Abstract
Intermittent hypoxia (IH) has been extensively studied during the last decade, primarily as a surrogate model of sleep apnea. However, IH is a much more pervasive phenomenon in human disease, is viewed as a potential therapeutic approach, and has also been used in other disciplines, such as in competitive sports. In this context, adverse outcomes involving cardiovascular, cognitive, metabolic, and cancer problems have emerged in obstructive sleep apnea-based studies, whereas beneficial effects of IH have also been identified. Those a priori contradictory findings may not be as contradictory as initially thought. Indeed, the opposite outcomes triggered by IH can be explained by the specific characteristics of the large diversity of IH patterns applied in each study. The balance between benefits and injury appears to primarily depend on the ability of the organism to respond and activate adaptive mechanisms to IH. In this context, the adaptive or maladaptive responses can be generally predicted by the frequency, severity, and duration of IH. However, the presence of underlying conditions such as hypertension or obesity, as well as age, sex, or genotypic variance, may be important factors tilting the balance between an appropriate homeostatic response and decompensation. Here, the two possible facets of IH as derived from human and experimental animal settings will be reviewed.
Keywords: intermittent hypoxia, sleep apnea, beneficial and pathological effects
Definition of Intermittent Hypoxia
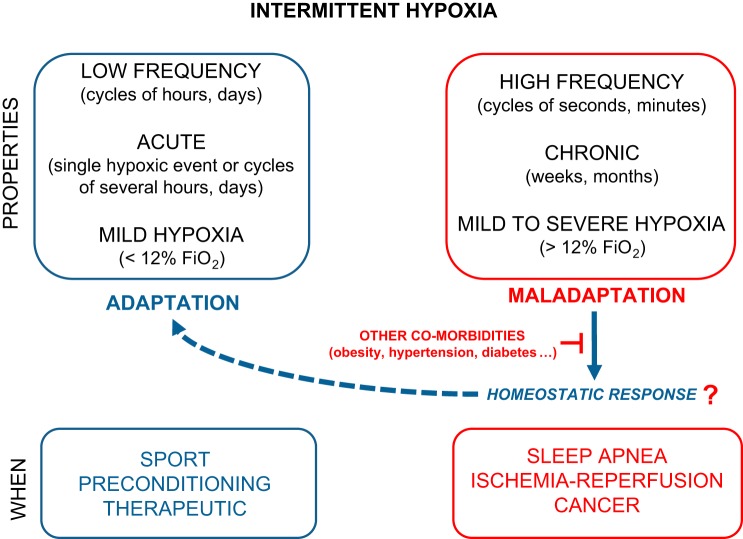
sleep apnea (sa) is characterized by repetitive central apneic episodes or by repetitive occlusions of the upper airway (obstructive sleep apnea, OSA) that lead to intermittent hypoxia (IH), thus manifesting as recurrent blood oxygen desaturations. Although IH in patients with SA is typically characterized by short cycles of hypoxia and reoxygenation, the patterns of IH vary greatly across patients with SA. Thus, to enable comparisons across subjects or experimental conditions, IH needs to be defined by the duration of each cycle, the severity of hypoxemia in each cycle, the duration of IH per day, and the total number of days to years in which IH is present (Fig. 1). From these considerations, it becomes immediately apparent that IH patterns imposed during experimental protocols vary considerably between research groups and are primarily dictated by technical and biological reasons. For example, the rates of IH or cyclic hypoxia have ranged from minutes or hours to multiple events per hour throughout the circadian or ultradian cycle or during the sleeping period of rodents. The selection of those variables in translational studies has depended on the intent to mimic a specific human disease condition associated with IH (e.g., chronic obstructive pulmonary disease, OSA, asthma, hypoxia-reperfusion, obesity, cancer, etc.). Considering the high variability of IH paradigms employed in the experimental literature, we will here delineate each of those paradigms as we address several a priori contradictory consequences of IH, namely the beneficial and deleterious effects of IH.
Fig. 1.
The protective and detrimental effects of intermittent hypoxia (IH) can be explained by the adaptation or maladaptation of the whole organism. Short- and low-frequency exposures to mild IH paradigms can afford protection to specific cells, tissues, or organs against more severe hypoxia. In addition, some homeostatic or adaptive responses elicited by IH have been described, such as release of stem cells. However, those mechanisms could be partially or totally abolished by other coexisting factors or diseases.
Adaptation to IH: Beneficial Effects
Several studies have reported that IH-induced adaptations or interval hypoxic exercise training can provide some measurable protection in some disease states or enable improvements in selected sport-related performances. Globally, the protective effects of IH can be explained by the activation and propagation of homeostatic or adaptive responses elicited by the IH stimulus, usually through a process that has been generally termed preconditioning. Thus short exposures to mild IH paradigms can afford protection to specific cells, tissues, or organs against more severe hypoxia and ischemia. In addition, mild or training IH exposures have also been implicated in enhancements of both physical and mental capacities (81, 168). As indicated above, the IH patterns used in most of the following studies were characterized by relatively milder hypoxic exposures (>12% FiO2) and by single sessions per day or by brief exposures ranging from minutes to several hours. In the following sections, we will briefly provide illustrative examples of IH-induced beneficial effects.
Preconditioning effects.
Animals subjected to various paradigms of acute IH become more resistant to injury in subsequent exposures to severe hypoxic/ischemic insults. For instance, compared with controls, mice treated with brief episodes of low-frequency IH (8% O2 × 10 min/21% O2 × 10 min, 6 cycles) survived substantially longer when exposed to lethal hypoxia. This phenomenon was accompanied by attenuated cellular and tissue injury in important organs, such as the lung and the brain (207). More detailed analyses revealed that IH-induced beneficial effects in the lung involved protection of the respiratory membrane integrity, especially hypoxia-sensitive type I epithelial cells, and preservation of gas-exchange function (207, 208). Furthermore, myocardium from mice treated with a similar IH paradigm (6% O2 × 6 min/21% O2 × 6 min, 5 cycles) or from rats treated with an IH paradigm with higher frequency but short duration (10% O2 × 40 s/21% O2 × 20 s, for 4 h) was shown to be protective from ischemia-induced infarction (17, 18, 23). Such IH-induced cardioprotection seemed to involve activation of pathways similar to those described in models of ischemic cardiac preconditioning (17, 18, 137) and required sufficient expression and activity of hypoxia-inducible factor 1α (HIF-1α) (23). Interestingly, IH paradigms of a longer period (7 days) but less severe FiO2 (12% O2) have been attempted in a recent study for postconditioning treatment against ischemic brain injury in rats (187).
Exercise training.
IH has been extensively used for exercise training in an attempt to improve physical performance. Interval training in hypoxic conditions, a form of IH, promotes the induction of erythropoietin production and is associated with improved aerobic performance capacity in athletes (153). It has been suggested that this adaptation to IH exposures is mediated, at least in part, by increased peripheral chemosensitivity to hypoxia (82). In animals models, respiratory adaptations to IH have been studied in great detail (146) with purported increases in the acute hypoxic ventilatory response (82, 143, 146), ventilatory long-term facilitation (see below) (115), and improved maximal oxygen uptake at high altitude or sea level (52). Although all the physiological adaptations induced by short-term or chronic IH have been used to develop therapies with IH for exercise performance in athletes, there is still substantial controversy on the mechanisms underlying the putative IH-induced changes, the optimal IH schedules required for any specific goal, and the overall magnitude of changes elicited by IH (70). These discrepancies are likely due to the huge diversity of IH-exposure paradigms used and the large variety of exercise training schedules and performance targets studied. As such, no specific mechanistic inferences can be drawn from these empirical IH exposures.
Long-term facilitation of respiratory output and facilitation.
