Abstract
Cytotoxic T lymphocytes (CTLs) rapidly kill target cells via the release of lytic granules into the immunological synapse, a process directed by the docking of the centrosome at the plasma membrane. New evidence highlights how signal strength and avidity influence the recruitment of cytolytic machinery to the synapse, and the role of each synaptic compartment. Release of cytolytic effector proteins, including perforin and FasL, is controlled at multiple levels and is also influenced by the avidity of the interaction. New imaging technologies and the use of photoactivatable peptides have allowed the dissection of signalling molecules involved in each step of the cytolytic process. This review highlights the important role of avidity in controlling how a T cell kills its target.
Introduction
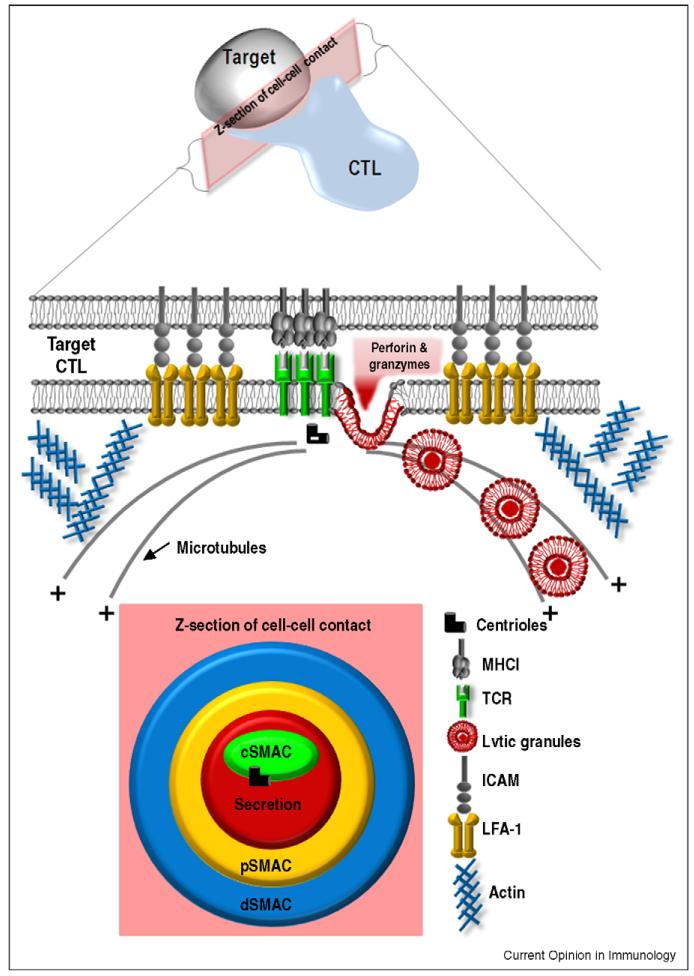
In response to antigenic challenge, naïve T cells undergo extensive cell division and acquire effector capabilities within the lymph node, before disseminating to the periphery. Here, activated CD8+ cytotoxic T cells (CTLs) mediate efficient and rapid killing of target cells either by the secretion of lytic granules or by ligation of death receptors. Lytic granules are secretory vesicles containing the pore-forming protein perforin and a family of serine proteases, called granzymes. Upon T cell receptor (TCR) engagement, the centrosome (also known as the microtubule organising centre (MTOC) of T cells) relocates to the point of TCR signalling within the immunological synapse (IS). Granules, which are associated with the microtubules, then migrate in a ‘minus-end’ direction towards the centrosome, where they dock and release their contents at the plasma membrane [1] (Figure 1). Many advances have been made in our understanding of the roles of the IS since the characteristic micron-scale bull’s eye organisation of cell surface receptors at the IS was first described by Kupfer in CD4+ T cells [2] (Figure 1). In this review we will discuss recent data exploring the intracellular machinery and signalling cascades involved in the recruitment of the cytoskeleton and lytic granules to the synapse.
Figure 1. The immunological synapse: a highly organised interface.
The interface between CTL and target is organised with an adhesion ring: the peripheral supramolecular activation complex (pSMAC) with leucocyte function associated antigen 1 (LFA-1) on the CTL and intercellular adhesion molecule (ICAM) on the target cell. Within the pSMAC is the central SMAC, where TCR accumulates, and beside it the secretion domain where lytic granules fuse to release their content. The centrioles of the centrosome dock beside the cSMAC, which acts as a focal point for minus end microtubule-mediated delivery of lytic granules. Actin accumulation occurs in a distal SMAC (dSMAC).
