Abstract
We sought to test experimentally whether maternal stress can promote susceptibility to development of asthma-like allergic airways disease in offspring. Normal pregnant mice (day 15) were subjected to a single restraint stress exposure. We subsequently tested their offspring for the development of airway hyperreactivity (AHR) and allergic airway inflammation (AI), after an intentionally suboptimal sensitization protocol. The offspring of stressed mothers showed levels of AI and enhanced airway responses to methacholine comparable to those seen in fully sensitized and challenged positive control animals; in contrast, minimal effects were seen in control offspring. Restraint stress caused a rapid and large increase in plasma corticosterone levels. Maternal treatment with dexamethasone on day 15 of pregnancy mimicked the stress effect and reproduced the AI and AHR outcomes, whereas blockade of the stress-induced corticosterone surge with metyrapone pretreatment of pregnant mice abrogated the effect. We conclude that stress-triggered glucocorticoids during pregnancy can increase susceptibility to allergy in offspring. Because inflammation typically includes a stress hormone response, the results also suggest a common pathway by which various injurious exposures during pregnancy might increase offspring susceptibility to asthma.
Keywords: pregnancy exposure, maternal transmission of asthma risk, dexamethasone
human epidemiological studies find that stress plays a significant role in exacerbations of already-established childhood asthma (38, 41, 46, 47). Murine studies also show that stress can increase allergen-induced airway inflammation (2, 25). One mechanism may be the capability of glucocorticoid (GC) stress hormones to promote proallergic Th2-type responses (16, 31). These proallergic effects of GCs are surprising and counterintuitive, given the efficacy of GC therapy in dampening allergic inflammation. However, animal models using chronic stress conditions, a scenario in which GCs are persistently elevated, observe a skew toward Th2-type responses (8, 21, 34).
Although stress in asthma patients can exacerbate disease, its role in asthma initiation has not been experimentally tested. Several observations suggest that stress responses may promote initiation of asthma. First, human studies suggest that caregiver stress in the perinatal period may predispose offspring to asthma/allergy phenotypes (11, 35, 37, 49, 50). A recent study has also demonstrated that maternal anxiety during pregnancy increased asthma risk in offspring (11). Second, it is clear that the in utero environment can affect the development of the fetal immune system (5, 14, 36, 43). Indeed, two groups (33, 36), using rodent models, showed that prenatal stress increased the relative severity of asthma-like outcomes in adult offspring, i.e., increased eosinophilia after induction of airway allergic responses.
Using a mouse model, we have found that maternal allergy (22), maternal exposure to pollutant particles (diesel exhaust) (18, 23) during gestation, and premating exposure to specific chemical contact irritants (27) all can induce asthma susceptibility in offspring. These exposures share induction of active inflammation in the pregnant mother, and active inflammation in turn triggers a stress response (9, 10, 40). Relatively transient maternal stresses can cause durable changes in offspring, as reported for offspring behavior, circadian rhythms, and hypothalamic-pituitary-adrenal (HPA) changes (12). Hence, we sought to directly test the hypothesis that maternal stress during gestation can promote the initiation of asthma in offspring, i.e., increased asthma susceptibility.
After using a mild restraint stress (RS) exposure on pregnant mice, we have monitored the development of an asthma-like phenotype [airway hyperreactivity (AHR) and allergic airway inflammation (AI)] after an intentionally suboptimal allergy induction protocol. This protocol produces minimal effects in normal neonatal mice, but leads to an asthma-like phenotype in offspring of mother mice with ovalbumin (OVA)-induced “chronic asthma” (22). We used these groups as negative and positive controls, respectively, and tested whether RS in normal pregnant mice would lead to asthma susceptibility in their offspring.
MATERIALS AND METHODS
Pregnant BALB/c mice (day 12 of pregnancy) were obtained commercially from Charles River Laboratories (Cambridge, MA). Mice were housed and fed standard laboratory chow ad libitum in a pathogen-free barrier facility that was maintained at 22–24°C with a 12:12-h dark-light cycle. Animal experiments were conducted under a protocol approved by our institutional review board.
Maternal stress and drug treatments.
On day 15 of pregnancy (E15) the females were placed in 60-ml syringes with the plunger inserted behind them. Air holes were cut at the tip and sides of the syringe for ventilation. The type of restraint is similar to the technique used by Kumlien Georen et al. (26); the mice had very limited movement but were not in pain. The duration of restraint was 1 h. Unstressed mice were kept without food or water for an equal duration. In a separate group of experiments, unstressed E15 females were injected subcutaneously with dexamethasone (125 μg/kg) (see Fig. 1). In other experiments, stressed mothers were preinjected with metyrapone intraperitoneally to inhibit corticosterone (CORT) synthesis (24, 39). All stresses and injections were conducted at the same time of day to eliminate circadian variation in CORT as a variable. Litter sizes for these experimental groups were six to eight pups, similar to control groups.
