Abstract
Tobacco smoke exposure, the major cause of chronic obstructive pulmonary disease (COPD), instigates a dysfunctional clearance of thick obstructive mucus. However, the mechanism underlying the formation of abnormally viscous mucus remains elusive. We investigated whether nicotine can directly alter the rheological properties of mucin by examining its physicochemical interactions with human airway mucin gels secreted from A549 lung epithelial cells. Swelling kinetics and multiple particle tracking were utilized to assess mucin gel viscosity change when exposed to nicotine. Herein we show that nicotine (≤50 nM) significantly hindered postexocytotic swelling and hydration of released mucins, leading to higher viscosity, possibly by electrostatic and hydrophobic interactions. Moreover, the close association of nicotine and mucins allows airway mucus to function as a reservoir for prolonged nicotine release, leading to correlated pathogenic effects. Our results provide a novel explanation for the maltransport of poorly hydrated mucus in smokers. More importantly, this study further indicates that even low-concentration nicotine can profoundly increase mucus viscosity and thus highlights the health risks of secondhand smoke exposure.
Keywords: mucin swelling kinetics, mucociliary clearance, viscosity, tobacco smoke, environmental tobacco smoke, chronic obstructive pulmonary disease
exposure to cigarette smoking or environmental tobacco smoke (ETS) is the predominant risk factor in developing chronic obstructive pulmonary disease (COPD) (12, 57). Conventional pathogenic theory considers smoking-induced chronic airway inflammation to be a vital component in the etiology of COPD (3, 9). Supporting clinical research data have indeed found a close association between the presence of inflammatory cells (2, 23), and abnormally high oxidative stress (9, 32), with disease progression. However, the assumption that airway inflammation accounts for all important pathological aspects risks oversimplification of the multifaceted nature of this disease and potentially limits the progress of COPD therapies (3, 12). Small airway occlusion with viscous mucus is one of the major clinical manifestations that is closely associated with accelerated decline in lung function, morbidity, and mortality in COPD patients (23–24, 55). Various models such as submucosal gland hypertrophy, hyperplasia, and metaplasia have been proposed that converge on mucin hypersecretory response, underhydration of airway surfaces, and an impaired mucociliary clearance (10, 15, 17, 29, 32, 42, 56). Hypersecretion of MUC5AC and 5B mucins has been shown to critically contribute toward COPD (28, 29). Excessive mucus built up in the airway surface layer may further disturb the periciliary liquid volume and ciliary beat frequency thus contributes to mucociliary clearance dysfunction (29, 38). All these results suggest that airway mucus plays a defining role in COPD pathophysiology (17, 38).
Aberrant mucin concentration due to the hypersecretion may lead to deviation from optimal mucus viscoelasticity and amplification of COPD-associated viscous obstructive mucus production (38, 56). Supporting this premise, macrorheological analysis of COPD sputum unveiled greater inherent viscosity (45). Although an increased release of the low-charge MUC5B in COPD patients has been postulated to form less-expanded networks that may be inefficiently transported (17, 28), it remains elusive how mucin oversecretion affects mucus rheology and disease development. More importantly, whether cigarette smoking can directly affect rheological properties of airway mucus is unknown. Nicotine, one of the major addictive constituents in tobacco, has been shown to elicit mucus hypersecretion (31), but the possibility that nicotine could directly alter the rheological properties of mucus by hampering hydration and increasing mucus viscosity has not, heretofore, been considered.
Mucus network has a characteristic tangled topology, the rheological properties of which are governed mainly by the density of mucin polymers that decreases with the square of the volume of the mucin matrix (54). Therefore, the degree of hydration primarily dictates mucus rheological properties (54). We utilized polymer-swelling kinetics to calculate the diffusivity of mucin matrices, which is closely related to mucin viscosity (13, 41, 49). The slow hydration rate of a mucin matrix (low mucin gel diffusivity) is associated with more viscous, poorly hydrated, and less-transportable mucus that characterizes the thick, viscous occlusion commonly found in COPD (13, 38).
Our research reports for the first time that nicotine can directly modulate the rheological properties of mucins released from airway cells through interacting with mucin matrices. This study provides much needed understanding of the interactions between nicotine and mucins that will, in turn, shed light on the pathogenesis of COPD and may facilitate the development of possible therapeutic interventions.
MATERIALS AND METHODS
A549 cell culture.
The human airway A549 was obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in F-12 medium (Invitrogen, Carlsbad, CA) supplemented with l-glutamine, penicillin/streptomycin, and 10% heat-inactivated fetal bovine serum (13). Cultures were incubated in a humidified incubator at 37°C/5% CO2.
Porcine primary airway epithelial cells.
The isolation and culture protocol of porcine primary airway epithelial cells was adapted from our previously published protocol (34). In brief, pig tracheas were obtained from a local slaughterhouse, washed quickly with ice-cold PBS, and transferred to the laboratory on ice in the HBSS buffer. Epithelial tissue was carefully removed on ice-cold HBSS used immediately for primary epithelial cell isolation.
Secretory granule labeling.
The presence of secretory granules of A549 cells was identified by staining with quinacrine (10 μM; Sigma-Aldrich, St. Louis, MO) (13). The nucleus was counterstained with Hoechst (1:1,000; Sigma-Aldrich) (13). Expression of MUC5AC in A549 cells was confirmed by immunostaining (data not shown).
Swelling kinetics and A549 cell preparation.
