Abstract
We conducted a phase I study to assess safety, pharmacokinetics, pharmacodynamics and activity of lonafarnib plus gemcitabine. Subjects received oral lonafarnib twice daily and gemcitabine on days 1, 8 and 15 every 28 days; multiple dose levels were explored. Lonafarnib had no apparent effect on gemcitabine PK. Mean lonafarnib half-life ranged from 4 to 7 hours; median Tmax values ranged from 4 to 8 hours. Two patients had partial response; 7 patients had stable disease ≥6 months. Oral lonafarnib at 150 mg AM/100 mg PM plus gemcitabine at 1000 mg/m2 is the maximum tolerated dose with acceptable safety and tolerability.
Keywords: Lonafarnib, Gemcitabine, Phase I, Advanced Cancer
Introduction
Protein prenylation is the process of post-translational lipid modification via the covalent addition of a hydrophobic isoprenoid residue, either farnesyl pyrophosphate or geranylgeranyl pyrophosphate, to the carboxyl terminus[1, 2]. This process is mediated by three enzymes, farnesyl transferase and geranylgeranyl transferase I and II. Prenylation is critical for the proper membrane localization and function of many proteins involved in a wide variety of cellular functions[3, 4].
Interest in protein prenylation intensified in the 1980s with the discovery that Ras proteins undergo farnesylation[3, 5]. Ras proteins serve as molecular switches that transmit cell surface signals to the nucleus and belong to the small guanosine triphosphate binding protein (G protein) superfamily[6]. Activating point mutations in RAS genes are found in 30% of human cancers, including 90% of pancreatic cancers and 50% of colon and thyroid cancers[7, 8]. Preclinical studies have demonstrated that farnesylation is essential for the transforming properties of activated Ras, making this step a promising target for anti-neoplastic drug development[9–11].
Lonafarnib (Sarasar, Merck & Company, formerly Schering Plough Research Institute, Kenilworth, NJ, USA, previously known as SCH 66336) is a potent and specific orally bioavailable tricyclic non-peptide farnesyl transferase inhibitor (FTI). In vitro, lonafarnib inhibits H-Ras processing in whole cells and blocks the anchorage independent growth properties of fibroblasts and human tumor cell lines expressing activated K-Ras proteins[12, 13]. In the nude mouse, lonafarnib potently inhibits growth of a wide array human tumor xenograft models[14, 15]. Multiple single agent Phase I studies with lonafarnib indicate the drug is well-tolerated and exhibits clinical activity[16–19].
Gemcitabine is a nucleoside analog that, after metabolic activation, inhibits DNA synthesis, thereby leading to cell cycle-specific cytotoxicity in S phase and blockade of cell cycle progression through the G1/S phase boundary. Gemcitabine is indicated for the treatment of advanced breast, lung, pancreatic and ovarian cancer.
Preclinical data suggests the clinical activity of FTIs combined with gemcitabine may be augmented when compared to each agent alone[20]. In addition, gemcitabine demonstrates non-overlapping mechanism of action and toxicities with lonafarnib. For these reasons, we performed a phase I dose escalation study to determine the maximum tolerated dose (MTD), pharmacokinetics and to preliminarily evaluate the clinical activity of lonafarnib plus gemcitabine in advanced solid tumors.
Materials and Methods
Patient Eligibility
Eligibility criteria included histologically confirmed solid malignancy refractory to standard therapy; presence of measurable disease; age ≥ 18; World Health Organization Performance Status of 0, 1 or 2; baseline toxicity grade ≤ 1; adequate end organ function assessed within 14 days prior to commencement of therapy [absolute neutrophil count (ANC) > 1.5 × 109/L, platelet count ≥ 100 × 109/L, hemoglobin ≥ 10 g/dL, serum creatinine ≤ 1.5 times the upper limit of normal (ULN), bilirubin ≤ 2.0 mg/dL, alanine aminotransaminase or aspartate aminotranaminase ≤ 3 times ULN (5 fold if elevations are due to liver metastases)]; willingness to comply with treatment and follow-up; and life expectancy ≥ 12 weeks. Exclusion criteria included hematological malignancies; poorly controlled systemic illness or infection; inability to take oral medication; chemotherapy, radiation or biologic or investigational therapy concurrently or within four weeks prior to administration of lonafarnib (6 weeks for mitomycin C or nitrosourea); previous wide field radiation therapy to ≥25% of bone marrow such as pelvic radiation; prior bone marrow or peripheral stem cell transplantation; pregnancy or lactation; HIV positivity or AIDS-related illness; and active central nervous system metastasis. Men or women of childbearing potential were required to use an effective contraception throughout the study period, and women of childbearing potential were required to have a negative urine or serum pregnancy test within 24 hours prior to first administration of lonafarnib. The study protocol was approved by the institutional review boards of the respective study centers and followed the guidelines of the Helsinki Declaration. Written informed consent was obtained prior to study-related procedures. The study was conducted from October 1998 to August 2001.
Treatment
This was an open-label, dose-escalation, multiple-dose study. Lonafarnib was administered twice daily with food continuously, and gemcitabine was dosed as a 30 minute intravenous infusion on days 1, 8, and 15. Both drugs were given on 28 day cycles. The dose escalation scheme is outlined in Table 1. The starting dose for lonafarnib was 150 mg orally twice daily, 75% of the established continuous monotherapy dose[21]. The starting dose of weekly gemcitabine was 750 mg/m2.
Table 1.
Dose escalation scheme and cycle 1 dose-limiting events (DLTs).
| Dose Level | Lonafarnib | Gemcitabine* | No. Patients | No. Patients with DLTs |
DLT events |
|---|---|---|---|---|---|
| −1 | 100 mg BID | 750 mg/m2 | 4 | 0 | - |
| 1 | 150 mg BID | 750 mg/m2 | 4 | 2 | Grade 3 headache, anorexia, nausea, and vomiting. |
| Grade 3 anorexia, diarrhea, dehydration, and fatigue | |||||
| Intermediate | |||||
| A | 100 mg BID | 1000 mg/m2 | 3 | 0 | - |
| B | 150 mg AM, 100 mg PM | 1000 mg/m2 | 7 | 1 | Grade 4 neutropenia and thrombocytopenia |
| C | 200 mg BID | 600 mg/m2 | 7 | 0 | - |
Intravenously days 1, 8, 15 every 28 days
Cycles were repeated until unacceptable toxicities or disease progression. Patients with stable disease or disease response ≥ 8 months were given the option of discontinuing gemcitabine and continuing lonafarnib monotherapy. The use of concomitant colony stimulating factors including erythropoietin was prohibited.