As will be subsequently discussed, the central nervous system (CNS) undergoes degenerative changes in the context of long-term IH. However, attempts to minimize pathology by increasing the expression of growth/trophic factors that confer neuroprotection and neuroplasticity are also part of the response armamentarium of the CNS. For example, short-term IH (3 to 10 short cycles lasting a few minutes each and not more than 1–2 h/day) elicits respiratory motor plasticity, increasing the strength of respiratory contractions and breathing, a phenomenon that has been termed long-term facilitation (LTF). This type of short IH paradigm upregulates the expression and function of hypoxia-sensitive growth/trophic factors within respiratory motor neurons and is void of any detectable end-organ pathologies such as hippocampal cell death, neuroinflammation, or systemic hypertension. One of the most studied models of respiratory plasticity is LTF of hypoglossal and phrenic motor output following acute intermittent hypoxia (39, 55, 109, 117, 147). LTF is characterized by a progressive increase in respiratory motor output and is associated with increases in phrenic, hypoglossal, or carotid sinus nerve inspiratory-modulated discharge. This phenomenon has been described in several species, including humans, and can be influenced by other factors such as sex and age (199). Serotonin is currently the most characterized signaling mediator during IH-mediated LTF (103). However, LTF-driven increases in ventilatory output are mediated in part by slight increases in oxidative stress and other signaling pathways (106–108) and have been successfully harnessed as an approach aiming to rehabilitate patients after spinal cord injury (69, 128, 129). However, LTF-generating paradigms could lead to long-term muscle fatigue with remodeling of the upper airway muscles and diaphragm and ultimately deleterious effects on motor control (53, 175–178). This dysfunction emerges as highly dependent on factors such as age (177) or sex (176).
Cardiovascular protection and improvement.
IH can protect the heart against ischemia-reperfusion injury (197) by improving against ischemia-induced contractile dysfunction (31, 127), endothelial dysfunction (110, 111), arrhythmias (110, 116, 209), and cell death (45, 89). Also, IH treatment can promote higher resistance to arrhythmias during acute myocardial ischemia and prevent the development of atherosclerosis in rabbits (88). This protection has been ascribed to the higher myocardial vascularity, coronary blood flow, cardiomyoglobin, and expression of antioxidant proteins induced by IH (211). Other investigators have reported antihypertensive effects of conditioning IH exposures (9–10% O2, 5–10 min, 5–8 times per day) in young spontaneous hypertensive rats, which have been associated with prevention of endothelial dysfunction and with increased accumulation of nitric oxide (NO) in vasculature (111). In addition, IH appears to provide a therapeutic effect on permanent coronary artery ligation-induced myocardial infarction by reducing the infarct size, myocardial fibrosis, and apoptosis (202). Due to the benefits reported by IH on myocardial infarction and its simple intervention with fewer adverse effects, IH paradigms have been proposed as treatment for patients suffering from these conditions.
The early phase of reperfusion after myocardial ischemia can generate a large amount of reactive oxygen species (ROS), which can contribute to increase in the myocardial injury (14, 189, 212). However, at the same time, ROS also can trigger cardioprotection induced by ischemic or pharmacological conditioning (34, 68, 188). The possible role of ROS has been explained as depending on concentration, such that ROS emerge as cardioprotective at low levels but detrimental at high levels (36, 165). In support of such assumptions, Naghshin and colleagues (118, 119) reported that chronic IH treatment is sufficient to improve cardiac function in healthy mice and transgenic mice with underlying heart failure.
Neurovascular protection and cognitive improvement.
The vascular protective role of IH is not only confined to the heart. IH-mediated adaptations can also lead to improved cerebral flow responses to ischemia or stroke. Previous studies have reported that preconditioning with long hypoxic periods (hours) can minimize cortical infarction (102) as well as dampen learning and memory impairments related to severe CNS ischemia in rats (163, 164). These benefits have been related to an increased proliferation of neural stem cells in the subventricular zone and dentate gyrus observed after rats were subjected to hypoxia 4 h per day for 2 wk (210) and have also been related to the upregulation of c-Fos expression (163). However, as discussed below, higher frequency or duration of IH can induce oxidative stress and neuronal apoptosis promoting detrimental effects on memory (201).
Low-frequency IH in rats after brain ischemia can decrease infarct volume (186), which in turn can attenuate ischemia-induced spatial learning and memory deficits in those animals (187). As occurs with IH preconditioning, IH interventions following stroke occurrence induced hippocampal neurogenesis that ameliorated memory losses (187). Furthermore, application of postischemia IH can upregulate hippocampal brain-derived neurotrophic factor, suggesting that IH induces synaptogenesis (187). As a corollary to these findings, in a recent study carried out in patients with OSA, Hoth et al. (72) found that more severe hypoxemic events may result in memory improvements.
Newborns can be subjected to transient oxygen fluctuations and deprivation during birth (acute IH). Interestingly, noninjurious severe short-lasting neonatal hypoxia conferred resistance to brain senescence in aged male rats. Indeed, Martin et al. (113) reported in rats at 21 days of age increased cortical thickness and cell densities with strong synapsin activation in several brain subregions that were potentially associated with improved preservation of cognitive functioning.
Other potential benefits of IH.
Application of brief IH (cycles of 5 min, hypoxia 10%) in humans has been tested as immunotherapy. IH appears to enhance innate immunity by mobilizing hematopoietic progenitors, which activate immune cells such as neutrophils and increase circulating immunoglobulins (172). The authors suggested that IH can increase immune defenses without exacerbating inflammation. However, the establishment of IH treatment in patients is potentially fraught with substantial difficulties because the application of IH in humans needs to be exhaustively controlled and monitored. Another study carried out in humans showed similar results, whereby IH promoted the release of progenitor cells (191), such that short-term IH with muscle electrostimulation increased the number of hematopoietic CD34+ stem cells in circulation and could help increase hematopoietic stem cell homing in injured tissues (191). We should remark that short-lasting IH exposures mimicking SA were associated with recruitment of bone-marrow derived pluripotent stem cells that exhibited upregulation of stem cell differentiation pathways, particularly involving CNS development and angiogenesis (58, 59).
Maladaptation to IH: Pathological Effects
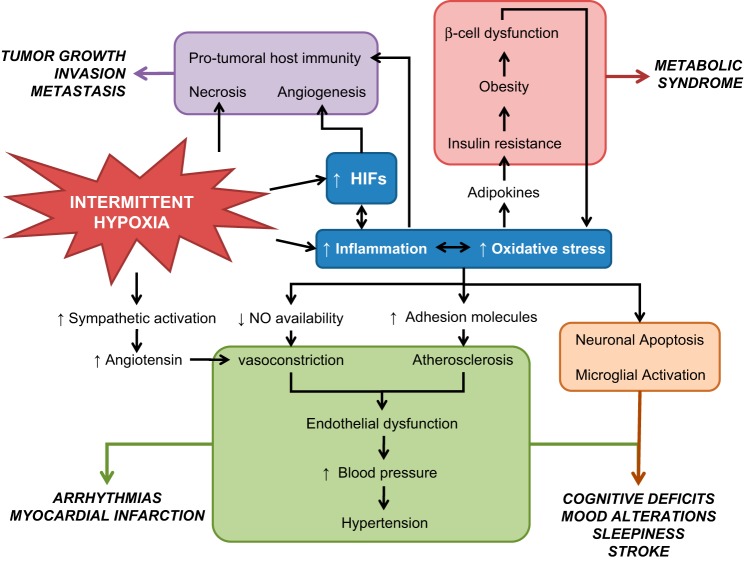
There is increasing evidence that IH plays a mechanistic role in the development of cardiovascular, metabolic, and cognitive consequences in the context of the OSA through activation of oxidative stress and inflammatory pathways (Fig. 2). The IH pattern in most of the animal-based studies aiming to replicate OSA included higher cycle frequency (from seconds to few minutes), lower FiO2 being applied, and restriction of IH to the sleep or rest period (149). As mentioned, the selected IH parameters aim to recapitulate some characteristics of OSA, as defined by the apnea hypopnea and oxygen desaturation indices usually seen in the clinical practice settings (161).
Fig. 2.