The experimental procedures follow the protocols published previously (13). In brief, A549 cells were detached and resuspended with HBSS (Invitrogen) in glass-bottom dishes (MatTek, Ashland, MA) and briefly equilibrated in 37°C incubator prior to adding nicotine (5 nM, 10 nM, 50 nM, 100 nM, and 1 μM) (Sigma-Aldrich). Both HBSS and nicotine were buffered with Tris·HCl/MES (Sigma-Aldrich) at pH 7.3 throughout the experiments (13). Degranulation of A549 cells was induced by 1 μM ionomycin (VWR, Brisbane, CA) (1, 13). To assess the swelling kinetics of nonpreloaded cells, A549 cells were bathed in HBSS containing 5 nM–1 μM nicotine throughout measurement. The nicotine-preloaded cells were evaluated by incubating with 10 nM–1 μM nicotine in HBSS for 15 min at 37°C followed by replenishment with fresh HBSS before stimulating exocytosis. To measure the swelling kinetics of nicotine-preloaded isolated granules, A549 secretory granules were extracted (according to our previous protocol), resuspended in intracellular buffer (maintained at pH 7.3 with Tris·HCl/MES), and allowed to settle on poly-l-lysine-coated dishes with 100 nM and 1 μM nicotine for 15 min at 37°C (34). Isolated granules were then replenished with fresh intracellular buffer before stimulation of mucin matrix release with 10 μM monensin (20) and 20 μM valinomycin (Sigma-Aldrich) (37). Video recordings of mucin network swelling were captured at 30 frames/s with a Nikon Eclipse TE2000U inverted microscope (Technical Instrument, Burlingame, CA), and all dishes were mounted on the 37°C thermoregulated stage.
Mucin viscosity determination by multiple particle tracking.
Particle transport rates were measured by analyzing trajectories of red-fluorescent carboxylate-modified microspheres (500 nm; Invitrogen) by using Auto Video Spot Tracker software (University of North Carolina, Chapel Hill, NC) and recorded by using a fast and highly sensitive Q-Imaging scientific camera (RETIGA-SRV, Technical Instruments) mounted on the Nikon Eclipse TE2000U microscope. The images were taken at room temperature, ∼30 frames/s for 5 s with a ×20 objective. Experiments were conducted in MatTek glass-bottom dishes where diluted particles solutions (1:500 vol/vol) were applied to 1% porcine gastric mucin (reconstructed in PBS, pH 7.3) (Sigma-Aldrich) and incubated for 20 min before microscopy. Trajectories of n ≥ 24 particles were analyzed for each experiment with different nicotine concentrations (50 nM–50 μM). The intensity-weighted centroid of each particle was tracked with ∼33-ms temporal resolution. The coordinates of microsphere centroids were transformed into time-averaged mean-square displacements (MSDs), <Δr2(τ)> = [x(t + τ) − x(t)]2 + [y(t + τ) − y(t)]2 (τ = time scale or time lag), from which distributions of MSDs and effective diffusivities <D(τ)> = <Δr2(τ)>/4τ were calculated (30). The viscosity values of mucin solutions were subsequently determined from the Stokes-Einstein equation D = KBT/6πηr (30).
Particle sizing and mucin quantification.
The aggregation of mucin gels was monitored with homodyne dynamics laser scattering using the goniometer of a Brookhaven laser spectrometer (Brookhaven Instruments, Holtville, NY) (13). Particle size distribution was calculated by the CONTIN method (13). Briefly, cultured A549 cells were stimulated with ionomycin to secrete mucin in HBSS at 37°C. Supernatant was filtered through a 5-μm PVDF syringe filter (Fisher Scientific, Pittsburgh, PA) into clean scintillating vials (VWR). Nicotine and/or EGTA (Sigma-Aldrich) filtered with a 0.22-μm syringe filter was then added to filtered mucin solution. Samples of human airway mucin were also prepared with 0.6 M NaCl containing HBSS. Mucin gel aggregation measurements were recorded at 0, 1, 24, 48, and 72 h, and gel dispersion induced by 2 mM EGTA was monitored at 74 and 96 h by detecting the scattering fluctuations at a 45° scattering angle. The pH was maintained with HBSS (Tris·HCl/MES, pH 7.3) during the experiments.
Filtered mucin was quantified by using human mucin-5 subtype AC (MUC5AC) ELISA kit (Novatein Biosciences, Cambridge, MA). MUC5AC assessment procedures were conducted in accord to the protocol provided by vendor. In brief, mucin samples were incubated in a 96-well kit at 37°C for 30 min followed by rinsing and additional incubation with horseradish peroxidase-conjugated antibody at 37°C for 30 min. After secondary rinsing, samples were reacted with substrates for 15 min at 37°C, terminated, and read at the optical density of 450 nm. A standard curve was used to calculate the mucin sample concentration.
Nicotine accumulation measurement.
Nicotine accumulation within isolated granules and granules in A549 cells was measured with fluorescence microscopy. A549 granules were isolated by using our previous protocol (34). Different nicotine concentrations (1 and 10 μM) were prepared in the intracellular buffer for isolated granules and in HBSS solution for A549 cells. Cells and isolated granules were carefully rinsed to remove remnants of background nicotine. The amount of nicotine accumulated was fluorescently measured by recording emitted fluorescence of nicotine (λex = 340 nm, λem = 398 nm) excited with UV at 0, 5, 15, 30, and 60 min (21). Nicotine standard was made in HBSS (Tris·HCl/MES, pH 7.3) containing 10 μM to 6 mM.
Mucin-controlled nicotine release measurement.
Samples of porcine gastric mucin at 1 and 2% (wt/vol; Sigma-Aldrich) were dissolved in HBSS (pH 7.3) and dialyzed (molecular weight cutoff <1,000; Spectrum Laboratories, Rancho Dominguez, CA) overnight in HBSS. Dialyzed gastric mucin samples were independently added with nicotine to reach a final concentration of 50 mM. The nicotine-containing mucin samples in dialysis tubes were then immersed in HBSS bathing solution. The concentration of nicotine diffused out into HBSS was determined at intervals of 0, 1, 5, 10, 15, 20, and 30 min with a spectrophotometer (at λabs = 290 nm). Values from control (nicotine-only) and nicotine-containing mucin samples were compared and the released nicotine concentrations were extrapolated from calibrations made (0–100 mM).
Statistical analysis.
Data were presented as means ± SE or means ± SD. Statistical analyses were carried out with Prism 4 and Instat 3 (GraphPad Software, San Diego, CA). Two-tailed Student's t-tests and one-way ANOVA analysis (Kruskal-Wallis test) were performed. The results were considered significantly different when P < 0.05.
RESULTS
Nicotine reduces mucin matrix hydration and increases mucin viscosity.