Patient Monitoring
History, physical examination, performance status, laboratory tests and urinalysis were performed at baseline and every 28 days. Electrocardiography was performed at baseline and every 28 days to monitor for QTc abnormalities. Because of documented retinal toxicity with high dose lonafarnib in animal studies, direct ophthalmoscopy, visual acuity and color vision testing were performed by an ophthalmologist at baseline, end of cycle 1 and end of study. Retinal photography was obtained at baseline and as clinically indicated.
Adverse events were assessed using National Cancer Institute Common Toxicity Criteria (NCI CTC) version 2.0. Lonafarnib was held for ANC <0.5 × 109/L or platelet count <50,000 × 109/L and restarted one dose level lower when ANC >0.5 × 109/L and platelet count >50 × 109/L. Lonafarnib was held for serum creatinine ≥2X ULN (twice baseline level if serum creatinine elevated at baseline) and resumed at the next lower dose level when serum creatinine returned to baseline. If QTc increased to >500 msec and to >130% of baseline QTc, the dose of lonafarnib was decreased by one level.
Gemcitabine was administered at full dose if ANC ≥1.0 × 109/L and platelet count ≥100 × 109/L; at 75% dose if ANC ≥ 0.5–1.0 × 109/L or platelet ≥ 50–100 × 109/L; and held if ANC <0.5 × 109/L or platelet count < 50 × 109/L. In the event that dose reduction or holding was required, gemcitabine was administered at one dose level lower for the following cycle. Lonafarnib was decreased by one dose level for occurrence of grade 3 nausea, vomiting or diarrhea despite optimal supportive therapy. If grade 3 diarrhea persisted longer than 48 hours despite lonafarnib dose reduction, gemcitabine dose was reduced by 25%.
Tumor evaluation by computed tomography or magnetic resonance imaging was performed at baseline and every 2 cycles. Clinically evaluable lesions were measured every cycle. Evaluation of tumor response was performed using modified World Health Organization reporting of response criteria.
Dose Escalation
A rule-based traditional 3+3 dose escalation design was used to enroll a maximum of 6 patients per dose level and a total of 6 patients at the MTD level[22]. The MTD was determined as the dose level where 0 or 1 out of 6 patients experienced DLT and at least 2 patients experienced DLT at the next higher level (DLT dose level). With the design and the sample size used for this study, doses with high toxicity rates (>40%) had a high probability >0.70 of being declared as DLT. Patients who withdrew from study prior to completion of cycle 1 other than for serious adverse events or dose limiting toxicity were replaced.
DLTs were defined as ANC <0.5 × 109/L for longer than 5 days; ANC <0.5 × 109/L with fever (≥38.3°C); platelets <25 × 109/L; hemoglobin <6.5 g/dL; any treatment-emergent grade ≥3 non-hematologic toxicity; grade ≥3 nausea/vomiting while receiving an optimal anti-emetic regimen for prophylaxis and management (i.e., consisting of a 5-HT3 antagonist on an optimal dose-schedule); grade ≥3 diarrhea while receiving an optimal anti-diarrheal regimen; or treatment delay for toxicity lasting greater than 2 weeks.
Protocol amendments were carried out during the course of the trial to facilitate the evaluation of doses intermediate to the initial scheme in order to define more accurately the recommended phase II dose.
Endpoints
The primary objective of this study was to characterize the safety, tolerability, maximum tolerated dose (MTD) and dose-limiting toxicity (DLT) of lonafarnib when administered orally in combination with gemcitabine in subjects with advanced malignancy. Secondary objectives of this study were to characterize the multiple-dose pharmacokinetics of oral lonafarnib; to characterize the pharmacokinetics of gemcitabine when administered in combination with lonafarnib; preliminary evaluation of the anti-tumor activity of lonafarnib in combination with gemcitabine; and preliminary evaluation of prelamin A as a marker of farnesyl transferase inhibition.
Pharmacokinetic studies
At each dose level, multi-dose pharmacokinetic evaluation of lonafarnib was performed on day 15 in cycles 1 and 2. Blood samples for lonafarnib concentration were collected just prior to the morning dose (0 hr) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 20 and 24 hours. On day 14, evening doses of lonafarnib were withheld to evaluate pharmacokinetics over 24 hours. In addition, a single blood sample was drawn prior to each infusion of gemcitabine.
Gemcitabine pharmacokinetics were determined on cycle 1 days 1 and 15. Blood samples for gemcitabine concentration were collected just prior to infusion, at 15 minutes, end of infusion (30 minutes), 45 minutes, 1 hour, 1.5, 2, 3, 4, 6, 8, 10 and 12 hours after the beginning of the infusion from the arm opposite the infusion. On cycle 1 day 1, the morning dose of lonafarnib was withheld to evaluate gemcitabine pharmacokinetics.
Plasma lonafarnib and gemcitabine concentrations were determined using validated liquid chromatography with tandem mass spectrometric detection (LC-MS/MS) assays. The lower limits of quantitation were 5.00 and 10.1 ng/mL for lonafarnib and gemcitabine, respectively. The corresponding linear concentration ranges were 5.00–2500 ng/mL and 10.1–2535 ng/mL, respectively. The analyses for lonafarnib were performed at Taylor Technology, Princeton, NJ; the analyses for gemcitabine were performed at MDS Pharma Services, Inc., Saint-Laurent, Quebec, Canada.
Individual plasma lonafarnib and gemcitabine concentrations were analyzed using model-independent methods. The maximum plasma concentration (Cmax) and time of maximum plasma concentration (Tmax) were the observed values. The terminal phase rate constant (k) was calculated as the negative slope of the log:linear terminal portion of the plasma concentration-time curve using linear regression. The terminal phase half-life, t½, was calculated as 0.693/k. The area under the plasma concentration-time curve during the 12 hour dosing interval, AUC(τ), and from time 0 to the time of final quantifiable sample, AUC(tf), were calculated using the linear trapezoidal method.
Total body clearance (CL/F) for lonafarnib following multiple-dose oral administration was calculated by the following equation: CL/F= Dose/AUC(τ).
