Abstract
Airway epithelial cells are the primary cell type involved in respiratory viral infection. Upon infection, airway epithelium plays a critical role in host defense against viral infection by contributing to innate and adaptive immune responses. Influenza A virus, rhinovirus, and respiratory syncytial virus (RSV) represent a broad range of human viral pathogens that cause viral pneumonia and induce exacerbations of asthma and chronic obstructive pulmonary disease. These respiratory viruses induce airway epithelial production of IL-8, which involves epidermal growth factor receptor (EGFR) activation. EGFR activation involves an integrated signaling pathway that includes NADPH oxidase activation of metalloproteinase, and EGFR proligand release that activates EGFR. Because respiratory viruses have been shown to activate EGFR via this signaling pathway in airway epithelium, we investigated the effect of virus-induced EGFR activation on airway epithelial antiviral responses. CXCL10, a chemokine produced by airway epithelial cells in response to respiratory viral infection, contributes to the recruitment of lymphocytes to target and kill virus-infected cells. While respiratory viruses activate EGFR, the interaction between CXCL10 and EGFR signaling pathways is unclear, and the potential for EGFR signaling to suppress CXCL10 has not been explored. Here, we report that respiratory virus-induced EGFR activation suppresses CXCL10 production. We found that influenza virus-, rhinovirus-, and RSV-induced EGFR activation suppressed IFN regulatory factor (IRF) 1-dependent CXCL10 production. In addition, inhibition of EGFR during viral infection augmented IRF1 and CXCL10. These findings describe a novel mechanism that viruses use to suppress endogenous antiviral defenses, and provide potential targets for future therapies.
Keywords: innate immunity, epidermal growth factor receptor, interferon regulatory factor 1, CXCL10
the airway epithelium plays a critical role in host defense. Upon exposure to pathogens, airway epithelial cells contribute to innate immune responses and influence adaptive immune effector cells. Respiratory viral infections, which cause pneumonia and exacerbations of chronic lung diseases [e.g., asthma and chronic obstructive pulmonary disease (COPD)], are responsible for significant morbidity and mortality. Despite substantial disease burden, there are limited therapies for treating virus-induced pulmonary disease. Therefore, improving our understanding of host responses, and relevant signaling mechanisms, may contribute to identifying novel targets for therapy.
Airway epithelial cells are the primary cell type involved in respiratory viral infection. Upon infection, viruses induce airway epithelial production of interleukin (IL)-8 [also known as CXCL8 (10, 44)], which has been shown to require epidermal growth factor receptor (EGFR) activation (22, 25, 31). EGFR (ErbB1/HER1), a tyrosine kinase receptor present in epithelial cells, is activated in a ligand-dependent manner (41). In airway epithelial cells, EGFR activation involves an integrated signaling pathway that includes NADPH oxidase (Nox) activation of a metalloproteinase (MP), which cleaves an EGFR proligand that is released to bind to, and to activate, EGFR (7, 40). Recently, respiratory viruses have been shown to activate EGFR via this signaling pathway in airway epithelium (4, 22, 59). Therefore, we investigated the effect of virus-induced EGFR activation on airway epithelial antiviral responses.
Recruitment of natural killer (NK) cells and other lymphocytes (e.g., CD8+ T cells) to target and kill virus-infected cells is a critical component of pulmonary antiviral defense. CXCL10 [also known as interferon (IFN)-γ-inducible protein of 10 kDa (IP-10)] is a member of a family of chemokine ligands for CXCR3, a receptor that is expressed on B and T lymphocytes and NK cells, as well as dendritic cells, macrophages, and nonhematopoietic cells. Originally identified from IFN-γ-stimulated monocytes, CXCL10 was found to be the highest expressed CXCR3 ligand in airway epithelial cells (38). CXCL10 is elevated during COPD exacerbations (3) and was implicated as a potential biomarker for virus-induced asthma exacerbations (55). A variety of molecules have been implicated in CXCL10 regulation, including IFN-stimulated response element (34), signal transducer and activator of transcription 1 (30), NF-kβ (43), and IFN regulatory factors (IRFs) (58).
Influenza A virus (H1N1), rhinovirus (RV), and respiratory syncytial virus (RSV) are single-stranded RNA viruses that represent a broad range of human viral pathogens. Each virus may cause viral pneumonia or induce exacerbations of asthma and COPD (19). In addition, RSV causes bronchiolitis in children, which contributes significantly to childhood morbidity (15). Importantly, each of these viruses has been shown to activate EGFR (13, 25, 31), and H1N1 and RV were shown to activate EGFR via Nox and MP-induced release of EGFR ligand (4, 13, 25, 59). Airway epithelial cell culture experiments have shown increased CXCL10 in response to respiratory viral infections (9, 32, 43). In addition, IRF have been implicated in RV-induced airway epithelial CXCL10 production (58). However, the interaction between CXCL10 and EGFR signaling pathways is unclear, and the potential for EGFR signaling to suppress CXCL10 has not been explored. In addition, the interaction between these two signaling pathways may have implications for effective antiviral host defense. Here, we examined the interaction between virus-induced EGFR signaling and CXCL10 production in airway epithelium.
MATERIALS AND METHODS
Reagents.
EGFR tyrosine kinase inhibitor AG-1478, platelet-derived growth factor (PDGF) receptor tyrosine kinase inhibitor AG-1295, tumor necrosis factor (TNF)-α proteinase inhibitor-1 (TAPI), EGFR neutralizing antibody (Ab) diphenyleneiodonium chloride (DPI), epidermal growth factor (EGF), and transforming growth factor (TGF)-α were obtained from EMD Millipore (Billerica, MA). N-propyl galatte (nPG) was obtained from Sigma-Aldrich (St. Louis, MO). Gefitinib was purchased from Tocris Biosciences (Bristol, UK). The synthetic dsRNA polyinosine-polycytidylic acid (poly I:C) was purchased from Invivogen (San Diego, CA).
Viruses.
Purified influenza A/PR/8/34 (H1N1) was purchased from Advanced Biotechnologies (Columbia, MD) and was used for in vitro and in vivo experiments. Influenza A virus (H1N1) titers were determined by tissue culture infectious dose 50 (TCID50%) and plaque assays (50). Rhinovirus (RV) 16 was a generous gift from Dr. William Busse, (Madison, WI), and RV1B was purchased from American Type Culture Collection (ATCC, Manassas, VA). RV16 and -1B were grown in HeLa cells (ATCC) and purified by centrifugation through sucrose gradient, as previously described (58). RV titers were determined by TCID50% and plaque assay (50). RSV A2 was purchased from ATCC, and RSV titers were determined by TCID50% and plaque assay. Supernatants collected from mock-infected BEAS-2b cells did not induce CXCL10 above serum-free medium alone (data not shown).
Cell culture.
