Abstract
Background
Survivors of head and neck squamous cell carcinoma (HNSCC) face excess mortality from multiple causes.
Methods
We used population-based Surveillance, Epidemiology, and End Results (SEER) cancer registry data to evaluate the causes of death in patients with non-metastatic HNSCC diagnosed between 1992 and 2005 who survived at least three years from diagnosis (long-term survivors). We used competing-risks proportional hazards regression to estimate probabilities of death from causes: HNSCC, second primary malignancy (SPM) excluding HNSCC, cardiovascular disease (CVD), and other causes.
Results
We identified 35,958 3-year survivors of HNSCC with a median age at diagnosis of 60 years (range 18 to 100 years) and a median follow-up of 7.7 years (range 3 to 18 years). There were 13,120 deaths during the study period. Death from any cause at 5 and 10 years was 15.4% (95% confidence interval [CI], 15.0% to 15.8%) and 41.0% (95% CI, 40.4% to 41.6%), respectively. There were 3,852 HNSCC deaths including both primary and subsequent head and neck tumors. The risk of death from HNSCC was greater in patients with nasopharynx or hypopharynx cancer and in patients with locally advanced disease. SPM was the leading cause of non-HNSCC death, and the most common sites of SPM death were lung (53%), esophagus (10%), and colorectal (5%) cancer.
Conclusion
Many long-term HNSCC survivors die from cancers other than HNSCC and from non-cancer causes. Routine follow-up care for HNSCC survivors should expand beyond surveillance for recurrence and new head and neck cancers.
Keywords: competing mortality, head and neck cancer, survivorship, second primary malignancy, competing risk
Background
More than 40,000 cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed each year, and nearly 250,000 survivors are living with this diagnosis in the United States.1, 2 The epidemiology of HNSCC has changed over the last two decades, with a decrease in the average age at diagnosis mostly attributable to a rise in disease associated with human papillomavirus (HPV) and a decline in tobacco-related disease.3, 4 These epidemiological changes and advances in treatment have improved the 5-year disease-specific survival rate from 55% in 1992–1996 to 66% in 2002–2006.5 However, these survivors face excess mortality beyond 5 years.6 Although less well studied in HNSCC, in other adult malignancies late excess risk has been attributed to both disease recurrence and to alternative causes of death associated with risk factors including lifestyle behaviors, genetic predisposition or treatment toxicity.7–9
Current guidelines for following HNSCC survivors generally focus on the early detection of recurrent disease and new primary head and neck tumors, advocating a schedule of head and neck physical exams that is more frequent immediately after treatment and then less frequent over time.7, 10 The vast majority of recurrences are detected within 3 years of treatment completion, but HNSCC survivors continue to face an increased risk of death compared to age- and sex-matched counterparts in the general population.11, 12
In prior studies, competing mortality analyses were used to evaluate the benefit of aggressive multimodality therapy in patients diagnosed with locally advanced disease.13, 14 Common causes of death other than HNSCC included second primary malignancy (SPM), cardiovascular and pulmonary disease.13–15 The experience of long-term survivors, or patients who survive their initial cancer treatment and the period of highest risk of recurrence has not been well characterized. Our objectives were to evaluate the timing and causes of death in a large cohort of patients diagnosed with HNSCC who survived at least 3 years from diagnosis and to identify demographic and clinical factors associated with specific causes of death to inform survivorship care.
Subjects and Methods
Data Source
We analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer registry program, a consortium of population-based cancer registries. We identified patients diagnosed between 1992 and 2000 from 13 registries and patients diagnosed between 2000 and 2005 from 18 registries covering 14% and 28% of the US population in each time period, reflecting an expansion of the SEER program after 2000. The SEER registries collect information regarding site and extent of disease, first course of cancer-directed therapy, and sociodemographics, with active follow-up for date and cause of death for all incident cancers.
Study Cohort
We identified patients aged 18 or older diagnosed with HNSCC of the oral cavity, oropharynx, nasopharynx, hypopharynx or larynx. We excluded patients who had metastatic disease at diagnosis or a prior cancer diagnosis in SEER, were missing staging information, or had no record of surgery or radiation therapy. We also excluded patients missing cause of death information (n=812). We excluded patients who died or were lost to follow-up in the first 3 years after diagnosis (n=18,889).
