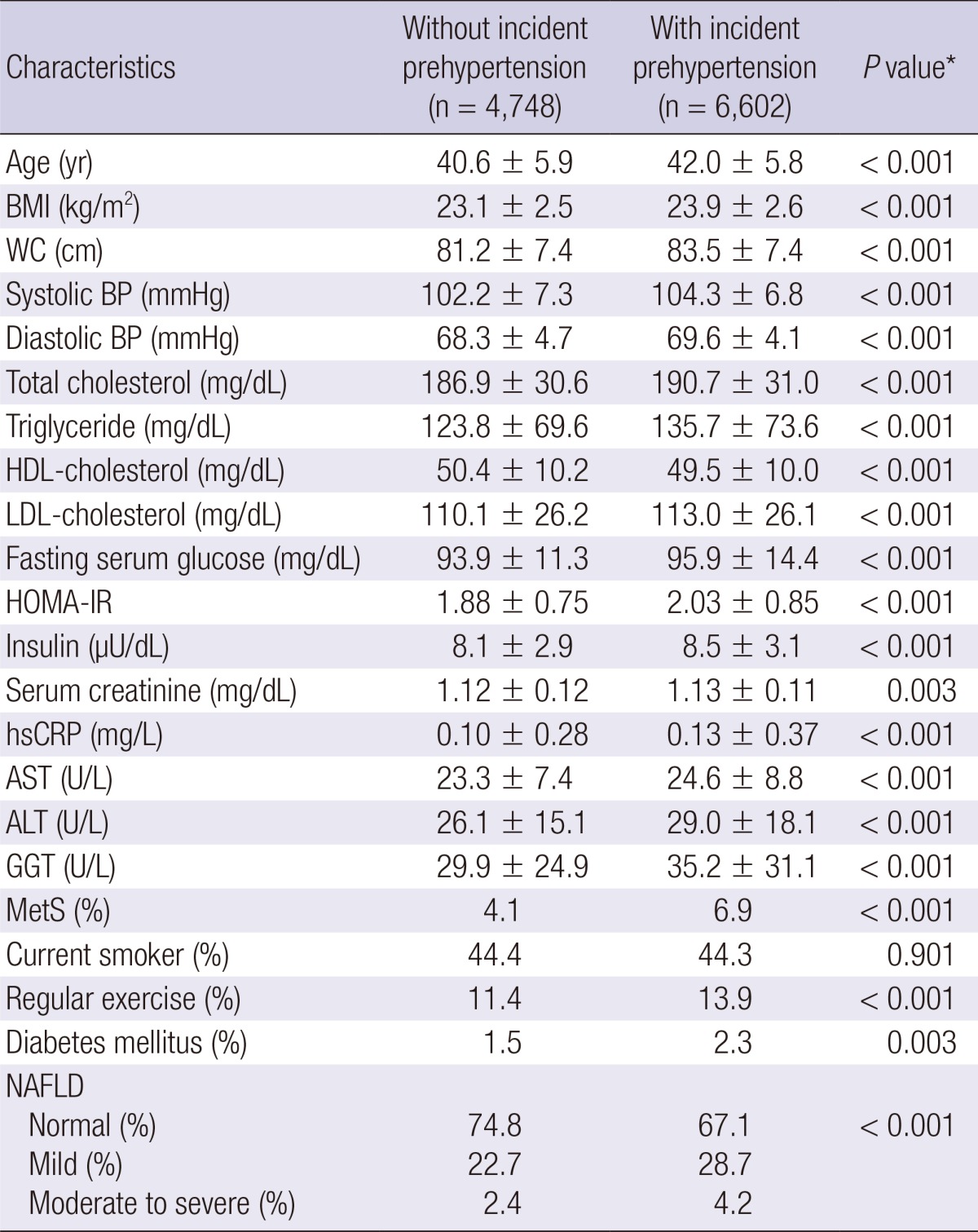
Data are are means±standard deviation, medians or percentages. *P value by ANOVA-test for continuous variables and Chi square test for categorical variables. BMI, body mass index; WC, waist circumference; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity c-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; MetS, metabolic syndrome.
Table 4 shows the hazard ratios and 95% CI for prehypertension according to the NAFLD categories. In unadjusted model, the hazard ratios and 95% CI for prehypertension increased according to the degree of NAFLD (mild NAFLD, 1.26 [1.20-1.33]; moderate to severe NAFLD, 1.40 [1.24-1.58], respectively [P for trend<0.001]). These associations remained statistically significant, even after further adjustments for covariates in model 1 and 2. In model 2, the adjusted hazard ratios and 95% CI for prehypertension were 1.18 (1.07-1.31) and 1.62 (1.21-2.17), respectively (P for trend<0.001).
Table 4.
Hazard ratios and 95% confidence intervals for the incidence of prehypertension according to NAFLD categories

Model 1 was adjusted for age, HDL-cholesterol, log (hsCRP) and serum creatinine. Model 2 was adjusted for model 1 plus recent smoking status, regular exercise, MetS and diabetes mellitus. HDL, high density lipoprotein; hsCRP, high-sensitivity c-reactive protein; MetS, metabolic syndrome.
DISCUSSION
This study demonstrated a significant association between degree of NAFLD and the subsequent development of prehypertension in Korean men. This association was independent of age, HDL-cholesterol, log (hsCRP), serum creatinine, recent smoking status, regular exercise, MetS and diabetes.
