Abstract
Importance
Long-acting injectable (LAI) antipsychotics are used to reduce medication non-adherence and subsequent relapse in schizophrenia-spectrum disorders. The relative effectiveness of LAI versions of second-generation (atypical) and older antipsychotics has not been assessed.
Objective
To compare the effectiveness of the second-generation LAI antipsychotic paliperidone palmitate (PP) to the older LAI antipsychotic haloperidol decanoate (HD).
Design, Setting, and Participants
Multisite, double-blind, randomized clinical trial conducted at 22 clinical research sites in the U.S. The 311 randomized patients (PP=157, HD=154) were adults diagnosed with schizophrenia or schizoaffective disorder who were clinically assessed to be at risk of relapse and likely to benefit from a LAI antipsychotic.
Interventions
Intramuscular injections of HD 25–200 mg or PP 39–234 mg every month for up to 24 months.
Main Outcome Measures
Efficacy failure, which reflected inadequate control of psychopathology by the study medication, as determined by a blinded adjudication committee. Key secondary outcomes were common adverse effects of antipsychotic medications.
Results
There was no statistically significant difference in the rate of efficacy failure for PP compared to HD (adjusted hazard ratio 0.98, 95% confidence interval [CI] 0.65–1.47). On average, patients on PP gained and those on HD lost weight; after six months the least squares mean weight change on PP was +2.17 kg (1.25 to 3.09) and on HD was −0.96 kg (−1.88 to −0.04). Patients taking PP had significantly greater increases in serum prolactin (men 34.56 µg/L (29.75 to 39.37) vs. 15.41 (10.73 to 20.08), p<0.001; women 75.19 (63.03 to 87.36) vs. 26.84 (13.29 to 40.40), p<0.001). Patients taking HD had significantly larger increases in global ratings of akathisia (0.73 [0.59 to 0.87] vs. 0.45 [0.31 to 0.59], p=0.006).
Conclusions and Relevance
Among adults with schizophrenia or schizoaffective disorder, treatment with paliperidone palmitate compared with haloperidol decanoate did not result in a statistically significant difference in efficacy failure, but was associated with more weight gain and greater increases in serum prolactin, whereas haloperidol was associated with more akathisia. However, based on the 95% confidence limits, a clinically meaningful difference in efficacy failure between treatments cannot be ruled out.
Trial Registration
clinicaltrials.gov identifier NCT01136772
Long-acting injectable (LAI) antipsychotic medications are prescribed to reduce non-adherence and relapse in people diagnosed with a schizophrenia-spectrum disorder. LAI versions of older or “typical” antipsychotics have been available for decades but their use has been limited in part due to their propensity to cause extrapyramidal side effects (EPS), including tardive dyskinesia. Beginning in 1989, oral forms of newer “atypical” antipsychotic medications considered to entail lower risk of EPS were introduced. Due to rapid acceptance of the newer oral antipsychotics, LAI versions of these medications were anticipated to gain widespread use. The first of these, risperidone microspheres, was introduced in 2003. Risperidone microspheres, however, must be refrigerated before use, reconstituted with a diluent provided by the manufacturer, and administered bi-weekly. In 2009, a long-acting version of risperidone’s active metabolite, paliperidone, was brought to market. Paliperidone palmitate can be administered monthly and does not require refrigeration or reconstitution. Because of these logistical advantages, paliperidone palmitate was considered to be an important advance in LAI antipsychotics, although its high acquisition cost made its role uncertain.1
In recent years head-to-head trials and meta-analyses have called into question the advantages of the atypical antipsychotic medications over typical antipsychotics.2–5 The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial showed that when an older drug, perphenazine, was used at moderate doses that several newer ones were not superior in safety or effectiveness.3 A recent secondary analysis provided evidence that perphenazine is non-inferior to olanzapine, quetiapine, and risperidone with respect to symptom scores.6 Moreover, some newer antipsychotics were shown to cause significant weight gain and to be associated with dyslipidemias and diabetes mellitus.7,8
This investigation compared the effects of LAI paliperidone palmitate (PP) and haloperidol decanoate (HD), an older, widely used LAI antipsychotic. Based on an earlier comparison of oral risperidone to oral haloperidol,9 we hypothesized that PP would be associated with lower rates of efficacy failure and EPS than HD, but that HD would cause less weight gain and less increase in serum prolactin levels.
Method
Study setting and design
A Comparison of Long-acting Injectable Medications for Schizophrenia (ACLAIMS) was a multisite, parallel-group, double-blinded randomized clinical trial. The study was conducted at 22 U.S. clinical sites affiliated with the National Institute of Mental Health-supported Schizophrenia Trials Network. Each site obtained institutional review board approval to conduct the study.
Patients
Patients were adults aged 18–65 with a diagnosis of schizophrenia or schizoaffective disorder as defined by DSM-IV-TR criteria and confirmed by the Structured Clinical Interview for DSM-IV (SCID). Patients were eligible if judged by their clinician and study psychiatrist as likely to benefit from treatment with PP or HD and to be at risk of efficacy failure based on a history of medication non-compliance and/or significant substance abuse. All patients demonstrated adequate decisional capacity to participate and provided written informed consent.
Patients with the following characteristics were excluded: currently stable and doing well on an antipsychotic regimen; not expected to benefit from the study medications due to past experience with risperidone, haloperidol, or paliperidone due to adverse effects or no improvement of severe symptoms in spite of an adequate treatment trial of at least 6 weeks duration; moderate or severe tardive dyskinesia; presence of any medical condition that might preclude safe completion of the study; or intellectual disability. Women who were pregnant or breastfeeding were also excluded.
Patients attended a screening visit. If potentially eligible, a baseline visit was scheduled within 21 days. If determined eligible at the baseline visit, patients were then randomized on a 1:1 basis to PP or HD using an internet-based system.