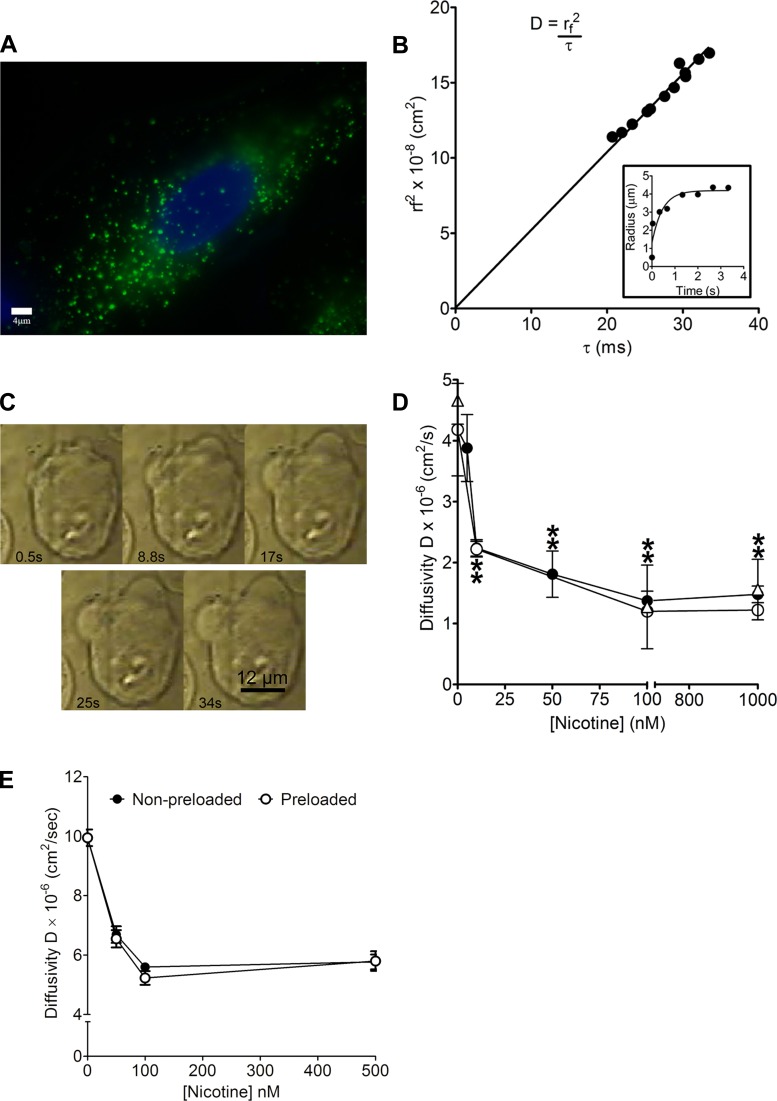
To study the effect of nicotine on mucin gel hydration we first measured the swelling kinetics of mucin matrices when exposed to nicotine of both intact and isolated granules of cultured A549 cells, a model human respiratory epithelium expressing representative mucins (13, 39, 58). Fluorescent quinacrine images (green) confirmed the presence of secretory granules in A549 cells (Fig. 1A). Figure 1B, inset shows a representative plot of mucin gel swelling from which we derived an expression for characteristic relaxation time that is proportional to the second power of the mucin matrix radius expansion (Fig. 1B). This correlation yields the hydrated mucin network diffusivity (D) (cm2/s), which is used to evaluate the impact of the surrounding solution's properties such as ionic species, organic polycations, and hydrophobic molecules on mucin swelling (Fig. 1B) (13, 54). Several phase-contrast images illustrating the process of mucin matrix gel expansion were also presented (Fig. 1C). We then conducted swelling kinetics experiments on mucin secreted from three separate A549 samples: nonpreloaded cells, preloaded cells, and preloaded isolated granules with nicotine. The resultant values of D in three experimental conditions are summarized in Table 1. The magnitude of D in nicotine preloaded cells and preloaded-isolated granules decreased by ∼70% compared with the control (HBSS) and intracellular buffer only correspondingly (Fig. 1D). Similarly, nonpreloaded cells exposed to 1 μM nicotine in HBSS resulted in a diminished D that is about 65% lower than in the control (Fig. 1D). We further investigated the effect of nicotine on mucin diffusivity from isolated primary porcine airway epithelial cells (nonpreloaded cells and preloaded cells). Data from Fig. 1E show that exposure to 100 nM nicotine in HBSS significantly decreased porcine mucin D by ∼45–50% in contrast to the control.
Fig. 1.
Identification of secretory granules and measurement of mucin matrix swelling kinetics. A: loading of quinacrine dye showed the presence of green secretory granules in the cytosol of A549 cells. The nucleus was stained blue with Hoechst dye. B: linear function between the characteristic time τ and r2 with an inset depicting the first-order swelling kinetics of the mucin granule matrix. Inset: notice that the radial expansion followed a characteristic first-order kinetics of the form r(t) = rf − (rf − ri)e−t/τ. The diffusivity (D) representing mucin polymer network swelling is given by D = (rf)2/τ (cm2/s) (13, 54). C: digital image composition of the expansion of 2 mucin matrices from a single A549 cell during exocytosis, recorded by videomicroscopy. The volume of mucin expansion increased as a function of time and reached a radius of ∼6 μm in ∼30 s. The same method was utilized for measuring the swelling kinetics of isolated mucin granules based on quinacrine fluorescence images. D: nicotine-induced changes in mucin network diffusivity (D). D values of secretory matrices from nonpreloaded intact granules of A549 cells (●; P < 0.0001; n ≥ 7; Student's t-test) were significantly lower with increasing nicotine concentration (10 nM–1 μM) than control (nicotine-free; ●; n = 10). Preloading intact granules in A549 cells with increasing nicotine concentrations (10 nM–1 μM) also generated significantly greater reductions in D values (○; P < 0.0001; n ≥ 9; Student's t-test) than control (nicotine-free; ○; n = 10). Equilibrating isolated granules in intracellular buffer containing increasing concentration of nicotine (100 nM and 1 μM) yielded markedly more retarded D (△; P < 0.0001; n ≥ 13; Student's t-test) than control (nicotine-free; △; n = 11). Data points were plotted in accord to the equation r(t) = rf − (rf − ri)e−t/τ and were presented as means ± SD; n represents the number of cells or granules investigated in the experiments. Additional details are summarized in Table 1. E: nicotine-induced changes in mucin network D. D values of secretory matrices from nonpreloaded intact granules of primary porcine airway epithelial cells (●; P < 0.0001; n ≥ 8; Student's t-test) were significantly lower with increasing nicotine concentration (50 nM–500 nM) than control (nicotine-free; ●; n = 36). Preloading intact granules in primary porcine airway epithelial cells with increasing nicotine concentrations (50 nM–500 μM) also generated significantly greater reductions in D values (○; P < 0.0001; n ≥ 20; Student's t-test) than control (nicotine-free; ○; n = 36). Data points were plotted in accord to the equation r(t) = rf − (rf − ri)e−t/τ and were presented as means ± SD; n represents the number of cells investigated in the experiments.
