Abstract
BACKGROUND
Higher intake of fruits and vegetables appears to protect against inflammation, poor physical performance, and disability, but their relationship with muscle strength is unclear. We examined the association between total plasma carotenoids, an indicator of fruit and vegetable intake, and changes in muscle strength over a 6-year follow-up in the participants aged 65 years and older in the InCHIANTI study, a population-based study in Tuscany, Italy.
METHODS
Plasma carotenoids were measured at enrollment (1998–2000). Hip, knee and grip strength were measured at enrollment and 6 years later (2004–2006) in 628 of the 948 participants evaluated at baseline. Poor muscle strength was defined as the lowest sex-specific quartile of hip, knee and grip strength at enrollment. The main outcome was poor muscle strength at the six-year follow-up visit among those originally in the upper three quartiles of strength at enrollment.
RESULTS
Overall, 24.9% (110/441), 25.0% (111/444) and 24.9% (118/474) participants developed poor hip, knee and grip strength, respectively. After adjusting for potential confounders, participants in the lowest vs. the highest quartile of total plasma carotenoids at enrollment were at higher risk of developing poor hip (O.R. 2.25, 95% C.I. 1.13–4.48, P = 0.02), knee (O.R. 2.12, 95% C.I. 1.08–4.09, P = 0.03) and grip (O.R. 1.85, 95% C.I. 0.95–3.61, P = 0.07) muscle strength at the six-year follow-up visit.
CONCLUSION
These findings suggest that older community-dwelling adults with lower plasma carotenoids levels, a marker of poor fruit and vegetable intake, are at a higher risk of decline in skeletal muscle strength over time.
Keywords: carotenoids, InCHIANTI study, fruit, vegetable, sarcopenia
Introduction
Sarcopenia, a condition characterized by loss of skeletal muscle mass and strength with aging, is considered a key factor in the disablement process (1). It is widely recognized that age-related sarcopenia is caused by a combination of intrinsic factors involving changes at the energetic molecular and cellular levels, and extrinsic or environmental factors such as nutrition and exercise (2, 3). Several lines of research suggest that excessive oxidative stress caused by the accumulation of reactive oxygen species (ROS) is a causal factor in sarcopenia. ROS levels increase when the respiratory chain is malfunctioning and/or antioxidant cellular defense mechanisms is insufficient. Oxidative stress can damage macromolecules such as DNA, proteins, lipids, etc., thereby causing significant damage to the cells and tissue (4, 5).
Recent studies suggest that higher intake of fruits and vegetables, rich sources of antioxidants, including carotenoids, vitamin C, flavonoids, and other polyphenols, is correlated with muscle strength, physical performance, functional limitation and disability (6, 7, 8). In the Women’s Health and Aging Study, low serum carotenoids was associated with poor muscle strength among older moderately-severely disabled women living in the community (6). Low dietary β-carotene intake was associated with impaired lower extremity performance in older community-dwelling adults in Italy (7). Among older adults in the Atherosclerosis Risk in Communities study, fruit and vegetable intake was inversely correlated with functional limitations and disability (8). Serum carotenoids are considered the most valid indicator of fruit and vegetable intake (9), and thus, can be considered biomarkers for fruit and vegetable consumption.
Most studies looking at carotenoid intake or circulating levels and their relation with physical function have been cross-sectional. Thus, it is not known whether carotenoids deficiency is a significant predictor of accelerated functional loss, or simply reflect a global deterioration of functional status, with little or no effect on the risk of losing physical function.
To address the hypothesis that low serum carotenoids may predict a greater decline in skeletal muscle strength, we examined the relationship between plasma total carotenoids at enrollment and the decline in hip, knee and grip muscle strength over a six-year interval among participants in the InCHIANTI study, a population-based study of older adult living in the Chianti region of Tuscany, Italy.
Subjects and Methods
The study participants consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy (www.inchiantistudy.net). The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability (10). Briefly, in August 1998, 1270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (population 11,709) and Bagno a Ripoli (population 4,704), and of 1256 eligible subjects, 1155 (90.1%) agreed to participate. Of the 1155 participants, 1055 (91.3%) participated in the blood drawing. Participants received an extensive description of the study and participated after written, informed consent. The participants were seen again for a three-year follow-up visit (2001–2003) and a six-year follow-up visit (2004–2006), at which times they underwent a repeat phlebotomy and laboratory testing and assessment of physical performance. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee.