Different technologies are used to investigate CTL–target interactions
CTLs make challenging subjects for high resolution imaging, as they are very small and highly motile. Modern imaging technology and different model systems have been important for our current understanding of the immunological synapse and the recruitment of cytolytic machinery.
Many studies have taken advantage of the high resolution of light microscopy provided by confocal laser scanning microscopy (CLSM). The use of laser light that is focussed onto a detector, excluding out-of-focus light from specimens, allows optical sectioning and the reconstruction of 3D images. Multi-photon technology permits in vivo imaging in lymph nodes allowing CD8 synapse formation to be visualised in vivo [3-5].
The use of total internal reflection fluorescence microscopy (TIRFM) in conjunction with glass supported planar lipid bilayers, has provided a great deal of information about the synapse. TIRF provides laser light at an acute angle causing an evanescent wave, thereby exciting only the small interface between a coverslip and the cell. The light is reflected off the interface and penetrates <100 nm into the cell. The advantage of this system is the high resolution at the surface within the narrow illuminated plane. This allows events at the plasma membrane to be imaged, but provides very limited information about intracellular events. Since the diameter of a lytic granule can be up to 1 μm, TIRF only provides information about granules contacting the membrane.
The combination of TIRF with phospholipid monolayers on glass has been powerful in allowing the study of the movement of receptors, introduced into the lipid bilayer artificially by a number of methods, which couple the extracellular domains of the proteins of interest to the head groups of the outer leaflet of the phospholipid bilayer (reviewed in Ref. [6]).
Each of these methods has advantages and limitations for examining events at the synapse, and this needs to be kept in mind when comparing results from the different systems.
The structure and function of the immunological synapse
At the CTL–target cell junction, a cascade of activation signals causes rapid segregation of cell surface receptors into three concentric compartments, called the central, peripheral and distal supramolecular activation complex (SMAC) (see Figure 1) [2] and reviewed in Ref. [7]. Although the structure of the IS is well documented, its function has been controversial. The cSMAC is enriched in TCR associated signalling proteins including TCRζ, Lck, ZAP-70 and PKCθ, and was assumed to be the site of TCR signalling. However, studies using lipid bilayers revealed that signalling occurred in actin-dependent peripheral microclusters at the distal SMAC (dSMAC), which coalesce in the cSMAC where TCR can be downregulated [8]. Cemerski et al. [9 ••] showed that signalling in response to weak peptides was enhanced by increasing the strength of cSMAC formation artificially by introducing NKG2D and DAP10 into T cells stimulated with weak peptides. The authors suggest that the cSMAC serves both as a site of signalling and degradation with the quality and avidity of antigen recognition dictating the fate of the TCR [10].
Some studies have questioned whether the formation of a stable synapse is important for cytotoxicity. Single molecule labelling showed that killing required the ligation of only three TCR molecules and occurred in the absence of molecular segregation at the synapse [11]. One possible explanation could be that the re-organisation of receptors occurred, but not at high enough levels to be visualised, another might be that since these studies used high avidity TCRs, lower avidity antigens might require the formation of a stable cSMAC to concentrate signalling molecules and elicit a response.
The docking of the MTOC at the immunological synapse
Once signalling has occurred, the actin and microtubule cytoskeleton rapidly polarise towards the synapse, a crucial step in cytotoxicity (reviewed in Ref. [12]).