Dr. John Fahy [University of California, San Francisco (UCSF), San Francisco, CA] generously provided bronchial epithelial (BEAS-2b) cells. Drs. Patrick Hayden (MatTek, Ashland, MA) and Walter Finkbeiner (UCSF) generously provided primary normal human bronchial epithelial (NHBE) cells from healthy donors. Cells were seeded at 0.5–2 × 105 cells/ml and grown in bronchial epithelial growth medium (Lonza, Walkersville, MD) supplemented with growth factors, penicillin (100 U/ml), and streptomycin (100 μg/ml). Sixteen hours before viral infection, EGF and hydrocortisone were removed from cell culture medium. After preliminary experiments were completed with different H1N1, RV, and RSV concentrations at 24 h to determine CXCL10 production, subsequent experiments used H1N1 at a multiplicity of infection (MOI) of 0.5, RV1b at MOI of 1, RV16 at MOI of 2, and RSV at MOI of 1 in BEAS-2b cells (50). To maximize viral infection, NHBE cell cultures were infected at 80–90% confluence at MOI = 10, as previously described (11, 50). Chemical inhibitors were added to cell cultures at the time of viral infection. AG-1478 and gefitinib were used at 10 μM because experiments have shown this concentration to inhibit virus-induced inflammation (16, 24, 25, 50), and neither inhibitor induced cell toxicity as measured by lactate dehydrogenase (LDH) production at this concentration (50). In addition, we used EGFR siRNA to confirm selectivity for EGFR (Fig. 1B; for details see below). For experiments using the EGFR ligands EGF and TGF-α, we used 10 ng/ml because we, and other investigators, have shown that this concentration increased the effect of respiratory viruses in airway epithelial cells (45, 50).
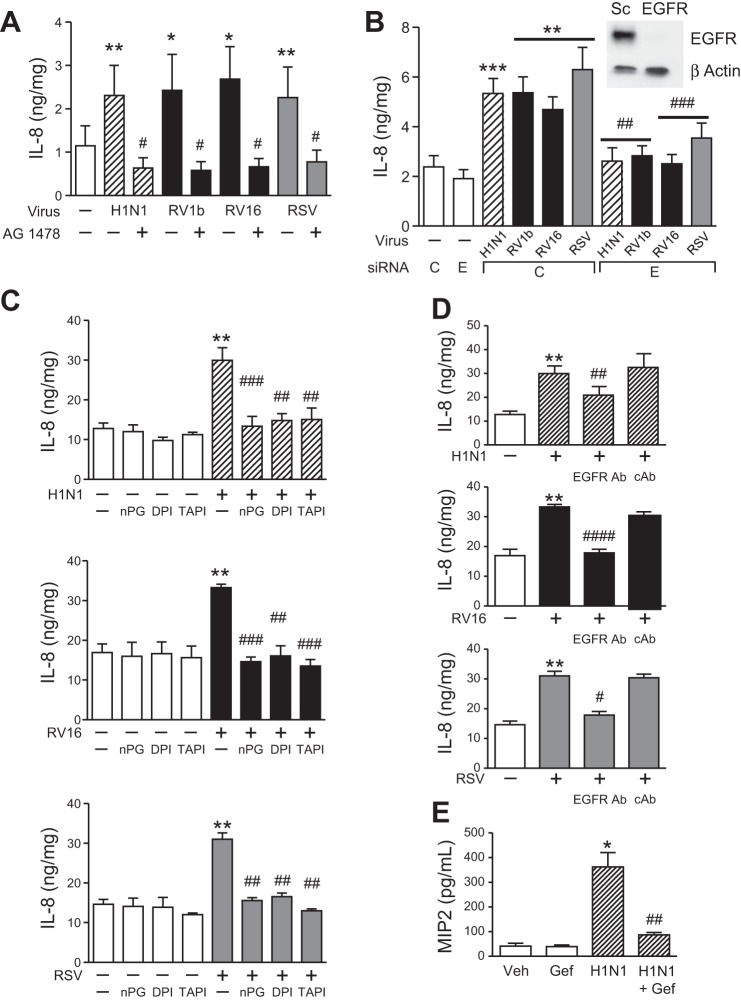
Fig. 1.
Role of epidermal growth factor receptor (EGFR) in respiratory virus-induced inflammation. A: normal human bronchial epithelial (NHBE) cells were treated with serum-free medium alone (white bars), AG-1478 (10 μM), influenza A virus (H1N1, hatched bars), rhinovirus (RV) 1b and RV16 (black bars), and respiratory syncytial virus (RSV, gray bars) alone, or with AG-1478, and secreted interleukin (IL)-8 was measured by ELISA at 24 h (n = 6 independent experiments, means ± SE; *P < 0.05 and **P < 0.01 vs. control; #P < 0.05 vs. each virus alone). B: BEAS-2b cells were treated with serum-free medium alone or transfected with EGFR or control (C) siRNA for 24 h and treated with serum-free medium alone (white bars), or H1N1 (hatched bars), RV1b (black bars), RV16, and RSV (gray bars). After viral infection (24 h), secreted IL-8 was measured by ELISA (n = 8 independent experiments, means ± SE; **P < 0.001 and ***P < 0.0005 vs. serum-free medium and C siRNA; ##P < 0.001 and ###P < 0.0005 vs. C siRNA + virus). BEAS-2b cells were transfected with EGFR siRNA, and EGFR protein was assessed by Western blot (representative of 3 independent experiments). C: BEAS-2b cells were treated with serum-free medium alone (white bars), N-propyl galatte (nPG, 100 μM), diphenyleneiodonium chloride (DPI, 3 μM), tumor necrosis factor-α proteinase inhibitor-1 (TAPI, 10 μM), H1N1 (hatched bars), RV1b and RV16 (black bars), and RSV (gray bars) alone, or with nPG, DPI, and TAPI, and secreted IL-8 was measured by ELISA at 24 h (n = 3–5 independent experiments, means ± SE; **P < 0.005 vs. control; ##P < 0.005 and ###P < 0.0001 vs. each virus alone). D: BEAS-2b cells were treated with serum-free medium alone (white bars), H1N1 (hatched bars), RV1b and RV16 (black bars), and RSV (gray bars) alone, or with an EGFR Ab or isotype-matched control Ab, and secreted IL-8 was measured by ELISA at 24 h (n = 3–5 independent experiments, means ± SE; **P < 0.005, ####P < 0.0001 vs. control; #P < 0.05, ##P < 0.005, and ####P < 0.0001 vs. each virus alone). E: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with H1N1 (104.5 TCID50%) or H1N1 + gefitinib. After 24 h, bronchoalveolar lavage (BAL) was collected, and MIP2 was measured by ELISA in BAL (n = 5 mice/group repeated two times, means ± SE; *P < 0.01 vs. vehicle alone; ##P < 0.005 vs. virus alone).
Cell cultures were incubated at 37°C, and cell culture homogenates and supernatants were harvested at the indicated time points. CXCL10 and IL-8 protein production in cell culture supernatants was measured at 24 h by ELISA (eBioscience). BEAS-2b cells cultured in serum-free medium, treated with chemical inhibitors, or siRNA were assessed for cytotoxicity by using a LDH assay (Roche, Indianapolis, IN), and no significant differences were found (50).
CXCL10 and IRF1 mRNA expression was assessed by quantitative RT-PCR, as previously described (14, 52). Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). RT-PCR was evaluated with an Applied Biosystems model 7900 sequence detector [CXCL10 (forward): GAAATTATTCCTGCAAGCCAATTT; CXCL10 (reverse): TCACCCTTCTTTTTCATTGTAGCA; IRF1 (forward): CCTGATACCTTCTCTGATGGAC TCA; IRF1 (reverse): TGCATGTAGCCTGGAACTGTGT]. The housekeeping gene GAPDH was used as an internal control.