Demographic information and information on smoking and medication use were collected using standardized questionnaires. Average daily intakes of energy (kcal), carbohydrates, total protein, total lipids, etc. were estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire which has been validated for use in the older population (11). All participants were examined by trained geriatricians, and diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study for coronary heart disease, diabetes mellitus, chronic obstructive pulmonary disease, osteoarthritis, and cancer (12). Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Mini-Mental Status Examination (MMSE) was administered at enrollment (13). The level of physical activity in the year prior to the interview was classified on an ordinal scale based on responses to a modified standard questionnaire (10) into: 1) hardly any physical activity; 2) mostly sitting (occasionally walks, easy gardening); 3) light exercise (no sweat) 2–4 h/week; 4) moderate exercise (sweat) 1–2 h/week (level 4); 5) moderate exercise >3 h/week; 6) intense exercise (at the limits) > 3 times/week. For analytical purposes, we grouped the participants as: 1–3) inactive or having light physical activity; 4–5 having moderate physical activity; 6) having intense activity.
In this study, the process that leads to sarcopenia was operationalized as decline of muscle strength. Low muscle strength appears to be more important than low muscle mass as predictor of disability and mortality (1, 14). Therefore, in the analysis presented here we focused on the effect of baseline carotenoid levels on change over time in three different measures of muscle strength.
At enrollment and at the six-year follow-up visit, isometric muscle strength was assessed on eight muscle groups of the lower extremity by a hand held dynamometer, using a standard protocol (15). All measures of lower extremity muscle strength were highly correlated (Pearson’s correlation coefficients ranging from 0.87 to 0.92). Therefore, only hip flexion and knee extension strength were considered in the analysis to indicate lower extremity muscle strength. Measures of upper extremity muscle strength available in the InCHIANTI study were isometric shoulder adduction and handgrip. Between them, we selected the handgrip for the present analysis because the assessment of handgrip is easy, reliable, and has been utilized by a larger number of studies (1, 16). There is strong evidence in the literature that handgrip is a strong predictor of disability and mortality (16, 17). We included both measures of lower and upper extremity muscle strength because previous studies suggested that the rate of age-associated decline in muscle strength is quite different in these two anatomical regions (1). In fact, the correlation between handgrip and isometric strength of the lower extremity muscle groups was only moderately high, ranging from 0.70 to 0.72. Some participants were unable to come to the testing center and were evaluated only for grip strength during their home visit.
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at −80° C. Aliquots of plasma were shipped on dry ice to Dr. Semba’s laboratory for measurements of plasma carotenoids. Carotenoids were measured using high performance liquid chromatography (HPLC) (18). Total carotenoids were calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene in μmol/L. Within-run and between-run coefficients of variation, respectively, were 7.3 and 9.6% for α-carotene, 4.5 and 5.4% for β-carotene, 2.7 and 3.5% for β-cryptoxanthin, 2.6 and 7.1% for lutein, 6.2 and 6.8% for zeaxanthin, and 7.5 and 7.8% for lycopene.
Variables are reported as means (standard deviations) for normally distributed parameters or as percentages. The present analysis was limited to participants who were seen at enrollment and 6-year follow-up visits. Quartiles of total plasma carotenoids at enrollment were defined at the following cut-offs: <1.37, 1.37–1.74, 1.75–2.16, and >2.16 μmol/L. Poor hip, grip, and knee strength were defined by the lowest sex-specific quartile of hip, knee and grip strength at enrollment using the following cut-off values: 13.0, 10.45 and 16.0 kg for women, and 20.5, 16.45 and 28.0 kg for men. The longitudinal analyses included participants who were in the upper three quartiles of hip, knee and grip strength, for each analysis. The outcome measure was a decrease in hip (n=441), knee (n=444) and grip (n=474) strength below the cut-off for the lowest sex-specific quartile of baseline strength by the six-year follow-up visit.