Table 1.
Summary of mucin matrix diffusivities when exposed to varying concentrations of nicotine
| Preloading Buffer for 15 Min | Bathing Buffer for Swelling Kinetics Measurement | Diffusivity, Cm2/S | |
|---|---|---|---|
| Nonpreloaded cells | Not preloaded | HBSS buffer | |
| [Nicotine] = 0 μM | 1. [Nicotine] = 0 nM | 1. 4.18 ± 0.76 (n = 10) | |
| 2. [Nicotine] =5 nM | 2. 3.88 ± 0.55 (n = 36) | ||
| 3. [Nicotine] =10 nM | 3. 2.24 ± 0.14* (n = 14) | ||
| 4. [Nicotine] =50 nM | 4. 1.81 ± 0.38* (n = 19) | ||
| 5. [Nicotine] =100 nM | 5. 1.37 ± 0.16* (n = 14) | ||
| 6. [Nicotine] =1 μM | 6. 1.48 ± 0.14* (n = 7) | ||
| Nicotine preloaded cells | HBSS buffer | HBSS buffer | |
| 1. [Nicotine] = 0 nM | [Nicotine] = 0 μM | 1. 4.18 ± 0.76 (n = 10) | |
| 2. [Nicotine] = 10 nM | 2. 2.22 ± 0.13* (n = 21) | ||
| 3. [Nicotine] = 100 nM | 3. 1.20 ± 0.025* (n = 12) | ||
| 4. [Nicotine] = 1 μM | 4. 1.22 ± 0.042* (n = 9) | ||
| Nicotine preloaded isolated granules | Intracellular buffer | Intracellular buffer | |
| 1. [Nicotine] = 0 nM | [Nicotine] = 0 μM | 1. 4.66 ± 0.39 (n = 11) | |
| 2. [Nicotine] = 100 nM | 2. 1.27 ± 0.69* (n = 21) | ||
| 3. [Nicotine] = 1 μM | 3. 1.56 ± 0.50* (n = 13) |
Diffusivities of means±s.d. are provided for each nicotine concentration.
Significantly different from control (nicotine-free) (P < 0.0001; Student's t-test); n represents the number of cells or granules investigated in the experiments.
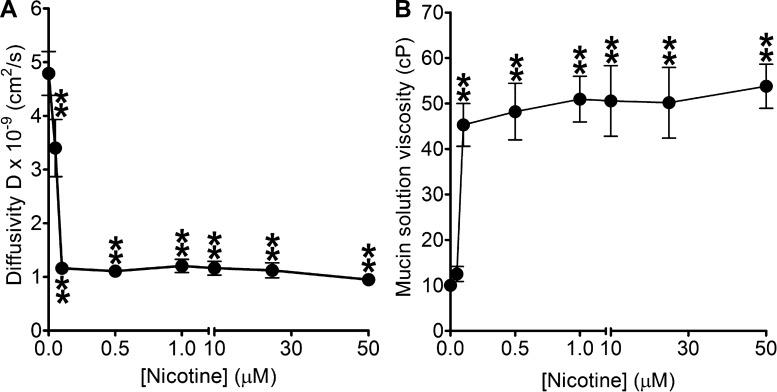
Experiments using high-resolution multiple particle tracking (MPT) showed a drastic decrease in particle transport in mucin solutions containing nicotine that parallels an elevation in viscosity (Fig. 2, A and B). The elevation in mucin solution viscosity starts to plateau between 0.1 and 50 μM. Moreover, the trends shown in Fig. 2, A and B correspond to the drop in mucin matrix diffusivity illustrated in Fig. 1. These results suggest that at low nicotine concentrations (100 nM) mucus hydration and transportability are also likely to be significantly compromised in smokers.
Fig. 2.
Measurements of fluorescent particle displacement and microviscosity changes. A: multiple particle tracking was used to calculate mean-square displacements needed to derive diffusivity of 500-nm-diameter carboxylated polystyrene particles in 1% porcine gastric mucin solution with increasing nicotine concentration (0–50 μM) in PBS. The mucin content used in this experiment is consistent with the range found in cervical, nasal, and lung mucus (1–3% of by weight) (30). Diffusion coefficients of particles in nicotine (50 nM to 50 μM)-treated mucin samples (P < 0.0001; n ≥ 24; Student's t-test) decreased significantly when compared with the mucin-only control (n = 32). Data were presented as means ± SE; n represents the number of independent experimental samples investigated. B: diffusivity was then used to derive the values for mucin solution viscosity. The mucin solution viscosity values increased significantly from ∼10 cP (mucin-only control; n = 32) to ∼50 cP (100 nM to 50 μM nicotine; P < 0.0001; n ≥ 24; Student's t-test). Data were presented as means ± SE; n represents the number of independent experimental samples investigated.
Nicotine promotes airway mucin self-aggregation.