Western blot was used to measure IRF1. Briefly, after stimulation of cell cultures, cells were lysed using RIPA buffer (Thermo Fisher Scientific, Lafayette, CO) supplemented with phosphatase and protease inhibitors. Equivalent amounts of protein were loaded on Mini-PROTEAN TGX 10% gels (Bio-Rad Laboratories, Hercules, CA). After electrophoresis and blocking with TBST (Bio-Rad Laboratories) containing 5% BSA, blots were then incubated with anti-IRF1 Ab (D5E4; Cell Signaling) overnight. Membranes were stripped and reprobed with mouse anti-β-actin Ab (sc-47778; Santa Cruz Biotechnology).
siRNA was used to knock down EGFR and IRF1 in BEAS-2b cells, as previously described (50, 53). Scrambled (control) and EGFR siRNA were purchased from Santa Cruz Biotechnology (sense: CUCUGGAGGAAAAGAAAGU; antisense: ACUUUCUUUU CCUCCAGAG). Scrambled (control) and IRF1 (duplex UCCCAAGACGUGGAAG GCCAACUUU) siRNA were purchased from Invitrogen (Grand Island, NY). siRNA transfection was carried out using Lipofectamine (Invitrogen) in subconfluent cells, and 24 h after transfection cell cultures were treated with dsRNA or infected with virus. siRNA knock down of EGFR and IRF1 was confirmed by Western blot using anti-EGFR (1005; Santa Cruz Biotechnology) and anti-IRF1 (D5E4; Cell Signaling) Abs.
To measure IRF1 transcriptional activity, BEAS-2b cells were transfected using TransIT-2020 Reagent (Mirus, Madison, WI) with 250 ng IRF1 luciferase reporter and the appropriate negative and positive controls (SABiosciences, Frederick, MD). After 24 h, cells were stimulated before cell lysates were prepared, and IRF1 luciferase activity was assayed by a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. Unless stated, gefitinib was added at the same time as viral infection. Twenty-four hours after viral infection, cell culture homogenates were prepared to measure viral titers, and cell culture supernatants were collected to measure IL-8 and CXCL10.
Mice and in vivo and ex vivo experiments.
C57BL/6 mice were purchased from NCI. All procedures were reviewed and conducted under approved Institutional Animal Care and Use Committee protocols from the University of California, San Francisco and Yale University. All experiments were performed using 6- to 8-wk-old female mice. For H1N1 infection, 50 μl of H1N1 (104.5 TCID50%) or sterile PBS were given intranasally after sedation with isoflurane. Systemic gefitinib (50 mg/kg), at a dose previously used to suppress lung inflammation (17, 50), was given 16 h before infection and continued daily. Forty-eight hours after viral infection, bronchoalveolar lavage (BAL) and lungs were collected. Total cell count, cell differential, and CXCL10 and MIP2 were measured by ELISA (eBioscience) in BAL. Lung homogenates were analyzed for IRF1 protein by Western blot (monoclonal antibody D5E4; Cell Signaling) and perforin mRNA by quantitative RT-PCR [perforin (forward): AGCACAAGTTCGTGCCAGG, (reverse): GCGTCTCTCATTAGGGAGTTTTT]. For some experiments, primary mouse tracheal epithelial cells (pMTECs) were cultured at air-liquid interface as previously described (6), before perturbation.
NK cell migration assay.
NKL cells, a human leukemia-derived NK cell line (12) (2 × 105), were placed in a Transwell filter in a standard migration assay. Supernatants, collected from BEAS-2b cells stimulated with dsRNA (25 μg/ml), AG-1478 (10 μM), and TGF-α (10 ng/ml) for 24 h, were treated with RNase to inactivate any residual dsRNA (20). The addition of RNase had no effect on NKL cell migration (data not shown). Five hundred microliters of BEAS-2b cell supernatant were placed in migration assay. To determine nonspecific or background migration, NKL cells were treated with supernatant from unstimulated BEAS-2b cells. After 3 h of incubation, upper chambers were removed. The percentage of migrated cells was calculated by subtracting the value of spontaneously migrating cells from the number of migrating cells, and afterward the result was divided by the total number of cells that was initially loaded on the Transwell filter.
Statistical analysis.
Results are presented as both individual data points and means ± SE. To determine significance, two-tailed Student's t-test, ANOVA, and Mann-Whitney test were used as appropriate (GraphPad Prism version 5; La Jolla, CA). P values ≤0.05 were considered to be statistically significant.
RESULTS
Role of EGFR in respiratory virus-induced inflammation.
Respiratory viruses activate EGFR (13, 25, 31), a result that we have recently confirmed for H1N1, RV, and RSV (50 and data not shown). EGFR activation induces airway inflammation via production of IL-8 (36), and EGFR has been implicated in airway epithelial IL-8 production in response to RSV and RV (25, 31). Therefore, we investigated the effect of EGFR in H1N1-induced IL-8 production and compared this with RV- and RSV-induced IL-8 production. We observed that NHBE cells infected with H1N1, RV (both RV1b and RV16), and RSV produced IL-8, and the addition of AG-1478, a selective EGFR tyrosine kinase inhibitor, suppressed this effect significantly (Fig. 1A). To confirm the specificity of the chemical inhibitor, we treated BEAS-2b cells with EGFR siRNA, which suppressed EGFR protein significantly (Fig. 1B, top right). Respiratory virus infection-induced IL-8 production in BEAS-2b cells was inhibited with EGFR siRNA, compared with cells stimulated with H1N1, RV (both RV1b and RV16), and RSV treated with control siRNA (Fig. 1B). In airway epithelium, EGFR activation involves Nox and MP cleavage of EGFR proligand that is released to activate EGFR (7, 40). Components of this pathway have been investigated previously for H1N1 and RV (4, 13, 25, 50, 59). Here, we implicated a role for Nox, MP, and EGFR ligand in H1N1-, RV-, and RSV-induced IL-8 production, which we showed is EGFR dependent (Fig. 1, A and B). First, we found that BEAS-2b cells infected with H1N1, RV, and RSV treated with a reactive oxygen species (ROS) scavenger (nPG), a Nox inhibitor (DPI), and a MP inhibitor (TAPI) decreased IL-8 production significantly (Fig. 1C). Second, the addition of an EGFR Ab that prevents ligand binding to EGFR suppressed respiratory virus-induced IL-8 production (Fig. 1D). Finally, to test the effect of EGFR inhibition on an in vivo model of respiratory viral infection, C57BL/6 mice were infected with H1N1 and treated with systemic gefitinib, a selective EGFR tyrosine kinase inhibitor that is used clinically, 16 h before viral infection and then continued daily. MIP2 (a mouse counterpart of IL-8) was measured by ELISA at 48 h, and we found that gefitinib inhibited H1N1-induced MIP2 production (Fig. 1E). These results confirm that respiratory viruses activate EGFR to induce IL-8 production, which contributes to airway inflammation in response to viral infection.
EGFR activation suppresses respiratory virus-induced CXCL10 production.