Means were compared using t-test and percentages were compared using chi-square tests. Logistic regression models were was used to examine relationships between total plasma carotenoids and other risk factors and the development of poor hip, grip, and knee strength at the six-year follow-up visit. All analyses were performed using SAS (v. 8.2, SAS Institute, Inc., Cary, NC) with a statistical significance level set at P <0.05.
Results
Of the 1155 participants ≥65 years seen at enrollment, 1055 (91.3%) participated in the blood drawing. There were 948 (82.1%) participants at enrollment that had both plasma carotenoids and at least one of the three measures of strength (hip, knee, and/or grip strength) available for this analysis. The subjects who did not participate in the blood drawing were generally older and had greater comorbidity than the subjects who participated in the blood drawing, as reported elsewhere (19). The characteristics of the study population at enrollment are shown in Table 1.
Table 1.
Characteristics the Study Population at Baseline
| Characteristic1 | Whole Study Population (n = 948) | Population with Measure of Strength both at Baseline and at the 6-year Follow-up | ||
|---|---|---|---|---|
| Hip Strength2 (n = 441) | Knee Strength2 (n = 444) | Grip Strength2 (n = 474) | ||
| Age (years) | 74.9 (7.0) | 75.00(5.7) | 74.4 (6.2) | 76.1 (6.4) |
| Sex (% female) | 55.7 | 46.8 | 46.9 | 48.6 |
| Education (years) | 5.4 (3.3) | 5.4 (2.3) | 5.3 (2.4) | 5.0 (2.7) |
| Current smokers (%) | 27.4 | 33.9 | 28.8 | 32.3 |
| Body mass index (kg/m2) | 27.5 (4.0) | 26.8 (4.3) | 26.5 (3.8) | 26.7 (4.2) |
| Total plasma carotenoids (μmol/L) | 1.84 (0.68) | 1.70 (0.68) | 1.78 (0.64) | 1.74 (0.61) |
| Total energy intake (kcal/day) | 1926 (562) | 2001 (566) | 2034 (553) | 1992.3 (580.1) |
| Hip strength (kg) | 20.2 (7.1) | 20.6 (4.5) | 20.7 (5.6) | 19.3 (6.5) |
| Grip strength (kg) | 26.2 (11.6) | 26.8 (10.2) | 27.8 (9.9) | 26.5 (7.2) |
| Knee strength (kg) | 15.9 (6.1) | 16.5 (5.5) | 16.8 (5.2) | 15.7 (6.0) |
| Coronary heart disease (%) | 4.6 | 6.4 | 6.3 | 4.8 |
| Congestive heart failure (%) | 4.8 | 2.7 | 2.7 | 2.1 |
| Peripheral artery disease (%) | 5.4 | 6.4 | 4.9 | 4.8 |
| Stroke (%) | 4.0 | 3.6 | 3.6 | 3.2 |
| Diabetes mellitus (%) | 10.5 | 11.0 | 10.8 | 10.5 |
| Chronic obstructive pulmonary disease (%) | 7.4 | 7.3 | 6.3 | 8.9 |
| Depression (%) | 19.9 | 20.2 | 18.9 | 21.0 |
| Hip osteoarthritis (%) | 5.7 | 5.5 | 4.7 | 4.1 |
| Knee osteoarthritis (%) | 7.5 | 10.1 | 9.0 | 9.7 |
Mean (SD) for continuous variables or percentages as noted
Only participants in the 3 upper quartile of strength are reported here, because they represent the population at risk of developing poor muscle strength
There were 628 participants who had measurements of muscle strength conducted at the 6-year follow-up visit. Of 328 people who were not seen at the 6-year follow-up visit, 179 had died, 122 refused to participate, and 14 moved out of the study area. After including only participants who at baseline were in the upper three quartiles of hip, knee and grip strength, 24.9% (110/441) had poor hip strength, 25.0% (111/444) had poor knee strength and 24.9% (118/474) had poor grip strength. Participants who had died or refused to participate in the 6-year follow-up visit had lower values of total plasma carotenoids at baseline compared to those analyzed in this study (1.75 ± 0.65 vs 1.87 ± 0.68, respectively; P=0.034).
General characteristics of the population at enrollment and of those evaluated at 6-years follow-up are shown in Table 1. Participants evaluated after 6-years follow-up had a mean age of 73.0, 54.8% were women and with an average BMI of 27.6 Kg/m2.