Outcomes
The primary outcome was cause of death, classified as (1) HNSCC, including the primary cancer or a new HNSCC; (2) a second primary malignancy (SPM) arising in a specified non-head and neck site; (3) cardiovascular disease (CVD) including coronary and cerebrovascular disease; (4) cancer of unspecified site; or (5) other cause of death (e.g., pulmonary disease, infection, and accidental deaths). The SEER registries record the underlying cause of death listed on state death certificates, based on International Classification of Diseases (ICD)-9 codes for deaths before 1999 and ICD-10 codes for deaths in 1999 and later. Death records were complete through December 2009. Cause of death was classified as HNSCC if (1) SEER coded death as due to the primary cancer; (2) cause of death was listed as HNSCC, not the primary cancer, and the patient had a record of being diagnosed with a subsequent head and neck cancer (i.e., the patient had a record of a primary oral cavity cancer and a record of a subsequent oropharynx cancer which was also the cause of death) or (3) cause of death was coded as ‘miscellaneous malignant cancer’ or, ‘in situ, benign or unknown behavior neoplasm’ and the only cancer recorded in SEER was the HNSCC. Records were compared to ensure that all patients who had cause of death classified as a specific cancer arising from a non-head and neck site or SPM had a matching cancer diagnosis recorded in SEER. Patients with more than one cancer diagnosis in SEER whose cause of death was recorded as ‘miscellaneous malignant cancer’ or ‘in situ, benign or unknown behavior neoplasm’ were categorized as death due to cancer of unspecified site.
Predictors
Demographic and disease characteristics included age, sex, race, socioeconomic status (SES), marital status, urban or rural residence, geographic region, year of diagnosis, primary site of disease, tumor grade and stage, and information about the first course of cancer-directed therapy. In the absence of individual-level information about SES, we used median income in the census tract of residence at the time of diagnosis. We classified disease stage as early or locally advanced based on the American Joint Committee on Cancer (AJCC) TNM staging schema. Early-stage disease included AJCC stage I or II, and locally advanced disease included AJCC stages III, IVa or IVb.16 Primary treatment was defined as surgery, radiation therapy or both. Chemotherapy is often administered in the outpatient setting and not routinely captured in hospital records. As a result, receipt of chemotherapy is less reliably recorded in SEER and therefore not included in the analysis.17
Statistical Analysis
We used Gray’s method for competing risks to estimate cause-specific cumulative incidence of death starting at three years after diagnosis.18, 19 Competing-risks regression models were used to predict death from HNSCC, SPM, and CVD, with observations censored at the end of follow-up and death from other causes treated as competing events. Adjusted hazard ratios (HR), 95% confidence intervals (CI) and 2-sided p-values were calculated for each predictor. We also performed several stratified analyses to assess the presence of effect modification by sex, clinical stage (early vs. locally advanced) and age (<50 years vs. 50 and older). Analysis was performed using SAS® (version 9.2, SAS Institute, Cary, NC) and R software (R Foundation for Statistical Computing, Vienna, Austria; www.rproject.org).
Results
We identified 35,958 patients diagnosed with non-metastatic HNSCC who survived at least 3 years after diagnosis (Table 1). The median age at diagnosis was 60 years (range 18 to 100) and the median follow-up from diagnosis was 7.7 years (range 3.0 to 17.9). A majority of patients were white (82%) and male (73%). After surviving the first three years following diagnosis, 36% of patients subsequently died during our follow-up period.
Table 1.
Characteristics of cohort of long-term HNSCC survivors diagnosed 1992–2005*
| All Patients | ||
|---|---|---|
| N=35,958 | % | |
| Age | ||
| Under 50 | 7,408 | 21% |
| 50–59 | 10,435 | 29% |
| 60–69 | 9,920 | 28% |
| 70+ | 8,195 | 23% |
| Sex | ||
| Male | 26,114 | 73% |
| Female | 9,844 | 27% |
| Race | ||
| White | 29,526 | 82% |
| Black | 3,528 | 10% |
| Other/unknown | 2,904 | 8% |
| Marital status | ||
| Married | 21,903 | 61% |
| Not married | 14,055 | 39% |
| Urban/rural | ||
| Metropolitan | 30,853 | 86% |
| Non-metropolitan | 5,105 | 14% |
| Geographic region | ||
| Northeast | 4,765 | 13% |
| West | 19,091 | 53% |
| Midwest | 5,588 | 15% |
| South | 6,514 | 18% |
| Income quartile | ||
| Lowest quartile | 8,155 | 23% |
| Second quartile | 10,058 | 28% |
| Third quartile | 8,979 | 25% |
| Fourth quartile | 8,766 | 24% |
| Primary site | ||
| Oral cavity | 10,826 | 29% |
| Oropharynx | 8,615 | 25% |
| Nasopharynx | 1303 | 7% |
| Hypopharynx | 2,135 | 4% |
| Larynx | 13,079 | 35% |
| Comprehensive stage** | ||
| Early stage | 16,977 | 47% |
| Locally advanced | 18,981 | 53% |
| Treatment | ||
| Surgery | 10,205 | 28% |
| Radiation | 13,251 | 37% |
| Surgery and radiation | 12,502 | 35% |
| Tumor grade | ||
| I or II | 20,797 | 58% |
| III or IV | 9,179 | 26% |
| Unknown | 5,982 | 17% |
| Year of diagnosis | ||
| 1992–1995 | 6,811 | 19% |
| 1996–2000 | 10,678 | 30% |
| 2001–2005 | 18,469 | 51% |
Survived at least 3 years after diagnosis of head and neck squamous cell carcinoma.