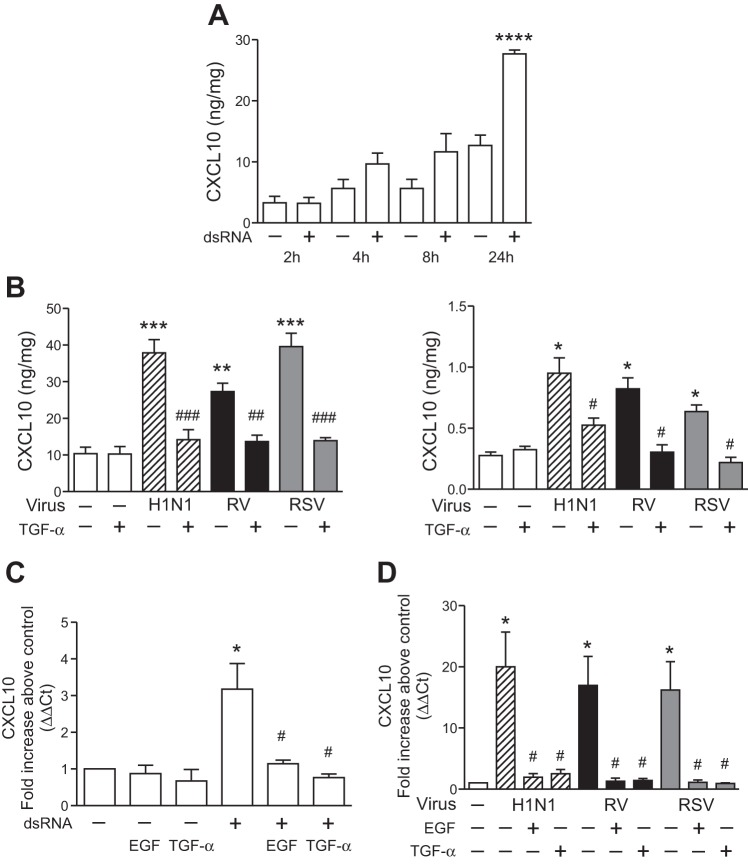
CXCL10 is a chemokine produced by airway epithelial cells in response to H1N1, RV, and RSV (37, 43, 51). However, the effect of EGFR activation on virus-induced CXCL10 production has not been explored. First, we examined the kinetics of airway epithelial CXCL10 production using synthetic dsRNA (poly I:C), an intermediate of ssRNA viral replication that is a common model of ssRNA viral infection. In BEAS-2b cells, dsRNA increased CXCL10 protein production at 24 h (Fig. 2A). To investigate a role for EGFR signaling in epithelial CXCL10 production, we investigated the effect of the EGFR ligand TGF-α on respiratory virus-induced CXCL10 protein production. BEAS-2b and NHBE cells infected with H1N1, RV, and RSV were treated with TGF-α, and CXCL10 protein production was measured. H1N1, RV, and RSV each stimulated epithelial CXCL10 production, and the addition of TGF-α significantly suppressed CXCL10 production in BEAS-2b (Fig. 2B, left) and NHBE (Fig. 2B, right) cells. Next, we investigated the effect of EGFR on CXCL10 mRNA expression. dsRNA-induced CXCL10 mRNA was suppressed by the addition of TGF-α and EGF (Fig. 2C). In addition, we found that H1N1-, RV-, and RSV-induced CXCL10 mRNA was suppressed significantly by the addition of TGF-α and EGF in NHBE cells (Fig. 2D). These results reveal a novel role for EGFR signaling to suppress respiratory virus-induced airway epithelial cell CXCL10 mRNA and protein production.
Fig. 2.
EGFR activation suppresses respiratory virus-induced CXCL10 production. A: BEAS-2b cells were treated with serum-free medium alone or the addition of polyinosine-polycytidylic acid (poly I:C, 25 μg/ml; dsRNA), and secreted CXCL10 protein was measured by ELISA at the indicated time points (n = 3–6 independent experiments, means ± SE; ****P < 0.0001 vs. control). B: BEAS-2b (left) and NHBE (right) cells were treated with serum-free medium alone (white bars), transforming growth factor (TGF)-α (10 ng/ml), H1N1 (hatched bars), RV (black bars), and RSV (gray bars), or virus + TGF-α, and secreted CXCL10 protein was measured by ELISA at 24 h (n = 3–4 independent experiments, means ± SE; *P < 0.05, **P < 0.01, and ***P < 0.005 vs. control; #P < 0.05, ##P < 0.01, and ###P < 0.005 vs. each virus alone). C: BEAS-2b cells were treated with serum-free medium alone, epidermal growth factor (EGF, 10 ng/ml), TGF-α (10 ng/ml), or poly I:C (100 μg/ml; dsRNA) alone, or poly I:C + EGF and TGF-α. CXCL10 mRNA was analyzed by quantitative RT-PCR (n = 6 independent experiments; *P < 0.05 vs. serum-free medium; #P < 0.05 vs. dsRNA alone). D: NHBE cells were treated with serum-free medium alone (white bars), H1N1 (hatched bars), RV (black bars), RSV (gray bars), and virus + EGF (10 ng/ml) or TGF-α, and CXCL10 mRNA was analyzed at 8 h by quantitative RT-PCR (n = 4 independent experiments; *P < 0.05 vs. control; #P < 0.05 vs. each virus alone).
EGFR activation suppresses IRF1-dependent CXCL10 production.
IFNs, which potently induce CXCL10, were shown to require IRF1 for CXCL10 production (8, 42). Our laboratory, and other investigators, have found that H1N1, RV, and RSV each activate IRF1 in airway epithelial cells (21, 26, 46, 50, 51, 58). In addition, H1N1 and RV were shown to involve IRF1 in CXCL10 production (21, 51, 58). Here, we confirmed that respiratory viruses induce CXCL10 production via IRF1 by treating BEAS-2b cells with IRF1 siRNA, which significantly suppressed IRF1 protein (Fig. 3A, right). Respiratory virus-induced CXCL10 production in BEAS-2b cells was inhibited with IRF1 siRNA compared with cells stimulated with H1N1, RV, and RSV treated with control siRNA (Fig. 3A). Because we found that respiratory virus-induced CXCL10 production involved IRF1, we hypothesized that EGFR activation may inhibit IRF1 as a mechanism for EGFR-induced suppression of CXCL10. We have consistently observed that IRF1 mRNA is increased significantly at 2 h in BEAS-2b cells stimulated with dsRNA (50). Next, we found that treatment of BEAS-2b cells with the EGFR ligands EGF and TGF-α suppressed dsRNA-induced IRF1 mRNA production in BEAS-2b cells at 2 h (Fig. 3B, left). In addition, we found that H1N1-, RV-, and RSV-induced IRF1 mRNA was suppressed with the addition of TGF-α and EGF in NHBE cells (Fig. 3B, right). To measure the effect of EGFR activation on IRF1 function in BEAS-2b cells, we used an IRF1 luciferase reporter assay. The addition of EGF decreased dsRNA-induced IRF1 transcriptional activity (Fig. 3C). Finally, we measured the effect of EGFR ligands on dsRNA-induced IRF1 protein production in pMTECs. dsRNA-induced IRF1 protein production was suppressed by the addition of EGF in pMTECs (Fig. 3D). These experiments provide evidence that airway epithelial CXCL10 production involves IRF1, and they suggest that EGFR activation suppresses IRF1 mRNA, transcriptional activity, and protein production.