Between enrollment and the 6-year follow-up visit, the overall mean declines (SD) in hip strength, knee strength and grip strength were −2.28 (5.24) kg (P <0.0001), −0.82 (5.60) kg (P <0.0001) and −1.44 kg (P <0.0001), respectively.
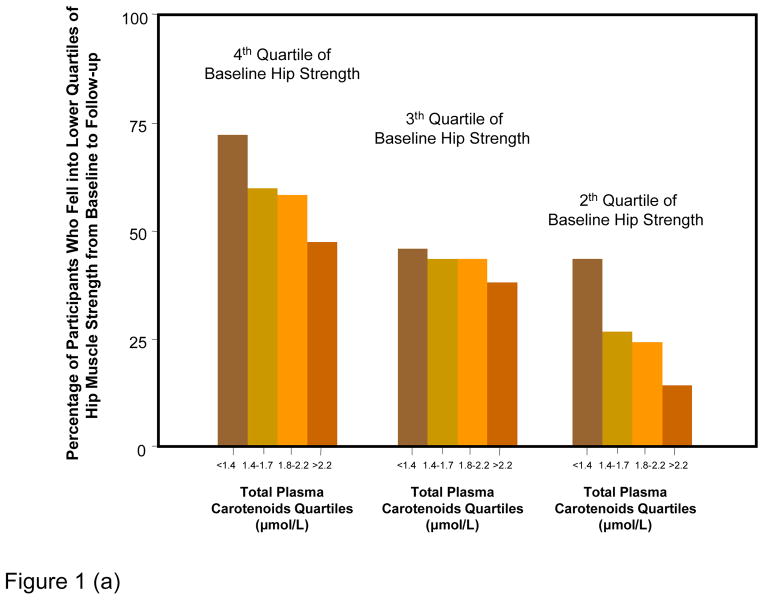
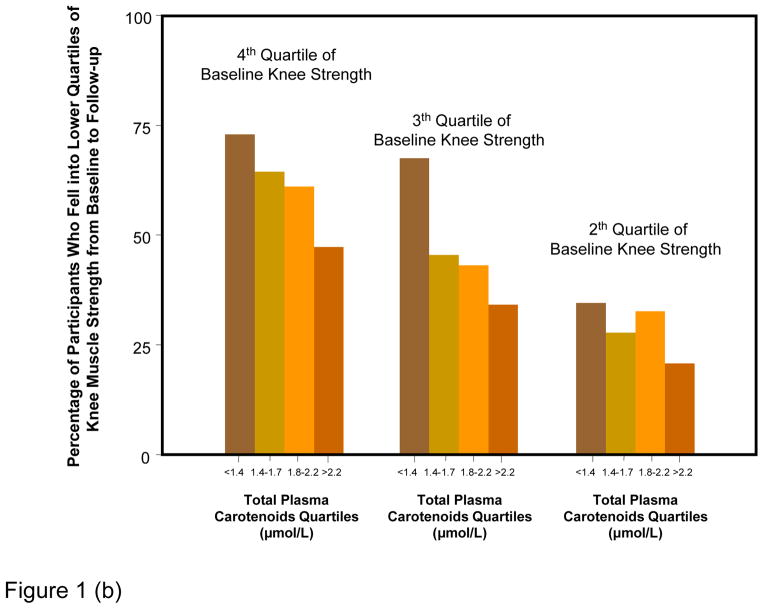
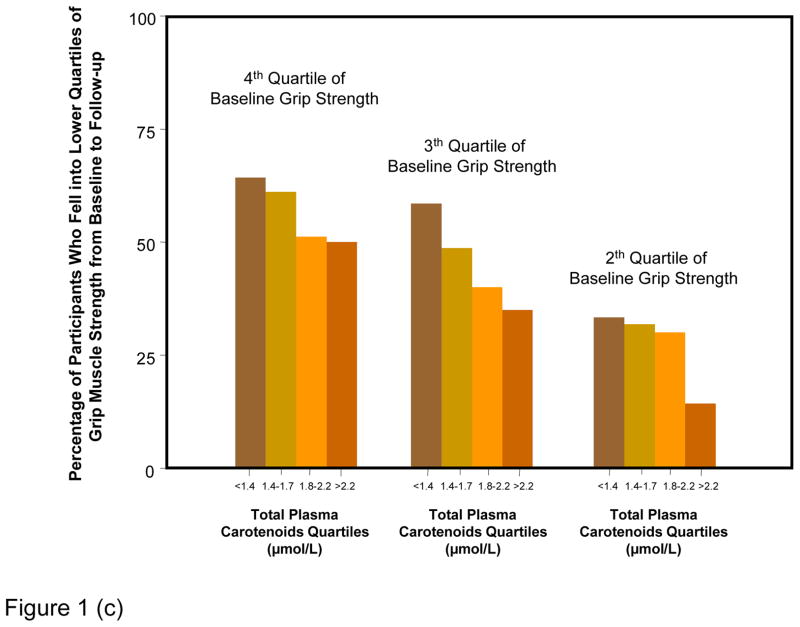
Figure 1 shows percentages of participants whose strength declined of at least one quartile over the follow-up, according to baseline muscle strength and carotenoid quartiles. Results are strikingly consistent for the three measures of strength. Regardless of the initial quartile of strength, lower carotenoids levels were associated with progressively higher rates of strength decline from baseline to 6-years follow-up. Considering strength as a continuous variable, the average decline in hip, knee and grip strength by quartile of total plasma carotenoids at enrollment, adjusting for age, sex and strength at enrollment, are shown in Figure 2. Participants in the lowest quartile of total plasma carotenoids showed the largest declines in hip, knee and grip strength, whereas those in the highest quartile showed the least decline in the respective strength measurements (Tests for linear trend, P = 0.0007 for hip strength, P = 0.0006 for knee strength and P = 0.04 for grip strength).
Figure 1.