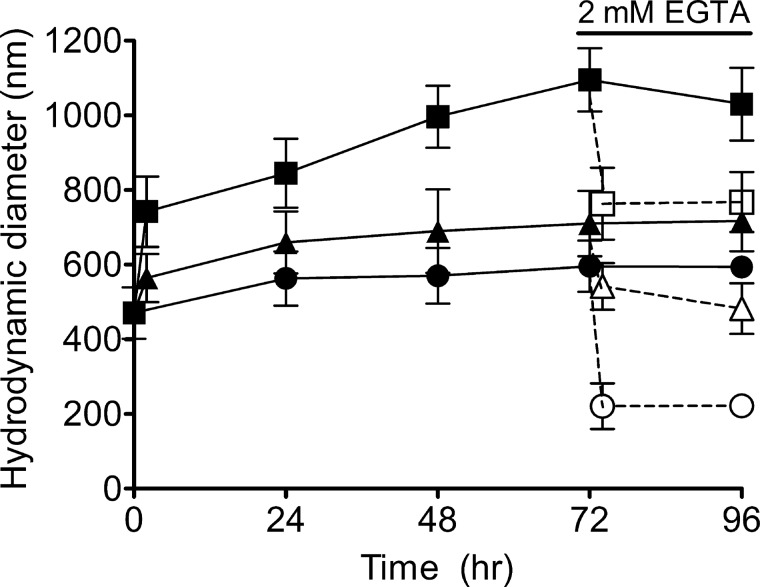
Since nicotine and mucin polymers contain both hydrophobic and complementary ionic moieties (at physiological pH, mucins are largely negative charged and nicotine is positive charged), we tested whether nicotine can directly interact with the mucin network by forming cross-links to aggregate mucins. To verify the biochemical interaction, we measured the effect of nicotine on the spontaneous self-assembly properties of diluted human airway mucin secreted from A549 cells (expressing both MUC5AC and 5B) (13, 58). Our results show that mucins (∼70 μg/l) can self-assemble to form small gels (500–600 nm) (Fig. 3). Although 50 μM nicotine dramatically increased the equilibrium size of the assembled mucin gels by ∼1.85 fold (1.1 μm), removing Ca2+ cross-linkers with EGTA diminished the gel size (∼700 nm). Shielding mucin charges (high salt) yielded similar gel size (∼700 nm). Under a Ca2+-free control (EGTA chelated) without nicotine, the mucin aggregate dimension (∼220 nm) was similar to sizes reported for human respiratory mucus (6, 11). Our data demonstrated that nicotine promotes mucin aggregation and can function as a potential cross-linker for mucin gel aggregates.
Fig. 3.
Nicotine changes the sizes of mucin self-assembly gels. Nicotine increased the equilibrium mucin gel particle sizes. After addition of 50 μM nicotine to 5 μm-filtered human airway mucin (70 μg/l) prepared in HBSS (Tris·HCl/MES, pH 7.3) for 24, 48, 72, and 96, the sizes of mucin gels (■; P < 0.001; n ≥ 14; 1-way ANOVA) were significantly larger than the control (mucin-only; ●; n ≥ 14). The nicotine-treated mucin gel sizes (■, P < 0.01; n ≥ 14; 1-way ANOVA) were also markedly larger than mucin gels prepared in 0.6 M NaCl HBSS with 50 μM nicotine (Tris·HCl/MES, pH 7.3) (▲; n ≥ 10) after 24 h of incubation. Hydrodynamic mucin gel sizes of EGTA-nicotine-treated (◻ P < 0.01; n ≥ 8; 1-way ANOVA) and EGTA-high-salt-nicotine-treated (△; P < 0.01; n ≥ 12; 1-way ANOVA) samples were larger than that of EGTA-treated mucin control (○; n ≥ 14) after 24 h of EGTA treatment. Data were presented as means ± SD; n represents the number of independent experimental samples investigated.
Nicotine accumulation in intracellular mucin granules.
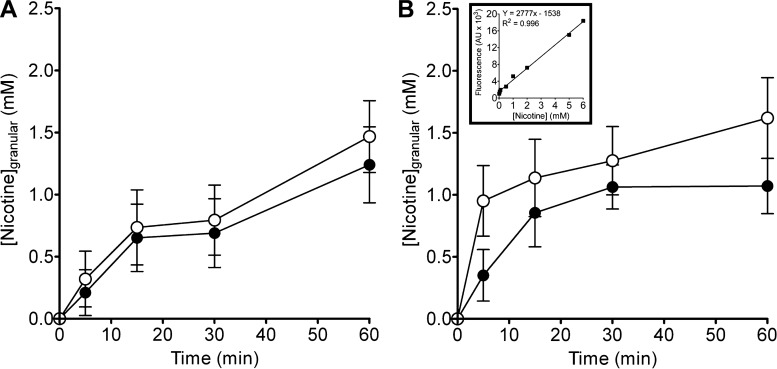
Prior to exocytosis, nicotine can infiltrate cytosolic granules, associate with matrices, and be sequestered in vesicles (35, 36). To demonstrate this possibility we tested the storage capacity of nicotine in secretory granules. Both intact cells and isolated secretory granules were used. Equilibration of A549 cells in HBSS containing 1 or 10 μM nicotine leads to rapid nicotine accumulation in intact secretory granules (Fig. 4A). Nicotine partition kinetics in isolated granules produced even more accumulation (Fig. 4B). These results indicate that nicotine, like other tertiary amines, can permeate and concentrate in granules against substantial concentration gradients that is consistent with other studies (16, 36, 40).
Fig. 4.
Nicotine accumulation in intact and isolated granules of A549 cells. Nicotine accumulation within intact and isolated granules ([Nicotine]granular) increased in a time- and dose-dependent manner. A: both 1 μM (●; n ≥ 24) and 10 μM (○; n ≥ 60) nicotine exposure resulted in similar rate of accumulation in intact granules of A549 cells. B: exposure of isolated granules to nicotine 1 μM (●; n ≥ 15) and 10 μM (○; P < 35) resulted in accelerated accumulation similar to intact granules. Inset displays equivalent fluorescent yields of a nicotine standard made in intracellular solution. Data were presented as means ± SD; n represents the number of cells or granules investigated in the experiments.