Comprehensive stage categorized as early if AJCC stage I or II and locally advanced if stage III, IVa or IVb.
Note: Percentages do not always sum to 100 due to rounding.
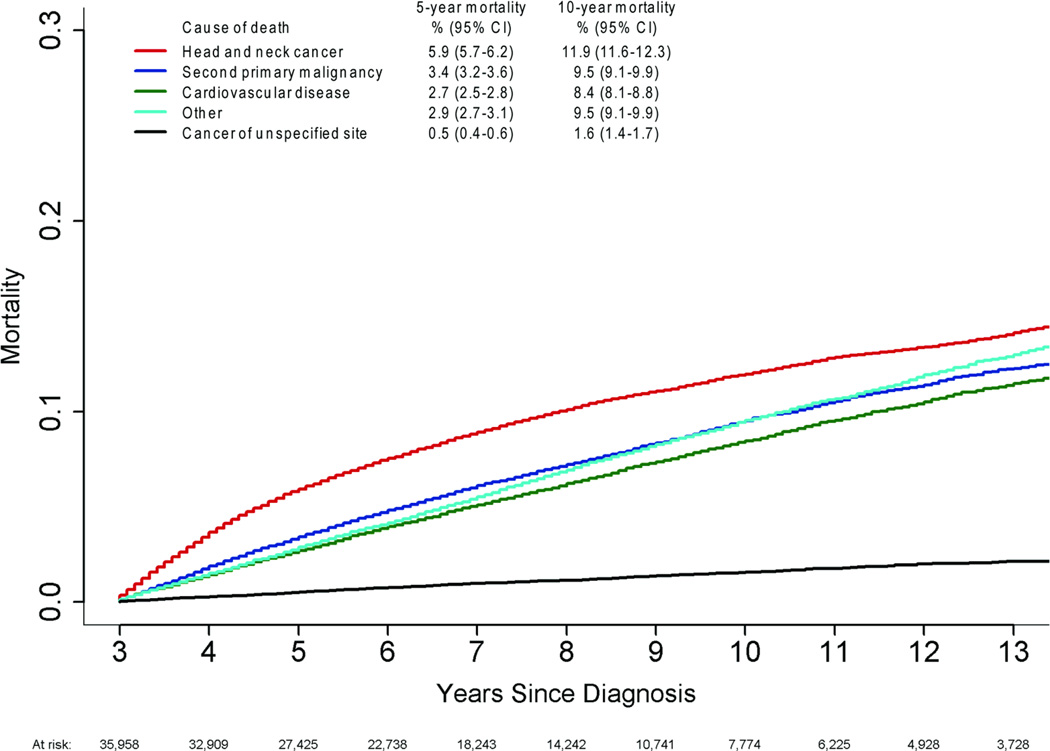
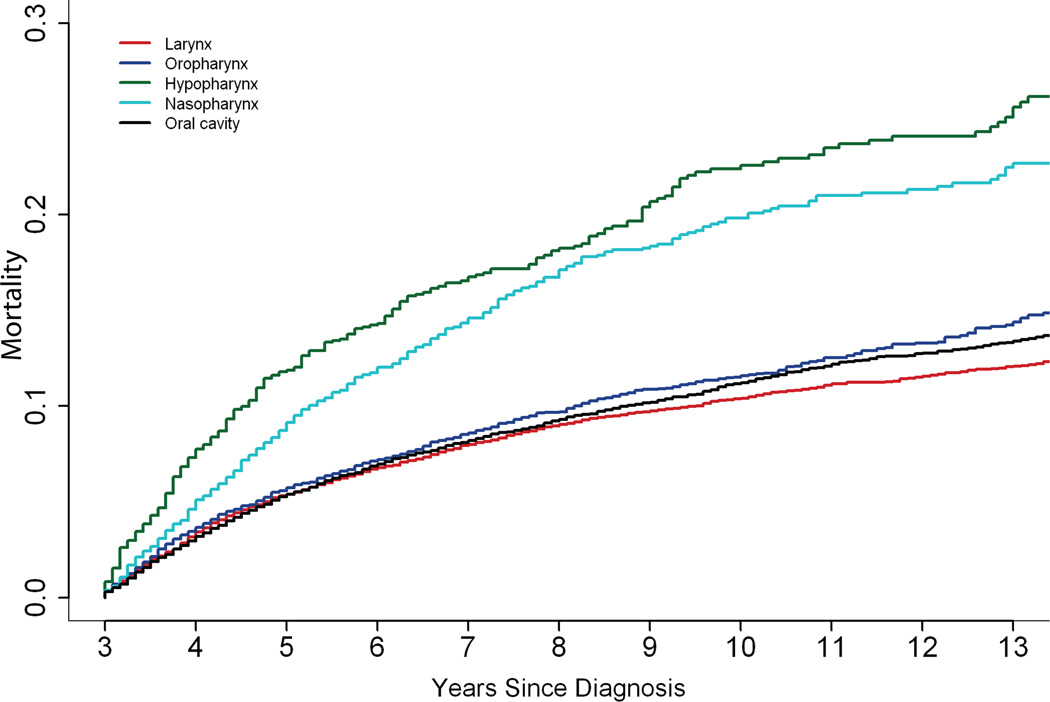
Of the 13,120 deaths, 3,852 (29%) were attributed to HNSCC, 3,007 (23%) to SPM, 2,716 (21%) to CVD, 506 (4%) to unspecified cancer, and 3,039 (23%) to all other causes (Table 2). The most commonly identified other causes of death included chronic obstructive pulmonary disease (n=777) and pneumonia and influenza (n=288). Although the risk of death from HNSCC was greatest in the first 5 years and appeared to stabilize, it remained the leading cause of death over time, with mortality rates of almost 6% at 5 years and nearly 12% at 10 years (Figure 1). Death due to a SPM, CVD or other cause continued to rise during the follow-up period. Patients with hypopharynx and nasopharynx cancer had a greater risk of death from HNSCC compared to those with other head and neck cancers (Figure 2).
Table 2.
Causes of death in long-term HNSCC survivors*
| All Deaths | Deaths in years 3–5 |
Deaths in years 5–10 |
||||
|---|---|---|---|---|---|---|
| N=13,120 | % | N=5,406 | % | N=5,890 | % | |
| Causes of death | ||||||
| Head and neck cancer | 3,852 | 29% | 2,088 | 39% | 1,433 | 24% |
| Non-head and neck cancer | 3,007 | 23% | 1,196 | 22% | 1,399 | 24% |
| Cardiovascular disease | 2,716 | 21% | 939 | 17% | 1,314 | 22% |
| Other cause of death | 3,039 | 23% | 1,003 | 19% | 1,506 | 26% |
| Unknown cancer | 506 | 4% | 180 | 3% | 238 | 4% |
Percent of deaths shown from each cause within the given time period.
Survived at least 3 years after diagnosis of head and neck squamous cell carcinoma.
Figure 1.
Cumulative incidence curves are shown for causes of death in long-term survivors of head and neck cancer.
Note: Cumulative death by cause is shown at 5 and 10 years post-diagnosis
Figure 2.
Death due to head and neck squamous cell carcinoma by primary site of disease.
The likelihood of death from HNSCC was influenced by several patient and disease characteristics. It was greater among patients with cancers of the hypopharynx, nasopharynx and oral cavity, compared to those with cancer of the larynx (Table 3). Older age and black race were also associated with an increased risk of death from HNSCC, although stratified analysis suggested an interaction between race and sex. Black men had an increased risk of death due to HNSCC compared to white men (adjusted HR 1.34, 95% CI 1.10–151), but there was no difference between black and white women.
Table 3.