Percentage of participants who fell in a worse quartile of hip (a), knee (b) and grip (c) muscle strength from baseline to 6-year follow-up according to baseline strength and carotenoids level quartile.
Figure 2.
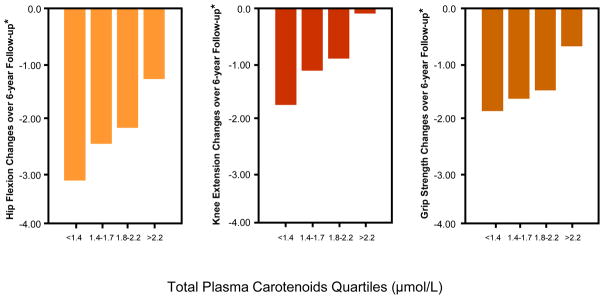
Relationship between total plasma carotenoids (in quartiles) with change in hip, knee and grip knee strength between enrollment and 6-year follow-up visit, adjusted by age, sex, and strength at enrollment (Tests for linear trend, P = 0.0007 for hip strength, P = 0.0006 for knee strength and P = 0.04 for grip strength).
* Age- Sex- and Baseline Muscle Strength adjusted.
The univariate relationships between plasma carotenoids and other risk factors at enrollment with the development of poor hip, knee and grip strength at the six-year follow-up (defined as a decline in strength below the thresholds that define the lowest baseline quartile) visit are shown in Table 2. Age, education, and BMI were significantly associated with the development of poor hip, knee and grip strength at the six-year follow-up. Hip and knee osteoarthritis were significantly associated with higher risk of developing poor hip and knee strength, respectively. Depression, current smoking and low physical activity was significantly associated with higher probability of developing poor grip strength. Coronary artery disease, congestive heart failure, peripheral artery disease, and diabetes mellitus were not significantly related to the risk of developing poor strength in any of the three measures.
Table 2.