Apparent volume of distribution (Vdarea/F) for lonafarnib following multiple-dose oral administration was calculated as: Vdarea/F = [Dose/AUC(τ)]/k.
Pharmacodynamics
Prelamin A is a polypeptide dependent on farnesylation for processing; its accumulation in buccal mucosal cells serves as an in-vivo marker of farnesyl transferase inhibition. Buccal swabs were obtained on cycle 1 days 1 and 15 and fixed with acetone. Double label immunohistochemistry using mouse-anti-lamin A and rabbit anti-prelamin A antibodies followed by fluorochrome-labeled secondary antibodies was performed as previously described[16].
Results
Patient Demographics
Twenty-five subjects were enrolled in the present study. Baseline demographic and clinical characteristics are summarized in Table 2. The median age was 56 years (range 26–74). The majority of patients had performance status 0–1. Ten patients had received 2 or more prior lines of systemic chemotherapy for advanced disease.
Table 2.
Baseline Clinical and Demographic Characteristics
| Patients (N=25) | ||
|---|---|---|
| Characteristics | No. | % |
| Gender | ||
| Female | 12 | 48 |
| Male | 13 | 52 |
| Ethnicity | ||
| White | 23 | 92 |
| Asian | 1 | 4 |
| Native American Indian | 1 | 4 |
| Age, years | ||
| Median | 56 | |
| Range | 26–74 | |
| ECOG performance status | ||
| 0 | 1 | 4 |
| 1 | 22 | 88 |
| 2 | 2 | 8 |
| Primary tumor | ||
| Pancreatic adenocarcinoma | 8 | 32 |
| Non-small cell lung cancer | 6 | 24 |
| Renal cell carcinoma | 2 | 8 |
| Sarcoma | 2 | 8 |
| Others# | 7 | 28 |
| Prior chemotherapy | ||
| 0 | 9 | 36 |
| 1 | 5 | 20 |
| 2 | 8 | 32 |
| ≥3 | 2 | 8 |
| unknown | 1 | 4 |
ECOG: Eastern Cooperative Group
one each of adenocarcinoma unknown primary, adenoid cystic carcinoma, ampullary carcinoma, hepatocellular carcinoma, mesothlioma, small cell lung cancer, squamous cell carcinoma ethmoid sinus
Dose Escalation and MTD
The dose escalation schedule and DLTs are summarized in Table 1.
Two patients in dose level 1 (lonafarnib 150 mg BID, gemcitabine 750 mg/m2) developed DLTs consisting of grade 3 anorexia, diarrhea, dehydration, and fatigue; and grade 3 headache, anorexia, nausea, and vomiting, respectively. Four patients recruited to the next dose level lower (dose level -1: lonafarnib 100 mg BID, gemcitabine 750 mg/m2) completed cycle 1 without DLT. Because it was felt that both lonafarnib and gemcitabine could potentially be subtherapeutic at dose level -1, intermediate doses A (lonafarnib 100 mg BID) and B (lonafarnib 150 mg AM and 100 mg PM) were then examined (Table 1). Intermediate level C was also added to explore full dose lonafarnib 200 mg BID with low dose gemcitabine 600 mg/m2. Hence, intermediate cohorts A, B and C provided further guidance with regard to drug dosing, safety and tolerability. No DLTs were observed at intermediate levels A and C. One of 7 patients developed dose limiting grade 4 neutropenia and thrombocytopenia at intermediate level B. The MTD was thus determined to be lonafarnib 150 mg AM and, 100 mg PM with gemcitabine 1000 mg/m2 on days 1, 8 and 15 every 28 days. This was also the RPTD given the tolerability profile and clinically relevant dose of gemcitabine.
Safety
The incidence of treatment-related toxicities by dose level is shown in Table 3.
Table 3.
Common Treatment Related Toxicities
| Toxicity | Total | Level -1 (100+750) |
Level 1 (150+750) |
Intermediate A (100+1000) |
Intermediate B (150/100+1000) |
Intermediate C (200+600) |
|---|---|---|---|---|---|---|
| (N=25) | (N=4) | (N=4) | (N=3) | (N=7) | (N=7) | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Anemia | ||||||
| Grade 1–2 | 12 (48) | 2 (50) | 3 (75) | 1 (33) | 4 (57) | 2 (29) |
| Grade 3–4 | 3 (12) | 0 | 0 | 1 (33) | 2 (29) | 0 |
| Anorexia | ||||||
| Grade 1–2 | 17 (68) | 4 (100) | 1 (25) | 3 (100) | 4 (57) | 5 (71) |
| Grade 3–4 | 3 (12) | 0 | 3 (75) | 0 | 0 | 0 |
| Diarrhea | ||||||
| Grade 1–2 | 12 (48) | 2 (50) | 2 (50) | 2 (67) | 4 (57) | 2 (29) |
| Grade 3–4 | 3 (12) | 1 (25) | 1 (25) | 0 | 0 | 1 (14) |
| Fatigue | ||||||
| Grade 1–2 | 19 (76) | 3 (75) | 3(75) | 2 (67) | 5 (71) | 6 (86) |
| Grade 3–4 | 5 (20) | 1 (25) | 1 (25) | 1 (33) | 1 (14) | 1 (14) |
| Headache | ||||||
| Grade 1–2 | 11 (44) | 1 (25) | 2 (50) | 1 (33) | 5 (71) | 2 (29) |
| Grade 3–4 | 3 (12) | 0 | 2 (50) | 1 (33) | 0 | 0 |
| Nausea | ||||||
| Grade 1–2 | 20 (80) | 3 (75) | 2 (50) | 3 (100) | 5 (71) | 7 (100) |
| Grade 3–4 | 2 (8) | 0 | 2 (50) | 0 | 0 | 0 |
| Neutropenia | ||||||
| Grade 1–2 | 9 (36) | 1 (25) | 2 (50) | 1 (33) | 2 (29) | 3 (43) |
| Grade 3–4 | 6 (24) | 1 (25) | 1 (25) | 0 | 3 (43) | 1 (14) |
| Thrombocytopenia | ||||||
| Grade 1–2 | 9 (36) | 0 | 2 (50) | 1 (33) | 2 (29) | 4 (57) |
| Grade 3–4 | 4 (16) | 0 | 0 | 1 (33) | 2 (29) | 1 (14) |
| Vomiting | ||||||
| Grade 1–2 | 13 (52) | 0 | 3 (75) | 3 (100) | 3 (43) | 4 (57) |
| Grade 3–4 | 2 (8) | 1 (25) | 1 (25) | 0 | 0 | 0 |
| Weight Loss | ||||||
| Grade 1–2 | 9 (36) | 2 (50) | 1 (25) | 0 | 2 (29) | 4 (57) |
| Grade 3–4 | 0 | 0 | 0 | 0 | 0 | 0 |
Considering cycle 1 only, treatment-emergent adverse events (AE) were reported by 92% (23/25) of patients. The most commonly reported (≥33% incidence) events were: anorexia (72%), fatigue (72%), nausea (72%), diarrhea (52%), neutropenia (48%), vomiting (48%), and headache (40%). Grade 3 and 4 AEs were reported by 36% (9/25) of patients, most commonly (≥10% incidence) anorexia (12%), fatigue (12%), and neutropenia (12%).