Multivariable Analysis of Factors Associated with Death from Head and Neck Cancer
| Head and Neck Deaths | |||
|---|---|---|---|
| HR | 95% CI | P | |
| Age at diagnosis | |||
| Under 50 | Ref | ||
| 50–59 | 1.31 | (1.18–1.44) | <0.0001 |
| 60–69 | 1.49 | (1.35–1.65) | <0.0001 |
| 70+ | 1.62 | (1.46–1.80) | <0.0001 |
| Sex | |||
| Male | Ref | ||
| Female | 0.96 | (0.89–1.04) | NS |
| Race | |||
| White | Ref | ||
| Black | 1.31 | (1.19–1.45) | <0.0001 |
| Other/unknown | 1.09 | (0.98–1.25) | NS |
| Marital status | |||
| Married | Ref | ||
| Not married | 1.29 | (1.21–1.38) | <0.0001 |
| Geographic region | |||
| Northeast | Ref | ||
| West | 0.97 | (0.87–1.07) | NS |
| Midwest | 0.90 | (0.79–1.02) | NS |
| South | 0.94 | (0.82–1.07) | NS |
| Urban/rural | |||
| Metropolitan | Ref | ||
| Non-metropolitan | 1.02 | (0.92–1.14) | NS |
| Income quartile | |||
| Lowest | Ref | ||
| Second | 0.99 | (0.91–1.09) | NS |
| Third | 0.94 | (0.84–1.04) | NS |
| Fourth | 0.93 | (0.83–1.06) | NS |
| Primary site | |||
| Larynx | Ref | ||
| Oral Cavity | 1.42 | (1.29–1.57) | <0.0001 |
| Oropharynx | 1.03 | (0.93–1.14) | NS |
| Hypopharynx | 1.82 | (1.59–2.09) | <0.0001 |
| Nasopharynx | 1.64 | (1.42–1.90) | <0.0001 |
| Comprehensive stage* | |||
| Early stage | Ref | ||
| Locally advanced | 1.57 | (1.45–1.69) | <0.0001 |
| Treatment | |||
| Surgery only | Ref | ||
| Radiation only | 1.85 | (1.66–2.07) | <0.0001 |
| Surgery and radiation | 1.65 | (1.49–1.83) | <0.0001 |
| Tumor grade | |||
| III or IV | Ref | ||
| I or II | 1.03 | (0.94–1.12) | NS |
| Unknown | 0.92 | (0.84–1.01) | NS |
| Year of diagnosis | 0.97 | (0.96–0.98) | <0.0001 |
Comprehensive stage categorized as early if AJCC stage I or II or locally advanced if stage III, IVa or IVb.
About a quarter (23%) of all deaths during the study period were attributed to a SPM. The leading sites of death due to a non-HNSCC malignancy were lung and bronchus (N=1,605, 53%), esophagus (N=286, 10%), and colon and rectum (N=164, 5%). Factors associated with death due to an SPM included age greater than 60 years at HNSCC diagnosis, black race, locally advanced stage HNSCC, and receipt of radiation. Patients with oral cavity and hypopharynx cancers had a greater risk of death from SPM, and patients with nasopharynx and oropharynx cancers had a lower risk of death from SPM (Table 4). Deaths due to cancers of unspecified site, in patients with multiple malignancies recorded in SEER, accounted for 4% of all deaths over the study period.
Table 4.
Multivariable Analysis of Factors associated with Death from Cardiovascular Disease or a Second Primary Malignancy
| Cardiovascular Deaths | Second Malignancy Deaths | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age at diagnosis | ||||||
| Under 50 | Ref | Ref | ||||
| 50–59 | 2.65 | (2.16–3.26) | <0.001 | 2.19 | (1.89–2.52) | <0.001 |
| 60–69 | 4.79 | (3.95–5.82) | <0.001 | 3.19 | (2.78–3.66) | <0.001 |
| 70+ | 11.17 | (9.27–13.54) | <0.001 | 2.91 | (2.53–3.36) | <0.001 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 0.8 | (0.73–0.88) | <0.001 | 0.89 | (0.82–0.97) | <0.05 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 1.21 | (1.06–1.37) | <0.01 | 1.17 | (1.04–1.32) | <0.01 |
| Other/unknown | 0.77 | (0.65–0.92) | <0.01 | 0.71 | (0.59–0.85) | <0.001 |
| Marital status | ||||||
| Married | Ref | Ref | ||||
| Not married | 1.28 | (1.19–1.39) | <0.001 | 1.11 | (1.03–1.20) | <0.001 |
| Geographic region | ||||||
| Northeast | Ref | Ref | ||||
| West | 0.99 | (0.87–1.12) | NS | 1.02 | (0.90–1.15) | NS |
| Midwest | 1.19 | (1.03–1.38) | <0.05 | 1.02 | (0.88–1.18) | NS |
| South | 0.97 | (0.84–1.17) | NS | 1.17 | (1.01–1.36) | <0.05 |
| Urban/rural | 0.97 | (0.84–1.17) | NS | 1.14 | (0.98–1.32) | NS |