Fig. 3.
EGFR activation suppresses interferon regulatory factor (IRF) 1-induced CXCL10 production. A: BEAS-2b cells were treated with serum-free medium alone, or transfected with IRF1 or control (C) siRNA for 24 h and treated with serum-free medium alone (white bars), or H1N1 (hatched bars), RV (black bars), and RSV (gray bars). After viral infection (24 h), secreted CXCL10 was measured by ELISA (n = 5 independent experiments, means ± SE; *P < 0.05 vs. serum-free medium; #P < 0.05 vs. serum-free medium and C siRNA; +P < 0.05 vs. C siRNA + virus). BEAS-2b cells were transfected with IRF1 siRNA, and IRF1 protein was assessed by Western blot (representative of 3 independent experiments). B: left, BEAS-2b cells were treated with serum-free medium alone, dsRNA, dsRNA + EGF (10 ng/ml) and TGF-α (10 ng/ml), and IRF1 mRNA was analyzed at 2 h by quantitative RT-PCR (n = 5 independent experiments; *P < 0.05 vs. serum-free medium; ##P < 0.005 vs. dsRNA alone). NHBE cells were treated with serum-free medium alone (white bars), H1N1 (hatched bars), RV (black bars), RSV (gray bars), and virus + EGF (10 ng/ml), and IRF1 mRNA was analyzed at 2 h by quantitative RT-PCR (n = 3 independent experiments in duplicate; *P < 0.05 vs. control; #P < 0.05 vs. each virus alone). C: BEAS-2b cells were transfected with IRF1 luciferase reporter and after 24 h treated with serum-free medium alone, EGF (10 ng/ml), dsRNA (100 μg/ml), and dsRNA + EGF for 3 h before luciferase activity was measured (n = 4 independent experiments in duplicate; ***P < 0.0005 vs. serum-free medium; ##P < 0.01 vs. dsRNA alone). D: IRF1 protein was measured in primary mouse tracheal epithelial cells (pMTECs) by Western blot 2 h after treatment with serum-free medium, dsRNA (100 μg/ml), dsRNA + EGF (10 ng/ml), and EGF alone (data shown are representative of 3 independent experiments).
EGFR inhibition increases IRF1-induced CXCL10 production in vitro and in vivo.
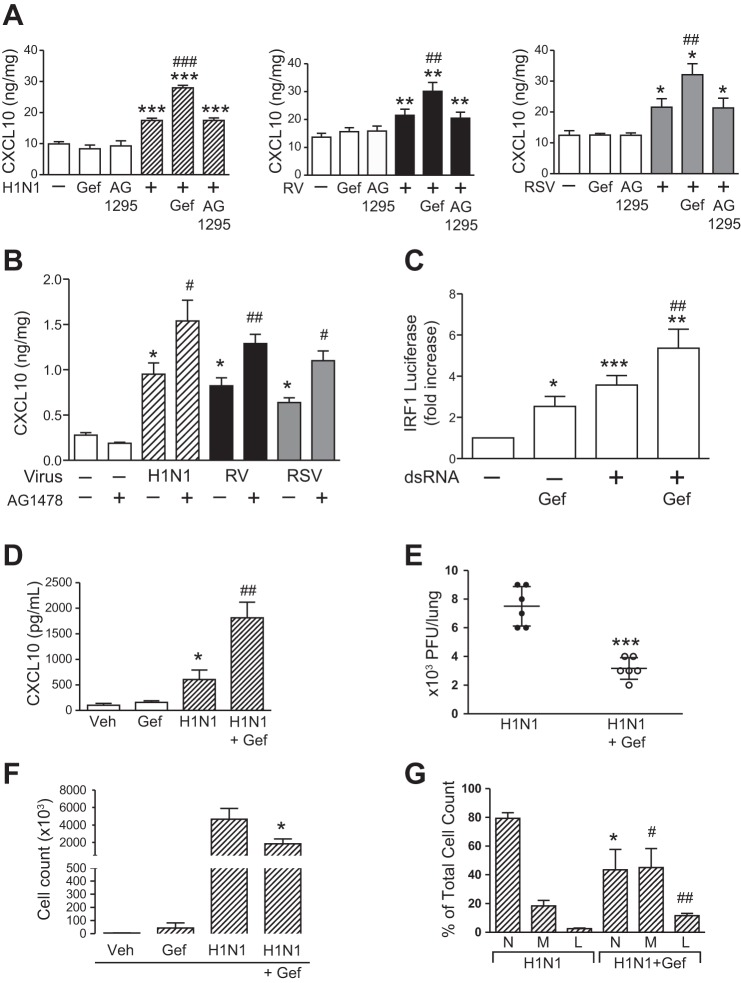
Because we found that EGFR activation suppressed IRF1 and CXCL10, we investigated the effect of EGFR inhibition on virus-induced IRF1 and CXCL10 production in airway epithelial cells. Here, we used gefitinib and measured CXCL10 production in BEAS-2b and NHBE cells infected with H1N1, RV, and RSV. The addition of gefitinib exaggerated virus-induced CXCL10 production in BEAS-2b cells, an effect that was not seen with another tyrosine kinase inhibitor that selectively targets the PDGF receptor (AG-1295; Fig. 4A). These results were confirmed in NHBE cells with H1N1, RV, and RSV (Fig. 4B). The addition of gefitinib increased dsRNA-induced IRF1 transcriptional activity in BEAS-2b cells (Fig. 4C). To test the effect of EGFR inhibition on an in vivo model of respiratory viral infection, C57BL/6 mice were infected with H1N1, and CXCL10 protein was measured by ELISA. Gefitinib, given systemically before viral infection, and then continued daily, increased H1N1-induced CXCL10 production (Fig. 4D). The addition of gefitinib also decreased viral titers (Fig. 4E), an effect that we found previously in vitro and in vivo (50). In addition, we found that EGFR inhibition in vivo decreased the total number of inflammatory cells found on BAL (Fig. 4F). This effect was associated with decreased neutrophils (Fig. 4G), which reflects decreased MIP2 production that we found with EGFR inhibition (Fig. 1E), and an increase in monocyte/macrophages and lymphocytes (Fig. 4G), which is consistent with increased CXCL10 production observed with EGFR inhibition (Fig. 4D). Therefore, these results showed that: 1) EGFR inhibition increased CXCL10 production in vitro and in vivo, and 2) EGFR inhibition results in decreased viral infection, decreased neutrophils, and increased monocyte/macrophages and lymphocytes in vivo.
Fig. 4.