Interventions
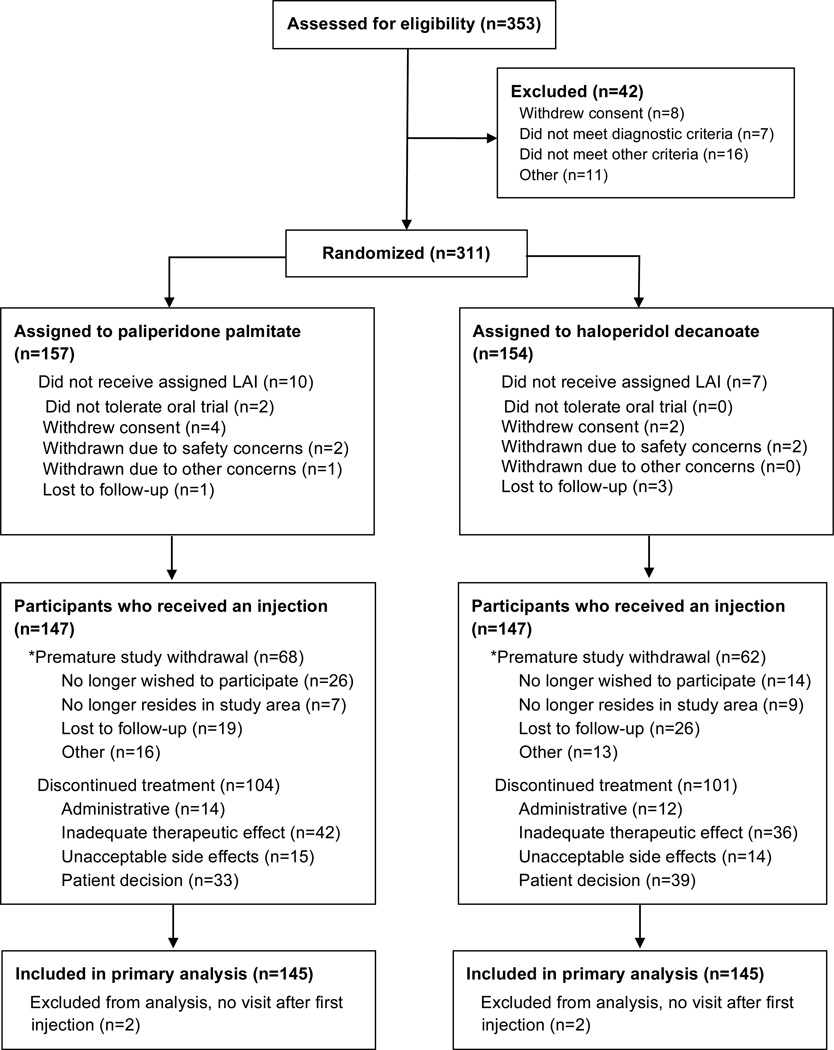
A total of 353 patients enrolled for screening; 311 were eligible and randomized to study treatment. Study treatments were long-acting injectable PP supplied in dosages of 39 mg, 78 mg, 117 mg, 156 mg and 234 mg; and HD supplied in vials of 50 mg/ml or 100 mg/ml for injection. Each participant received a blinded trial of the oral version of the assigned medication prior to receiving an injection. In the case of PP the oral trial was with risperidone in accordance with the product label. The oral trial lasted from 4–7 days, with each patient recommended to receive 2 mg of either haloperidol or risperidone on days 1 and 2 and 4 mg thereafter. Haloperidol 2 mg and risperidone 2mg were supplied in identical-appearing capsules. Oral benztropine 1 mg was supplied to treat EPS if needed. Patients who demonstrated allergy, EPS not relieved by benztropine, or other intolerability to the oral trial were dropped from the trial. Seventeen randomized patients never received the assigned LAI antipsychotic; only two of these were due to intolerability to the oral medication trial. The first injection was given 4–7 days after the baseline visit. Subsequent visits were at weeks 1, 2, 4, 6, 8, 10, and 12, then monthly (i.e., every four weeks) for up to 24 months.
Study physicians and all other personnel were blinded to treatment condition. Study physicians wrote orders for both of the potential LAI antipsychotic medications, for example: “If HD, administer 50 mg IM; If PP, administer 117 mg IM.” Each patient was then injected with only the randomly assigned drug. A clinician not otherwise involved in the trial administered the injection and concealed the identity of the medication from the patient and study personnel.
The loading strategy schedule described in the PP prescribing information was recommended for both drugs. The recommended starting dose of PP was 234 mg IM on day 1 followed on day 8 with 156 mg IM. The recommended standard monthly dose of PP was 117 mg. The recommended starting dose of HD was 50 mg IM on day 1 followed on day 8 with 50 mg IM. On day 28 the recommended dose of HD was 75 mg IM to be followed on day 56 and on subsequent monthly visits with 50 mg IM. The first two injections were given in the deltoid (day 1 and day 8). Subsequent monthly injections were given in the deltoid or gluteal muscle. The recommended injection schedules were adjusted according to the clinical situation. For the first 8 weeks clinicians were allowed to supplement the LAI with any oral antipsychotic as needed.
Investigators were required to consult with the project’s safety officer, who was a physician blinded to treatment assignment, if there was a desire to continue the patient on study treatment when the following criteria were met: new onset diabetes mellitus, weight gain ≥ 15 pounds, increase in low-density lipoprotein (LDL) cholesterol ≥ 20 mg/dl, worsening tardive dyskinesia, hospitalization, clinical worsening as indicated by the Clinical Global Impressions scale, or any serious adverse event. After the safety officer reviewed the case, study medication was continued if the clinician considered it in the best interest of the patient to continue and the patient and safety officer concurred.