These findings can be epidemiologic evidence sustaining the causative relation between NAFLD and CVD. As aforementioned, there is only limited data explaining the association between NAFLD and CVD. Although several studies have indicated the independent role of NAFLD on CVD (16, 17, 18, 19), the pathophysiologic mechanism remains unclear. However, our study findings may be one of pathophysiologic mechanism for these studies. Especially, considering the disease progression from prehypertension through hypertension to CVD, our study suggests the role of NAFLD in the development of CVD.
As the mechanism of our findings, we suggest theories concerning the effects of NAFLD on circulatory system. The first is insulin resistance identified as the critical factor for the development of NAFLD. Insulin resistance can induce dyslipidemia and provoke the secretion of proinflammatory cytokine such as tumor necrosis factor-α and interleukin-6, which accelerates the arteriosclerosis (20, 21, 22). These conditions can decrease the vascular elasticity and luminal width to increase blood pressure. The second is the oxidative stress, and chronically potential inflammation associated with NAFLD (23, 24). NAFLD is related with the increased oxidative stress and hazardous cytokine as well as decreased anti-atherogenic factor like adiponectin (25, 26). These hazardous conditions can provoke the inflammatory response in the arterial inside to deteriorate the arteriosclerosis. Considering the significant association between arteriosclerosis and hypertension, these theories may be a probable background to explain the mechanism of the present study.
When interpreting our results, some limitations should be considered. First, the presence of NAFLD was assessed by ultrasonographic method instead of pathologic method. Although US is regarded reasonably accurate, it cannot identify fatty infiltration of the liver below the threshold of 30% (27). However, it is inappropriate to perform invasive tests in a population-based epidemiological study (28). In addition, all examinations were carried out by experienced radiologists using widely established methods, and US examination of our group has credible inter- and intra-observer reliability. Thus, US might be clinically appropriate and reasonable modality to diagnose NAFLD in this study.
Second, we could not count obesity into adjusting covariates. Especially, considering the significance of obesity in the development of hypertension, this limitation should be addressed. However, when body mass index (BMI) was included in adjusting covariates, we found that statistical significance disappeared. These findings suggest the pivotal role of obesity on the development of NAFLD and prehypertension. Actually, when study participants were divided to 2 groups of BMI<28 and BMI≥28, we could ascertain the interesting results. While people with BMI<28 showed the statistically significant HR for prehypertension, people with BMI≥28 did not have the significant HR. This finding shows the stronger effect of obesity on prehypertension than that of NAFLD. Thus, we could not be sure that NAFLD was a risk factor for prehypertension independently of obesity. Nonetheless, it does not seem that NAFLD has no contributory relationship with the development of prehypertension. In this study, NAFLD was significantly associated with the risk for prehypertension even after adjusting for diabetes, fasting glucose level and MetS. Diabetes, fasting glucose and metabolic syndrome are medical conditions encompassing metabolic conditions of insulin resistance. As aforementioned, insulin resistance plays a significant role in elevation of blood pressure. Accordingly, the finding that statistical significance remained even after adjusting for parameters of insulin resistance is thought to be showing the significant association between NAFLD and prehypertension.
Third, our study population was only limited to Korean men. Although prevalence of NAFLD is different between two genders, including women may be better to show the effect of NAFLD on IR. Especially, the prevalence of NAFLD is comparatively lower in woman than man, and known to increase in postmenopausal state (29, 30). In addition, Bedogni et al. (31) showed that gender was the significant predictor of NAFLD. Thus, these results cannot be necessarily extrapolated to women and other ethnic groups and further studies are needed.
In conclusion, our findings, which were obtained from large number of cohort, showed that the risk of prehypertension increased in proportion to the degree of NAFLD during a 5-yr' follow-up. However, when BMI was counted into adjusting covariates, the statistical significance was not maintained. These findings suggest not only the availability but also the limitation of NAFLD as a risk factor for prehypertension. Therefore, to reveal the more correct association between NAFLD and prehypertension, consecutive studies should be conducted to reveal the effect of NAFLD on the blood pressure.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Targher G, Bertolini L, Padovani R, Poli F, Scala L, Tessari R, Zenari L, Falezza G. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2006;23:403–409. doi: 10.1111/j.1464-5491.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 5.Brea A, Mosquera D, Martín E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 6.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72–78. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 8.Donati G, Stagni B, Piscaglia F, Venturoli N, Morselli-Labate AM, Rasciti L, Bolondi L. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut. 2004;53:1020–1023. doi: 10.1136/gut.2003.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun H, Park SK, Ryoo JH. Association of serum gamma-glutamyltransferase level and incident prehypertension in Korean men. J Korean Med Sci. 2013;28:1603–1608. doi: 10.3346/jkms.2013.28.11.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 12.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 13.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Sung KC, Ryan MC, Wilson AM. The severity of nonalcoholic fatty liver disease is associated with increased cardiovascular risk in a large cohort of non-obese Asian subjects. Atherosclerosis. 2009;203:581–586. doi: 10.1016/j.atherosclerosis.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, Kim YS, Kim CH, Choi SH, Kim W, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104:1953–1960. doi: 10.1038/ajg.2009.238. [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005;11:4838–4842. doi: 10.3748/wjg.v11.i31.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 21.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 22.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 24.Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 26.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 27.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 28.Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol. 2003;15:539–543. doi: 10.1097/01.meg.0000059112.41030.2e. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 31.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]