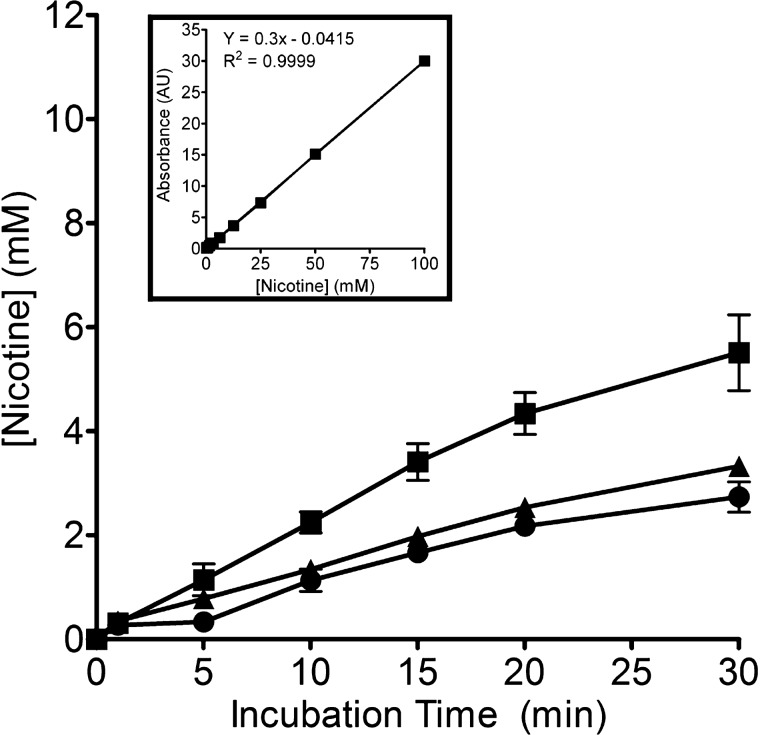
The fact that nicotine interacts with mucin networks suggests that it not only can be stored in mucin matrices but can also be gradually released into the airway. We examined whether mucin gels could modulate nicotine release. Figure 5 shows that nicotine released from 1 and 2% mucin was correspondingly ∼35 and ∼50% lower than the mucin-free control at the 30-min period. After 24 h, the ratio of nicotine released from mucin containing nicotine samples when compared with the control remained similar (data not shown). The data suggest strongly that higher mucin content enables more nicotine-mucin association, modulating the amount of free nicotine available for sustained release.
Fig. 5.
Mucin controls nicotine release rate. Nicotine (50 mM) was mixed with HBSS-only and mucin solutions with different concentrations. Nicotine released from 1% mucin at 5 min (▲; P < 0.05; n = 12; 1-way ANOVA) and 2% mucin (●; P < 0.001; n ≥ 8; 1-way ANOVA) after 5 min of dialysis were significantly lower than HBSS-only control (■; n ≥ 8). As more nicotine molecules become trapped in mucin network by interacting with mucin polymers, the amount of freely diffusible nicotine through dialysis membrane was reduced, indicating that mucin exerted a control-release mechanism for nicotine. Data were presented as means ± SD; n represents the number of independent experimental samples investigated.
DISCUSSION
Airway mucin gels serve myriad roles in maintaining pulmonary integrity (17, 51). These multifaceted functions rely immensely on the harmonious orchestration of optimal mucus rheology, hydration, and mucociliary action, which is fundamentally regulated by Donnan equilibrium (38, 51, 54, 56). Poor mucus hydration leads to improper dispersion and luminal obstruction as cardinally found in COPD (38, 51). We are the first to demonstrate that nicotine can directly alter mucin rheological properties by hindering hydration and increasing viscosity. This intriguing discovery is a distinct departure from the conventional model of hypersecretion-induced viscous mucus formation (29, 38, 56) and necessitates alternative therapeutic strategies for treating COPD.
Network hydration fundamentally determines mucus rheological properties (13, 38, 54). Measurements of mucin matrix diffusivity provide direct assessment of deviation in mucin viscosity released from cells under both physiological and pathological conditions (13, 54). Like many pulmonary disorders, particularly in COPD, cigarette smoking has been shown to induce mucin hypersecretion; however, whether it directly affects mucin rheological property remains elusive. Although cigarette smoke extract has been commonly utilized in respiratory disease related studies (4, 22), the complexity of its composition (more than 4,500 molecules) prevents us from identifying a clear mechanism (47). Nicotine, on other hand, is a common major addictive compound in cigarette smoke that has well-established cellular and molecular mechanisms relating to pulmonary mucus hypersecretion disorder (31). In this study, that nicotine can directly influence mucus rheological properties via physicochemical processes is to be investigated. We tested whether nicotine could limit mucin matrix hydration at typical inhalation concentrations. First, our data showed that, at nicotine concentrations equivalent to environmental exposure (≤50 nM) (5), mucin network swelling rates were reduced by ∼2- and ∼1.5-fold as shown by human and porcine airway epithelial cells, correspondingly (Fig. 1, D and E). At just 100 nM, the decrease in diffusivity reached a maximum for nonpreloaded cells, preloaded cells and preloaded isolated granules (Fig. 1D) in human airway respiratory cells. The same nicotine concentration also engendered a similar maximal reduction of D in both nonpreloaded and preloaded primary porcine airway cells (Fig. 1E). Both data suggested that the nicotine-induced mucin swelling hindrance may be conserved throughout different species, as a result of comparable mucin molecules and architecture (46, 50) and further underlined the hypersensitivity of mucin rheological properties to nicotine exposure. In addition to the data generated from human A549 cells and primary porcine airway epithelium, our results can be strengthened with the usages of primary human airway epithelium and to compare mucin network hydration rates between normal subjects and COPD patients. Outcomes from Fig. 1, D and E further indicated that the blood nicotine concentration (∼300–500 nM) obtained during direct cigarette smoking is highly likely to hinder mucin hydration (31). Modified mucus hydration may potentially be a critical concern for nicotine replacement therapy (NRT) users. A popular option of NRT is electronic cigarette (E-cigarette). A brief delivery of nicotine by electronic cigarette through inhalation has been shown to increase respiratory impedance and peripheral airway flow resistance in healthy adult smokers when compared with a control group (53). At the same time, ceasing its usage has also revealed significant improvement of cough, sputum production, and breathlessness (25). Therefore, it is a reasonable concern that the exposure of nicotine to airway via such route may equally hinder proper mucin hydration and alter rheological properties. Similar undesirable effects are implicated in other NRT including nicotine nasal spray, vapor inhaler, and nebulizer (8, 44). Safety assessment and monitoring the nonconventional adverse effects are very much needed for various NRT products. Our laboratory results might be limited by themselves; for example mucus extrusion might become more complicated in vivo. However, animal experimental data indicated that expelled mucus remained adherent at the secretory site, and, when dislodged, the mucin masses had slower transport rate than the control (smoke-free) (31). These data further suggested that mucus extrusion in cigarette smoke-exposed rodents is hindered. Moreover, in vitro and in vivo evidences have shown that exposure to cigarette smoke could possibly generate stagnant mucus and impair mucociliary clearance by inducing airway surface liquid dehydration (15). These studies supported the notion of cigarette smoke-induced improper gel hydration and altered rheological characteristics in smokers (26).