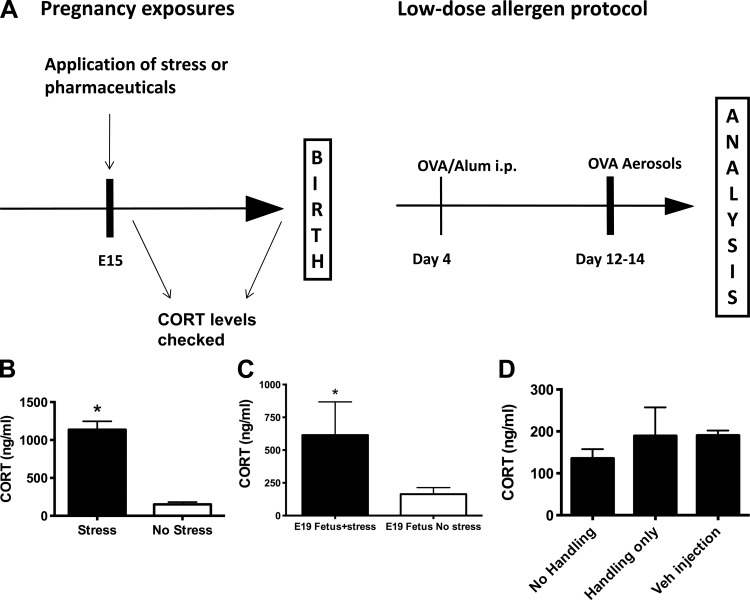
Fig. 1.
A: schematic of experimental protocol. Fifteen-day pregnant mice (E15) were subjected to 1 h of restraint stress and allowed to deliver normally. Offspring received one intraperitoneal injection of ovalbumin (OVA)/Al(OH)3 on day 4 of life and 10 min aerosol challenges with 3% OVA on days 12–14 (Intentionally suboptimal protocol) followed by analysis on days 15 and 16. B: maternal serum corticosterone (CORT) levels were evaluated by ELISA immediately after the stress (RS) vs. unstressed controls (n ≥ 5/group, *P < 0.05). C: after maternal stress, fetal E19 serum CORT levels were elevated significantly vs. the levels seen in control fetuses, tested as a pool of samples from a pregnant mouse (n = 3/group, *P < 0.05). D: maternal serum CORT levels were not affected by handling or vehicle injection (n = 3/group).
Allergen sensitization and challenge.
Offspring of treated/untreated mother mice were subjected to an intentionally low-dose suboptimal asthma induction protocol (see Fig. 1) that features a single intraperitoneal injection of 5 μg OVA in 1 mg Al(OH)3 on day 4 of life, followed by 3% OVA aerosols on days 12–14. Although the formal description of the outcome is an OVA sensitization-dependent asthma-like phenotype with AHR and allergic AI, we will use the term asthma in this manuscript to avoid repeating this cumbersome phrase, recognizing that mice do not get the human disease asthma. This protocol is known to induce a robust asthmatic phenotype in offspring of allergic mothers but has minimal effect on normal pups (18, 19, 23). Positive controls received an additional injection of OVA plus Al(OH)3 on day 9 of life: recipients of the two injection protocols develop an asthmatic phenotype regardless of parental allergy. Final physiological and pathological analyses were performed on days 15 and 16, respectively, i.e., next day after the last OVA aerosol. To summarize the designation of groups: negative controls are offspring of normal (unstressed) mothers who were subjected to the suboptimal asthma induction protocol; positive controls are offspring of normal (unstressed) mothers who were subjected to a full (optimal) asthma induction protocol; and the stress group is offspring of stressed mothers who were subjected to the suboptimal asthma induction protocol.
Lung function testing.
Airway responsiveness of mice to increasing concentrations of aerosolized methacholine was measured using whole body plethysmography (Buxco, Sharon, CT). Briefly, each mouse was placed in a chamber, and continuous measurements of box pressure/time wave were calculated via a connected transducer and associated computer data acquisition system. The main indicator of airflow obstruction, enhanced pause (Penh), which shows strong correlation in BALB/c mice with the airway resistance examined by standard evaluation methods, was calculated from the box waveform. After measurement of baseline Penh, aerosolized PBS or methacholine (Sigma-Aldrich) in increasing concentrations (6, 12, 25, 50, and 100 mg/ml) was nebulized through an inlet of the chamber for 1 min, and Penh measurements were taken for 9 min after each dose. Penh values for the first 2 and the last 2 min after each nebulization were discarded, and the values for 5 min in between were averaged and used to compare results. Increased Penh values were interpreted as evidence of increased AHR (see discussion).