Outcome Measures
The primary outcome was efficacy failure, which reflected inadequate control of the psychopathology of schizophrenia or schizoaffective disorder. Efficacy failure was determined for each study participant by an Outcome Adjudication Committee (OAC) consisting of three research psychiatrists who were blind to treatment assignment and not otherwise involved in the study. A majority vote of the committee determined whether and when a participant experienced efficacy failure. The criteria considered for efficacy failure included psychiatric hospitalization; a need for crisis stabilization; a clinically meaningful increase in frequency of outpatient visits; a clinician’s decision that oral antipsychotic could not be discontinued within 8 weeks after starting the LAI; a clinician’s decision to discontinue the assigned LAI due to inadequate therapeutic benefit; or, for patients successfully transitioned to study LAI within 8 weeks, ongoing or repeated need for adjunctive oral antipsychotic medication.
Secondary outcome measures included change in weight from baseline and worst changes in fasting blood glucose, glycosolated hemoglobin, cholesterol, triglycerides and prolactin. The worst changes (e.g., highest recorded level of triglycerides, lowest recorded levels of HDL) were used for these laboratory-measured outcomes because interventions to treat abnormalities were allowed. Other important secondary outcomes included measures of abnormal involuntary movements, akathisia, Parkinsonism, and sexual functioning. Weight and measures of neurologic side effects were obtained at all study visits. Laboratory blood tests were obtained at screening, months 3 and 6, and then every 6 months. Patients were systematically queried about 12 adverse effects commonly associated with antipsychotic medications at each visit. Symptoms were measured using the Positive and Negative Syndrome Scale (PANSS) at baseline, month 1, and then every three months.
Statistical methods
The primary analysis was conducted among the modified intent-to-treat (mITT) population, which consisted of all patients who received at least one injection and at least one post-baseline assessment. The Kaplan-Meier (KM) method was used to estimate the proportion without efficacy failure by time since first injection and a site stratified (two-sided) log-rank test for the primary comparison of time until efficacy failure. In the primary analysis, we censored patients 90 days after their last injection to account for the time most likely affected by the long-acting study medications. Planned supporting and sensitivity analyses included 1) estimating the hazard ratio (and 95% CI) using a Cox proportional hazards model (controlling for baseline PANSS score and site), 2) repeating the site stratified log-rank test without censoring 90 days after last injection, and 3) conducting an unstratified log-rank test. We expanded the Cox model to test for site by treatment interaction and to test whether the hazard ratio for treatment was equal across three predefined time intervals: months 1–3, months 4–12, and months 13–24.10 We repeated the site stratified log-rank test for one subgroup defined a priori, participants who were not in an exacerbated state (i.e., not hospitalized) at randomization. All main effects of treatment and treatment by site interactions for safety analyses were tested at the 2–sided α=0.05 level. For efficacy, interactions were tested at the α=0.10 level.
Safety analyses excluded data collected more than 6 weeks after a participant’s last injection. Mixed effect linear models (with spatial power covariance structure) were used to compare weight change over time. Fixed effects were included for assigned treatment, clinical site, baseline weight, time (months since first injection) and treatment by time interaction. The proportion of patients whose weight ever increased at least 15 pounds from baseline was compared using a Mantel-Haenszel chi-square test. ANCOVA was used for metabolic analyses, with the worst case as the outcome and treatment, site, and baseline value as covariates.
The same ANCOVA approach was used for comparisons of worst AIMS global score, Barnes Akathisia Scale (BAS) global score and Simpson-Angus Extrapyramidal Scale (SAS) score. Incidence of clinically significant scores on three different assessments (i.e., AIMS global score ≥2, Barnes Akathisia Scale global score ≥3, and Simpson-Angus Extrapyramidal Scale score ≥1)11 were compared between treatment groups using Mantel-Haenszel chi-square tests excluding patients who had a clinically significant score at baseline. As a post hoc analysis, the proportions meeting Schooler-Kane criteria for tardive dyskinesia (at least moderate dyskinetic movements in one body area or mild dyskinetic movements in two body areas)12 were compared using Mantel-Haenszel chi-square tests, excluding patients who met criteria at baseline.
For prolactin and associated adverse effects, separate analyses were planned for men and women. The key comparisons used ANOVA to compare the highest recorded prolactin level as the response, with treatment and site as covariates. Supporting analyses compared incidence of associated abnormalities (e.g, gynecomastia or galactorrhea) between treatment groups using a Mantel-Haenszel chi-square test or Barnard’s exact test (if fewer than 10 events were observed) and highest Arizona Sexual Experiences (ASEX) scale score using ANOVA.
The original plan to randomize a total of 360 patients and follow for 2 years was modified due to resource constraints. The recruitment period was March 2011–July 2012; follow-up ended in July 2013. Ultimately 311 individuals were randomized. The earliest enrollees were followed for up to 24 months and the last enrollees for up to 12 months. The planned sample size was expected to provide at least 80% power to detect a difference in survival curves (two-sided log rank test, alpha=0.05) assuming efficacy failure rates of 0.56 and 0.40 for the HD and PP groups, respectively (i.e., a hazard ratio of 1.6).
Analyses were performed using SAS version 9.3 (SAS Institute, Inc.).
Results
Figure 1 summarizes the progress of patients who were screened and randomly assigned to each group. Baseline demographic and clinical characteristics of the 145 PP and 145 HD patients in the primary analysis are in Table 1. Patients were followed for a median of 488 days (25th–75th percentile: 225–645).
Figure 1. Participant enrollment and follow-up.