Considering all cycles, treatment-related AEs were reported by 96% (24/25) of patients. The most commonly reported (≥33% incidence) events were fatigue (96%), nausea (88%), anorexia (80%), vomiting (60%), diarrhea (60%), neutropenia (60%), headache (56%), anemia (52%), thrombocytopenia (48%), and weight decrease (36%). Grade 3 or 4 AEs occurred in 68% (17/25) of patients, most commonly (≥10% incidence) neutropenia (24%), fatigue (20%), thrombocytopenia (16%), anorexia (12%), headache (12%), and diarrhea (12%). There were two on-study deaths resulting from disease progression and deemed unrelated to the study treatment; there were no deaths attributed to study treatment.
Efficacy
Twenty-five patients were evaluable for efficacy. There were no complete responses. Two patients experienced partial responses: a 37-year old female with sarcoma (response duration of 22 months), and a 56-year old male with pancreatic adenocarcinoma (response duration of 2 months). Four patients had stable disease for over a year (13, 14, 16, and 20 months, respectively), and eight additional subjects had stable disease ranging from 3 months to 10 months (Table 4). Only one patient had known prior gemcitabine treatment.
Table 4.
Characteristics of Patients with Partial Response and Prolonged Stable Disease ≥ 6 months
| Age/Sex | Lonafarnib + Gemcitabine Dose |
Primary Tumor | Response | Duration (Months) |
Prior Treatment |
|---|---|---|---|---|---|
| F/37 | 150/100+1000 | Sarcoma | PR | 22 | Ifosphamide, MAID, radiotherapy |
| M/56 | 100+1000 | Pancreatic adenocarcinoma | PR | 2 | Not available |
| F/34 | 150+750 | Pancreatic cancer | SD | 20 | Gemcitabine, 5 FU, SU5416, immunotherapy |
| F/73 | 150/100+1000 | Non small cell lung cancer | SD | 16 | Paclitaxel carboplatin, vinorelbine |
| M/48 | 200+600 | Adenocarcinoma unknown primary | SD | 14 | Radiotherapy |
| F/69 | 150/100+1000 | Malignant mesothelioma | SD | 13 | Gene therapy |
| F/55 | 200+600 | Small cell lung cancer | SD | 10 | Etoposide carboplatin, topotecan |
| F/63 | 150+750 | Non small cell lung cancer | SD | 8 | Paclitaxel carboplatin, Vinorelbine, thalidomide |
| M/70 | 150+750 | Renal cell carcinoma | SD | 8 | IL-2, interferon, 5-FU, KW2189, Radiotherapy |
F= Female; M= Male PR= Partial response;
SD= Stable disease
5-FU= 5-Fluorouracil; KW2189 = Duocamycin B2 Analog, MAID= Mesna, Adriamycin, Ifosphamide, Dacarbazine; SU5418 = Semaxanib
Lonafarnib Pharmacokinetics
Lonafarnib was slowly absorbed following oral administration with food. Median Tmax values ranged from 4 to 8 hours. Following twice daily multiple doses of lonafarnib in combination with weekly gemcitabine, mean half-life (t1/2) ranged from 4 to 7 hours, and mean total body clearance (CL/F) was 163–594 mL/min. Dose dependency in systemic exposure was confounded by high inter-subject variability in total body clearance. Nonetheless, the greatest AUC and Cmax values were observed at the highest dose level (Table 5).
Table 5.
Lonafarnib plus Gemcitabine Pharmacokinetic Parameter Estimates
| Lonafarnib | |||||||
|---|---|---|---|---|---|---|---|
| Lonafarnib+Gemcitabine Dose |
Cycle | No. of subjects |
Cmax (ng/mL) |
Tmax*** (hour) |
AUC (τ) (ng*hr/mL) |
CL/F (nL/min) |
T½ (hour) |
| 100+750 | 1 | 4 | 717 | 5.0 | 5995 | 504 | 5.21 |
| 2 | 3 | 757 | 4.0 | 6033 | 571 | 3.50 | |
| 100+1000 | 1 | 3 | 1007 | 4.0 | 8201 | 577 | 3.80 |
| 2 | 2 | 645 | 5.5 | 5945 | 370 | 3.79 | |
| 150+750 | 1 | 2 | 779 | 5.5 | 5790 | 437 | 3.67 |
| 2 | 1 | 1500 | 8.0 | 15325 | 163 | 6.68 | |
| 150/100**+1000 | 1 | 6 | 1078 | 6.0 | 8947 | 413 | 5.57 |
| 2 | 6 | 736 | 5.0 | 5634 | 440 | 4.68 | |
| 200+600 | 1 | 5 | 2044 | 6.0 | 16812 | 594 | 5.32 |
| 2 | 4 | 1885 | 5.0 | 14544 | 261 | 4.78 | |
| Gemcitabine | |||||
|---|---|---|---|---|---|
| Lonafarnib+Gemcitabine Dose |
Day | No. of subjects |
Cmax (ng/mL) |
Tmax*** (hour) |
AUC (τ) (ng*hr/mL) |
| 100+750 | 1 | 4 | 11307 | 0.50 | 5382 |
| 15 | 4 | 14953 | 0.50 | 7889 | |
| 100+1000 | 1 | 2 | 10163 | 0.38 | 5075 |
| 15 | 3 | 9797 | 0.25 | 5064 | |
| 150+750 | 1 | 2 | 12495 | 0.25 | 5948 |
| 15 | 2 | 11653 | 0.50 | 8005 | |
| 150/100**+1000 | 1 | 7 | 15083 | 0.50 | 8801 |
| 15 | 6 | 17471 | 0.50 | 10111 | |
| 200+600 | 1 | 6 | 7934 | 0.38 | 4141 |
| 15 | 7 | 11191 | 0.25 | 4966 | |
Cmax, peak plasma concentration, Tmax, time to peak concentration, AUC(τ), area under the plasma concentration - time curve over the dosing interval (12 hours), CL/F, apparent total body clearance
Lonafarnib dose (mg) BID + gemcitabine dose (mg/m2)
Lonafarnib dose 150 mg AM, 100 mg PM
Median values
There was no apparent difference in AUC(τ) values between Cycle 1 Day 15 and Cycle 2 Day 15 based on log-transformed data. The point estimate was 105% when comparing Cycle 2 Day 15 to Cycle 1 Day 15. The 90% confidence interval for the point estimate was 76.2–145%, which suggests that steady state was attained by Cycle 1 Day 15.