General diagram of the detrimental effects of IH on cognitive, metabolic, cardiovascular, and cancer outcomes. IH can promote oscillations of oxygen levels in the whole organism. Some tissues, concretely those that are more dependent on oxygen, could experience apoptosis during IH as occurs in some neurons or cardiomyocytes. Also, IH can induce necrosis in tumor tissues where there is a high metabolism and oxygen demand. Although the activation of hypoxia-inducible factor (HIF) by intermittent hypoxia is not clear among the different studies, the recurrent oxygen oscillations can promote inflammation and/or oxidative stress, which in turn could activate HIF pathways. IH-induced chronic inflammation has been related to the majority of consequences of sleep apnea and promoting cardiovascular, metabolic, and cognitive disorders. Very recently, inflammation and oxygen oscillations have been associated to increased tumor aggressiveness. NO, nitic oxide.
Link between IH and inflammation.
Recurrent blood oxygen desaturation is the prototypic feature of IH. The changes of oxygen supply in circulation can elicit swings in oxygen partial pressure in tissues and more markedly in those with higher metabolic rate and perfusion (i.e., brain, liver, kidney) (5, 9). Although some homeostatic responses to IH (and hypercapnia) may develop over time, the oscillating changes in tissue oxygen availability are widely considered as an important source of ROS. These molecules can be generated from different subcellular compartments and organelles, such as mitochondria, cellular membrane, lysosomes, peroxisomes, and the endoplasmic reticulum (11, 15, 51, 91, 167).
The HIF family can account, at least in part, for some of the processes linking IH to ROS production, activation of inflammation, and downstream upregulation of other molecules involved in angiogenesis and homeostatic responses. HIF-1 is a commonly studied transcriptional activator, which comprises an O2-regulated α-subunit and a constitutive β-subunit (193). Thus HIF-1α can be induced by hypoxia as a consequence of decreased O2-dependent degradation of HIF-1α (35, 204). HIF-2α is another well-studied member of the HIF family induced by continuous hypoxia, which also can interact with HIF-1α (182). Although continuous hypoxia promotes the increased expression and activity of both molecules (71), acute IH upregulates HIF-1α and downregulates HIF-2α protein via calpains (125). Furthermore, contrary to sustained hypoxia, long-term IH during sleep has been reported to induce early activation of HIF-1α in the brain that is then followed by reductions in HIF-1α expression or its target genes over time (41, 99, 122). If we consider that HIF-2α regulates the transcription of several antioxidant enzymes including SOD-2 (169), the downregulation of HIF-2α in the context of acute IH may also contribute to increases in ROS via insufficient transcription of antioxidative enzymes. Therefore, the differential regulation of both transcriptional factors in acute IH and the potential biphasic or multiphasic temporal trajectories of HIF in chronic IH may account for the higher levels of ROS in IH compared with sustained hypoxia. Peng et al. (141) showed that IH enhances carotid body responses to hypoxia and elevates local ROS production that is mediated by HIF-1α accumulation, and such responses appear to underlie some of the cardiorespiratory responses to IH.
In addition, most cells will respond to increased ROS by upregulating the redox-sensitive transcriptional factor NF-κB, a pivotal molecule in the proinflammatory response. In the context of hypoxia, NF-κB can be also modulated by HIF-1α (60). In the nucleus, NF-κB upregulates the transcription of several proinflammatory genes responsible for encoding inflammatory cytokines (tumor necrosis factor-α, interleukin-6, and interleukin-8), chemokines, surface adhesion molecules, and other enzymes such cyclooxygenase-2 (COX-2). Interestingly, the redox-sensitive NF-κB can activate endothelial cells, leukocytes, and platelets expressing adhesion molecules and proinflammatory cytokines and contribute to the endothelial dysfunction and other cardiovascular morbidities that have been ascribed to long-term IH (21).
Metabolic consequences.
An increased risk for developing metabolic syndrome has been widely associated with the presence of OSA in obese adults and children (20, 37, 50, 62, 126, 139, 162, 183, 185). For example, when comparing obese people with and without OSA, Vgontzas et al. (190) showed that the major difference between both groups was the higher amount of visceral fat depots in patients with OSA. This increase in visceral fat content was strongly correlated with the degree of respiratory disturbance or severity of nocturnal intermittent hypoxemia (190). However, the potential mechanisms implicated in the pathogenesis of metabolic syndrome in the context of OSA and IH remain incompletely defined in part due to the high prevalence of obesity in patients with OSA. Therefore, the use of animal models has been key to better understand the role of IH for each of the metabolic alterations encompassed in the context of metabolic syndrome. In a mouse model of OSA, IH exposures induced dyslipidemia by upregulating lipid biosynthesis in the liver through HIF activation, increasing lipolysis within the adipose tissue, and inhibiting lipoprotein clearance (47). In addition, the detrimental effects of IH on insulin sensitivity and glucose homeostasis have been illustrated, particularly in the concurrent presence of obesity (48, 73). Other studies carried out in cultured cells and reproduced in animals showed that IH-related intermediate mechanisms can alter insulin signaling in adipocytes (151). Also, IH stimulates pancreatic β-cell replication (203) via upregulation of Reg family genes and the hepatocyte growth factor gene (135). Similarly, coinciding with the increase in proliferation, the subcellular localization of the cell cycle regulator cyclin D2 was increased in the nucleus of pancreatic β-cells (200). In addition, pancreatic β-cell death was increased approximately fourfold. However, manganese superoxide dismutase transgenic overexpression did not alter the effects of IH on β-cell proliferation but completely abrogated the IH effects on cell death. Reinke et al. (70) reported that IH resulted in lower levels of tissue oxygenation, promoting insulin resistance in both lean and obese mice. In lean mice, IH increased serum leptin levels, oxidative stress markers, and adipose tissue inflammation (152). Similarly, Polak et al. (144, 194) found that 14 days of IH induced insulin resistance, impaired β-cell function, enhanced hepatocyte glucose output, and increased oxidative stress in the pancreas. However, those reported changes were not completely reversed by cessation of IH (144, 194). An additional aspect of IH is that it is associated with gestation, where it has been found to increase the risk of the offspring to metabolic diseases developing during adulthood (74). Cumulatively, the data suggest that IH can promote some components of the metabolic syndrome, playing an independent cofactor role in conjunction with obesity. However, we need to again emphasize that IH-reported effects could depend on the duration, severity, and frequency of IH. For instance, different frequencies of IH resulted in divergent patterns of metabolic dysfunction (29, 152).
Cardiovascular consequences.
Patients with OSA exhibit significantly higher risk of hypertension, arrhythmia, and myocardial infarction (131, 173). From murine models, it is well known that long-term IH can promote increased blood pressure, biventricular hypertrophy, left ventricle contractile dysfunction (30, 32, 54, 56, 198), and infarction (77) mainly through the previously mentioned ROS-mediated mechanisms and/or peripheral chemoreceptors (57). IH sympathetic-induced activation, alterations in carotid body, reduced NO bioavailability, and/or increased myogenic tone have been proposed as potential mechanisms promoting hypertension (75, 97, 134, 148). Similar to IH and metabolic disorders (145), short-term exposures to IH in rats (1 and 4 days) seem to be protective (95). However, longer periods (>1 wk of IH exposures) resulted in detrimental effects. The outcomes observed by different authors are highly varied and likely depend on the particular IH parameters employed, such as duration, frequency, and severity of hypoxia. With consideration of these factors, long-term IH exposures in murine models showed increased right ventricular systolic pressure, right ventricular mass, neovascularization of distal pulmonary vessels (54), cardiomyocyte diameter (133), systemic and pulmonary vascular pressures (24, 132), interstitial space (33), cardiac hypertrophy, cardiac and perivascular fibrosis (133), inflammation and dysfunction (198), and a decrease in endothelial nitric oxide synthase (eNOS) expression in ventricular and aortic tissues (133, 192).