*Patients were intended to stay in the study for 24 months or until the study was terminated even if treatment was discontinued.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients in the Modified Intent to Treat Population
| Characteristic | Paliperidone palmitate (n=145) |
Haloperidol decanoate (n=145) |
|---|---|---|
| Demographic characteristics | ||
| Age, mean (SD), y | 43 (12.6) | 45 (12.3) |
| Sex, No. (%) | ||
| Male | 106 (73.1) | 110 (75.9) |
| Female | 39 (26.9) | 35 (24.1) |
| Race, No. (%) | ||
| White | 56 (38.6) | 54 (37.2) |
| Black | 83 (57.2) | 83 (57.2) |
| Othera | 6 (4.1) | 8 (5.5) |
| Spanish, Hispanic, or Latino ethnicity, No. (%) | 6 (4.1) | 8 (5.5) |
| Baseline clinical characteristics | ||
| In hospital, No. (%) | 24 (16.6) | 28 (19.3) |
| Weight, mean (SD), kg | 90 (21.7) | 90 (22.5) |
| BMI, mean (SD) | 30 (7.4) | 30 (7.3) |
| HbA1c (%) (SD) | 5.9 (1.3) | 5.6 (0.6) |
| Blood glucose (mg/dL) (SD) | 104.0 (31.5) | 94.6 (17.6) |
| Total Cholesterol (mg/dL) (SD) | 179.7 (38.5) | 181.5 (41.9) |
| LDL-cholesterol (mg/dL) (SD) | 104.6 (35.1) | 108.1 (33.0) |
| Triglycerides (mg/dL) (SD) | 123.2 (85.4) | 119.9 (80.5) |
| Prolactin (µg/L) men (SD) | 17.4 (20.8) | 17.8 (13.5) |
| Prolactin (µg/L) women (SD) | 35.9 (35.2) | 32.2 (38.5) |
| PANSS total score, mean (SD)b | 73 (15.3) | 70 (15.7) |
| CGI severity score, mean (SD)c | 4.0 (0.8) | 3.8 (0.9) |
| AIMS global severity score, median (range)d | 0.0 (0–2) | 0.0 (0–2) |
| SAS mean score, median (range)e | 0.0 (0–1.5) | 1.0 (0–1.5) |
| BAS global score, median (range)f | 0.0 (0–3) | 0.0 (0–3) |
| Psychiatric history, mean (SD) | ||
| Age at 1st treatment for any behavioral or emotional problem, years | 23 (9.3) | 24 (10.9) |
| Age at 1st antipsychotic medication, years | 26 (9.0) | 27 (10.1) |
| SCID diagnosis, No. (%) | ||
| Schizophrenia – lifetime | 103 (71.0) | 107 (73.8) |
| Schizoaffective disorder – lifetime | 56 (38.6) | 53 (36.6) |
| Major depression – past 5 years | 36 (24.8) | 40 (27.6) |
| Alcohol dependence – past 5 years | 27 (18.6) | 27 (18.6) |
| Alcohol abuse – past 5 years | 42 (29.0) | 43 (29.7) |
| Drug dependence – past 5 years | 33 (22.8) | 36 (24.8) |
| Drug abuse – past 5 years | 50 (34.5) | 45 (31.0) |
| Antisocial personality disorder – past 5 years | 17 (11.8) | 16 (11.0) |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; BAS, Barnes akathisia scale; BMI, body mass index; CGI, Clinical Global Impressions Scale; PANSS, Positive and Negative Syndrome Scale; SAS, Simpson-Angus Scale; SCID, Structured Clinical Interview for DSM-IV; SD, standard deviation.
Race was self-reported. “Other” includes American Indian or Alaska Native, Asian, or Native Hawaiian or other Pacific Islander and two or more races.
PANSS range is 30–210, with higher scores reflecting greater severity of psychopathology
CGI range is 1–7 with higher scores reflecting greater severity of illness
AIMS global score range is 0–4 higher scores reflecting greater severity of abnormal movements
SAS mean score range is 0–4 with higher scores reflecting greater severity of Parkinson’s symptoms
BAS global score range is 0–3 with higher scores reflecting greater severity of akathisia
Dose
In the initial month of LAI treatment, which included doses on day 1 and day 8, the mean dose of PP was 325 mg and of HD 94 mg. Subsequently the mean monthly dose of PP ranged from 129–169 mg and the mean monthly dose of HD ranged from 67–83 mg.