Univariate Relationship between Baseline Carotenoids and Other Risk Factors and the Development of Poor Muscle Strength at the Six-year Follow-up
| Characteristic | Poor Hip Strength1 | Poor Knee Strength1 | Poor Grip Strength1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
| Age (years) | 1.13 | 1.08–1.18 | <0.0001 | 1.08 | 1.04–1.12 | <0.0001 | 1.15 | 1.11–1.20 | <0.0001 |
| Sex (% female) | 0.91 | 0.60–1.40 | 0.67 | 0.92 | 0.60–1.40 | 0.69 | 0.97 | 0.65–1.46 | 0.88 |
| Education (years) | 0.92 | 0.83–0.98 | 0.02 | 0.91 | 0.84–0.98 | 0.02 | 0.85 | 0.70–0.93 | 0.0003 |
| Current smokers (%) | 1.10 | 0.61–1.95 | 0.76 | 1.23 | 0.71–2.15 | 0.46 | 1.04 | 0.60–1.80 | 0.90 |
| Body mass index (kg/m2) | 0.93 | 0.87–0.98 | 0.01 | 0.90 | 0.84–0.95 | 0.0004 | 0.93 | 0.88–0.98 | 0.01 |
| Total plasma carotenoids (μmol/L)2 | |||||||||
| Quartile 1 | 2.54 | 1.35–4.75 | 0.004 | 1.94 | 1.05–3.39 | 0.04 | 1.99 | 1.11–3.57 | 0.02 |
| Quartile 2 | 1.35 | 0.69–2.63 | 0.38 | 1.25 | 0.66–2.37 | 0.50 | 1.69 | 0.93–3.06 | 0.09 |
| Quartile 3 | 1.36 | 0.70–2.64 | 0.36 | 1.57 | 0.85–2.90 | 0.15 | 1.29 | 0.71–2.37 | 0.41 |
| Quartile 4 | 1.00 | --- | --- | 1.00 | --- | --- | 1.00 | --- | --- |
| Total energy intake (kcal/day) | 1.00 | 0.999–1.00 | 0.42 | 1.00 | 1.00–1.000 | 0.91 | 0.999 | 0.999–1.000 | 0.63 |
| Physical Activity | 0.66 | 0.40–1.09 | 0.11 | 0.92 | 0.54–1.57 | 0.32 | 0.32 | 0.19–0.53 | <.0001 |
| Coronary heart disease | 1.96 | 0.76–5.05 | 0.16 | 1.84 | 0.72–4.67 | 0.20 | 1.25 | 0.47–3.29 | 0.66 |
| Congestive heart failure | 1.03 | 0.77–1.38 | 0.83 | 1.11 | 0.83–1.48 | 0.48 | 1.27 | 0.98–1.64 | 0.07 |
| Peripheral artery disease | 1.13 | 0.78–1.63 | 0.53 | 1.16 | 0.81–1.66 | 0.42 | 1.05 | 0.74–1.49 | 0.78 |
| Stroke | 1.28 | 0.80–2.07 | 0.31 | 1.16 | 0.74–1.83 | 0.50 | 1.23 | 0.79–1.93 | 0.35 |
| Diabetes mellitus | 1.01 | 0.57–1.78 | 0.98 | 0.98 | 0.56–1.73 | 0.95 | 0.76 | 0.42–1.36 | 0.36 |
| Chronic obstructive pulmonary disease | 0.86 | 0.51–1.47 | 0.59 | 0.93 | 0.56–1.57 | 0.79 | 1.01 | 0.63–1.60 | 0.98 |
| Depression | 1.40 | 0.81–2.41 | 0.23 | 1.45 | 0.83–2.52 | 0.19 | 1.85 | 1.09–3.14 | 0.02 |
| Osteoarthritis3 | 1.39 | 0.99–1.95 | 0.06 | 1.26 | 0.98–1.62 | 0.07 | --- | --- | --- |
Defined as a decline in strength below the thresholds that define the lowest baseline quartile.
Highest quartile of total plasma carotenoids is the reference quartile.
Hip osteoarthritis for poor hip strength model; knee osteoarthritis for poor knee strength model.