EGFR inhibition increases IRF1-induced CXCL10 production in vitro and in vivo. A: BEAS-2b cells were treated with serum-free medium alone (white bars), gefitinib (Gef; 10 μM), AG-1295 (10 μM), H1N1 (hatched bars), RV (black bars), and RSV (gray bars), or virus + Gef and AG-1295, and secreted CXCL10 protein was measured by ELISA at 24 h (n = 3–6 independent experiments, means ± SE; *P < 0.05, **P < 0.005, and ***P < 0.0005 vs. control; ##P < 0.005 and ###P < 0.0005 vs. each virus alone). B: NHBE cells were treated with serum-free medium alone (white bars), AG-1478 (10 μM), H1N1 (hatched bars), RV (black bars), and RSV (gray bars), or virus + AG-1478, and secreted CXCL10 protein was measured by ELISA at 24 h (n = 3 independent experiments, means ± SE; *P < 0.05, vs. control; #P < 0.05 and ##P < 0.005 vs. each virus alone). C: BEAS-2b cells were transfected with IRF1 luciferase reporter and after 24 h treated with serum-free medium alone (white bars), gefitinib (Gef; 10 μM), dsRNA (100 μg/ml), and dsRNA + Gef for 3 h before luciferase activity was measured (n = 4 independent experiments in duplicate; *P < 0.05, **P < 0.005, and ***P < 0.0005 vs. control; ##P < 0.005 vs. dsRNA alone). D: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with influenza A virus (IAV, 104.5 TCID50%) or IAV + gefitinib. After 24 h, BAL was collected, and CXCL10 was measured by ELISA in BAL (n = 5 mice/group representative of 2 independent experiments, means ± SE; *P < 0.05 vs. vehicle alone; ##P < 0.005 vs. virus alone). E: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with IAV (104.5 TCID50%) or IAV + gefitinib, and viral titers were quantified by plaque assay at 48 h (n = 6 mice/group representative of 2 independent experiments, means ± SE; ***P < 0.001 vs. virus alone). F: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with IAV (104.5 TCID50%) or IAV + gefitinib, and total cell counts in BAL were measured (n = 3–4 mice/group repeated two times, means ± SE; *P < 0.05 vs. virus alone). G: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with IAV (104.5 TCID50%) or IAV + gefitinib, and percent neutrophils (N), monocyte/macrophages (M), and lymphocytes (L) was measured (n = 3–4 mice/group repeated two times, means ± SE; *P < 0.05, #P < 0.05, ##P < 0.005 vs. virus alone).
Finally, we examined the role of IRF1 in the effect of EGFR inhibition to exaggerate virus-induced epithelial CXCL10 production. We compared BEAS-2b cells transfected with control or IRF1 siRNA that were stimulated with H1N1, RV, and RSV with cells transfected with IRF1 siRNA that were treated with gefitinib. IRF1 siRNA abrogated the effect of gefitinib to exaggerate CXCL10 protein production (Fig. 5A), a result that shows IRF1 is required for EGFR inhibition to increase CXCL10 production.
Fig. 5.

EGFR signaling affects virus-induced perforin and NK cell migration. A: BEAS-2b cells were treated with serum-free medium alone or transfected with IRF1 or control (C) siRNA for 24 h and treated with serum-free medium alone (white bars), H1N1 (hatched bars), RV (black bars), and RSV (gray bars), or each virus + gefitinib (Gef; 10 μM). After viral infection (24 h), secreted CXCL10 was measured by ELISA (n = 6 independent experiments, means ± SE; *P < 0.05 vs. control; #P < 0.05 vs. C siRNA + virus). B: C57BL/6 mice were treated with vehicle (DMSO) or gefitinib (50 mg/kg) or infected (intranasally) with IAV (104.5 TCID50%) or IAV + gefitinib. After 48 h, lung was collected, and perforin mRNA was measured by quantitative RT-PCR (n = 7 mice/group representative of 2 independent experiments, means ± SE; ***P < 0.001 vs. vehicle alone; ##P < 0.005 vs. virus alone). C: BEAS-2b cells were treated for 24 h with serum-free medium alone, dsRNA (25 μg/ml) alone, or with dsRNA and the addition of AG-1478 (10 μM), and TGF-α (10 ng/ml). Cell culture supernatants were collected and added to NKL cells in a standard cell migration assay for 3 h. Data are expressed as a percentage of serum-free medium (n = 5 independent experiments, means ± SE; *P < 0.05 and **P < 0.01 vs. control; #P < 0.05 vs. dsRNA alone).
CXCL10 activates CXCR3 expressed on B and T lymphocytes, NK cells, dendritic cells, and nonhematopoietic cells. Perforin is a cytotoxic protein secreted by cytotoxic T lymphocytes (CTLs) and NK cells. To implicate CXCL10-induced recruitment of CTLs and NK cells, we measured perforin mRNA in the in vivo model of respiratory viral infection. Gefitinib, given systemically before viral infection, and then continued daily, increased H1N1-induced perforin mRNA (Fig. 5B), which suggests an increase in CTLs and NK cells that is consistent with the increase in lymphocytes that we observed with EGFR inhibition in vivo (Fig. 4D). In addition, because of CXCL10's chemokine properties, in proof of concept experiments, we investigated the effect of airway epithelial EGFR signaling to influence NK cell migration in a standard cell migration assay. First, BEAS-2b cells were stimulated with dsRNA, and EGFR was activated by TGF-α or inhibited by AG-1478. Cell culture supernatants were collected and treated with RNase to remove residual dsRNA (20) and then added to a human NK cell line (NKL cells) in a migration assay. BEAS-2b cell culture supernatants induced NKL cell migration, and the addition of TGF-α, which suppressed airway epithelial cell CXCL10 production (Fig. 2), inhibited NKL cell migration (Fig. 5C). The addition of AG-1478, which exaggerated epithelial production of CXCL10 (Fig. 4), to BEAS-2b cell culture supernatants increased NKL cell migration (Fig. 5C). These results suggest that EGFR signaling in airway epithelial cells, which modulates CXCL10 production, may influence NK cell migration in response to a viral stimulus.
DISCUSSION
The airway epithelium plays a critical role in host response to viral infection. An essential component of this response is production of cytokines and chemokines that influence innate and adaptive antiviral immune responses. Here, we found that respiratory viruses stimulate IL-8 production via EGFR activation, which suppresses H1N1-, RV-, and RSV-induced IRF1 and CXCL10 production in airway epithelial cells. In addition, inhibition of EGFR augmented epithelial production of IRF1 and CXCL10, which led to increased lymphocytes, perforin, and NK cell migration.
In the study of airway epithelial responses to viral infection, one of the earliest observations was the induction of IL-8 in response to respiratory viruses (5, 10, 44). Airway epithelial production of IL-8 was found to involve EGFR signaling (36), and more recently EGFR was implicated in RV- and RSV-induced IL-8 production (25, 31). H1N1 activates EGFR in airway epithelium (13, 50), and H1N1-induced EGFR signaling stimulates airway epithelial mucin production (4). Surprisingly, airway epithelial EGFR-induced IL-8 production, in response to H1N1, has not been reported. Here, we found that blockade of EGFR tyrosine kinase activation suppressed H1N1-, RV16-, RV1b-, and RSV-induced IL-8 production in NHBE cells. To confirm the specificity of chemical inhibitors, we showed that EGFR siRNA suppressed virus-induced IL-8 production. In airway epithelium, EGFR activation involves an integrated signaling pathway that includes Nox-induced production of ROS that activate a MP [e.g., TNF-α-converting enzyme (TACE)], which cleaves an EGFR proligand that is released to bind to, and to activate, EGFR (22, 40). Recently, we implicated Nox as a shared epithelial signal in response to multiple respiratory viruses (50). In addition, here we found that a Nox inhibitor (DPI), ROS scavenger (nPG), MP inhibitor (TAPI) with some selectivity to TACE, and an EGFR Ab that prevents ligand binding to EGFR, each suppressed H1N1-, RV-, and RSV-induced IL-8 production. To test the effect of EGFR inhibition on respiratory virus-induced IL-8 production in vivo, we used a mouse model of H1N1 infection. We found that EGFR inhibition suppressed H1N1-induced MIP2, a mouse counterpart of human IL-8. Collectively, these experiments implicate EGFR signaling in virus-induced IL-8 production.