Efficacy failure
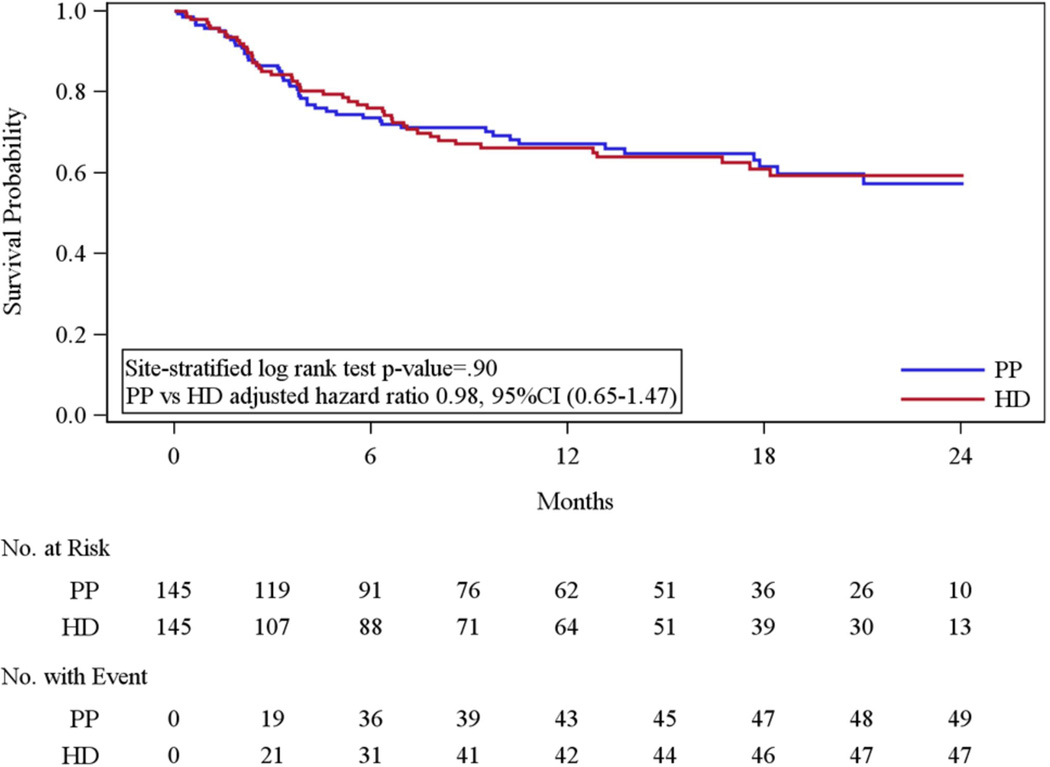
In the primary analysis, there was no statistically significant difference in the rate of efficacy failure (49 [33.8%] for PP vs. 47 [32.4%] for HD; site stratified log rank P=.90; site and baseline PANSS adjusted hazard ratio 0.98, 95% CI 0.65–1.47) among patients randomized to PP compared to HD (Figure 2). Results of all pre-planned sensitivity and supporting analyses led to similar conclusions (Supplementary eTable 1). Reasons for efficacy failure are in the Supplementary eTable 2. The most common reasons for efficacy failure noted by the OAC were psychiatric hospitalization (44 (89.8%) of PP and 34 (72.3%) of HD events) and clinician discontinuation of study medication due to inadequate therapeutic effect (34 (69.4%) PP and 28 (59.6%) HD events).
Figure 2.
Secondary outcomes
On average, patients taking PP gained weight progressively over time while those on HD lost weight. For example, at month 6 the least squares mean weight change for the PP group was +2.17 kg (1.25 to 3.09) and for the HD group was −0.96 kg (−1.88 to −0.04). The test of time-by-treatment interaction showed statistically significant treatment group differences (p<0.0001) (Table 2). Seven patients on PP (4.8%) compared to 2 (1.4%) on HD discontinued treatment due to weight gain.
Table 2.
Outcome Measures of Safety in the Modified Intent to Treat Population
| Outcome | Paliperidone palmitate (n=147) |
Haloperidol decanoate (n=147) |
P Value: PP vs HD |
|---|---|---|---|
| Weight change from baseline | |||
| Least squares mean at month 6, mean (95% CI) | 2.17 (1.25 to 3.09) | −0.96 (−1.88 to −0.04) | <.001a |
| Least squares mean at month 12, mean (95% CI) | 3.46 (1.83 to 5.09) | −1.93 (−3.56 to −0.31) | |
| Least squares mean at month 18, mean (95% CI) | 4.75 (2.36 to 7.14) | −2.91 (−5.28 to −0.53) | |
| Least squares mean at month 24, mean (95% CI) | 6.04 (2.88 to 9.20) | −3.88 (−7.02 to −0.73) | |
| Ever gained ≥ 15 lbs from baseline, No. (%) | 48 (33.1) | 32 (22.4) | 0.03b |
| Worst change from baseline in laboratory values | |||
| At least one laboratory assessment after first injection, No. of patients | 129 | 126 | |
| HbA1c, Least squares mean (95% CI) | 0.34 (0.17 to 0.52) | 0.23 (0.06 to 0.41) | 0.38c |
| Blood glucose, Least squares mean (95% CI) | 21.13 (12.59 to 29.67) | 20.96 (12.38 to 29.54) | 0.98c |
| Total cholesterol, Least squares mean (95% CI) | 12.42 (7.20 to 17.63) | 16.82 (11.56 to 22.07) | 0.25c |
| LDL, Least squares mean (95% CI) | 11.70 (7.06 to 16.34) | 13.49 (8.85 to 18.14) | 0.59c |
| Triglycerides, Least squares mean (95% CI) | 36.91 (22.40 to 51.43) | 46.57 (31.93 to 61.21) | 0.36c |
| HDL, Least squares mean (95% CI) | −5.28 (−6.74 to −3.83) | −4.52 (−5.98 to −3.05) | 0.47c |
| Neurologic effects | |||
| AIMS global severity score | |||
| Incidence of AIMS ≥ 2, No. (%) | 28 (21.4) | 30 (23.8) | 0.57b |
| Worst change from baseline, Least squares mean (95% CI) | 0.43 (0.31 to 0.55) | 0.50 (0.38 to 0.62) | 0.39c |
| Barnes akathisia rating scale | |||
| Incidence of BAS ≥ 3, No. (%) | 4 (2.8) | 15 (10.6) | 0.006b |
| Worst change from baseline, Least squares mean (95% CI) | 0.45 (0.31 to 0.59) | 0.73 (0.59 to 0.87) | 0.006c |
| Simpson-Angus Extrapyramidal Scale (SAS) | |||
| Incidence of SAS ≥ 1, No. (%) | 109 (79.0) | 101 (74.8) | 0.45b |
| Worst change from baseline, Least squares mean (95% CI) | 0.21 (0.16 to 0.27) | 0.25 (0.20 to 0.30) | 0.34c |
| Serum prolactin levels | |||
| Among males only | |||
| Highest level after baseline, Least squares mean (95% CI) | 34.56 (29.75 to 39.37) | 15.41 (10.73 to 20.08) | <.001d |
| Worst ASEX after baseline, Least squares mean (95% CI)f | 17.68 (16.36 to 19.00) | 17.95 (16.66 to 19.25) | 0.77d |
| ASEX score ≥ 19, No. (%) | 34 (37.8) | 37 (39.4) | 0.72b |
| Incidence of gynecomastia or galactorrhea, No. (%) | 5 (4.7) | 3 (2.8) | 0.46e |
| Among females only | |||
| Highest level after baseline, Least squares mean (95% CI) | 75.19 (63.03 to 87.36) | 26.84 (13.29 to 40.40) | <.001d |
| Worst ASEX after baseline, Least squares mean (95% CI)f | 23.41 (21.01 to 25.80) | 22.83 (20.12 to 25.54) | 0.75d |
| ASEX score ≥ 19, No. (%) | 24 (72.7) | 19 (73.1) | 0.88b |
| Incidence of gynecomastia or galactorrheag, No. (%) | 10 (38.5) | 5 (29.4) | 0.13b |
Abbreviations: AIMS, Abnormal Involuntary Movement Scale; ASEX, Arizona Sexual Side Effects; BAS, Barnes akathisia rating scale; CI, confidence interval; HDL, high density lipoprotein; LDL, low density lipoprotein; SAS, Simpson-Angus Scale.