Multivariate logistic regression models testing the effect of serum carotenoids on the risk of developing poor hip, knee and grip strength are shown in Table 3. Adjusting for age and sex (model 1), participants in the lowest quartile of total plasma carotenoids at enrollment were at higher risk of developing poor hip strength (O.R. 2.19, 95% C.I. 1.13–4.24, P =0.02), poor knee strength (O.R. 1.84, 95% C.I. 1.00–3.38, P = 0.051) and poor grip strength (O.R. 2.01, 95% C.I. 1.06–3.80, P = 0.03) compared to those in the highest quartile. Adjusting for age, sex, education, body mass index, current smoking, total energy intake and physical activity (model 2), participants in the lowest quartile of total plasma carotenoids were at higher risk of developing poor hip strength (O.R. 2.25, 95% C.I. 1.13–4.48, P = 0.02), knee strength (O.R. 2.12, 95% C.I. 1.08–4.09, P = 0.03) and grip strength (O.R. 1.85, 95% C.I. 0.95–3.61, P = 0.07) compared to those in the highest quartile. We also examined additional multivariate models that also included depression and chronic obstructive pulmonary disease in addition to all the other covariates included in model 2. In these additional models, participants in the lowest quartile of total plasma carotenoids were at higher risk of developing poor hip strength (OR. 2.23, 95% C.I. 1.12–4.44, P = 0.02), knee strength (O.R. 2.10, 95% C.I. 1.06–4.14, P = 0.03) and grip strength (O.R. 1.82, 95% C.I. 0.93–3.56, P = 0.07) compared to those in the highest quartile.
Table 3.
Multivariate Analyses of the Relationship between Baseline Carotenoids and the Risk of Developing Poor Muscle Strength at the Six-year Follow-up Visit, Adjusting for Multiple Confounders
| Characteristic | Poor Hip Strength1 | Poor Knee Strength1 | Poor Grip Strength1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
| Model 1 | |||||||||
| Total plasma carotenoids (μmol/L)2 | |||||||||
| Quartile 1 | 2.19 | 1.13–4.24 | 0.02 | 1.84 | 1.00–3.51 | 0.051 | 2.01 | 1.06–3.80 | 0.02 |
| Quartile 2 | 1.27 | 0.64–2.54 | 0.49 | 1.26 | 0.66–2.43 | 0.48 | 1.83 | 0.96–3.46 | 0.06 |
| Quartile 3 | 1.41 | 0.71–2.80 | 0.32 | 1.68 | 0.89–3.15 | 0.10 | 1.45 | 0.76–2.79 | 0.26 |
| Quartile 4 | 1.00 | --- | --- | 1.00 | --- | --- | 1.00 | --- | --- |
| Age (years) | 1.23 | 1.08–1.17 | <0.0001 | 1.08 | 1.04–1.12 | <0.0001 | 1.16 | 1.12–1.21 | <0.0001 |
| Sex (female) | 0.93 | 0.59–1.46 | 0.74 | 0.95 | 0.61–1.48 | 0.83 | 0.94 | 0.60–1.48 | 0.81 |
| Model 2 | |||||||||
| Total plasma carotenoids (μmol/L)2 | |||||||||
| Quartile 1 | 2.25 | 1.13–4.48 | 0.02 | 2.12 | 1.08–4.19 | 0.03 | 1.85 | 0.95–3.61 | 0.07 |
| Quartile 2 | 1.30 | 0.64–2.66 | 0.47 | 1.32 | 0.67–2.63 | 0.42 | 1.64 | 0.84–3.20 | 0.15 |
| Quartile 3 | 1.30 | 0.65–2.62 | 0.46 | 1.65 | 0.86–3.16 | 0.13 | 1.27 | 0.65–2.50 | 0.48 |
| Quartile 4 | 1.00 | --- | --- | 1.00 | --- | --- | 1.00 | --- | --- |
| Age (years) | 1.12 | 1.07–1.17 | <0.0001 | 1.08 | 1.04–1.13 | 0.0001 | 1.15 | 1.10–1.20 | <0.0001 |
| Sex (female) | 0.82 | 0.45–1.50 | 0.53 | 0.88 | 0.49–1.57 | 0.67 | 0.87 | 0.49–1.54 | 0.63 |
| Education (years) | 0.94 | 0.86–1.02 | 0.11 | 0.94 | 0.86–1.01 | 0.10 | 0.89 | 0.82–0.97 | 0.005 |
| Body mass index (kg/m2) | 0.91 | 0.86–1.02 | 0.02 | 0.88 | 0.83–0.95 | 0.003 | 0.63 | 0.33–1.32 | 0.23 |
| Current smoking | 1.10 | 0.78–1.56 | 0.59 | 1.02 | 0.73–1.44 | 0.89 | 1.14 | 0.81–1.61 | 0.45 |
| Total energy intake (kcal/day) | 1.00 | 0.99–1.00 | 0.80 | 1.00 | 1.00–1.00 | 0.83 | 1.00 | 1.00–1.00 | 0.23 |
| Physical Activity | 0.84 | 0.48–1.48 | 0.55 | 1.10 | 0.61–1.98 | 0.75 | 0.34 | 0.19–0.62 | 0.0003 |
| Osteoarthritis3 | 1.43 | 1.07–1.92 | 0.02 | 1.44 | 1.09–1.91 | 0.01 | --- | --- | --- |
Defined as a decline in strength below the thresholds that define the lowest baseline quartile.