A total of 65 pre-dose plasma samples from 7 patients were collected during Cycles 3–24. Mean pre-dose plasma lonafarnib concentrations ranged from 340 to 2355 ng/mL. The intra-subject variability in pre-dose plasma lonafarnib concentrations ranged from 25 to 118%.
Gemcitabine Pharmacokinetics
Plasma gemcitabine concentrations and derived pharmacokinetic parameters following administration of gemcitabine alone (Day 1) were similar to those in combination with lonafarnib (Day 15; Table 5). There was no statistically significant difference (p=0.363) in AUC(tf) values between Day 1 and Day 15 based on log-transformed data. The point estimate was 109% when comparing Day 15 to Day 1 and the 95% confidence interval for the point estimate was 93.2%–127%. AUC(tf) was used because AUC(I) values could not be determined in this study. Additionally, the distribution of individual AUC(tf) values following administration of gemcitabine in combination with 100 mg lonafarnib encompassed the same range as those in combination with 150 mg lonafarnib. Thus, multiple doses of lonafarnib had no important effect on the pharmacokinetics of gemcitabine.
Pharmacodynamics
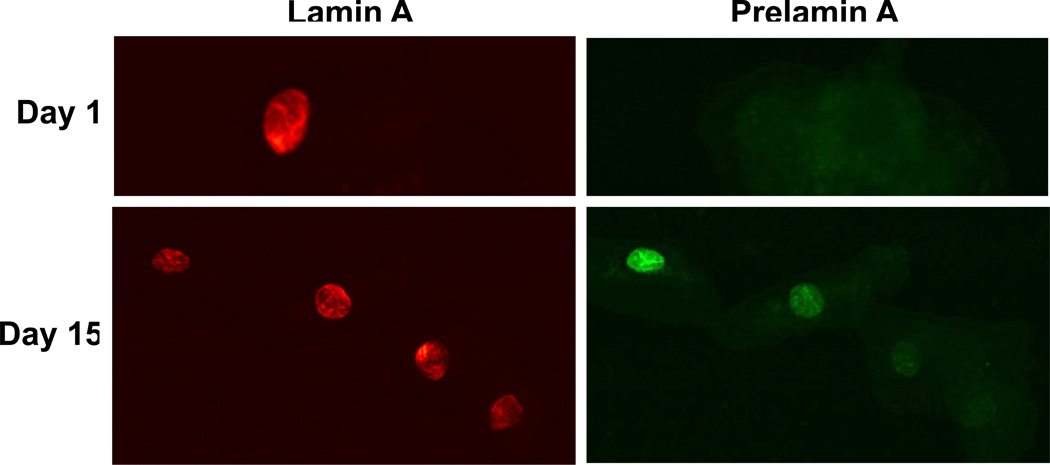
Of those buccal mucosal swabs available for prelamin A testing, 10 of 17 samples (59%) showed prelamin A accumulation after treatment with lonafarnib, indicating farnesyl transferase inhibition. Some degree of inhibition was seen across all dosing cohorts except level 1. At dose level 1, buccal cells where not found in 2 out of 4 patients, no post treatment specimen was available in the third patient, and no prelamin accumulation was demonstrated in the fourth patient. Figure 1A illustrates the accumulation of prelamin A. There was no apparent relationship between pre-dose plasma lonafarnib concentration on day 15 or AUC(τ) and prelamin A accumulation in buccal smears (data not shown).
Figure 1.
Prelamin A accumulation after treatment with Lonafarnib. A.) Buccal cells were reacted with anti-rabbit prelamin A and mouse anti-lamin followed by fluorochrome-labeled secondary antibodies.
Discussion
In this open-label, dose escalation, multiple-dose study of twice daily oral lonafarnib in combination with weekly intravenous gemcitabine given on days 1, 8 and 15 every 28 days, the MTD and recommended phase II dose for this regimen was determined to be lonafarnib 150 mg AM, 100 mg PM and gemcitabine 1000 mg/m2. At the recommended phase II dose, this regimen was well tolerated. However, in combination with full dose gemcitabine, lonafarnib needed to be given at reduced doses compared to its monotherapy dose. The adverse event profile of this combination consisted primarily of myelosuppression and gastrointestinal toxicities, which are overlapping toxicities of each agent.
The lonafarnib pharmacokinetic parameter estimates reflected high inter-subject variability, as also previously noted[23, 24]. There was no difference in plasma lonafarnib concentrations between cycle 1 day 15 and cycle 2 day 15. The Cmax and AUC values obtained in this study when either 100 or 200 mg lonafarnib was administered with gemcitabine were similar to those in previous phase I studies when lonafarnib was administered alone[23, 24] which suggest that gemcitabine had no apparent effect on the pharmacokinetics of lonafarnib and is consistent with prior studies[25]. Similarly, evaluation of gemcitabine pharmacokinetics on the first day of lonafarnib compared to after 15 days of dosing revealed no pharmacokinetic interaction between the drugs.
Modest clinical activity was seen in this generally heavily pretreated population (Table 4). Two subjects achieved a partial response and 7 patients had prolonged stable disease lasting longer than 6 months; most patients with clinical activity were gemcitabine naive. There appeared to be no relationship between the presence of prelamin A accumulation and pre-dose plasma concentrations of lonafarnib on the day of sampling, or with AUC(τ), suggesting the absence of any dose effect on this one marker of farnesylation at least in the evaluated dose ranges.