P Value is from test of time by treatment interaction.
P Value for the comparison of binary outcomes is from a Cochran-Mantel-Haenszel (CMH) test stratified by grouped-site.
P Value for overall comparison between treatment groups obtained from analysis of covariance adjusting for baseline value and pooled site. The least squares mean and standard error are from the corresponding analysis of covariance model.
P Value for overall comparison between treatment groups obtained from analysis of variance adjusting for pooled site. The least squares mean and standard error are from the corresponding analysis of variance model.
P Value is from Barndard’s exact test, due to low event counts in males.
Range of the Arizona Sexual Experiences Scale (ASEX) is 6–30 with higher scores representing worse sexual functioning.
Incidence among premenopausal females only.
There were no statistically significant differences between those treated with PP and HD in mean change to the highest recorded levels of HbA1c, glucose, total cholesterol, LDL cholesterol, and triglycerides; or in the lowest recorded levels of HDL cholesterol.
There were no statistically significant differences in changes in ratings of abnormal involuntary movements as indicated by change from baseline score in the Abnormal Involuntary Movement Scale (AIMS) global score (0.43 (0.31–0.55) for PP vs. 0.50 (0.38–0.62) for HD; p=0.39). (Table 2) There was no statistically significant difference in the incidence of probable TD (15 on PP (10.6%) and 21 on HD (15.4%), p=0.24). Patients taking HD experienced greater increases in Barnes Akathisia Scale (BAS) global scores (0.45 (0.31–0.59) for PP vs. 0.73 (0.59–0.87) for HD; p=0.006). There were no statistically significant difference in changes in ratings of Parkinsonism as measured by the mean Simpson-Angus Extrapyramidal Scale (SAS) scale score (0.21 (0.16–0.27) for PP vs. 0.25 (0.20–0.30) for HD; p=0.34). Fewer patients taking PP than HD started a medication to treat Parkinsonism (18 (15.8%) vs. 27 (29.3%), p=0.007) and akathisia (5 (3.6%) vs. 16 (11.0%), p=0.03). Treatment discontinuations due to neurologic side effects according to clinician judgment were as follows: 2 patients (1.4%) on HD and 1 (0.7%) on PP due to akathisia; 3 (2.0%) on HD compared to 1 (0.7%) on PP due to Parkinsonism, and 4 (2.7%) on HD compared to 1 (0.7%) on PP due to tardive dyskinesia.
Mean serum prolactin levels increased for men and women treated with both PP and HD. The mean highest prolactin level was higher for PP than HD for men (34.56 µg/L (1502.6 pmol/L) (SI unit conversion factor 43.478 to pmol/L) (29.75 to 39.37) vs. 15.41 µg/L (670 pmol/L)(10.73 to 20.08), p<0.001) and women (75.19 µg/L (3289.11 pmol/L)(63.03 to 87.36) vs. 26.84 µg/L (1166.95 pmol/L) (13.29 to 40.40), p<0.001). There were no statistically significant differences in the proportions taking PP or HD who had a score on the ASEX scale ≥ 19 indicating sexual dysfunction for men or women. There were no significant differences in the incidence of gynecomastia or galactorrhea for men or women.
Overall, 68.0% of patients on PP compared to 59.9% of those on HD reported at least one adverse effect rated as moderate or severe (Table 3). Among the individual items, 16.3% of patients taking PP compared to 10.9% on HD developed sialorrhea. This is the only item with a difference of 5% or more between the groups. Fifty-three (36.1%) of patients on PP experienced a total of 69 serious adverse events (SAEs) compared to 45 (30.6%) on HD, who experienced a total of 64 SAEs. One male participant in his sixties died of unknown causes approximately six weeks after his last HD injection.
Table 3.