Pathological analysis.
Animals were killed with pentobarbital sodium (Veterinary Laboratories, Lenexa, KS). The tracheas were cannulated, and bronchoalveolar lavage (BAL) was performed five times with 0.3 ml of sterile PBS instilled and harvested gently. Lavage fluid was collected and centrifuged at 1,200 rpm for 5 min, and the cell pellet was resuspended in 0.1 ml PBS. Total cell yield was quantified by hemocytometer. BAL differential cell counts were performed on cytocentrifuge slides prepared by centrifugation of samples at 800 rpm for 5 min (Cytospin 2; Shandon, Pittsburgh, PA). These slides were fixed in 95% methanol and stained with Diff-Quik (VWR, Boston, MA), a modified Wright-Giemsa stain, and a total of 200 cells were counted for each sample by microscopy. Total cells and eosinophils were enumerated. For histological analysis, lungs were inflated with 10% buffered formalin, removed, and immersed in the same solution. All of the tissue samples were embedded in paraffin, and sections for microscopy were stained with hematoxylin and eosin. Histopathological slides were evaluated for severity and extent of inflammation by a blinded scorer. Severity scores were from one to three. A score of 1, 2, or 3 corresponded to perivascular cell layer thickness of 1–3 cells, 3–10 cells, or >10 cells, respectively. Extent scores were from one to three. A score of 1, 2, or 3 corresponded to <25%, <50%, or >50% inclusion of inflamed areas in pathological sections, respectively. An index was calculated by multiplying the severity score by the extent score. The slides were also scored for goblet cell metaplasia (GCM) in the airways on a scale from one to three.
Serum collection and CORT assay.
Blood obtained for the CORT assays were obtained following anesthesia with isoflurane followed by cervical dislocation to achieve rapid death and to avoid the stress-related increases in CORT levels possible with slower methods (e.g., pentobarbital). Blood was collected via cardiac puncture. In an experiment to measure CORT levels in the fetuses of RS mothers, we used day 19 pregnancy mothers to allow collection of sufficient fetal blood for analysis. Serum was allowed to separate overnight at 4°C and collected after the blood was centrifuged at 3,000 rpm for 10 min at 4°C; serum samples were stored at −80°C. Serum CORT was determined using an ELISA kit (Immunodiagostik).
Statistical analysis.
All data are presented as means ± SE. Differences between groups were compared using Kruskal-Wallis ANOVA with posttests. Prism software (GraphPad.com) was used for all statistical analysis. A P value <0.05 was considered significant.
RESULTS
Increased asthma susceptibility in offspring of stressed mothers.
E15 mice were subjected to 1 h of RS, and their offspring were submitted to the low-dose “intentionally suboptimal” asthma induction protocol (Fig. 1). Negative controls consisted of offspring of nonstressed mothers who were subjected to the suboptimal protocol after birth. Positive controls were offspring of nonstressed mothers who received the two-injection asthma induction protocol. To determine if RS significantly increased CORT levels, some of the E15 mothers were killed immediately following RS, and blood was collected for CORT assay. Stressed pregnant mice had significantly elevated serum CORT levels compared with their nonstressed pregnant controls (1,136 and 151.5 ng/ml, respectively, P < 0.0001, Fig. 1B), indicating that the RS procedure was effective. Similarly, CORT levels were elevated in the fetuses of RS mothers (Fig. 1C). Simple handling of the mothers, or injections of vehicle, did not significantly affect maternal levels of CORT (Fig. 1D).
BAL samples from offspring of stressed animals showed markedly elevated total cell counts compared with offspring of nonstressed controls and were similar to positive controls (P < 0.05, Fig. 2A). Total BAL eosinophils were similarly increased in the experimental group compared with the negative control group (P < 0.05, Fig. 2B). The single stress at day E15 was chosen after pilot studies compared effects of maternal RS administered one (day E15), two (days E15 and E17), and three (days E15, E17, and E19) times. These initial experiments showed a similar effect of increased asthma susceptibility in offspring (data not shown). Based on these pilot data, the single day 15 time point was used.
Fig. 2.