Those adverse cardiovascular effects are not limited only to the heart. In addition to hypertension, IH can promote vascular dysfunction, which has been associated with a higher risk of atherosclerosis (49) and stroke in patients with OSA (40). The development of endothelial dysfunction has been ascribed to a variety of mechanisms including oxidative stress, inflammation, growth factors, adhesion molecules, as well as some of the pathways described above. A possible relationship between endothelial dysfunction and IH involves excessive formation and propagation of ROS species (46, 93). In fact, patients with OSA present with attenuated expression of eNOS and an increased expression of nitrotyrosine (an oxidative stress marker) in endothelial cells (76). Interestingly, treatment with continuous positive airway pressure (CPAP) for 4 wk was sufficient to improve endothelial function and reduce oxidative stress (76). Similarly, Del Ben et al. (43) showed higher levels of urinary 8-iso-PGF-2α and serum-soluble NADPH oxidase 2-derived peptide with lower NO metabolites nitrite and nitrate (NOx) in patients with severe OSA (43). They also found a negative association between flow-mediated brachial artery dilation and OSA severity. After 6 mo of CPAP treatment, oxidative stress and arterial dysfunction were partially reversed (43). In rats, inhibition of xanthine oxidase, a major source of superoxide in endothelium, is able to attenuate the endothelial dysfunction caused by IH (46).
Arterioles and pulmonary arteries from rats exposed to IH were more sensitive to endothelin-1 (ET-1) suggesting an impaired endothelium-dependent vasodilation and increased vasoconstrictor responsiveness (2, 181, 196). Allahdadi et al. (1, 3) suggested that hypertension derived from exposure to IH seems to cause alterations in ET-1 signaling, increasing calcium sensitivity during ET-A activation. This phenomenon, which is similar to that experienced in patients with SA (79, 83, 90), appears to involve PKC-β that mediates the augmented vasoconstrictor reactivity to ET-1 in the pulmonary circulation of IH rats (180). This is in contrast to the role of PKC-δ in systemic arteries (2). Capone et al. (27) showed that mice exposed to chronic IH exhibit altered key regulatory mechanisms of the cerebral circulation through ET-1 and NADPH oxidase-derived radicals. Also, a recent study by Marcus and colleagues (112) proposed that endothelial dysfunction associated with IH can be also dependent, at least in part, on renin-angiotensin system signaling (112). Dematteis et al. (44) found early functional cardiovascular remodeling in mice in response to IH presenting as delayed changes in peripheral vasoreactivity. However, Julien and collaborators (78) revealed that vasoconstriction is enhanced in mice exposed to IH but that no dysfunction of endothelium-relaxation occurred. In addition, IH can promote vessel inflammation, enhancing the progression of atherosclerosis, which has been suggested to be mediated by upregulation of inflammatory adhesion molecules (13, 92, 94). Cell type regulated on activation normal T cell expressed and secreted (RANTES)/CCL5 has been proposed as a determinant cytokine for IH-induced preatherosclerotic remodeling (12). In cerebral cortex, IH-induced ROS can produce important alterations of cerebrovascular regulation via endothelium-dependent vasodilation and neurovascular coupling, which seems mediated in part by increased levels of ET-1 (27). Phillips et al. (142) showed that chronic IH can impair endothelium-dependent dilation in cerebral and skeletal muscle resistance arteries in rats. Although OSA has been associated with endothelial dysfunction independently of other confounding factors such as obesity (124), it is well known that some metabolites released in the context of IH could also indirectly participate in this process. Also, experimental data obtained from in vitro and in vivo skin biopsies of patients with OSA showed a differential gene expression profile according to severity of hypoxemia and related to OSA-induced vascular dysfunction (80).
Recently, substantial interest has emerged in relation to the possibility that IH may harness the recruitment of progenitor and stem cell populations. We have previously shown that IH triggers the mobilization of some bone marrow-derived stem populations including very small embryonic-like pluripotent stem cells in mice (58). Similarly, in humans, bone-marrow derived hematopoietic stem cells are activated and appear in the circulation (172). In addition, acute exposures to recurrent apneas in rats promote the mobilization of bone-marrow mesenchymal stem cells (28). However, the implications of the increased number of progenitor cells are unclear. In patients with OSA, changes in endothelial progenitor cells (EPCs) (104) have emerged and been correlated to endothelial dysfunction (86). Current assumptions posit that EPCs could be released during acute IH exposures and facilitate repair mechanisms involved in restoration of endothelial injury but could also act as modulators of the inflammatory response. EPCs from healthy individuals were increased after exposures to intermittent hypoxia in vitro (19). However, the increase in EPCs in patients with OSA has been somewhat inconsistently reported. It is probable that the prolonged exposure to IH, either alone or in coexistence with underlying comorbidities, may have affected the global densities of stem cell populations in the circulation. Thus the high complexity of OSA in the context of time, severity, and coexistence of other diseases may account for the incongruence of findings and the controversy about the role of EPCs in the circulation of these patients (4, 19).
Cognitive consequences.
Increased awareness to the potential neurocognitive consequences of episodic hypoxia led to development of animal models aiming at replicating the cardiorespiratory and sleep patterns of patients with OSA, such as to enable investigation of the more specific roles played by each of these abnormalities on the CNS. Indeed, SA has been associated with a broad range of neurocognitive difficulties, including excessive daytime sleepiness, personality and psychosocial maladjustment patterns, and mental impairments in both adults and children (16, 84, 87), which have been corroborated by imaging evidence of structural and functional derangements (38, 61, 105). Regional reductions in gray matter that improved after CPAP treatment along with parallel amelioration in memory, attention, and executive functioning have also been reported (26).
Gozal and colleagues (63) reasoned that IH exposures would elicit the oxyhemoglobin desaturation patterns that closely resemble the patients with moderate to severe OSA, such that inferences could then be made on the anatomical and functional pathologies that such exposures would elicit. With the use of this IH paradigm in rats, exposures for 14 days to an IH profile consisting of alternating 90-s epochs of hypoxia (10% O2) and room air during the habitual sleep times induced substantial impairments on a hippocampal-dependent learning task, the spatial reference version of the Morris water maze (63). In contrast, no alterations emerged in IH-exposed animals on a simple cued version water maze task, indicating that these impairments could not be ascribed to alterations in sensorimotor function. Since these initial findings, multiple studies have confirmed that both rats and mice display cognitive deficits consistent with impaired functioning of the hippocampus and/or prefrontal cortex following exposures to IH (42, 157, 159). This suggests a reduced probability for long-term potentiation of field-evoked potentials following a titanic pulse train stimulation of Shaffer collaterals in the CA1 region of the hippocampus (138), where alterations in G protein-mediated signal transduction contribute to IH-related hippocampal dysfunction (67).
In developing rats, exposures to IH from postnatal day 10 to 25, a developmental period that corresponds to the peak prevalence of OSA in children, was not only associated with prominent neurobehavioral deficits in the spatial reference version of the Morris water maze (159) but also coincided with unique susceptibility to IH as reported by neuronal apoptosis cell counts (65). Furthermore, another period of increased vulnerability to IH occurs during aging, whereby aging rats exposed to room air or IH displayed significant spatial learning impairments compared with similarly exposed young rats, but decrements in performance associated with IH conditions were markedly greater in the aging rats (64). IH during sleep induced decreases in proteasomal activity that were particularly pronounced in the aging animals, indicating the formation of protein aggregates and attendant neuronal cell dysfunction and increased apoptosis.