CXCL10 is a CXCR3 ligand, produced by airway epithelium, that contributes to NK cell and T lymphocyte recruitment to target and kill virus-infected cells. We investigated the role of EGFR signaling on CXCL10 production in airway epithelium, and we found that EGFR activation suppressed respiratory virus-induced CXCL10 mRNA and protein production. EGFR has been implicated in keratinocyte CXCL10 production (28), but the mechanisms involved have not been identified. IRF1 has also been implicated in virus-induced CXCL10 production (21, 51, 58), and here we found that EGFR activation suppressed respiratory virus-induced IRF1 mRNA and protein production. IRF1 suppresses oncogenic transformation and induces apoptosis (47), which are opposing effects of EGFR-induced cell proliferation. However, prior studies have reported EGFR-dependent IRF1 production in a cancer cell line (2), but this effect required higher concentrations of EGFR ligand, and the timing of IRF1 was later than what we observed in airway epithelial cells. In addition, we found that inhibition of EGFR in airway epithelial cells increased virus-induced IRF1 and CXCL10. Furthermore, inhibition of both EGFR and ERK 1/2, a mitogen-activated protein kinase downstream from EGFR, increased IP-10 production in keratinocytes (28). More recently, Zaheer et al. reported that ERK inhibition increased RV-induced CXCL10 (57), a result that we have confirmed (data not shown). This result is consistent with our finding that EGFR inhibition exaggerates H1N1-, RV-, and RSV-induced CXCL10, because EGFR activation is a strong stimulus for ERK in airway epithelial cells. These results suggest that future experiments to investigate downstream EGFR-dependent ERK signaling will be informative to elucidate the signaling intermediates between IRF1 and EGFR.
By contributing to the recognition and response to inhaled pathogens, the airway epithelium plays a fundamental role in host defense. However, the inflammatory host response that is initiated to combat infection must be effectively regulated to prevent persistent damage. Therefore, components of the inflammatory response may be more or less productive to overall host defense. The regulation of these host responses is referred to as host tolerance (39). For example, airway epithelial IL-8 production results in recruitment of neutrophils into the lung, which is a critical innate immune response against bacterial infection. However, the role of neutrophil recruitment in response to viral infections is less clear. Neutrophils are present during viral exacerbations of asthma (49) and COPD (27) and contribute to exaggerated lung inflammation in these diseases. Virus-induced acute lung injury severity is associated with neutrophil recruitment (35), which is a critical component of the pathogenesis of acute lung injury (54). In addition, inhibition of neutrophils in models of acute lung injury was shown to be protective (1). However, recruited neutrophils secrete molecules that contribute to persistent inflammation, which causes epithelial damage. For example, neutrophils secrete elastase, which induces airway epithelial EGFR activation (23), and provides positive feedback for continued epithelial production of IL-8 and subsequent neutrophil recruitment. In addition, we have shown that EGFR activation suppresses CXCL10 production, which may contribute to less effective viral clearance because of impaired recruitment of NK cells and T lymphocytes that kill virus-infected epithelial cells. Importantly, airway epithelium produces both IL-8 and CXCL10 in response to viral infection. However, temporal differences in protein production may have significant implications for the downstream effects of these molecules. For example, airway epithelial cells quickly produce IL-8 in response to pathogens (e.g., within 4–6 h) in vitro (7), whereas CXCL10 production requires 24 h before it is significantly increased in vitro (Fig. 2A). The temporal differences between IL-8 and CXCL10 production may be a result of interactions between these two signaling pathways. However, we found that EGFR inhibition did not increase CXCL10 production before 24 h (data not shown). Therefore, the mechanisms that regulate earlier epithelial IL-8 production, compared with later CXCL10 secretion, remain to be explored.
Although we have shown that EGFR inhibition increases lymphocyte recruitment and results in decreased viral infection, targeting EGFR to suppress neutrophil recruitment and subsequent inflammation has potential limitations. First, removal of neutrophils in a model of influenza virus infection worsened outcomes, although this result required treatment before viral infection (48). Second, the chronic use of an inhaled EGFR inhibitor in COPD patients was not well tolerated (56). Third, recent studies have suggested that innate lymphoid cell-induced repair after influenza virus infection involves EGFR signaling (18, 33). However, the potential for EGFR inhibition (ideally using an inhaled small molecule inhibitor), for a short duration after respiratory viral infection, remains to be explored. As a potential mechanism to support this hypothesis, we found that treatment with an EGFR inhibitor, which increases CXCL10 production, increased: 1) lymphocyte recruitment in vivo, 2) perforin mRNA, which is found in CTL and NK cells, and 3) NK cell migration, which reflects the chemotactic properties of CXCL10. In addition, recently we showed that EGFR inhibition increased epithelial interferon production, which resulted in lower viral titers in vitro and in vivo (50), which we confirmed here (Fig. 4E).
In conclusion, these experiments show that respiratory viruses stimulate IL-8 production via EGFR activation, which suppresses IRF1-dependent CXCL10 production in airway epithelial cells. In addition, inhibition of EGFR augmented epithelial production of IRF1 and CXCL10, which led to increased lymphocytes, perforin, and NK cell chemotaxis (summarized in Fig. 6). These studies provide a novel mechanism that viruses activate to suppress antiviral defenses and implicates potential targets to explore for novel antiviral therapies.
Fig. 6.

Effect of EGFR signaling on IRF1-induced CXCL10 production. Top, respiratory viruses (e.g., influenza virus, RV, and RSV) stimulate airway epithelial NADPH oxidase (Nox), metalloproteinase (MP), and ligand-induced activation of EGFR, which leads to IL-8 production. EGFR activation suppresses IRF1-induced CXCL10. Bottom, in the presence of EGFR inhibition (e.g., gefitinib and AG-1478), IRF1-induced CXCL10 is increased.
GRANTS
J. Koff was supported National Institutes of Health (NIH) Grant No. K08-HL-0923288, G. Min-Oo was supported by a Bisby Fellowship from the Canadian Institutes of Health, and L. L. Lanier is an American Cancer Society Professor and is supported by NIH Grant AI-068129.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.K., I.U., L.L.L., J.A.N., and J.K. conception and design of research; A.K., I.U., G.M.-O., E.B.-L., D.K., B.G., and J.K. performed experiments; A.K., I.U., E.B.-L., D.K., B.G., and J.K. analyzed data; A.K., I.U., G.M.-O., D.K., B.G., L.L.L., and J.K. interpreted results of experiments; A.K., G.M.-O., B.G., L.L.L., J.A.N., and J.K. edited and revised manuscript; A.K., I.U., G.M.-O., E.B.-L., D.K., B.G., L.L.L., J.A.N., and J.K. approved final version of manuscript; J.K. prepared figures; J.K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Drs. Patrick Hayden and Walter Finkbeiner for generously providing primary cells.