Effect of maternal stress on offspring allergy phenotype. Following the intentionally suboptimal protocol, bronchoalveolar lavage (BAL) total cell counts and eosinophils were increased in offspring of stressed mothers compared with nonstressed mothers (n ≥ 6/group, *P < 0.05, A and B). Cell counts and eosinophils were similar in the experimental group and the positive control group (P > 0.05). Following the intentionally suboptimal protocol, BAL total cell counts and eosinophils were increased in offspring of dexamethasone-injected mothers compared with vehicle-injected mothers (n ≥ 16/group, *P < 0.05, C and D). Total cell counts and eosinophil counts were similar in the experimental dexamethasone (Dex) group and positive control group (P > 0.05).
Increased asthma susceptibility in offspring of dexamethasone-injected nonstressed mothers.
To investigate the potential role of maternal stress-induced corticosteroids, we sought as proof-of-principle to test whether administration of exogenous corticosteroid, e.g., dexamethasone, to pregnant mother mice would alter asthma susceptibility in their offspring. We chose dexamethasone because it readily crosses the placenta and enters fetal circulation (48). In this group of experiments, nonstressed pregnant E15 mice were injected subcutaneously with dexamethasone, and allowed to give birth normally. After birth, the offspring were subjected to the suboptimal protocol (Fig. 1). Positive controls were pups who received the two-injection protocol. For these experiments, there were three negative control groups, all subjected to the intentionally suboptimal protocol summarized in Fig. 1. The first negative control group was comprised of offspring of nonstressed mothers; the second negative control group consisted of offspring of vehicle-injected mothers; the third negative control group consisted of offspring of “handled” mothers. The “handling” consisted of taking the animal out of the cage but no injection. These negative controls were used because mere handling and injection may constitute stress in animals, which could potentially affect offspring.
The offspring of dexamethasone-injected mothers showed increased BAL total cell counts compared with all three negative controls (P < 0.05, Fig. 2C). These levels were similar to those seen in positive controls (P > 0.05). The experimental group also had increased BAL total eosinophils compared with negative controls (P < 0.05, Fig. 2D). The vehicle-injected and handling controls did not show statistically significant differences in BAL total cell counts and total eosinophilia compared with the offspring of nonstressed, noninjected, nonhandled mothers.
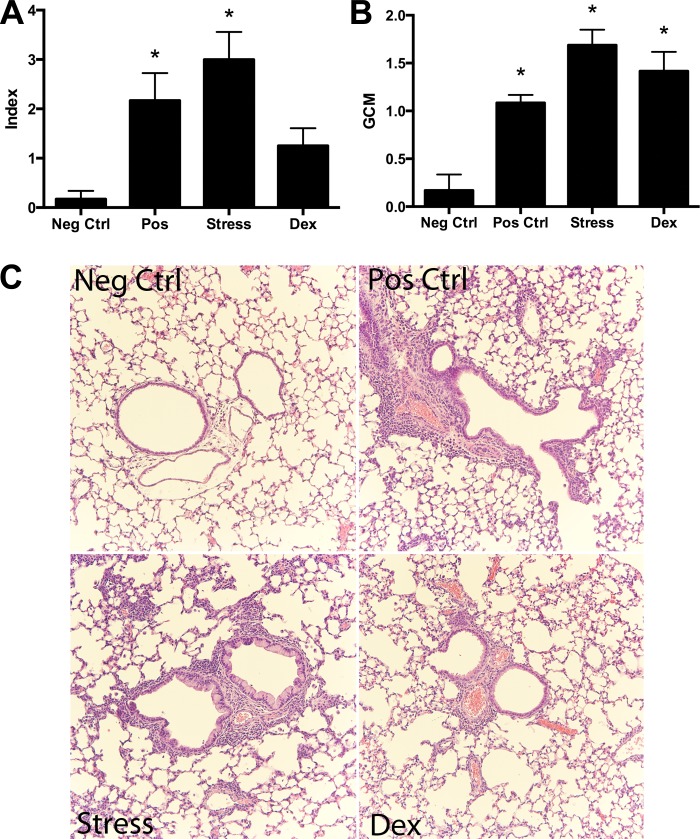
Histological evaluation also demonstrated increased allergic inflammation around airways and vessels in the lung samples of the offspring of stressed mothers compared with negative controls. The offspring of stressed mothers had increased index scores and GCM (see materials and methods) compared with negative controls (P < 0.05, Fig. 3).
Fig. 3.
Lung histopathology. A: quantitation showed and significantly increased allergic inflammation index and goblet cell metaplasia (GCM) scores in the offspring of stressed mothers (RS) and the positive controls compared with negative controls (n = 6/group, *P < 0.05, A and B). Offspring of dexamethasone-injected mothers (Dex) also had a significantly increased GCM score on histopathology compared with negative controls (n ≥ 6/group, *P < 0.05, B). Index scores for the Dex group also trended to be greater than negative controls but did not reach statistical significance. C: representative micrographs of histopathology show increased airway-centered inflammation in positive controls (Pos Ctrl), offspring of stressed mothers (RS), and in offspring of dexamethasone-injected mothers (Dex).