Adverse Events in the Intent to Treat Population
| Outcome | Paliperidone palmitate (n=147) |
Haloperidol decanoate (n=147) |
|---|---|---|
| Serious Adverse events, No. (%) | ||
| Any serious adverse event | 53 (36.1) | 45 (30.6) |
| Suicidal or homicidal ideation | 23 (15.6) | 21 (14.3) |
| Adverse events, No. (%) | ||
| Any moderate or severe adverse event from systematic inquiry | 100 (68.0) | 88 (59.9) |
| Insomnia | 49 (33.3) | 54 (36.7) |
| Sleepiness | 41 (27.9) | 44 (29.9) |
| Dry mouth | 40 (27.2) | 34 (23.1) |
| Increased appetite | 33 (22.4) | 26 (17.7) |
| Hypersomnia | 24 (16.3) | 20 (13.6) |
| Constipation | 21 (14.3) | 20 (13.6) |
| Sialorrhea | 24 (16.3) | 16 (10.9) |
| Orthostatic faintness | 14 (9.5) | 12 (8.2) |
| Incontinence/nocturia | 13 (8.8) | 8 (5.4) |
| Menstrual irregularities | 12 (8.2) | 5 (3.4) |
| Urinary hesitancy | 7 (4.8) | 9 (6.1) |
| Gynecomastia/galactorrhea | 4 (2.7) | 5 (3.4) |
Decreases in PANSS total scores from baseline were similar for both groups at each time point (See eFigure 1). For example, at month 6 the least squares mean PANSS change was −6.87 (−8.79, −4.94) for PP and −6.40 (−8.32, −4.48) for HD. In addition, as seen in Figure 1, rates of treatment discontinuation due to any cause (104/147 [70.7%] for PP and 101/147 [68.7%] for HD) and due to unacceptable side effects (15/146 [10.2%] for PP and 14/147 [9.5%] for HD) were similar.
Discussion
This randomized clinical trial found no evidence that long-acting injectable PP was superior to HD with respect to prevention of efficacy failure. However, based on the 95% confidence limits for the event rates, the results cannot rule out a clinically meaningful difference favoring one of the drugs.
Contrary to expectations, there was no statistically significant advantage for PP compared to HD in ratings of the severity abnormal involuntary movements and Parkinsonism, or in the incidence of TD. However, ratings of the severity of akathisia increased more for HD, and more medications to manage akathisia and Parkinsonism were started for patients on HD, partially confirming that PP has a lower propensity to cause EPS than HD. The current study was informed by studies from the 1980s that compared “standard” doses of typical LAI antipsychotic medication with lower doses and found that patients could be successfully maintained at these lower doses without relapse and without extrapyramidal toxicities.13–15 Similarly, the CATIE schizophrenia trial found that modest doses of typical oral antipsychotic medication could be used effectively without excessive EPS.3 The modest dose of haloperidol decanoate used here, about 75mg IM per month, is lower than the equivalent oral dosage used in a trial that found an advantage of oral risperidone over oral haloperidol.9 In that study, the average daily dose of haloperidol was 11.7±5.0 mg whereas in this study, using a standard conversion from oral haloperidol to haloperidol decanoate of 10–15 times the daily dose, the corresponding daily dose is approximately 5.0–7.5 mg. The modest dosing of haloperidol decanoate in this study is consistent with current recommendations and may help to account for its better-than-expected comparative tolerability. It was unexpected that the relapse rate was similar to that in the earlier study comparing oral risperidone to oral haloperidol considering that the current study enrolled people at increased risk of non-adherence and relapse. One reason may be that the outcome in the oral trial, relapse, was somewhat broader than the definition of efficacy failure used here. Another possible reason is that LAI antipsychotics are more useful than oral antipsychotics in preventing relapse, but this question was not addressed in our study. The higher doses of haloperidol in the prior study may have had a negative effect on its tolerability and, consequentially, its effectiveness.
Early termination of the study’s follow-up period, which meant that patients enrolled during the second year of the study were followed for at least one year but less than the planned two years, had little effect on statistical power for the primary outcome because the risk of efficacy failure during the second treatment year was low. However, the early termination may have resulted in less reliable estimates of weight change at later time points.
The study did not include a comparison to an oral antipsychotic medication. At the time this study was begun, two randomized clinical trials comparing oral and LAI antipsychotics were underway. Neither of these studies found an advantage of LAI antipsychotics over oral antipsychotics in reducing hospitalizations.16,17 The only of these to be published to date had rates of hospitalization (45% for oral medication and 39% for LAI over two years) that are similar to the current study.16 Nevertheless, the use of LAIs is supported by some systematic reviews18,19 and expert panels20,21 for outpatients at increased risk of relapse. A limitation is that the study did not include subjective measures of medication satisfaction or global well-being. In addition, we have not addressed current cost differences for payers, which may be substantial as PP is still on patent while HD is available as a generic.
Conclusion
Among adults with schizophrenia or schizoaffective disorder, treatment with paliperidone palmitate compared with haloperidol decanoate did not result in a statistically significant difference in efficacy failure, but the results do not rule out the possibility of a clinically meaningful difference. The results are consistent with previous research that has not found large differences in the effectiveness of newer and older antipsychotics.
Supplementary Material
Acknowledgment
The following investigators conducted the study:
Lawrence Adler, Glen Burnie, MD; Peter Buckley, Augusta, GA; Matthew Byerly, Dallas, TX; Stanley Caroff, Philadelphia, PA; Cherilyn DeSouza, Kansas City, MO; Dale D’Mello, Lansing MI; Deepak D’Souza, New Haven, CT; Fred Jarskog, Chapel Hill, NC; Venkata Jasty, Detroit, MI; Eric Konicki, Cleveland, OH; Matthew Macaluso, Wichita, KS; J. Steven Lamberti, Rochester, NY; Joshua Kantrowitz, New York, NY; Joseph McEvoy, Butner, NC; Del Miller, Iowa City, IA; Robert Millet, Durham, NC; Max Schubert, Waco, TX; Martin Strassnig, Miami, FL; Sriram Ramaswamy, Omaha, NE; Andre Tapp, Tacoma, WA; Sarah Yasmin, Palo Alto, CA.
Role of Funding Source
NIMH had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript. The funder had no role in the decision to submit the manuscript for publication. A Data and Safety Monitoring Board convened by NIMH monitored the study. NIMH grant funds paid for all study medications.
Footnotes
Authors Contributions
The study was funded by grants from NIMH to Drs. McEvoy and Stroup. Drs. Byerly, Buckley, McEvoy, and Lamberti collected data. Drs. Hamer and Dominik, Ms. Ray and Ms. Wilkins oversaw or conducted the analyses. Dr. Stroup wrote the first draft of the manuscript. All authors participated in the writing and editing of the manuscript.