The clinical development of FTIs has been hampered by the limited activity of these agents at tolerable doses. In a variety of solid tumor settings, FTIs have shown limited activity as monotherapy, with response rates ranging between 0–13%[26–34]. In addition, no survival benefit was demonstrated in a randomized phase III study of best supportive care with or without tipifarnib in chemo-refractory colorectal cancer[35]. In combination studies of FTI plus chemotherapy, several phase I and II trials have shown mixed results regarding safety and activity[25, 36–45]. In addition, randomized phase III studies to date have not been able to demonstrate clinically meaningful benefit from the addition of FTIs to chemotherapy in solid tumors[46, 47].
FTIs continue to be evaluated in the field of hematologic malignancies, where promising phase II activity has been demonstrated[48–53]. In addition, research is ongoing to elucidate the exact mechanisms of FTI action and resistance. It is now known that refractoriness to FTIs can result from alternate prenylation of K-Ras and N-Ras by geranylgeranyl transferase I[54–57]; and that the anti-neoplastic effects of FTIs may also involve inhibition of other prenylated proteins such as RHOB, RHEB and centromeric proteins[58–61]. Importantly, biomarkers to identify suitable tumor types and patients for FTI therapy are lacking as RAS mutations are not predictive of response[62].
Acknowledgements
This work was supported by Merck & Company, formerly known as Schering-Plough Research Institute. We gratefully acknowledge the invaluable contributions of the patients who participated in this study and their families. We also gratefully acknowledge the research clinical teams and health care providers at Duke, UCLA and Mayo clinic who participated in this clinical study.
Footnotes
Declaration of Interest
The following authors report no declarations of interest:
Nan Soon Wong
Kellen Meadows
Lee Rosen
Alex Adjei
Michael Morse
William Petros
Yali Zhu
Paul Statkevich
Herbert Hurwitz
The following authors report declaration of interest:
David Cutler: 1) Employment: Merck (formerly Schering-Plough); 2) Stock ownership: Merck (formerly Schering-Plough)
Scott Kaufmann: 1) Research Funding: Merck (formerly Schering-Plough 2) Advisory Board: Merck (formerly Schering-Plough)
Michael Meyers: 1) Stock Ownership: Merck (formerly Schering Plough); 2) Honoraria: Merck (formerly Schering-Plough; 3) Other Renumeration: Merck (formerly Schering-Plough)
References
- 1.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 2.Lobell RB. Prenylation of Ras GTPase superfamily proteins and their function in immunobiology. Adv. Immunol. 1998;68:145–189. doi: 10.1016/s0065-2776(08)60559-3. [DOI] [PubMed] [Google Scholar]
- 3.Schafer WR, Trueblood CE, Yang CC, Mayer MP, Rosenberg S, Poulter CD, Kim SH, Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science. 1990;249:1133–1139. doi: 10.1126/science.2204115. [DOI] [PubMed] [Google Scholar]
- 4.Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7:297–300. doi: 10.1016/j.ccr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Casey PJ, Solski PA, Der CJ, Buss JE. p21ras is modified by a farnesyl isoprenoid. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 8.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J. Natl. Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 9.Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 10.Willumsen BM, Norris K, Papageorge AG, Hubbert NL, Lowy DR. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984;3:2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JH, Cochrane CG, Bourne JR, Solski PA, Buss JE, Der CJ. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters DG, Hoover RR, Gerlach MJ, Koh EY, Zhang H, Choe K, Kirschmeier P, Bishop WR, Daley GQ. Activity of the farnesyl protein transferase inhibitor SCH66336 against BCR/ABL-induced murine leukemia and primary cells from patients with chronic myeloid leukemia. Blood. 2001;97:1404–1412. doi: 10.1182/blood.v97.5.1404. [DOI] [PubMed] [Google Scholar]
- 13.Petit T, Izbicka E, Lawrence RA, Bishop WR, Weitman S, Von Hoff DD. Activity of SCH 66336, a tricyclic farnesyltransferase inhibitor, against human tumor colony-forming units. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1999;10:449–453. doi: 10.1023/a:1008313232381. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Lipari P, Malkowski M, Ferrari E, Nielsen L, Prioli N, Dell J, Sinha D, Syed J, Korfmacher WA, Nomeir AA, Lin CC, Wang L, Taveras AG, Doll RJ, Njoroge FG, Mallams AK, Remiszewski S, Catino JJ, Girijavallabhan VM, Bishop WR, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer research. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 15.Reichert A, Heisterkamp N, Daley GQ, Groffen J. Treatment of Bcr/Abl-positive acute lymphoblastic leukemia in P190 transgenic mice with the farnesyl transferase inhibitor SCH66336. Blood. 2001;97:1399–1403. doi: 10.1182/blood.v97.5.1399. [DOI] [PubMed] [Google Scholar]
- 16.Adjei AA, Erlichman C, Davis JN, Cutler DL, Sloan JA, Marks RS, Hanson LJ, Svingen PA, Atherton P, Bishop WR, Kirschmeier P, Kaufmann SH. A Phase I trial of the farnesyl transferase inhibitor SCH66336: evidence for biological and clinical activity. Cancer research. 2000;60:1871–1877. [PubMed] [Google Scholar]
- 17.Eskens FA, Awada A, Cutler DL, de Jonge MJ, Luyten GP, Faber MN, Statkevich P, Sparreboom A, Verweij J, Hanauske AR, Piccart M. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J Clin Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 18.Castaneda C, Meadows KL, Truax R, Morse MA, Kaufmann SH, Petros WP, Zhu Y, Statkevich P, Cutler DL, Hurwitz HI. Phase I and pharmacokinetic study of lonafarnib, SCH 66336, using a 2-week on 2-week off schedule in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67:455–463. doi: 10.1007/s00280-010-1488-5. [DOI] [PubMed] [Google Scholar]