| Metropolitan | Ref | Ref | ||||
| Non-metropolitan | 1.12 | (1.00–1.28) | <0.05 | 1.12 | (0.99–1.26) | NS |
| Income quartile | ||||||
| Lowest | Ref | Ref | ||||
| Second | 1.07 | (0.96–1.19) | NS | 0.99 | (0.90–1.11) | NS |
| Third | 0.95 | (0.84–1.08) | NS | 0.96 | (0.85–1.08) | NS |
| Fourth | 0.91 | (0.78–1.06) | NS | 0.93 | (0.80–1.07) | NS |
| Primary site | ||||||
| Larynx | Ref | Ref | ||||
| Oral Cavity | 1.00 | (0.90–1.12) | NS | 0.96 | (0.87–1.07) | NS |
| Oropharynx | 0.79 | (0.70–0.90) | <0.001 | 0.8 | (0.72–0.90) | <0.001 |
| Hypopharynx | 1.02 | (0.84–1.12) | NS | 1.13 | (0.95–1.34) | NS |
| Nasopharynx | 0.89 | (0.70–1.12) | NS | 0.66 | (0.52–0.84) | <0.001 |
| Comprehensive stage* | ||||||
| Early stage | Ref | Ref | ||||
| Locally advanced | 1.03 | (0.95–1.12) | NS | 1.08 | (1.00–1.17) | NS |
| Treatment | ||||||
| Surgery only | Ref | Ref | ||||
| Radiation only | 1.1 | (0.99–1.25) | NS | 1.2 | (1.08–1.33) | <0.01 |
| Surgery and radiation | 1.02 | (0.92–1.14) | NS | 1.08 | (0.98–1.20) | NS |
| Tumor grade | ||||||
| III or IV | Ref | Ref | ||||
| I or II | 1.01 | (0.92–1.12) | NS | 1.03 | (0.94–1.13) | NS |
| Unknown | 0.97 | (0.88–1.08) | NS | 0.91 | (0.82–1.00) | NS |
| Year of diagnosis | 0.94 | (0.93–0.95) | <0.0001 | 0.95 | (0.94–0.96) | <0.0001 |
Comprehensive stage categorized as early if AJCC stage I or II or locally advanced if stage III, IVa or IVb.
The risk of death due to CVD increased over time. Age was the strongest predictor of CVD-related death (Table 4). Men and black patients had an increased risk of death from CVD, controlling for other characteristics. Patients with oropharynx cancers were 20% less likely to die of CVD than those with cancers of the larynx (adjusted HR 0.79, 95% CI 0.70–0.90). In analysis stratified by sex, black men had an increased risk of death due to CVD (adjusted HR 1.23, 95% CI 1.07–1.43) compared to white men, but race was not associated death due to CVD among women. In patients under age 50 at HNSCC diagnosis, women were about half as likely to die of CVD (adjusted HR 0.56, 95% CI 0.35–0.90) compared to men, but this difference between men and women was not observed in patients over 50.
Discussion
With evolving etiologies and advances in treatment, HNSCC survival has improved in recent decades, and the pool of HNSCC survivors is likely to continue to grow.5 While HNSCC was the leading cause of death in this population-based cohort of patients who survived at least 3 years from diagnosis, the risks of death due to SPM and CVD were considerable, and they varied with demographic characteristics, disease site and primary treatment. Across the cohort, the risk of dying from a cause other than HNSCC increased over time.
Compared with the general population, head and neck cancer survivors face an increased risk of death from any cause.11, 12 One study found an excess mortality risk of about 20% that stabilized at 3 or 4 years after diagnosis and persisted for at least a decade after.12 In addition to excess risk due to HNSCC, increased mortality may reflect tobacco use in the head and neck cancer population. Cigarette smoking is the most common risk factor for HNSCC and is also a major risk factor for other malignancies, cardiovascular and pulmonary disease. Similar to our study, the leading causes of death in the general US population vary by age. In 2010, cancer was leading cause of death for people between the ages of 45 and 64 years while heart disease became the most common cause of death in people aged 65 years or older.20 However, estimates of excess mortality by each cause in long-term HNSCC survivors should involve comparisons with peers of similar age, race and smoking history.