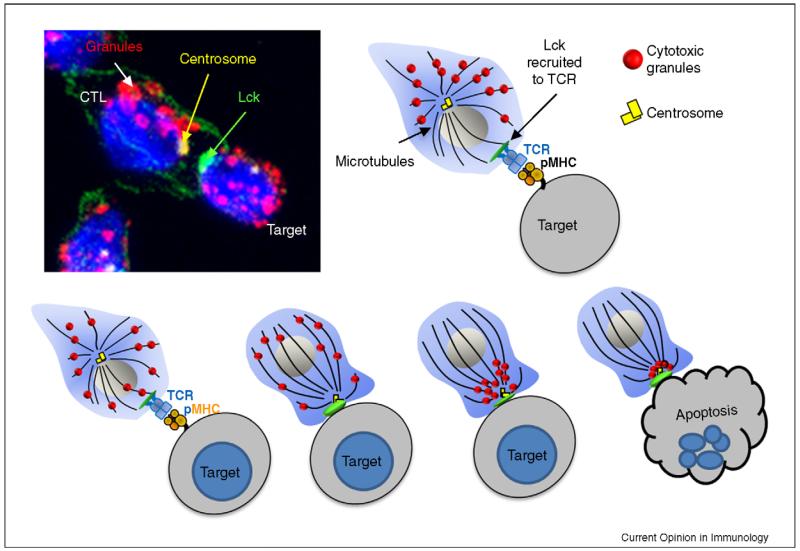
Microtubules that have a defined polarity, radiate from the MTOC (minus ends) to the cell periphery (plus ends), and granules migrate in a minus direction towards the MTOC. Remarkably, the combination of electron microscopy and fluorescence microscopy has revealed that the MTOC (or more accurately, the centrosome) always contacts the plasma membrane at the same place within the synapse, at the cSMAC (Figure 2). The centrioles appear to dock at the plasma membrane, although it is not yet clear if a physical attachment is formed. The granules are delivered to this point on the plasma membrane and release their contents into the secretory domain formed [1,13].
Figure 2. Mechanism of lytic granule release.
Recognition of the target MHC class I molecules bound to peptide antigen occurs via the TCR. Once the TCR is engaged, the most proximal signalling molecule to be recruited is Lck (shown in green). The centrosome (γ-tubulin, yellow), which is normally perinuclear, polarises towards the synapse and docks at the plasma membrane. The granules (LAMP-1, red) migrate towards the centrosome, where they dock and fuse with at the plasma membrane, releasing their contents into the synaptic cleft, causing target cell destruction.
Early studies by Weiss [14], carried out on Jurkat T cells using TCR cross-linking, identified Zap-70, LAT and SLP76 and calcium, as essential for MTOC polarisation to the IS.
The tyrosine kinase Lck and the LAT–SLP76 complex recruit PLC-γ to the plasma membrane, which hydrolyses phosphatidylinositol-4,5-bisphosphate (PIP2) to yield soluble inositol-1,4,5-tris-phosphate (IP3) and membrane-bound diacylglycerol (DAG). DAG, which is normally found on the plasma membrane, however accumulates at the synapse after T cell stimulation. Studies using planar lipid bilayers create an expansive interface between the T cell and the flat bilayer surface, making it difficult to define MTOC polarisation to a defined point. Huse and colleagues [15 ••] circumvented this issue by using a photoactivatable system in which the immobilised MHC1 (I-Ek) containing an agonist peptide derived from moth cytochrome C in a form that is non-stimulatory to the 5C.C7 transgenic TCR, until irradiated with UV light. In this way, a small and localised region of activated pMHC could be formed, triggering the relocation of the MTOC towards the activated spot within minutes. Blocking DAG production with chemical inhibitors, impeded MTOC recruitment towards the activated pMHC. Conversely, generating a localised DAG signal, by photoactivation of a caged form of DAG, the MTOC polarised towards the signal [14].
Delivery of the lethal hit
Once the MTOC/centrosome is polarised to the synapse, the granules move to the plasma membrane, dock and release their contents into a specialised cleft. Lytic granules are specialised secretory lysosomes, containing cytolytic proteins such as perforin and granzymes, in addition to lysosomal hydrolases such as cathepsins B and D, β-hexosaminidase and the lysosomal membrane proteins, LAMP-1, LAMP-2 and LAMP-3. Additionally trans-membrane receptors are also delivered, including Fas ligand, which is packaged into lytic granules with perforin.
The lysosomal membrane protein, LAMP-1 (CD107a), is commonly used as a marker of degranulation. Upon degranulation, LAMP-1 is exposed at the plasma membrane and rapidly endocytosed. Measuring the uptake of labelled anti-LAMP-1 antibodies therefore provides a measure of the level of degranulation. Using this technique combined with TIRF microscopy, Liu et al. [16 ••] followed degranulation of lytic granules from NK cells into the synapse by following LAMP-1 internalisation. When intercellular adhesion molecule (ICAM)-1 was incorporated into the lipid bilayers, LAMP-1 remained at a focussed point at the plasma membrane, adjacent to intracellular lysosomal compartments, while it rapidly diffused away over a large area of synapse in controls lacking ICAM-1. These results suggest that leucocyte function associated antigen 1 (LFA-1) in the peripheral SMAC (pSMAC) serves to focus both exocytic and endocytic events within the synapse. Recently a kinase associated with lytic granules exocytosis was identified. PKCδ localises to granzyme B+ granules, becomes activated upon TCR engagement and is involved in selective cytotoxic granule movement towards the IS [17 •,18].