Increased expression of oxidative stress markers occurs in the brains of rodents exposed to IH (150, 160, 174, 201) and has been mechanistically implicated in the cognitive and behavioral deficits described heretofore (120, 195). In addition, increased inflammation as evidenced by increased prostaglandin E2 neural tissue concentrations, a marker for the expression and activity of the inflammatory protein COX-2, is evident in hippocampal and cortical regions following exposures to IH (100) and also accompanied by lipid peroxidation of polyunsaturated fatty acids. In addition, increased carbonylation and nitrosylation-induced oxidative injury emerges in IH-susceptible brain regions and promotes increased somnolence (166, 205). Although the exact cellular sources of such IH-induced detrimental effects are still incompletely defined, it is likely that a portion of such deleterious effects is ascribable to activation of astroglia and subsequent loss of buffering functions that ultimately contribute to pathological processes, such as increased glial proliferation and microglial activation (63, 179). Astroglial and microglial cells play critical roles in regional blood flow regulation and inflammatory processes in the brain as well as critical coordination of bioenergetics through lactate transport (22, 140, 155). Activated microglia express high levels of the inducible isoform of NOS (iNOS) and COX-2, with both enzymes initiating pathways ultimately leading to generation and propagation of ROS. Furthermore, cytokine release magnifies the production of ROS superoxide and hydrogen peroxide as well as the activity of iNOS and COX-2 by microglia, thus perpetuating inflammation and aggravating ongoing oxidative stress (66). Consistent with these findings, experiments in a murine model of IH demonstrated that both pharmacological and genetic inhibition of iNOS afforded protection against IH-induced learning deficits (101), and pharmacological inhibition of COX-2 markedly attenuated IH-induced spatial learning deficits in rats (158).
There is now compelling support for the role played by the pro- and antioxidant cellular systems in neuronal injury associated with IH. In addition to the aforementioned considerations, we should note that transgenic mice overexpressing Cu,Zn-superoxide dismutase that were exposed to chronic IH conditions had a lower level of steady-state ROS production and reduced neuronal apoptosis compared with wild-type mice (174). Furthermore, NADPH oxidase-dependent production of superoxide radical (O2·−) has been identified as a major contributor to oxidative injury in the brain and other target organs under conditions of inflammation and severe hypoxia. Long-term IH increases the expression of NADPH oxidase (i.e., its p47phox subunit), the major enzyme underlying oxygen radical production, suggesting that activation of NADPH oxidase may, at least in part, underlie the increased neuronal inflammation and oxidative stress observed in animal models of OSA (120, 121, 123, 206).
Alterations in neurotransmitter systems may also play a role in the neurobehavioral disturbances seen after IH exposures and have implicated disruption of norepinephrine and dopaminergic pathways in the development of hyperactivity and working memory dysfunction (42, 85, 98, 159). The available evidence suggests that dysregulation of dopaminergic function is of significance in the context of IH-induced neurobehavioral deficits (156).
Cancer.
IH is becoming a focus of great interest in cancer research because most solid tumor types develop intratumoral IH in the context of episodic changes in vascular supply to rapidly proliferating tumor regions (114, 154, 184). In two recent seminal epidemiological studies, OSA has been associated with an increased incidence (25) and enhanced mortality (130) in cancer. In this context, the cyclical hypoxia that characterizes OSA has been proposed as the major correlate of processes involving tumor invasion and metastasis (96, 154). Previous studies in mice showed that IH exposures lead to accelerated melanoma tumor growth (6) and metastatic potential (8), thereby lending biological plausibility to the epidemiological studies and further supporting the putative role of episodic hypoxia in tumor biology (136, 170, 171). Specifically, IH was able to accelerate the tumor growth in a melanoma model and also increased the metastatic potential from two different experimental metastatic models (intravenous and subcutaneous tumor injection). Although the preliminary data suggest that hypoxia-inducible genes could participate in the increased levels of VEGF and therefore in tumor vascularization (7), the potential mechanisms involved in IH-induced changes in tumor growth remain to be explored in further studies. Very recent work from our laboratory further suggests that IH-induced alterations in innate immunity, more specifically in tumor-associated macrophage polarity may account for some of the changes in tumor biology (10).
Summary
The current evidence supports the notion that IH elicits divergent responses that are stringently dependent on the contextual setting in which IH occurs. The characteristics of IH exposures, particularly focused around severity of hypoxia, duration, and cycle frequency, emerge as the fundamental determinants of IH-related outcomes. As such, short, mild, and lower cycle frequency would be generally anticipated to generate beneficial and adaptive responses, whereas chronic, moderate to severe, and high frequency IH will induce maladaptive disruption of homeostatic mechanisms, leading to end-organ dysfunction (Fig. 2). Efforts are clearly needed to refine and delineate the specific characteristics of IH that account for the wide spectrum and divergence of responses to harness the potential benefits of IH while developing therapeutic targets against its deleterious consequences.
GRANTS
D. Gozal is supported by National Institutes of Health grants HL-65270, HL-086662, and HL-107160. I. Almendros is supported by Beatriu de Pinós fellowship from Generalitat de Catalunya (2010 BP_A 00238).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.A. prepared figures; I.A. and Y.W. drafted manuscript; I.A., Y.W., and D.G. approved final version of manuscript; D.G. edited and revised manuscript.
REFERENCES
- 1. Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol 295: H434–H440, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol 294: H920–H927, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45: 705–709, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Almendros I, Carreras A, Montserrat JM, Gozal D, Navajas D, Farre R. Potential role of adult stem cells in obstructive sleep apnea. Front Neurol 3: 112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep 34: 1127–1133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Almendros I, Montserrat JM, Ramirez J, Torres M, Duran-Cantolla J, Navajas D, Farre R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J 39: 215–217, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Almendros I, Montserrat JM, Torres M, Bonsignore MR, Chimenti L, Navajas D, Farre R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med 13: 1254–1260, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Almendros I, Montserrat JM, Torres M, Dalmases M, Cabanas ML, Campos-Rodriguez F, Navajas D, Farre R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol 186: 303–307, 2013 [DOI] [PubMed] [Google Scholar]
- 9. Almendros I, Montserrat JM, Torres M, Gonzalez C, Navajas D, Farre R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res 11: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, Schoenfelt KS, Carreras A, Hakim F, Zhang SX, Farre R, Gozal D. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med 189: 593–601, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Angermuller S, Islinger M, Volkl A. Peroxisomes and reactive oxygen species, a lasting challenge. Histochem Cell Biol 131: 459–463, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Arnaud C, Beguin PC, Lantuejoul S, Pepin JL, Guillermet C, Pelli G, Burger F, Buatois V, Ribuot C, Baguet JP, Mach F, Levy P, Dematteis M. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med 184: 724–731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arnaud C, Poulain L, Levy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis 219: 425–431, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 61: 461–470, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Beguin PC, Belaidi E, Godin-Ribuot D, Levy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol 42: 343–351, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Beguin PC, Joyeux-Faure M, Godin-Ribuot D, Levy P, Ribuot C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J Appl Physiol 99: 1064–1069, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Berger S, Aronson D, Lavie P, Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med 187: 90–98, 2013 [DOI] [PubMed] [Google Scholar]