Second primary malignancies contribute to decreased survival in HNSCC survivors, with an estimated annual incidence of 3–5% that varies by tobacco and alcohol history.21–23 In our study, SPM in sites other than the head and neck accounted for about a quarter of deaths by 10 years after HNSCC diagnosis. Field cancerization of the entire upper aerodigestive tract due to tobacco or alcohol exposure is the cause of increased SPM risk in HNSCC survivors.24 Our findings support this, with lung and esophageal cancer accounting for nearly two-thirds of deaths due to SPM. Patients with primary head and neck cancers with possible viral etiologies were less likely to die of SPM than larynx cancer which mostly due to smoking, supporting the field cancerization theory.24, 25 Colorectal cancer was the third leading site of SPM death and has also been linked to prior smoking history, although to a lesser degree.26 Screening for colorectal cancer is routinely recommended for adults age 50 and older and should not be overlooked in HNSCC survivors. Breast and cervical cancer, for which routine screening is also recommended, accounted for 3% and 1% of non-HNSCC cancer deaths in women, respectively. Prostate cancer accounted for 3% of non-HNSCC cancer deaths in men.
Cardiovascular disease is a major source of morbidity in cancer survivors, particularly those with unhealthy lifestyle behaviors and cardiotoxic exposures.27, 28 We found that older age at diagnosis was the strongest predictor of CVD mortality. Patients who were 70 year or older had an 11 times greater risk than those younger than 50 years. Although prior studies have identified an increased risk of cerebrovascular disease following radiation to the head and neck, we were unable to confirm this association due to the low number of deaths attributed to cerebrovascular events.29, 30 However, we found a substantial incidence of CVD mortality, accounting for 21% of all deaths by the end of the follow-up period. The CVD deaths in our cohort were likely a result of an interaction between traditional cardiovascular risk factors such as age, tobacco use, and comorbid conditions and treatment toxicity. In other highly curable cancers diagnosed in younger patients such as testicular cancer and Hodgkin’s lymphoma, late effects of treatment become increasingly significant as patients live longer.9, 28 Longer follow-up will be needed to better understand the interaction between behavioral, comorbidity, and treatment in subsequent cardiovascular death.
Patients with oropharynx cancer resulting from an infection with HPV are the fastest growing subset of HNSCC survivors over the last decade.3 These patients are younger, have less comorbidity, and can expect better tumor control at the time of diagnosis than patients with non-HPV-related oropharynx cancer.31, 32 As a result of a longer survivorship phase, patients with HPV-related oropharynx cancer are at an elevated risk of late toxicities. Although we do not know the HPV status of tumors in our study, patients with oropharynx cancer were less likely to die from both SPM and CVD. However, longer follow-up and information about HPV status will be needed to fully assess the possible long-term impact of surgery, radiation and specific chemotherapy regimens on excess morbidity and cause of death in this subset of HNSCC survivors.
The methodologic strengths of our study include the very large sample and the detailed information included in the SEER database.33 However, several limitations should be noted. The SEER registries record the underlying cause of death listed in state death certificates, which may be subject to misattribution.34 Although prior studies support the use of information from death certificates to study cancer mortality rates, they also suggest that death due to coronary artery disease may be overrepresented as a cause of death on death certificates, especially in the oldest patients.35–37 Additionally, without information about chemotherapy, we could not be sure whether our estimate of the impact of radiation therapy on cause-specific mortality reflects radiation alone or combined chemoradiation, a common treatment strategy for many head and neck cancers.10 Finally, information regarding important potential confounders, such as performance status, comorbidity, HPV-status, and smoking history was not available in the dataset.
Long-term head and neck cancer survivors are a heterogeneous group who face an increased risk of death from multiple causes that vary by demographic, health, lifestyle, and disease characteristics including time since diagnosis. Although head and neck cancer is the leading cause of death and surveillance remains the cornerstone of survivorship care, our analysis suggests that those patients who survived the first three years following diagnosis face a considerable risk of death from non-HNSCC causes including SPM and CVD. Primary care addressing screening for other cancers, modification of known risk factors and management of comorbid illnesses should play an important role in the care of long-term survivors.
Acknowledgments
Funding: This work was supported by the Survivorship Initiative at Memorial Sloan-Kettering Cancer and Chanel, Inc (Baxi, PI), and the National Institutes of Health (Oeffinger KC, PI: K05-CA-160724).
Footnotes
The authors have no financial interests, disclosures, conflicts of interest.
We evaluated the cause and timing of death in a large cohort of long-term survivors of head and neck squamous cell carcinoma. Using a competing risk model, we identified varying risk of death based on demographic and clinical factors including age, primary site of disease and race.