- 19.Awada A, Eskens FA, Piccart M, Cutler DL, van der Gaast A, Bleiberg H, Wanders J, Faber MN, Statkevich P, Fumoleau P, Verweij J. Phase I and pharmacological study of the oral farnesyltransferase inhibitor SCH 66336 given once daily to patients with advanced solid tumours. Eur J Cancer. 2002;38:2272–2278. doi: 10.1016/s0959-8049(02)00379-9. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Blaskovich MA, Knowles D, Qian Y, Ohkanda J, Bailey RD, Hamilton AD, Sebti SM. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999;59:4919–4926. [PubMed] [Google Scholar]
- 21.Eskens FA, Awada A, Cutler DL, de Jonge MJ, Luyten GP, Faber MN, Statkevich P, Sparreboom A, Verweij J, Hanauske AR, Piccart M. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J. Clin. Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 22.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J. Natl. Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A phase I study of cyclic two-week oral administration of SCH 66336 in patients with advanced cancer. Kenilworth NJ: Schering-Plough Research Institute; 2001. Schering-Plough Clinical document 1715018. [Google Scholar]
- 24.Phase I study to determine the safety of SCH 66336 in patients with a solid tumor on twice daily oral schedule for 28 days. Kenilworth, NJ: Schering-Plough Research Institute; 2001. Clinical document 1760976. [Google Scholar]
- 25.Theodore C, Geoffrois L, Vermorken JB, Caponigro F, Fiedler W, Chollet P, Ravaud A, Peters GJ, de Balincourt C, Lacombe D, Fumoleau P. Multicentre EORTC study 16997: feasibility and phase II trial of farnesyl transferase inhibitor & gemcitabine combination in salvage treatment of advanced urothelial tract cancers. Eur J Cancer. 2005;41:1150–1157. doi: 10.1016/j.ejca.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Adjei AA, Mauer A, Bruzek L, Marks RS, Hillman S, Geyer S, Hanson LJ, Wright JJ, Erlichman C, Kaufmann SH, Vokes EE. Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2003;21:1760–1766. doi: 10.1200/JCO.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 27.Hanrahan EO, Kies MS, Glisson BS, Khuri FR, Feng L, Tran HT, Ginsberg LE, Truong MT, Hong WK, Kim ES. A Phase II Study of Lonafarnib (SCH66336) in Patients With Chemorefractory, Advanced Squamous Cell Carcinoma of the Head and Neck. Am. J. Clin. Oncol. 2009;3:274–279. doi: 10.1097/COC.0b013e318187dd57. [DOI] [PubMed] [Google Scholar]
- 28.Heymach JV, Johnson DH, Khuri FR, Safran H, Schlabach LL, Yunus F, DeVore RF, 3rd, De Porre PM, Richards HM, Jia X, Zhang S, Johnson BE. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann. Oncol. 2004;15:1187–1193. doi: 10.1093/annonc/mdh315. [DOI] [PubMed] [Google Scholar]
- 29.Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, Salter J, Michiels B, Perez-Ruixo JJ, Palmer P, Howes A. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J. Clin. Oncol. 2003;21:2492–2499. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade JL, 3rd, Giguere JK, Abbruzzese JL. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest. New Drugs. 2005;23:485–487. doi: 10.1007/s10637-005-2908-y. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg JE, von der Maase H, Seigne JD, Mardiak J, Vaughn DJ, Moore M, Sahasrabudhe D, Palmer PA, Perez-Ruixo JJ, Small EJ. A phase II trial of R115777, an oral farnesyl transferase inhibitor, in patients with advanced urothelial tract transitional cell carcinoma. Cancer. 2005;103:2035–2041. doi: 10.1002/cncr.21023. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Kemeny N, Kelsen DP, Ilson D, O'Reilly E, Zaknoen S, Baum C, Statkevich P, Hollywood E, Zhu Y, Saltz LB. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann. Oncol. 2002;13:1067–1071. doi: 10.1093/annonc/mdf173. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead RP, McCoy S, Macdonald JS, Rivkin SE, Neubauer MA, Dakhil SR, Lenz HJ, Tanaka MS, Abbruzzese JL. Phase II trial of R115777 (NSC #70818) in patients with advanced colorectal cancer: a Southwest Oncology Group study. Invest. New Drugs. 2006;24:335–341. doi: 10.1007/s10637-005-4345-3. [DOI] [PubMed] [Google Scholar]
- 34.Winquist E, Moore MJ, Chi KN, Ernst DS, Hirte H, North S, Powers J, Walsh W, Boucher T, Patton R, Seymour L. A multinomial Phase II study of lonafarnib (SCH 66336) in patients with refractory urothelial cancer. Urol Oncol. 2005;23:143–149. doi: 10.1016/j.urolonc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, Kourteva G, Iveson T, Andre T, Dostalova J, Illes A, Belly R, Perez-Ruixo JJ, Park YC, Palmer PA. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J. Clin. Oncol. 2004;22:3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 36.Adjei AA, Croghan GA, Erlichman C, Marks RS, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Bruzek LM, Atherton P, Thibault A, Palmer PA, Kaufmann SH. A Phase I trial of the farnesyl protein transferase inhibitor R115777 in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2003;9:2520–2526. [PubMed] [Google Scholar]
- 37.Awada A, Zhang S, Gil T, de Valeriola D, Lalami Y, De Porre P, Piccart-Gebhart MJ. A phase I clinical and pharmacokinetic study of tipifarnib in combination with docetaxel in patients with advanced solid malignancies. Curr Med Res Opin. 2007;23:991–1003. doi: 10.1185/030079907x178810. [DOI] [PubMed] [Google Scholar]
- 38.Gore L, Holden SN, Cohen RB, Morrow M, Pierson AS, O'Bryant CL, Persky M, Gustafson D, Mikule C, Zhang S, Palmer PA, Eckhardt SG. A phase I safety, pharmacological and biological study of the farnesyl protein transferase inhibitor, tipifarnib and capecitabine in advanced solid tumors. Ann Oncol. 2006;17:1709–1717. doi: 10.1093/annonc/mdl282. [DOI] [PubMed] [Google Scholar]
- 39.Khuri FR, Glisson BS, Kim ES, Statkevich P, Thall PF, Meyers ML, Herbst RS, Munden RF, Tendler C, Zhu Y, Bangert S, Thompson E, Lu C, Wang XM, Shin DM, Kies MS, Papadimitrakopoulou V, Fossella FV, Kirschmeier P, Bishop WR, Hong WK. Phase I study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in solid tumors. Clin Cancer Res. 2004;10:2968–2976. doi: 10.1158/1078-0432.ccr-03-0412. [DOI] [PubMed] [Google Scholar]