- 20. Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev 22: 353–364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosc LV, Resta T, Walker B, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med 14: 3–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brix B, Mesters JR, Pellerin L, Johren O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1alpha-mediated target gene activation. J Neurosci 32: 9727–9735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Pena ML, Masdeu MJ, Gonzalez M, Campo F, Gallego I, Marin JM, Barbe F, Montserrat JM, Farre R. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 187: 99–105, 2013 [DOI] [PubMed] [Google Scholar]
- 26. Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, Alemanno F, Ferini-Strambi L. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 183: 1419–1426, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension 60: 106–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farre R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep 32: 117–119, 2009 [PMC free article] [PubMed] [Google Scholar]
- 29. Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol 303: R700–R709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Chen L, Zhang J, Gan TX, Chen-Izu Y, Hasday JD, Karmazyn M, Balke CW, Scharf SM. Left ventricular dysfunction and associated cellular injury in rats exposed to chronic intermittent hypoxia. J Appl Physiol 104: 218–223, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Chen LM, Kuo WW, Yang JJ, Wang SG, Yeh YL, Tsai FJ, Ho YJ, Chang MH, Huang CY, Lee SD. Eccentric cardiac hypertrophy was induced by long-term intermittent hypoxia in rats. Exp Physiol 92: 409–416, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning's success. Basic Res Cardiol 103: 464–471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. Essays Biochem 43: 1–15, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem 281: 20801–20808, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 25: 735–741, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Cross RL, Kumar R, Macey PM, Doering LV, Alger JR, Yan-Go FL, Harper RM. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep 31: 1103–1109, 2008 [PMC free article] [PubMed] [Google Scholar]
- 39. Dale EA, Ben MF, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Das AM, Khan M. Obstructive sleep apnea and stroke. Expert Rev Cardiovasc Ther 10: 525–535, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, Gozal D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci 13: 77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Decker MJ, Hue GE, Caudle WM, Miller GW, Keating GL, Rye DB. Episodic neonatal hypoxia evokes executive dysfunction and regionally specific alterations in markers of dopamine signaling. Neuroscience 117: 417–425, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Del BM, Fabiani M, Loffredo L, Polimeni L, Carnevale R, Baratta F, Brunori M, Albanese F, Augelletti T, Violi F, Angelico F. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med 12: 36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 177: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13: 385–391, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Dopp JM, Philippi NR, Marcus NJ, Olson EB, Bird CE, Moran JJ, Mueller SW, Morgan BJ. Xanthine oxidase inhibition attenuates endothelial dysfunction caused by chronic intermittent hypoxia in rats. Respiration 82: 458–467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24: 843–851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 19: 2167–2174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 140: 534–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 62: 569–576, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Dufour SP, Ponsot E, Zoll J, Doutreleau S, Lonsdorfer-Wolf E, Geny B, Lampert E, Fluck M, Hoppeler H, Billat V, Mettauer B, Richard R, Lonsdorfer J. Exercise training in normobaric hypoxia in endurance runners. I. Improvement in aerobic performance capacity. J Appl Physiol 100: 1238–1248, 2006 [DOI] [PubMed] [Google Scholar]
- 53. El-Khoury R, Bradford A, O'Halloran KD. Chronic hypobaric hypoxia increases isolated rat fast-twitch and slow-twitch limb muscle force and fatigue. Physiol Res 61: 195–201, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Fagan KA. Selected Contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 90: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Fletcher EC, Lesske J, Behm R, Miller CCIII, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72: 1978–1984, 1992 [DOI] [PubMed] [Google Scholar]
- 58. Gharib SA, Dayyat EA, Khalyfa A, Kim J, Clair HB, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep 33: 1439–1446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gharib SA, Khalyfa A, Kucia MJ, Dayyat EA, Kim J, Clair HB, Gozal D. Transcriptional landscape of bone marrow-derived very small embryonic-like stem cells during hypoxia. Respir Res 12: 63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gorlach A, Bonello S. The cross-talk between NF-kappaB and HIF-1: further evidence for a significant liaison. Biochem J 412: e17-e19, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Gozal D. CrossTalk proposal: the intermittent hypoxia attending severe obstructive sleep apnoea does lead to alterations in brain structure and function. J Physiol 591: 379–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 177: 1142–1149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem 86: 1545–1552, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett 305: 197–201, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem Sci 31: 509–515, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Hambrecht VS, Vlisides PE, Row BW, Gozal D, Baghdoyan HA, Lydic R. Hypoxia modulates cholinergic but not opioid activation of G proteins in rat hippocampus. Hippocampus 17: 934–942, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Hausenloy DJ, Wynne AM, Yellon DM. Ischemic preconditioning targets the reperfusion phase. Basic Res Cardiol 102: 445–452, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82: 104–113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Holliss BA, Fulford J, Vanhatalo A, Pedlar CR, Jones AM. Influence of intermittent hypoxic training on muscle energetics and exercise tolerance. J Appl Physiol 114: 611–619, 2013 [DOI] [PubMed] [Google Scholar]
- 71. Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Pahlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 10: 413–423, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Hoth KF, Zimmerman ME, Meschede KA, Arnedt JT, Aloia MS. Obstructive sleep apnea: impact of hypoxemia on memory. Sleep Breath 17: 811–817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Iqbal W, Ciriello J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol 2013 [DOI] [PubMed] [Google Scholar]
- 75. Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res 108: 1439–1447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med 18: 253–260, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Joyeux-Faure M, Stanke-Labesque F, Lefebvre B, Beguin P, Godin-Ribuot D, Ribuot C, Launois SH, Bessard G, Levy P. Chronic intermittent hypoxia increases infarction in the isolated rat heart. J Appl Physiol 98: 1691–1696, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Julien C, Bayat S, Levy P. Vascular reactivity to norepinephrine and acetylcholine after chronic intermittent hypoxia in mice. Respir Physiol Neurobiol 139: 21–32, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Jurado-Gamez B, Fernandez-Marin MC, Gomez-Chaparro JL, Munoz-Cabrera L, Lopez-Barea J, Perez-Jimenez F, Lopez-Miranda J. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur Respir J 37: 873–879, 2011 [DOI] [PubMed] [Google Scholar]
- 80. Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, Tecilazich F, O'Donnell CP, Ferran C, Malhotra A. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One 8: e70559, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Katayama K, Matsuo H, Ishida K, Mori S, Miyamura M. Intermittent hypoxia improves endurance performance and submaximal exercise efficiency. High Alt Med Biol 4: 291–304, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Katayama K, Shima N, Sato Y, Qiu JC, Ishida K, Mori S, Miyamura M. Effect of intermittent hypoxia on cardiovascular adaptations and response to progressive hypoxia in humans. High Alt Med Biol 2: 501–508, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 102: 2607–2610, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Kheirandish L, Gozal D. Neurocognitive dysfunction in children with sleep disorders. Dev Sci 9: 388–399, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res 58: 594–599, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Kheirandish-Gozal L, Bhattacharjee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med 182: 92–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kielb SA, Ancoli-Israel S, Rebok GW, Spira AP. Cognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatment. Neuromolecular Med 14: 180–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kitaev MI, Aitbaev KA, Liamtsev VT. [Effect of hypoxic hypoxia on development of atherosclerosis in rabbits]. Aviakosm Ekolog Med 33: 54–57, 1999 [PubMed] [Google Scholar]
- 89. Kolar F, Neckar J, Ostadal B. MCC-134, a blocker of mitochondrial and opener of sarcolemmal ATP-sensitive K+ channels, abrogates cardioprotective effects of chronic hypoxia. Physiol Res 54: 467–471, 2005 [PubMed] [Google Scholar]
- 90. Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol 89: 493–498, 2000 [DOI] [PubMed] [Google Scholar]