The influence of signal strength on events at the immunological synapse
It is well established that the strength of TCR signal affects the proliferation, and differentiation fate of CTL and new studies highlight the effect of the strength of TCR signal on polarised secretion by CTL. Despite MTOC polarisation being absolutely essential for the delivery of granules to the synapse, it is not a predictive measure of cytotoxicity. Rather, the recruitment of lytic granules to the cSMAC is the decisive step in cytotoxicity. It is now possible to begin to dissect the individual different steps leading to granule mediated exocytosis.
We used transgenic OVA-specific OT-I CTL [19] with different peptide ligands to ask how different avidity interactions control polarisation of the MTOC (centrosome) and lytic granules to the synapse. High avidity interactions triggered by the OVA peptide trigger MTOC and granule polarisation, with highly effective degranulation and target cell killing. By contrast, low avidity interactions triggered by the altered peptide ligand, G4, triggered MTOC polarisation to the cSMAC, but not granule polarisation and consequently both degranulation and target cell killing were poor [20 ••]. Interestingly cSMAC activation was observed in response to both strong and weak avidity interactions, but was rapidly downregulated by phosphatases when the weak peptide agonist was used. This study shows that while the MTOC can polarise readily in response to both strong and relatively weak or short-lived signals, the recruitment of granules to the cSMAC requires a higher threshold of TCR signalling.
Although CD4+ T cells are predominantly involved in cytokine secretion a number of studies have shown that these cells can also express cytolytic proteins, including perforin, and exert cytolytic activity, albeit at a much lower level than CD8+ CTL [21,22]. Beal et al. [23 ••] have used TIRF microscopy combined with planar lipid bilayers to compare granule release by CD8+ and CD4+ T cells, using Lysotracker or LAMP-1 antibody-binding to detect granules. These methods detect endocytic compartments at the plasma membrane, with lysotracker labelling acidic compartments within the TIRF focus, and LAMP-1 antibodies endocytic compartments internalising LAMP-1. They show that CTLs differ in the recruitment of lytic granules to the synapse, with the lysotracker labelled-granules from CD8+ T cells clustering tightly to the cSMAC, while CD4+ granules remain scattered at the peripheral actin ring (dSMAC). They used strong or weak agonist-peptide-loaded Quantum dots to stimulate the CD4+ and CD8+ clones and found that granule sequestration to the periphery of the synapse correlated with reduced kinetics of TCR-triggered calcium fluxes.
There is also evidence for differential secretion of FasL in response to strong and weak signalling thresholds. FasL expression is tightly regulated by CTL and stored in lytic granules [24] although whether or not it is stored with perforin and granzyme B is debated [24,25]. Two waves of FasL cell surface expression have been reported: rapid release of pre-stored FasL (15–30 min) and delayed de novo synthesis. Recently, Ostergaard and colleagues [26] have used OT-I transgenic TCR CTL to show that stored FasL translocation required a lower signalling threshold, while a higher avidity signal was required to induce de novo FasL synthesis. In this study, they also showed that a higher avidity response induced killing of bystander cells. This highlights how signal strength plays an important role in regulating how and when a CTL will kill its target. In another recent study using an influenza model, the lower avidity DbNP366-specific CTLs display shorter contact times with target cells, compared to the higher avidity DbPA224-specific CTLs, and when recovered directly from the infected lungs of mice and assayed on peptide pulsed-target cells [27]. It is possible that despite equivalent expression of cytolytic proteins [28] and equivalent levels of killing in vivo [29], avidity may influence the contribution of FasL mediated killing compared to granule exocytosis.
Concluding remarks
With an increasing understanding of the signalling events controlling CTL killing as well as the mechanisms controlling polarised secretion from these cells, it is now becoming possible to understand how CTLs control target cell destruction so precisely. New technologies and different biological systems all contribute to our understanding of how CTL organise and control secretion towards target cells. While each system and approach has its own limitations, each provides new insights into our understanding of the cytolytic synapse.