Drs. Stroup and Dominik had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
Dr. McEvoy reports personal fees from Lilly, grants and personal fees from Merck, grants and personal fees from Sunovion, personal fees from Otsuka, personal fees from Alkermes, grants and personal fees from Roche/Genentech, personal fees from Envivo, grants from GlaxoSmithKline, grants from Psychogenics, outside the submitted work. Dr. Byerly reports research support from Otsuka, outside the submitted work. Dr. Dominik reports no competing interests. Dr. Hamer reports grants from NIMH, during the conduct of the study; personal fees from Novartis, personal fees from Lilly, personal fees from Roche, personal fees from Pfizer, personal fees from Winston & Strawn, personal fees from Protein Sciences, personal fees from Sheppard Mullin, personal fees from Alkermes, personal fees from Allergan, personal fees from Rakoczy Molino Mazzochi Siwik, personal fees from AstraZeneca, personal fees from Veterans Administration, personal fees from Abbot / Abvie, personal fees from Bioline, personal fees from Titan, personal fees from Duke University, personal fees from Columbia University, personal fees from Goldberg Segalla, personal fees from Cenerx, personal fees from National University of Singapore / Duke, personal fees from Neurogex, outside the submitted work. Dr. Rosenheck reports personal fees from testifying expert in Jones ex rel. the State of Attorney Genera of Texas in Texas v. Janssen Phamaceutica et al, personal fees from Otsuka outside the submitted work Dr. Swartz reports personal fees from Med-IQ outside the submitted work. Ms. Wilkins reports no competing interests. Ms. Ray reports no competing interests. Dr. Buckley reports grants and personal fees from National Institute of Mental Health, grants from Ameritox, and grants from Posit Science outside the submitted work; Dr. Lamberti reports no competing interests. Dr. Stroup reports participation in CME activities funded by Genentech, outside the submitted work.
Contributor Information
Joseph P. McEvoy, Georgia Regents University.
Matthew Byerly, UT Southwestern Medical Center.
Robert M. Hamer, University of North Carolina.
Rosalie Dominik, University of North Carolina.
Marvin S. Swartz, Duke University.
Robert A. Rosenheck, Yale University
Neepa Ray, University of North Carolina.
J. Steven Lamberti, University of Rochester.
Peter F. Buckley, Georgia Regents University.
Tania M. Wilkins, University of North Carolina.
T. Scott Stroup, Columbia University.
References
- 1.Citrome L. Paliperidone palmitate - review of the efficacy, safety and cost of a new second-generation depot antipsychotic medication. Int J Clin Pract. 2010 Jan;64(2):216–239. doi: 10.1111/j.1742-1241.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 2.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta- analysis. Lancet. 2009 Jan 3;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia research. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Rosenheck R, Perlick D, Bingham S, et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. Jama. 2003 Nov 26;290(20):2693–2702. doi: 10.1001/jama.290.20.2693. [DOI] [PubMed] [Google Scholar]
- 5.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006 Oct;63(10):1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 6.Rosenheck R, Lin H. Assessment of Non-Inferiority of Perphenazine and Three Second Generation Antipsychotics in Chronic Schizophrenia. Journal of Nervous and Mental Disease. doi: 10.1097/NMD.0000000000000065. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citrome LL. The increase in risk of diabetes mellitus from exposure to second-generation antipsychotic agents. Drugs Today (Barc) 2004 May;40(5):445–464. doi: 10.1358/dot.2004.40.5.850492. [DOI] [PubMed] [Google Scholar]
- 8.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 9.Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002 Jan 3;346(1):16–22. doi: 10.1056/NEJMoa002028. [DOI] [PubMed] [Google Scholar]
- 10.O'Quigley J, Pessione F. Score tests for homogeneity of regression effect in the proportional hazards model. Biometrics. 1989 Mar;45(1):135–144. [PubMed] [Google Scholar]
- 11.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 12.Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Archives of general psychiatry. 1982 Apr;39(4):486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]
- 13.Kane JM, Rifkin A, Woerner M, et al. Low-dose neuroleptic treatment of outpatient schizophrenics. I. Preliminary results for relapse rates. Arch Gen Psychiatry. 1983 Aug;40(8):893–896. doi: 10.1001/archpsyc.1983.01790070083010. [DOI] [PubMed] [Google Scholar]
- 14.Marder SR, Van Putten T, Mintz J, Lebell M, McKenzie J, May PR. Low- and conventional-dose maintenance therapy with fluphenazine decanoate. Two- year outcome. Arch Gen Psychiatry. 1987 Jun;44(6):518–521. doi: 10.1001/archpsyc.1987.01800180028005. [DOI] [PubMed] [Google Scholar]
- 15.Hogarty GE, McEvoy JP, Munetz M, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry. 1988 Sep;45(9):797–805. doi: 10.1001/archpsyc.1988.01800330021002. [DOI] [PubMed] [Google Scholar]
- 16.Rosenheck RA, Krystal JH, Lew R, et al. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. The New England journal of medicine. 2011 Mar 3;364(9):842–851. doi: 10.1056/NEJMoa1005987. [DOI] [PubMed] [Google Scholar]
- 17.Buckley PF, Schooler NR, Kane J. PROACTIVE. Paper presented at: New Clinical Drug Evaluation Unit Annual Meeting; Boca Raton, FL. 2012. [Google Scholar]
- 18.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013 Oct;74(10):957–965. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]
- 19.Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia--a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011 Apr;127(1–3):83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007 Nov;68(11):1751–1762. doi: 10.4088/jcp.v68n1115. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010 Jan;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.