- 91. Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, Takeuchi T. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem 285: 667–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin 23: 1059–1075, 2005 [DOI] [PubMed] [Google Scholar]
- 93. Lavie L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front Biosci 4: 1391–1403, 2012 [DOI] [PubMed] [Google Scholar]
- 94. Lavie L, Dyugovskaya L, Lavie P. Sleep-apnea-related intermittent hypoxia and atherogenesis: adhesion molecules and monocytes/endothelial cells interactions. Atherosclerosis 183: 183–184, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Lee SD, Kuo WW, Wu CH, Lin YM, Lin JA, Lu MC, Yang AL, Liu JY, Wang SG, Liu CJ, Chen LM, Huang CY. Effects of short- and long-term hypobaric hypoxia on Bcl2 family in rat heart. Int J Cardiol 108: 376–384, 2006 [DOI] [PubMed] [Google Scholar]
- 96. Lee SL, Rouhi P, Dahl JL, Zhang D, Ji H, Hauptmann G, Ingham P, Cao Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA 106: 19485–19490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997 [DOI] [PubMed] [Google Scholar]
- 98. Li R, Bao G, el-Mallakh RS, Fletcher EC. Effects of chronic episodic hypoxia on monoamine metabolism and motor activity. Physiol Behav 60: 1071–1076, 1996 [DOI] [PubMed] [Google Scholar]
- 99. Li RC, Guo SZ, Raccurt M, Moudilou E, Morel G, Brittian KR, Gozal D. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 196: 237–250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR, Jr, Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 168: 469–475, 2003 [DOI] [PubMed] [Google Scholar]
- 101. Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis 17: 44–53, 2004 [DOI] [PubMed] [Google Scholar]
- 102. Lin AM, Dung SW, Chen CF, Chen WH, Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann NY Acad Sci 993: 168–178, 2003 [DOI] [PubMed] [Google Scholar]
- 103. Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lui MM, Tse HF, Mak JC, Lam JC, Lam DC, Tan KC, Ip MS. Altered profile of circulating endothelial progenitor cells in obstructive sleep apnea. Sleep Breath 17: 937–942, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 166: 1382–1387, 2002 [DOI] [PubMed] [Google Scholar]
- 106. MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. MacFarlane PM, Vinit S, Mitchell GS. Spinal nNOS regulates phrenic motor facilitation by a 5-HT2B receptor- and NADPH oxidase-dependent mechanism. Neuroscience 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 110. Manukhina EB, Belkina LM, Terekhina OL, Abramochkin DV, Smirnova EA, Budanova OP, Mallet RT, Downey HF. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp Biol Med (Maywood) 238: 1413–1420, 2013 [DOI] [PubMed] [Google Scholar]
- 111. Manukhina EB, Jasti D, Vanin AF, Downey HF. Intermittent hypoxia conditioning prevents endothelial dysfunction and improves nitric oxide storage in spontaneously hypertensive rats. Exp Biol Med (Maywood) 236: 867–873, 2011 [DOI] [PubMed] [Google Scholar]
- 112. Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, Morgan BJ. Effect of AT1 receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir Physiol Neurobiol 183: 67–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martin N, Bossenmeyer-Pourie C, Koziel V, Jazi R, Audonnet S, Vert P, Gueant JL, Daval JL, Pourie G. Non-injurious neonatal hypoxia confers resistance to brain senescence in aged male rats. PLoS One 7: e48828, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martinive P, Defresne F, Bouzin C, Saliez J, Lair F, Gregoire V, Michiels C, Dessy C, Feron O. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for anticancer therapies. Cancer Res 66: 11736–11744, 2006 [DOI] [PubMed] [Google Scholar]
- 115. McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol 95: 1499–1508, 2003 [DOI] [PubMed] [Google Scholar]
- 116. Meerson FZ, Ustinova EE, Orlova EH. Prevention and elimination of heart arrhythmias by adaptation to intermittent high altitude hypoxia. Clin Cardiol 10: 783–789, 1987 [DOI] [PubMed] [Google Scholar]
- 117. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 118. Naghshin J, McGaffin KR, Witham WG, Mathier MA, Romano LC, Smith SH, Janczewski AM, Kirk JA, Shroff SG, O'Donnell CP. Chronic intermittent hypoxia increases left ventricular contractility in C57BL/6J mice. J Appl Physiol 107: 787–793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Naghshin J, Rodriguez RH, Davis EM, Romano LC, McGaffin KR, O'Donnell CP. Chronic intermittent hypoxia exposure improves left ventricular contractility in transgenic mice with heart failure. J Appl Physiol 113: 791–798, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 6: e19847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nair D, Ramesh V, Gozal D. Adverse cognitive effects of high-fat diet in a murine model of sleep apnea are mediated by NADPH oxidase activity. Neuroscience 227: 361–369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nair D, Ramesh V, Li RC, Schally AV, Gozal D. Growth hormone releasing hormone (GHRH) signaling modulates intermittent hypoxia-induced oxidative stress and cognitive deficits in mouse. J Neurochem 127: 531–540, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, Gozal D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med 184: 1305–1312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Namtvedt SK, Hisdal J, Randby A, Agewall S, Stranden E, Somers VK, Rosjo H, Omland T. Impaired endothelial function in persons with obstructive sleep apnoea: impact of obesity. Heart 99: 30–34, 2013 [DOI] [PubMed] [Google Scholar]
- 125. Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA 106: 1199–1204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nannapaneni S, Ramar K, Surani S. Effect of obstructive sleep apnea on type 2 diabetes mellitus: A comprehensive literature review. World J Diabetes 4: 238–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Neckar J, Papousek F, Novakova O, Ost'adal B, Kolar F. Cardioprotective effects of chronic hypoxia and ischaemic preconditioning are not additive. Basic Res Cardiol 97: 161–167, 2002 [DOI] [PubMed] [Google Scholar]
- 128. Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med 187: 535–542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nichols NL, Johnson RA, Satriotomo I, Mitchell GS. Neither serotonin nor adenosine-dependent mechanisms preserve ventilatory capacity in ALS rats. Respir Physiol Neurobiol 197: 19–28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 186: 190–194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA 283: 1829–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 132. Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nishioka S, Yoshioka T, Nomura A, Kato R, Miyamura M, Okada Y, Ishizaka N, Matsumura Y, Hayashi T. Celiprolol reduces oxidative stress and attenuates left ventricular remodeling induced by hypoxic stress in mice. Hypertens Res 36: 934–939, 2013 [DOI] [PubMed] [Google Scholar]
- 134. Norton CE, Jernigan NL, Kanagy NL, Walker BR, Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J Appl Physiol 111: 980–988, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Miyaoka T, Fujimura T, Tsujinaka H, Yoshimoto K, Nakagawara K, Tamaki S, Takasawa S, Kimura H. Pancreatic beta cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci 93: 664–672, 2013 [DOI] [PubMed] [Google Scholar]
- 136. Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 18: 1207–1213, 2012 [DOI] [PubMed] [Google Scholar]
- 137. Park AM, Nagase H, Vinod KS, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol Heart Circ Physiol 292: H751–H757, 2007 [DOI] [PubMed] [Google Scholar]
- 138. Payne RS, Goldbart A, Gozal D, Schurr A. Effect of intermittent hypoxia on long-term potentiation in rat hippocampal slices. Brain Res 1029: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 139. Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, Kramer MR. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med 101: 1696–1701, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int 43: 331–338, 2003 [DOI] [PubMed] [Google Scholar]
- 141. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 286: H388–H393, 2004 [DOI] [PubMed] [Google Scholar]
- 143. Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 180: 1002–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 144. Polak J, Shimoda LA, Drager LF, Undem C, McHugh H, Polotsky VY, Punjabi NM. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: Partial improvement with cessation of the exposure. Sleep 36: 1483–1490, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90: 1986–1994, 2001 [DOI] [PubMed] [Google Scholar]
- 147. Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol 281: L524–L528, 2001 [DOI] [PubMed] [Google Scholar]
- 148. Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32: 447–449, 2005 [DOI] [PubMed] [Google Scholar]
- 149. Quintero M, Gonzalez-Martin MC, Vega-Agapito V, Gonzalez C, Obeso A, Farre R, Agapito T, Yubero S. The effects of intermittent hypoxia on redox status, NF-kappaB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic Biol Med 65: 1143–1154, 2013 [DOI] [PubMed] [Google Scholar]