We evaluated whether vehicle injection and handling alone significantly affected maternal serum CORT levels compared with nonstressed, noninjected, nonhandled controls (P > 0.05, Fig. 1D). Whereas there was some minimal elevation, it was not statistically significant, and substantially less than levels observed in the RS-treated E15 pregnant mice (e.g., 1,136 ng/ml in stressed vs. 189 and 190 ng/ml in “handling only” and “vehicle injection,” respectively).
Inhibition of maternal GC synthesis with metyrapone abrogates the effect of maternal stress on offspring susceptibility to allergy.
To more directly test the role of endogenous corticosteroids, we used metyrapone, a steroid 11β-hydroxylase inhibitor that has been used to reduce excess GC production in human pregnancy (24). We first optimized the dose of metyrapone needed to eliminate the CORT surge after RS without eliminating baseline CORT levels. In Fig. 4A, the 100 and 200 mg/kg doses significantly decreased maternal CORT levels after RS; hence, the lower 100 mg/kg dose was selected for further experiments. The comparison of CORT levels in stressed mothers after metyrapone shows concentrations intermediate to those of nonstressed pregnant mice and nonstressed nonpregnant female mice (Fig. 4B). After exposure of offspring to the intentionally suboptimal protocol, we found that susceptibility to the allergy phenotype was abrogated by metyrapone pretreatment of RS mothers. The positive controls (double injected, or offspring of RS mothers injected with vehicle only) showed increased BAL eosinophilia. In contrast, the offspring of metyrapone-treated stressed mothers had significantly lower levels of BAL cells and eosinophils (Fig. 4, C and D) after the low-dose allergen protocol.
Fig. 4.
Metyrapone (MET) treatment abrogated maternal stress-induced effects on offspring susceptibility to allergy. Dose-response analysis in nonpregnant mice identifies efficacy of 100–200 mg/kg of MET blockade of stress-induced CORT increases (A). The 100 mg/kg MET dose also abrogated a rise in maternal CORT levels in blood after restraint stress (B, *P < 0.05) and was used in all further experiments; n ≥ 3/group. Total cells counts (C) and eosinophil counts (D) were decreased significantly in the offspring of stressed mothers after maternal MET pretreatment and were indistinguishable from negative controls (*P < 0.05). Similarly, neonatal airway responsiveness to methacholine was ameliorated significantly by maternal MET blockade after stress (n ≥ 6/group, *P < 0.05, E).
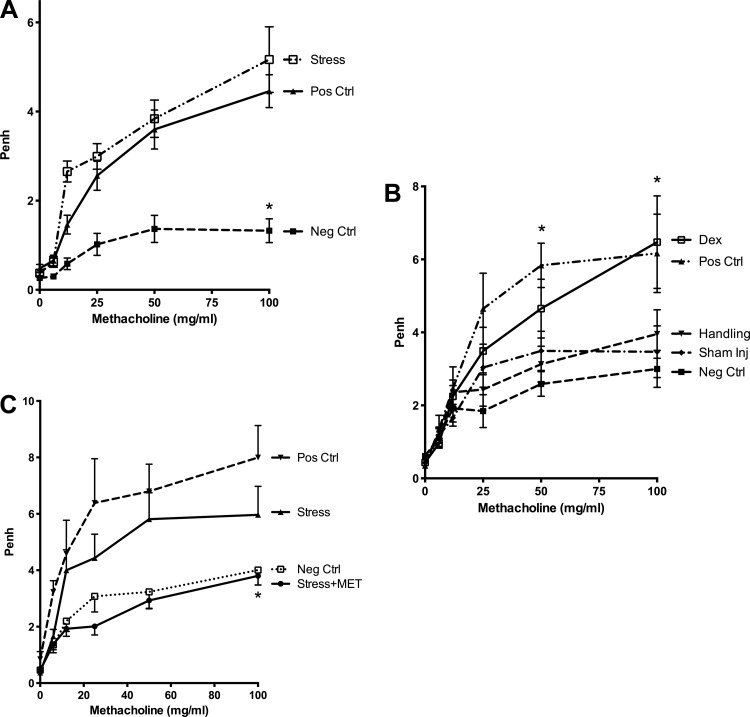
To complement the analyses of BAL cells and histology, we performed measurements of Penh response to inhaled methacholine in parallel to the pathological evaluations already reported. As shown in Fig. 5, airway responsiveness, as measured by unrestrained plethysmography, was also increased at the three highest concentrations of methacholine in the offspring of stressed mothers compared with negative controls (P < 0.05, Fig. 5A) and was similar to that seen in positive controls. Airway responses to increasing concentrations of methacholine (50 and 100 mg/ml) were also increased in the offspring of dexamethasone-injected mothers compared with the offspring of nonstressed, noninjected, nonhandled mothers (Fig. 5B). All negative controls had statistically similar minimal response to increasing concentrations of methacholine. In contrast, the offspring of metyrapone-treated stressed mothers had ameliorated responsiveness to methacholine (Fig. 5C) after the low-dose allergen protocol.