Acknowledgements
GMG is funded by the Wellcome Trust and MRJ is the recipient of an Australian National Health and Medical Research Council Fellowship. We would like to acknowledge Andy Tsun for the basis for Figure 1 and Maike de la Roche for critical review.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 2.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 3.Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groves JT, Dustin ML. Supported planar bilayers in studies on immune cell adhesion and communication. J Immunol Methods. 2003;278:19–32. doi: 10.1016/s0022-1759(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 7.Dustin ML. T-cell activation through immunological synapses and kinases. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 8.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [This study highlights the role of the synapse in enhancing signalling. By increasing the strength of the cSMAC artificially they show that signalling to weak peptides is amplified.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cemerski S, Das J, Locasale J, Arnold P, Giurisato E, Markiewicz MA, Fremont D, Allen PM, Chakraborty AK, Shaw AS. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–355. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 12.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 13.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 14.Kuhne MR, Lin J, Yablonski D, Mollenauer MN, Ehrlich LI, Huppa J, Davis MM, Weiss A. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J Immunol. 2003;171:860–866. doi: 10.4049/jimmunol.171.2.860. [DOI] [PubMed] [Google Scholar]
- 15••.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [Using photoactivatable peptides this paper demonstrates the role of DAG in directing MTOC movement.] [DOI] [PubMed] [Google Scholar]
- 16••.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [This paper shows that LFA-1 in the pSMAC constrains the diffusion of LAMP-1 upon degranulation, suggesting the pSMAC acts as a barrier to focus exocytic and endocytic events.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Ma JS, Haydar TF, Radoja S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J Immunol. 2008;181:4716–4722. doi: 10.4049/jimmunol.181.7.4716. [This paper identifies a role for PKCδ in lytic granule exocytosis.] [DOI] [PubMed] [Google Scholar]
- 18.Ma JS, Monu N, Shen DT, Mecklenbrauker I, Radoja N, Haydar TF, Leitges M, Frey AB, Vukmanovic S, Radoja S. Protein kinase Cdelta regulates antigen receptor-induced lytic granule polarization in mouse CD8+ CTL. J Immunol. 2007;178:7814–7821. doi: 10.4049/jimmunol.178.12.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20••.Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621–631. doi: 10.1016/j.immuni.2009.08.024. [The centrosome directs lytic granules to the synapse. By altering the strength of TCR signal, this study shows that while the centrosome/MTOC polarises to the synapse with weak signals, lytic granule movement requires a stronger threshold of signalling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasternack MS, Verret CR, Liu MA, Eisen HN. Serine esterase in cytolytic T lymphocytes. Nature. 1986;322:740–743. doi: 10.1038/322740a0. [DOI] [PubMed] [Google Scholar]
- 22.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 23••.Beal AM, Anikeeva N, Varma R, Cameron TO, Vasiliver-Shamis G, Norris PJ, Dustin ML, Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [This study compares signalling and lysosome recruitment to the synapse in CD4 and CD8 cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 25.He JS, Ostergaard HL. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J Immunol. 2007;179:2339–2348. doi: 10.4049/jimmunol.179.4.2339. [DOI] [PubMed] [Google Scholar]
- 26.He JS, Gong DE, Ostergaard HL. Stored Fas ligand (FasL), a mediator of rapid CTL-mediated killing, has a lower threshold for response than degranulation or newly synthesized FasL. J Immunol. 2010;184:555–563. doi: 10.4049/jimmunol.0902465. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins MR, La Gruta NL, Doherty PC, Trapani JA, Turner SJ, Waterhouse NJ. Visualizing CTL activity for different CD8+ effector T cells supports the idea that lower TCR/epitope avidity may be advantageous for target cell killing. Cell Death Differ. 2009;16:537–542. doi: 10.1038/cdd.2008.176. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins MR, Kedzierska K, Doherty PC, Turner SJ. Heterogeneity of effector phenotype for acute phase and memory influenza A virus-specific CTL. J Immunol. 2007;179:64–70. doi: 10.4049/jimmunol.179.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Stambas J, Doherty PC, Turner SJ. An in vivo cytotoxicity threshold for influenza A virus-specific effector and memory CD8(+) T cells. J Immunol. 2007;178:1285–1292. doi: 10.4049/jimmunol.178.3.1285. [DOI] [PubMed] [Google Scholar]