- 150. Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem 93: 47–52, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Regazzetti C, Peraldi P, Gremeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58: 95–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol 111: 881–890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rodriguez FA, Casas H, Casas M, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Viscor G. Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc 31: 264–268, 1999 [DOI] [PubMed] [Google Scholar]
- 154. Rofstad EK, Gaustad JV, Egeland TA, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer 127: 1535–1546, 2010 [DOI] [PubMed] [Google Scholar]
- 155. Rosafio K, Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1alpha-mediated transcriptional regulation. Glia 62: 477–490, 2014 [DOI] [PubMed] [Google Scholar]
- 156. Row BW. Intermittent hypoxia and behavior: is dopamine to blame? Sleep 28: 165–167, 2005 [PubMed] [Google Scholar]
- 157. Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res 177: 308–314, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Row BW, Kheirandish L, Li RC, Guo SZ, Brittian KR, Hardy M, Bazan NG, Gozal D. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem 89: 189–196, 2004 [DOI] [PubMed] [Google Scholar]
- 159. Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002 [DOI] [PubMed] [Google Scholar]
- 160. Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003 [DOI] [PubMed] [Google Scholar]
- 161. Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 32: 150–157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ryan S, Crinion SJ, McNicholas WT. Obesity and sleep-disordered breathing—when two ‘bad guys’ meet. QJM 2014 [DOI] [PubMed] [Google Scholar]
- 163. Rybnikova E, Glushchenko T, Tyulkova E, Baranova K, Samoilov M. Mild hypobaric hypoxia preconditioning up-regulates expression of transcription factors c-Fos and NGFI-A in rat neocortex and hippocampus. Neurosci Res 65: 360–366, 2009 [DOI] [PubMed] [Google Scholar]
- 164. Rybnikova E, Vataeva L, Tyulkova E, Gluschenko T, Otellin V, Pelto-Huikko M, Samoilov MO. Mild hypoxia preconditioning prevents impairment of passive avoidance learning and suppression of brain NGFI-A expression induced by severe hypoxia. Behav Brain Res 160: 107–114, 2005 [DOI] [PubMed] [Google Scholar]
- 165. Sadoshima J. Redox regulation of growth and death in cardiac myocytes. Antioxid Redox Signal 8: 1621–1624, 2006 [DOI] [PubMed] [Google Scholar]
- 166. Sanfilippo-Cohn B, Lai S, Zhan G, Fenik P, Pratico D, Mazza E, Veasey SC. Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep 29: 152–159, 2006 [DOI] [PubMed] [Google Scholar]
- 167. Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 168. Schega L, Peter B, Torpel A, Mutschler H, Isermann B, Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology 59: 316–323, 2013 [DOI] [PubMed] [Google Scholar]
- 169. Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340, 2003 [DOI] [PubMed] [Google Scholar]
- 170. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33: 207–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123: 3664–3671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT, Ishchuk VA. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol 12: 243–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 174. Shan X, Chi L, Ke Y, Luo C, Qian S, Gozal D, Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis 28: 206–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Shortt CM, Fredsted A, Chow HB, Williams R, Skelly JR, Edge D, Bradford A, O'Halloran KD. Reactive oxygen species mediated diaphragm fatigue in a rat model of chronic intermittent hypoxia. Exp Physiol 99: 688–700, 2014 [DOI] [PubMed] [Google Scholar]
- 176. Skelly JR, Bradford A, O'Halloran KD. Intermittent hypoxia impairs pharyngeal dilator muscle function in male but not female rats. Adv Exp Med Biol 669: 285–287, 2010 [DOI] [PubMed] [Google Scholar]
- 177. Skelly JR, O'Connell RA, Jones JF, O'Halloran KD. Structural and functional properties of an upper airway dilator muscle in aged obese male rats. Respiration 82: 539–549, 2011 [DOI] [PubMed] [Google Scholar]
- 178. Skelly JR, Rowan SC, Jones JF, O'Halloran KD. Upper airway dilator muscle weakness following intermittent and sustained hypoxia in the rat: effects of a superoxide scavenger. Physiol Res 62: 187–196, 2013 [DOI] [PubMed] [Google Scholar]
- 179. Smith SM, Friedle SA, Watters JJ. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One 8: e81584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Snow JB, Gonzalez Bosc LV, Kanagy NL, Walker BR, Resta TC. Role for PKCβ in enhanced endothelin-1-induced pulmonary vasoconstrictor reactivity following intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 301: L745–L754, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 90: 2007–2013, 2001 [DOI] [PubMed] [Google Scholar]
- 182. Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82, 1997 [DOI] [PubMed] [Google Scholar]
- 183. Tkacova R, Dorkova Z, Molcanyiova A, Radikova Z, Klimes I, Tkac I. Cardiovascular risk and insulin resistance in patients with obstructive sleep apnea. Med Sci Monit 14: CR438–CR444, 2008 [PubMed] [Google Scholar]
- 184. Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J 275: 2991–3002, 2008 [DOI] [PubMed] [Google Scholar]
- 185. Trzepizur W, Le VM, Meslier N, Pigeanne T, Masson P, Humeau MP, Bizieux-Thaminy A, Goupil F, Chollet S, Ducluzeau PH, Gagnadoux F. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest 143: 1584–1589, 2013 [DOI] [PubMed] [Google Scholar]
- 186. Tsai YW, Yang YR, Chen GH, Chang HC, Wang RY. The time window of intermittent hypoxia intervention after middle cerebral artery occlusion. Chin J Physiol 51: 324–328, 2008 [PubMed] [Google Scholar]
- 187. Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS One 6: e24001, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci 81: 1223–1227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol 29: 2441–2450, 1997 [DOI] [PubMed] [Google Scholar]
- 190. Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85: 1151–1158, 2000 [DOI] [PubMed] [Google Scholar]
- 191. Viscor G, Javierre C, Pages T, Ventura JL, Ricart A, Martin-Henao G, Azqueta C, Segura R. Combined intermittent hypoxia and surface muscle electrostimulation as a method to increase peripheral blood progenitor cell concentration. J Transl Med 7: 91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang B, Yan B, Song D, Ye X, Liu SF. Chronic intermittent hypoxia down-regulates endothelial nitric oxide synthase expression by an NF-kappaB-dependent mechanism. Sleep Med 14: 165–171, 2013 [DOI] [PubMed] [Google Scholar]
- 193. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wang N, Khan SA, Prabhakar NR, Nanduri J. Impairment of pancreatic beta-cell function by chronic intermittent hypoxia. Exp Physiol 98: 1376–1385, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wang Z, Li AY, Guo QH, Zhang JP, An Q, Guo YJ, Chu L, Weiss JW, Ji ES. Effects of cyclic intermittent hypoxia on ET-1 responsiveness and endothelial dysfunction of pulmonary arteries in rats. PLoS One 8: e58078, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wang ZH, Chen YX, Zhang CM, Wu L, Yu Z, Cai XL, Guan Y, Zhou ZN, Yang HT. Intermittent hypobaric hypoxia improves postischemic recovery of myocardial contractile function via redox signaling during early reperfusion. Am J Physiol Heart Circ Physiol 301: H1695–H1705, 2011 [DOI] [PubMed] [Google Scholar]
- 198. Williams AL, Chen L, Scharf SM. Effects of allopurinol on cardiac function and oxidant stress in chronic intermittent hypoxia. Sleep Breath 14: 51–57, 2010 [DOI] [PubMed] [Google Scholar]
- 199. Xing T, Fong AY, Bautista TG, Pilowsky PM. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40: 602–609, 2013 [DOI] [PubMed] [Google Scholar]
- 200. Xu J, Long YS, Gozal D, Epstein PN. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med 46: 783–790, 2009 [DOI] [PubMed] [Google Scholar]
- 201. Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004 [DOI] [PubMed] [Google Scholar]
- 202. Xu WQ, Yu Z, Xie Y, Huang GQ, Shu XH, Zhu Y, Zhou ZN, Yang HT. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res Cardiol 106: 329–342, 2011 [DOI] [PubMed] [Google Scholar]
- 203. Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol 586: 899–911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med 171: 1414–1420, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 172: 921–929, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhang SX, Miller JJ, Gozal D, Wang Y. Whole-body hypoxic preconditioning protects mice against acute hypoxia by improving lung function. J Appl Physiol 96: 392–397, 2004 [DOI] [PubMed] [Google Scholar]
- 208. Zhang SX, Miller JJ, Stolz DB, Serpero LD, Zhao W, Gozal D, Wang Y. Type I epithelial cells are the main target of whole-body hypoxic preconditioning in the lung. Am J Respir Cell Mol Biol 40: 332–339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhang Y, Zhong N, Zhu HF, Zhou ZN. [Antiarrhythmic and antioxidative effects of intermittent hypoxia exposure on rat myocardium]. Sheng Li Xue Bao 52: 89–92, 2000 [PubMed] [Google Scholar]
- 210. Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1055: 1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 211. Zhuang J, Zhou Z. Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. Biol Signals Recept 8: 316–322, 1999 [DOI] [PubMed] [Google Scholar]
- 212. Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 263: 1353–1357, 1988 [PubMed] [Google Scholar]