Fig. 5.
Airway responsiveness evaluation. A: following the protocol in Fig. 1, offspring of stressed mothers had increased enhanced pause (Penh) values in response to the 3 highest doses of methacholine compared with offspring of nonstressed mothers (n ≥ 13/group, *P < 0.05). Penh values were similar between offspring of stressed mothers and positive control pups. B: Penh values were elevated in offspring of dexamethasone-injected mothers compared with vehicle-injected mothers (n ≥ 9/group, *P < 0.05). C: metapyrone abrogated the effect of maternal stress on Penh in offspring (n ≥ 6/group, *P < 0.05, stress + MET vs. stress alone).
DISCUSSION
We sought to test the postulate that GCs generated by stress during pregnancy can induce asthma susceptibility in offspring. To test this hypothesis, we stressed mice on day 15 of pregnancy, allowed them to give birth, and assessed offspring for asthma susceptibility. There are multiple ways to experimentally stress mice (i.e., sound, cold, social conditions, restraint). For these experiments, we chose RS because it was found effective in prior murine asthma studies (26), it is considered a psychological stress (13, 28), it does not require specialized equipment, and is quantifiable (in terms of duration). Only the offspring of stressed mothers demonstrated increased asthma susceptibility (i.e., increased airway inflammation and increased Penh following intentionally suboptimal protocol) compared with offspring of nonstressed mothers. In addition, we also demonstrated that a single episode of RS significantly elevated maternal CORT levels. These results indicate that maternal stress can play a role in the initiation of asthma by increasing asthma susceptibility in offspring.
The mechanism for this phenomenon remains to be fully elucidated. However, we postulate that it may be related to the observed elevation of stress hormones (i.e., CORT). Maternal stress leads to the activation of the HPA axis, which, through the actions of hypothalamic corticotropin-releasing hormone and adrenocorticotropic hormone, causes release of stress hormones from the adrenal gland (44). To test this hypothesis, we injected nonstressed pregnant mice with dexamethasone, a stress hormone analog. Results from this group of experiments demonstrated that dexamethasone injection alone induces asthma susceptibility in offspring. Importantly, handled and vehicle-injected mothers did not have increased CORT levels, nor did their offspring have increased asthma susceptibility. Conversely, the effect of maternal RS was abrogated by pretreatment with metyrapone, an agent that inhibits CORT synthesis. This finding directly supports a critical role of steroid stress hormones in the observed effect.
Postulating that GC such as CORT can have proallergic/asthmatic effects may seem counterintuitive, especially given that they are the mainstays of highly effective therapeutics for asthma. However, GCs can act to promote Th2 responses, by upregulating IL-4, -10, and -13 production in vitro and by suppressing antigen-presenting cell production of IL-12 in vitro and in vivo (16). In vivo animal studies have also shown that experimental stress can have proallergic effects. A key point is that studies in asthma models have shown that chronic stress, but not acute stress, increases BAL total cell counts (8, 21, 34) and can increase BAL IL-4 and -5 levels (34). Interestingly, when RU-486, a potent GC receptor antagonist, was administered before OVA challenge in these stress studies, BAL cell counts remained normal without the usual eosinophilia (7). These data also indicate that chronic stress increases allergic AI in a GC-dependent manner.
The RS used in this model is an acute stress. However, pregnancy per se is already a state of chronic stress, at least as defined by the well-characterized elevation of serum CORT observed, compared with nonpregnant levels (3, 6, 15). Thus, our model could be viewed as an acute stress superimposed on a chronic stress model. We speculate that, in the setting of chronically elevated CORT levels seen in pregnancy, further acute elevations cross a threshold, thereby causing proallergic skewing of the developing immune system in offspring. It is noteworthy that elevations in maternal salivary cortisol during pregnancy were associated with children's wheeze in a recent human cohort study (51), consistent with other human epidemiological studies showing a link between maternal stress and increased incidence of asthma in children (11, 35, 37, 49, 50).