Since polymer diffusivity and viscosity are inversely proportional to each other (13), we examined whether nicotine could increase mucin viscosity. A representative porcine gastric mucin was used since it is a widely acceptable model to investigate the viscoelastic natures and biological applications of mucins (33, 48). Nicotine at 100 nM drastically elevates mucin solution viscosity (Fig. 2, A and B). Our data suggest that a very low level of sustained or transient exposures to nicotine can potentially hamper normal ciliary beat frequency, disrupting mucociliary clearance in both active smokers and populations exposed to ETS. Aligning with published reports, smoke-exposed rats showed zones of inactive cilia that have been associated with stagnant mucus (26). Impaired mucus clearance also leads to bacterial persistence, associated inflammation, and tissue damage (24, 38). Furthermore, a momentary exposure to secondhand smoke caused acute decrement in lung functions and can exert a substantiate role in eliciting chronic respiratory diseases (18).
Both nicotine and mucins are amphiphilic structures containing complementary features amenable to physicochemical interactions (35, 36). It is highly plausible that at neutral pH the polyanionic carbohydrate side chains and the nonglycosylated mucin core (6, 17, 54) can bind with monoprotonated and hydrophobic moieties of nicotine (35). It is also likely that nicotine can interact with the nodes of tightly bound protein domains in MUC5B mucins (27). Through these mechanisms, nicotine can limit mucin hydration at both the intra- and extracellular levels. We used dynamic laser scattering to authenticate that nicotine affects airway mucin self-assembly properties by enlarging the equilibrated mucin gel size (∼1.85-fold). This result is corroborated by the presence of mucus flakes in tobacco smoke-exposed rat airways (26). Data from EGTA-treated nicotine-containing mucin samples when compared with EGTA-treated control indicates that nicotine can replace conventional Ca2+ to function as cross-linkers for mucin networks (Fig. 3). Therefore, nicotine at concentrations found in smokers' airways (≤50 μM) (14) may readily link mucins together to form sizable aggregates. Differences between charge-shielded (high salt-nicotine sample; ∼710 nm) and nonshielded mucin aggregate sizes demonstrate that electrostatic interaction strongly potentiates hydrophobic binding among mucin and nicotine. Both these interactions probably contribute to retarding normal mucin hydration, yielding the thick mucus found in smokers. Moreover, the gel-on-brush model further complemented our data, suggesting that nicotine may interact with membrane-spanning mucins and large mucopolysaccharides tethered to cilia and epithelial surface to destabilize periciliary layer, possibly impeding mucus clearance in smokers (7).
To further prove that nicotine hinders network hydration through direct interaction with intracellular mucin matrices, Fig. 4 has validated nicotine concentrating in cytosolic vesicles (35, 43). This phenomenon can primarily be explained by pH partitioning. That is, chemically similar tertiary amine drugs (e.g., disobutamide, a cardiac antiarrhythmic drug) or weak alkaline compounds such as quinacrine (Atabrine, an antiprotozoal; Fig. 1A) or acridine orange are sequestered into acidic organelles and protonated, thus becoming trapped owing to granular pH gradients and the chemical pKa (35, 36, 40). Hence, it seems evident that nicotine at levels equivalent to ETS or cigarette smoking can result in significant accumulation in mucin granules of airway goblet cells.
Furthermore, our results indicate that mucin gels serve not only as a reservoir but also as a control-release modulator for nicotine (Fig. 5). Consequently, the body may experience prolonged exposure due to a sustained nicotine release from airway mucus beyond the period of active smoking and ETS exposures. Our data are supported by the lack of COPD decline among formerly heavy smokers (19). That chronic inflammation persisted after smoking cessation can be elucidated by the persistent nicotine released from mucus that attracts and activates neutrophils (32, 52). In addition, gradual release and retention of tobacco carcinogens and nicotine from mucus may be an additional mechanism linking lung carcinogenesis and COPD susceptibility (2).
This study has provided evidence supporting that nicotine directly retards mucin hydration through interactions with mucins possibly via cross-linking. The fact that low levels of tobacco smoke exposure may lead to nicotine accumulation in secretory granules highlights the hazardous potential of ETS for nonsmokers. Furthermore, airway mucus potentially sustains harmful blood nicotine concentration for extended time after tobacco inhalation. In addition, nicotine is likely to cause significant systemic effects by inducing widespread changes in the release kinetics of other secretory matrices (54) that may contribute to the complex pathology found in tobacco smokers or in individuals chronically exposed to ETS.
GRANTS
This study was supported in part by National Science Foundation (CBET-0932404), National Institutes of Health (1R15HL095039), and Linkou Chang Gung Memorial Hospital (CMRPD1C0031).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.Y.C. and W.-C.C. conception and design of research; E.Y.C., A.S., and C.-S.C. performed experiments; E.Y.C., A.S., and C.-S.C. analyzed data; E.Y.C., A.S., C.-S.C., A.J.M., and W.-C.C. interpreted results of experiments; E.Y.C., A.S., C.-S.C., and A.J.M. prepared figures; E.Y.C. and A.J.M. drafted manuscript; E.Y.C., A.J.M., and W.-C.C. edited and revised manuscript; E.Y.C., A.S., C.-S.C., A.J.M., and W.-C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Paul M. Quinton and John M. Mintz for critical suggestions and thorough editing.