Reference List
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer. Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 4.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the U.S.: 1998–2003. Cancer. 2008;113:2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 5.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15:994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skarsgard DP. Cancers of the upper aerodigestive tract in Ontario, Canada, and the United States. Cancer. 2000;88:1728–1738. doi: 10.1002/(sici)1097-0142(20000401)88:7<1728::aid-cncr29>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Bouillon K, Haddy N, Delaloge S, et al. Long-Term Cardiovascular Mortality After Radiotherapy for Breast Cancer. Journal of the American College of Cardiology. 2011;57:445–452. doi: 10.1016/j.jacc.2010.08.638. [DOI] [PubMed] [Google Scholar]
- 8.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Belt-Dusebout AW, Nuver J, de Wit R, et al. Long-Term Risk of Cardiovascular Disease in 5-Year Survivors of Testicular Cancer. Journal of clinical oncology. 2006;24:467–475. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 10.Pfister DG, Ang KK, Brizel DM, et al. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Head and neck cancers. J Natl Compr Canc Netw. 2011;9:596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 11.van der Schroeff MP, van de Schans SAM, Piccirillo JF, Langeveld TPM, Baatenburg de Jong RJ, Janssen-Heijnen MLG. Conditional relative survival in head and neck squamous cell carcinoma: Permanent excess mortality risk for long-term survivors. Head Neck. 2010;32:1613–1618. doi: 10.1002/hed.21369. [DOI] [PubMed] [Google Scholar]
- 12.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 13.Rose BS. Population-based study of competing mortality in head and neck cancer. Journal of clinical oncology. 2011;29:3503–3509. doi: 10.1200/JCO.2011.35.7301. [DOI] [PubMed] [Google Scholar]
- 14.Mell LK. Predictors of competing mortality in advanced head and neck cancer. Journal of clinical oncology. 2010;28:15–20. doi: 10.1200/JCO.2008.20.9288. [DOI] [PubMed] [Google Scholar]
- 15.Argiris A. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clinical cancer research. 2004;10:1956–1962. doi: 10.1158/1078-0432.ccr-03-1077. [DOI] [PubMed] [Google Scholar]
- 16.AJCC Cancer Staging Manual. 2010 [Google Scholar]
- 17.Warren Can Cancer Registry Data Be Used to Study Cancer Treatment? Medical care. 2003;41:1003–1005. doi: 10.1097/01.MLR.0000086827.00805.B5. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 19.Jason PF, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 20.Center for Disease Control (CDC) National Center for Health Statistics. CID_All_Deaths_By-Age_Group_2010-a. 2013 Oct 24;
- 21.Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343–1353. doi: 10.1002/1097-0142(19950315)75:6<1343::aid-cncr2820750617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Vikram B. Changing patterns of failure in advanced head and neck cancer. Arch Otolaryngol. 1984;110:564–565. doi: 10.1001/archotol.1984.00800350006003. [DOI] [PubMed] [Google Scholar]
- 23.Morris LGT. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. Journal of clinical oncology. 2011;29:739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Califano J, van der Riet P, Westra W, et al. Genetic Progression Model for Head and Neck Cancer: Implications for Field Cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 25.Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. An Updated Review of the Epidemiological Evidence that Cigarette Smoking Increases Risk of Colorectal Cancer. Cancer Epidemiology Biomarkers & Prevention. 2001;10:725–731. [PubMed] [Google Scholar]
- 27.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009:339. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular Status in Long-Term Survivors of Hodgkin's Disease Treated With Chest Radiotherapy. Journal of clinical oncology. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 29.Partap S. Stroke and Cerebrovascular Complications in Childhood Cancer Survivors. Seminars in Pediatric Neurology. 2012;19:18–24. doi: 10.1016/j.spen.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular Disease Risk in Older Head and Neck Cancer Patients After Radiotherapy. Journal of clinical oncology. 2008;26:5119–5125. doi: 10.1200/JCO.2008.16.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA: A Cancer Journal for Clinicians. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 32.Ang KK, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. New England Journal of Medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER program of the national cancer institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Kircher T, Nelson J, Burdo H. The autopsy as a measure of accuracy of the death certificate. N Engl J Med. 1985;313:1263–1269. doi: 10.1056/NEJM198511143132005. [DOI] [PubMed] [Google Scholar]
- 35.Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71:242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Percy C, Ries LG, Van Holten VD. The accuracy of liver cancer as the underlying cause of death on death certificates. Public Health Rep. 1990;105:361–367. [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of Death Certificates for Coding Coronary Heart Disease as the Cause of Death. Annals of internal medicine. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]