Although maternal CORT can be degraded to its inert form at the placental barrier by 11β-hydroxysteroid dehydrogenase type 2, the enzymatic barrier is not absolute. Maternal CORT can cross the placenta and significantly affect fetal CORT levels (reviewed in Ref. 45) during circadian peaks. Once across the placental barrier, this maternal CORT may have the same Th2-promoting effects seen in the adult chronic stress models (16), which could lead to increased vulnerability to asthma and allergies. Relatively transient maternal stress and increases in CORT can cause durable changes in offspring as reported for offspring behavioral, circadian rhythm, and HPA changes, most likely through epigenetic mechanisms (reviewed in Ref. 12). It has also been shown that GCs given to mothers during pregnancy can significantly and persistently alter offspring blood pressure and adult weight (4, 32). These data are consistent with our observation that a single stress during pregnancy can cause persistent changes in offspring. It should be noted that the magnitude of the CORT rise after the single RS in mice is quite high (∼6- to 8-fold), especially compared with human stress responses [e.g., <3-fold for social stresses, sky diving (20, 42)].
There are some limitations to this study that warrant discussion. First, a stress hormone analog was used (dexamethasone) rather than CORT. These compounds, although very similar, are not identical. In contrast to CORT, dexamethasone is more potent and crosses the placenta without degradation. Because of these differences, injection of dexamethasone may not exactly recapitulate the effects of increases in serum CORT following stress responses. Although this is true, dexamethasone was chosen precisely because of these differences. If the mechanism by which maternal stress induced offspring asthma susceptibility was transplacental passage of stress hormone then dexamethasone would likely give a stronger signal than CORT. Another shortcoming is that this model cannot differentiate between prenatal and postnatal effects of maternal stress. Whereas it is likely that maternal stress alters development of the fetal immune system leading to asthma susceptibility, it is also possible that maternal stress may cause postnatal changes leading to susceptibility. Stress may alter maternal behavior (29) or breast milk constituents, which could conceivably affect changes in the neonatal immune system. It is also equally possible that maternal stress can affect offspring immune systems both pre- and postnatally. However, to differentiate between pre- and postnatal effects is beyond the scope of the current study. Similarly, the molecular mechanism(s) by which GCs make developing offspring more susceptible to allergy remain unknown, although we speculate that changes in the epigenetic control of immune responses are likely to be involved.
A final topic that merits discussion is our use of Penh. The Penh measurement is controversial, since it is not a direct measure of airway resistance but rather a calculated dimensionless value used as a surrogate. However, it is a pulmonary function assay that is most easily applied to the small 15-day-old mice we study. In addition, the OVA-asthma model in the Balb/c strain is the only one where Penh actually correlates well with invasive tests of airway resistance [as detailed inside the major paper revealing problems with Penh in C57Bl/6 mice (1), and in work by others (30)]. For the interested reader, the limitations and advantages of this method have been extensively discussed elsewhere (17).
Despite these shortcomings, this study demonstrates that a relatively short-lived physical RS of pregnant mice can indeed promote initiation of an asthma-like phenotype in offspring. Furthermore, the stress effect can be recapitulated with a single injection of dexamethasone, a stress hormone analog, in a nonstressed pregnant female and blocked by inhibiting the endogenous production of a surge in CORT that follows RS. The findings suggest that inflammation-induced stress hormones may be a common pathway by which various toxic or stressful pregnancy exposures can increase offspring susceptibility to asthma.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Grants ES-017588 and ES-00002
DISCLOSURES
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: R.L. and L.K. conception and design of research; R.L. performed experiments; R.L., A.V.F., and L.K. analyzed data; R.L., A.V.F., and L.K. interpreted results of experiments; R.L., A.V.F., and L.K. prepared figures; R.L. and A.V.F. drafted manuscript; R.L., A.V.F., and L.K. edited and revised manuscript; R.L., A.V.F., and L.K. approved final version of manuscript.