REFERENCES
- 1. Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol Lung Cell Mol Physiol 273: L201–L210, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration 81: 265–284, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Barnes PJ. New treatments for COPD. Nat Rev Drug Discov 1: 437–446, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Betsuyaku T, Fuke S, Inomata T, Kaga K, Morikawa T, Odajima N, Adair-Kirk T, Nishimura M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. Eur Respir J 42: 42–53, 2013 [DOI] [PubMed] [Google Scholar]
- 5. Bolte G, Heitmann D, Kiranoglu M, Schierl R, Diemer J, Koerner W, Fromme H. Exposure to environmental tobacco smoke in German restaurants, pubs and discotheques. J Expo Sci Environ Epidemiol 18: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bromberg LE, Barr DP. Self-association of mucin. Biomacromolecules 1: 325–334, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337: 937–941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caldwell B, Sumner W, Crane J. A systematic review of nicotine by inhalation: is there a role for the inhaled route? Nicotine Tob Res 14: 1127–1139, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Cantin AM. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc Am Thorac Soc 7: 368–375, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Carlstedt I, Sheehan JK. Is the macromolecular architecture of cervical, respiratory and gastric mucins the same? Biochem Soc Trans 12: 615–617, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Cerveri I, Brusasco V. Revisited role for mucus hypersecretion in the pathogenesis of COPD. Eur Respir Rev 19: 109–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clunes LA, Bridges A, Alexis N, Tarran R. In vivo vs. in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol 32: 201–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duvvuri M, Krise JP. A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than pH-partitioning theory would predict. Mol Pharm 2: 440–448, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flouris AD, Metsios GS, Carrillo AE, Jamurtas AZ, Gourgoulianis K, Kiropoulos T, Tzatzarakis MN, Tsatsakis AM, Koutedakis Y. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med 179: 1029–1033, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax 57: 967–972, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goh GY, Huang H, Ullman J, Borre L, Hnasko TS, Trussell LO, Edwards RH. Presynaptic regulation of quantal size: K+/H+ exchange stimulates vesicular glutamate transport. Nat Neurosci 14: 1285–1292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gouaze V, Dousset N, Dousset JC, Valdiguie P. Effect of nicotine and cotinine on the susceptibility to in vitro oxidation of LDL in healthy non smokers and smokers. Clin Chim Acta 277: 25–37, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Heijink IH, de Bruin HG, van den Berge M, Bennink LJ, Brandenburg SM, Gosens R, van Oosterhout AJ, Postma DS. Role of aberrant WNT signalling in the airway epithelial response to cigarette smoke in chronic obstructive pulmonary disease. Thorax 68: 709–716, 2013 [DOI] [PubMed] [Google Scholar]
- 23. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Sharafkhaneh A, Luketich JD, Coxson HO, Elliott WM, Sciurba FC. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 176: 454–459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hureaux J, Drouet M, Urban T. A case report of subacute bronchial toxicity induced by an electronic cigarette. Thorax 69: 596–597, 2014 [DOI] [PubMed] [Google Scholar]
- 26. Iravani J, Melville GN. Long-term effect of cigarette smoke on mucociliary function in animals. Respiration 31: 358–366, 1974 [DOI] [PubMed] [Google Scholar]
- 27. Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol 298: L15–L22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton D. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 1033–1039, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv 23: 219–231, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA 104: 1482–1487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang M, Hummer B, Hahn HL. Effect of systemic nicotine on mucus secretion from tracheal submucosal glands and on cardiovascular, pulmonary, and hematologic variables. Klin Wochenschr 66, Suppl 11: 170–179, 1988 [PubMed] [Google Scholar]
- 32. Macnee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med 28: 479–513, v, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Mayol L, Quaglia F, Borzacchiello A, Ambrosio L, La Rotonda MI. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: rheological, mucoadhesive and in vitro release properties. Eur J Pharm Biopharm 70: 199–206, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen T, Chin WC, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+. Nature 395: 908–912, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Nielsen HM, Rassing MR. Nicotine permeability across the buccal TR146 cell culture model and porcine buccal mucosa in vitro: effect of pH and concentration. Eur J Pharm Sci 16: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 90: 656–664, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quesada I, Chin WC, Verdugo P. ATP-independent luminal oscillations and release of Ca2+ and H+ from mast cell secretory granules: implications for signal transduction. Biophys J 85: 963–970, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Randell SH, Boucher RC; University of North Carolina Virtual Lung Group. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35: 20–28, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose MC, Piazza FM, Chen YA, Alimam MZ, Bautista MV, Letwin N, Rajput B. Model systems for investigating mucin gene expression in airway diseases. J Aerosol Med 13: 245–261, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Ruben Z, Dodd DC, Rorig KJ, Anderson SN. Disobutamide: a model agent for investigating intracellular drug storage. Toxicol Appl Pharmacol 97: 57–71, 1989 [DOI] [PubMed] [Google Scholar]
- 41. Rubinstein M. Discretized model of entangled-polymer dynamics. Phys Rev Lett 59: 1946–1949, 1987 [DOI] [PubMed] [Google Scholar]
- 42. Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 161: 1016–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron 46: 595–607, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Schuh KJ, Schuh LM, Henningfield JE, Stitzer ML. Nicotine nasal spray and vapor inhaler: abuse liability assessment. Psychopharmacology (Berl) 130: 352–361, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Serisier DJ, Carroll MP, Shute JK, Young SA. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir Res 10: 63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sheehan JK, Oates K, Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J 239: 147–153, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2: 372–377, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Sriamornsak P, Wattanakorn N, Takeuchi H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydrate Polymers 79: 54–59, 2010 [Google Scholar]
- 49. Tanaka T, Fillmore D. Kinetics of swelling of gels. J Chem Phys 70: 1214–1218, 1979 [Google Scholar]
- 50. Thornton DJ, Davies JR, Kraayenbrink M, Richardson PS, Sheehan JK, Carlstedt I. Mucus glycoproteins from ‘normal’ human tracheobronchial secretion. Biochem J 265: 179–186, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc 1: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Totti N, 3rd, McCusker KT, Campbell EJ, Griffin GL, Senior RM. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science 223: 169–171, 1984 [DOI] [PubMed] [Google Scholar]
- 53. Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400–1406, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol 52: 157–176, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153: 1530–1535, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 135: 505–512, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Yin P, Jiang CQ, Cheng KK, Lam TH, Lam KH, Miller MR, Zhang WS, Thomas GN, Adab P. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet 370: 751–757, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol 170: 20–32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]