- 40.Kim ES, Kies MS, Fossella FV, Glisson BS, Zaknoen S, Statkevich P, Munden RF, Summey C, Pisters KM, Papadimitrakopoulou V, Tighiouart M, Rogatko A, Khuri FR. Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 41.Ready NE, Lipton A, Zhu Y, Statkevich P, Frank E, Curtis D, Bukowski RM. Phase I study of the farnesyltransferase inhibitor lonafarnib with weekly paclitaxel in patients with solid tumors. Clin Cancer Res. 2007;13:576–583. doi: 10.1158/1078-0432.CCR-06-1262. [DOI] [PubMed] [Google Scholar]
- 42.Siegel-Lakhai WS, Crul M, Zhang S, Sparidans RW, Pluim D, Howes A, Solanki B, Beijnen JH, Schellens JH. Phase I and pharmacological study of the farnesyltransferase inhibitor tipifarnib (Zarnestra, R115777) in combination with gemcitabine and cisplatin in patients with advanced solid tumours. Br J Cancer. 2005;93:1222–1229. doi: 10.1038/sj.bjc.6602850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparano JA, Moulder S, Kazi A, Coppola D, Negassa A, Vahdat L, Li T, Pellegrino C, Fineberg S, Munster P, Malafa M, Lee D, Hoschander S, Hopkins U, Hershman D, Wright JJ, Kleer C, Merajver S, Sebti SM. Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin Cancer Res. 2009;15:2942–2948. doi: 10.1158/1078-0432.CCR-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sparreboom A, Kehrer DF, Mathijssen RH, Xie R, de Jonge MJ, de Bruijn P, Planting AS, Eskens FA, Verheij C, de Heus G, Klaren A, Zhang S, Verhaeghe T, Palmer PA, Verweij J. Phase I and pharmacokinetic study of irinotecan in combination with R115777, a farnesyl protein transferase inhibitor. Br J Cancer. 2004;90:1508–1515. doi: 10.1038/sj.bjc.6601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow LQ, Eckhardt SG, O'Bryant CL, Schultz MK, Morrow M, Grolnic S, Basche M, Gore L. A phase I safety, pharmacological, and biological study of the farnesyl protein transferase inhibitor, lonafarnib (SCH 663366), in combination with cisplatin and gemcitabine in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2008;62:631–646. doi: 10.1007/s00280-007-0646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D. Phase III Trial of Gemcitabine Plus Tipifarnib Compared With Gemcitabine Plus Placebo in Advanced Pancreatic Cancer. Journal of Clinical Oncology. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 47. ClinicalTrials.gov Identifier: NCT00050336
- 48.Borthakur G, Kantarjian H, Daley G, Talpaz M, O'Brien S, Garcia-Manero G, Giles F, Faderl S, Sugrue M, Cortes J. Pilot study of lonafarnib, a farnesyl transferase inhibitor, in patients with chronic myeloid leukemia in the chronic or accelerated phase that is resistant or refractory to imatinib therapy. Cancer. 2006;106:346–352. doi: 10.1002/cncr.21590. [DOI] [PubMed] [Google Scholar]
- 49.Feldman EJ, Cortes J, DeAngelo DJ, Holyoake T, Simonsson B, O'Brien SG, Reiffers J, Turner AR, Roboz GJ, Lipton JH, Maloisel F, Colombat P, Martinelli G, Nielsen JL, Petersdorf S, Guilhot F, Barker J, Kirschmeier P, Frank E, Statkevich P, Zhu Y, Loechner S, List A. On the use of lonafarnib in myelodysplastic syndrome and chronic myelomonocytic leukemia. Leukemia. 2008;22:1707–1711. doi: 10.1038/leu.2008.156. [DOI] [PubMed] [Google Scholar]
- 50.Harousseau JL, Lancet JE, Reiffers J, Lowenberg B, Thomas X, Huguet F, Fenaux P, Zhang S, Rackoff W, De Porre P, Stone R. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109:5151–5156. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 51.Karp JE, Smith BD, Gojo I, Lancet JE, Greer J, Klein M, Morris L, Levis MJ, Gore SD, Wright JJ, Garrett-Mayer E. Phase II trial of tipifarnib as maintenance therapy in first complete remission in adults with acute myelogenous leukemia and poor-risk features. Clin. Cancer Res. 2008;14:3077–3082. doi: 10.1158/1078-0432.CCR-07-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lancet JE, Gojo I, Gotlib J, Feldman EJ, Greer J, Liesveld JL, Bruzek LM, Morris L, Park Y, Adjei AA, Kaufmann SH, Garrett-Mayer E, Greenberg PL, Wright JJ, Karp JE. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109:1387–1394. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravoet C, Mineur P, Robin V, Debusscher L, Bosly A, Andre M, El Housni H, Soree A, Bron D, Martiat P. Farnesyl transferase inhibitor (lonafarnib) in patients with myelodysplastic syndrome or secondary acute myeloid leukaemia: a phase II study. Ann. Hematol. 2008;87:881–885. doi: 10.1007/s00277-008-0536-2. [DOI] [PubMed] [Google Scholar]
- 54.James GL, Goldstein JL, Brown MS. Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J. Biol. Chem. 1995;270:6221–6226. doi: 10.1074/jbc.270.11.6221. [DOI] [PubMed] [Google Scholar]
- 55.Lerner EC, Zhang TT, Knowles DB, Qian Y, Hamilton AD, Sebti SM. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15:1283–1288. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]
- 56.Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J. Biol. Chem. 1997;272:14093–14097. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 57.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 58.Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J. Biol. Chem. 2000;275:30451–30457. doi: 10.1074/jbc.M003469200. [DOI] [PubMed] [Google Scholar]
- 59.Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J. Biol. Chem. 2005;280:31101–31108. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- 60.Lebowitz PF, Casey PJ, Prendergast GC, Thissen JA. Farnesyltransferase inhibitors alter the prenylation and growth-stimulating function of RhoB. J. Biol. Chem. 1997;272:15591–15594. doi: 10.1074/jbc.272.25.15591. [DOI] [PubMed] [Google Scholar]
- 61.Liu A, Du W, Liu JP, Jessell TM, Prendergast GC. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol. Cell. Biol. 2000;20:6105–6113. doi: 10.1128/mcb.20.16.6105-6113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feldkamp MM, Lau N, Roncari L, Guha A. Isotype-specific Ras.GTP-levels predict the efficacy of farnesyl transferase inhibitors against human astrocytomas regardless of Ras mutational status. Cancer Res. 2001;61:4425–4431. [PubMed] [Google Scholar]