REFERENCES
- 1. Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol 97: 286–292, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, Haczku A. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J Immunol 182: 7888–7896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barlow SM, Morrison PJ, Sullivan FM. Plasma corticosterone levels during pregnancy in the mouse (Abstract). Br J Pharmacol 48: 346P, 1973 [PMC free article] [PubMed] [Google Scholar]
- 4. Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 341: 339–341, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy 35: 397–402, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol 139: 416–422, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med 175: 316–322, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am J Respir Crit Care Med 175: 316–322, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chrousos GP. Stress and sex versus immunity and inflammation. Science Signaling 3: pe36, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109: 5995–5999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol 123: 847–853 e811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57: 571–585, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14: 1143–1152, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Devereux G, Seaton A, Barker RN. In utero priming of allergen-specific helper T cells. Clin Exp Allergy 31: 1686–1695, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology 144: 5268–5276, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann NY Acad Sci 1024: 138–146, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Fedulov A, Kobzik L. The Penh police are not infallible. Am J Respir Cell Mol Biol 45: 1272–1273, 2011. 22140201 [Google Scholar]
- 18. Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 38: 57–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fedulov AV, Leme AS, Kobzik L. Duration of allergic susceptibility in maternal transmission of asthma risk. Am J Reprod Immunol 58: 120–128, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev 35: 91–96, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Forsythe P, Ebeling C, Gordon JR, Befus AD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med 169: 220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 170: 1683–1689, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A 70: 688–695, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hana V, Dokoupilova M, Marek J, Plavka R. Recurrent ACTH-independent Cushing's syndrome in multiple pregnancies and its treatment with metyrapone. Clin Endocrinol (Oxf) 54: 277–281, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Joachim RA, Quarcoo D, Arck PC, Herz U, Renz H, Klapp BF. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom Med 65: 811–815, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Kumlien Georen S, Olgart Hoglund C, Tcacencu I, Wikstrom AC, Stierna P. Timing-dependent effects of restraint stress on eosinophilic airway inflammation in mice. Neuroimmunomodulation 15: 157–164, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lim RH, Arredouani MS, Fedulov A, Kobzik L, Hubeau C. Maternal allergic contact dermatitis causes increased asthma risk in offspring (Abstract). Respir Res 8: 56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madrigal JL, Caso JR, de Cristobal J, Cardenas A, Leza JC, Lizasoain I, Lorenzo P, Moro MA. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res 979: 137–145, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav 72: 473–479, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect 118: 273–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montoro J, Mullol J, Jauregui I, Davila I, Ferrer M, Bartra J, del Cuvillo A, Sastre J, Valero A. Stress and allergy. J Investig Allergol Clin Immunol 19, Suppl 1: 40–47, 2009 [PubMed] [Google Scholar]
- 32. Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav 88: 605–614, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Nogueira PJ, Ferreira HH, Antunes E, Teixeira NA. Chronic mild prenatal stress exacerbates the allergen-induced airway inflammation in rats. Mediators Inflamm 8: 119–122, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okuyama K, Ohwada K, Sakurada S, Sato N, Sora I, Tamura G, Takayanagi M, Ohno I. The distinctive effects of acute and chronic psychological stress on airway inflammation in a murine model of allergic asthma. Allergol Int 56: 29–35, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy 67: 545–551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, Wahn U, Hamelmann E, Arck PC. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 177: 8484–8492, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM, Hoepner L, Perera FP, Rauh V, Miller RL. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol 107: 42–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet 356: 982–987, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology 141: 2422–2428, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med 117: 854–866, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Strunk RC, Mrazek DA, Fuhrmann GS, LaBrecque JF. Physiologic and psychological characteristics associated with deaths due to asthma in childhood. A case-controlled study. J Am Med Assoc 254: 1193–1198, 1985 [PubMed] [Google Scholar]
- 42. Taverniers J, Smeets T, Lo Bue S, Syroit J, Van Ruysseveldt J, Pattyn N, von Grumbkow J. Visuo-spatial path learning, stress, and cortisol secretion following military cadets' first parachute jump: the effect of increasing task complexity. Cogn Affect Behav Neurosci 11: 332–343, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Uthoff H, Spenner A, Reckelkamm W, Ahrens B, Wolk G, Hackler R, Hardung F, Schaefer J, Scheffold A, Renz H, Herz U. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol 171: 3485–3492, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Vig RS, Forsythe P, Vliagoftis H. The role of stress in asthma: insight from studies on the effect of acute and chronic stressors in models of airway inflammation. Ann NY Acad Sci 1088: 65–77, 2006 [DOI] [PubMed] [Google Scholar]
- 45. von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol 109: 923–928, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Wade S, Weil C, Holden G, Mitchell H, Evans R, 3rd Kruszon-Moran D, Bauman L, Crain E, Eggleston P, Kattan M, Kercsmar C, Leickly F, Malveaux F, Wedner HJ. Psychosocial characteristics of inner-city children with asthma: a description of the NCICAS psychosocial protocol. National Cooperative Inner-City Asthma Study. Pediatr Pulmonol 24: 263–276, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics 104: 1274–1280, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med 165: 358–365, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol 113: 1051–1057, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, Coull BA. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med 187: 1186–1193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]