Current addresses: D. Knoff, Dana Farber Cancer Institute, Boston, MA; and J. Galen, Department of Medicine, Albert Einstein College of Medicine, NY.
REFERENCES
- 1. Abraham E. Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Andersen P, Pedersen MW, Woetmann A, Villingshoj M, Stockhausen MT, Odum N, Poulsen HS. EGFR induces expression of IRF-1 via STAT1 and STAT3 activation leading to growth arrest of human cancer cells. Int J Cancer 122: 342–349, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 184: 662–671, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Barbier D, Garcia-Verdugo I, Pothlichet J, Khazen R, Descamps D, Rousseau K, Thornton D, Si-Tahar M, Touqui L, Chignard M, Sallenave JM. Influenza A induces the major secreted airway mucin MUC5AC in a protease-EGFR-ERK-Sp1 dependent pathway. Am J Respir Cell Mol Biol 47: 149–157, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Becker S, Koren HS, Henke DC. Interleukin-8 expression in normal nasal epithelium and its modulation by infection with respiratory syncytial virus and cytokines tumor necrosis factor, interleukin-1, and interleukin-6. Am J Respir Cell Mol Biol 8: 20–27, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Britto CJ, Liu Q, Curran DR, Patham B, Dela Cruz CS, Cohn L. Short palate, lung, and nasal epithelial clone-1 is a tightly regulated airway sensor in innate and adaptive immunity. Am J Respir Cell Mol Biol 48: 717–724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgel PR, Nadel JA. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J 32: 1068–1081, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Buttmann M, Berberich-Siebelt F, Serfling E, Rieckmann P. Interferon-beta is a potent inducer of interferon regulatory factor-1/2-dependent IP-10/CXCL10 expression in primary human endothelial cells. J Vasc Res 44: 51–60, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chan RW, Yuen KM, Yu WC, Ho CC, Nicholls JM, Peiris JS, Chan MC. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS One 5: e8713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi AM, Jacoby DB. Influenza virus A infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett 309: 327–329, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12: 1023–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Drexler HG, Matsuo Y. Malignant hematopoietic cell lines: in vitro models for the study of natural killer cell leukemia-lymphoma. Leukemia 14: 777–782, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Eierhoff T, Hrincius ER, Rescher U, Ludwig S, Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog 6: e1001099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gencheva M, Chen CJ, Nguyen T, Shively JE. Regulation of CEACAM1 transcription in human breast epithelial cells (Abstract). BMC Mol Biol 11: 79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360: 588–598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, Kon OM, Papi A, Jeffery PK, Edwards MR, Johnston SL. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur Respir J 36: 1425–1435, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Hur GY, Lee SY, Lee SH, Kim SJ, Lee KJ, Jung JY, Lee EJ, Kang EH, Jung KH, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. Potential use of an anticancer drug gefinitib, an EGFR inhibitor, on allergic airway inflammation. Exp Mol Med 39: 367–375, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 340: 1230–1234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc 2: 150–156, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem 281: 36560–36568, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Koetzler R, Zaheer RS, Newton R, Proud D. Nitric oxide inhibits IFN regulatory factor 1 and nuclear factor-kappaB pathways in rhinovirus-infected epithelial cells. J Allergy Clin Immunol 124: 551–557, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol 294: L1068–L1075, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kohri K, Ueki IF, Nadel JA. Neutrophil elastase induces mucin production by ligand-dependent epidermal growth factor receptor activation. Am J Physiol Lung Cell Mol Physiol 283: L531–L540, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Langhammer S, Koban R, Yue C, Ellerbrok H. Inhibition of poxvirus spreading by the anti-tumor drug Gefitinib (Iressa). Antiviral Res 89: 64–70, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Liu K, Gualano RC, Hibbs ML, Anderson GP, Bozinovski S. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J Biol Chem 283: 9977–9985, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 279: 2461–2469, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183: 734–742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol 163: 303–312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayer ML, Blohmke CJ, Falsafi R, Fjell CD, Madera L, Turvey SE, Hancock RE. Rescue of dysfunctional autophagy attenuates hyperinflammatory responses from cystic fibrosis cells. J Immunol 190: 1227–1238, 2013 [DOI] [PubMed] [Google Scholar]
- 30. Mikhak Z, Fleming CM, Medoff BD, Thomas SY, Tager AM, Campanella GS, Luster AD. STAT1 in peripheral tissue differentially regulates homing of antigen-specific Th1 and Th2 cells. J Immunol 176: 4959–4967, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Monick MM, Cameron K, Staber J, Powers LS, Yarovinsky TO, Koland JG, Hunninghake GW. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem 280: 2147–2158, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol 179: 1648–1658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol 154: 5235–5244, 1995 [PubMed] [Google Scholar]
- 35. Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog 4: e1000115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richter A, O'Donnell RA, Powell RM, Sanders MW, Holgate ST, Djukanovic R, Davies DE. Autocrine ligands for the epidermal growth factor receptor mediate interleukin-8 release from bronchial epithelial cells in response to cigarette smoke. Am J Respir Cell Mol Biol 27: 85–90, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol 79: 3350–3357, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 162: 3549–3558, 1999 [PubMed] [Google Scholar]
- 39. Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8: 889–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 102: 767–772, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 100: 11618–11623, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shultz DB, Rani MR, Fuller JD, Ransohoff RM, Stark GR. Roles of IKK-beta, IRF1, and p65 in the activation of chemokine genes by interferon-gamma. J Interferon Cytokine Res 29: 817–824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 289: L85–L95, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest 96: 549–557, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Subauste MC, Proud D. Effects of tumor necrosis factor-alpha, epidermal growth factor and transforming growth factor-alpha on interleukin-8 production by, and human rhinovirus replication in, bronchial epithelial cells. Int Immunopharmacol 1: 1229–1234, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Takeuchi R, Tsutsumi H, Osaki M, Haseyama K, Mizue N, Chiba S. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1beta-converting enzyme gene expression but does not cause apoptosis. J Virol 72: 4498–4502, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26: 535–584, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 183: 7441–7450, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med 155: 1362–1366, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Ueki IF, Min-Oo G, Kalinowski A, Ballon-Landa E, Lanier LL, Nadel JA, Koff JL. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon lambda and antiviral defense in airway epithelium. J Exp Med 210: 1929–1936, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veckman V, Osterlund P, Fagerlund R, Melen K, Matikainen S, Julkunen I. TNF-alpha and IFN-alpha enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology 345: 96–104, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol 182: 1296–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol 183: 6989–6997, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, Zummo G, Holgate ST, Attia J, Thakkinstian A, Davies DE. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol 120: 586–593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, Criner GJ, Kim V, Prasse A, Nivens MC, Tetzlaff K, Heilker R, Fahy JV. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 438–445, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Zaheer RS, Koetzler R, Holden NS, Wiehler S, Proud D. Selective transcriptional down-regulation of human rhinovirus-induced production of CXCL10 from airway epithelial cells via the MEK1 pathway. J Immunol 182: 4854–4864, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Zaheer RS, Proud D. Human rhinovirus-induced epithelial production of CXCL10 is dependent upon IFN regulatory factor-1. Am J Respir Cell Mol Biol 43: 413–421, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol 40: 610–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]