Highest quartile of total plasma carotenoids is the reference quartile.
Hip strength model adjusted for hip osteoarthritis; knee strength model adjusted for knee osteoarthritis.
Discussion
This study shows that older community-dwelling men and women with low plasma carotenoid concentrations experience a greater decline in hip, knee and grip muscle strength over a period of six years compared to those with high plasma carotenoids. These findings support and expand the results of previous cross-sectional studies that showed that low carotenoid intake and serum level of carotenoids, natural antioxidants, are independent correlates of poor skeletal muscle strength and impaired physical performance (10, 11). In particular, our longitudinal analysis shows that older community-dwelling men and women with a total plasma carotenoids less than 1.37 μmol/L are at a higher risk of a decline in skeletal muscle strength over time.
To our knowledge, no prospective studies have previously documented the longitudinal relationship of carotenoids with accelerated decline of muscle strength. Our results support the hypothesis that plasma carotenoids are associated with sarcopenia in older adults and bear further support to the hypothesis that intake of this natural antioxidant may protect against the development of impaired muscle strength in older persons.
Given the considerable high number of older persons who have inadequate food intake (20) and a reduced intake of antioxidants (20), an inadequate antioxidant capability is probably highly prevalent and especially likely to contribute to sarcopenia when coupled with a mismatch between protein requirement and reduced intake. In fact, the decline in food intake that occurs even in healthy older persons, and is probably related to the typical ‘anorexia of aging’, could result in not only a reduction in protein intake (20), but also a reduction in consumption of fruits and vegetables. These are the most important natural sources of antioxidans, such as carotenoids and flavonoids, whose deficiency may have a particularly effect in muscle where the production of ROS is highest. If the fine physiological balance between oxidative reactions and antioxidants is continuously negative because of a chronically low intake of antioxidants, such as carotenoids, a detrimental effect of ROS on muscle tissue would be theoretically possible even if subjects maintain low levels of physical activity.
Many studies have reported that skeletal muscle, especially during aerobic exercise, dramatically increases oxygen uptake, which inevitably results in an increased production of reactive radical oxygen species (ROS). Usually, free radicals produced by the mitochondria of active muscle are removed or scavenged by endogenous antioxidants (21). However, if free radical formation exceeds antioxidant capacity, the radicals may escape from the mitochondria and oxidize lipids, proteins, sugars, and other cell components. For example, it has been shown that ROS cause lipid peroxidation of polyunsaturated fatty acids in biological membranes and blood, leading to impaired cell functions (22). Based on this assumption, it has been suggested that antioxidant supplementation might be appropriate for athletes before of intense physical activity (23).
Strengths of this investigation are that it is population-based and has available longitudinal measures of upper and lower extremity muscle strength. Noteworthy, the strong and consistent relationship between baseline total plasma carotenoids and decline in muscle strength was consistent across the three measures of strength.
The prevention of undernutrition may potentially reduce the risk of disability; however, such a hypothesis would need to be tested through randomized controlled trials. Given that the carotenoids are biological markers for fruit and vegetable intake (9) and that fruits and vegetables contain a complex mix of antioxidants, fiber, and vitamins, dietary modification toward an increased intake of fruits and vegetables should be evaluated as a potential intervention for disability prevention. Such an approach has been already undertaken with the Dietary Approaches to Stop Hypertension Studies (24) and, recently, a Mediterranean-style diet was shown in a short-term randomized trial to reduce cardiovascular risk factors (25). Although a Mediterranean-style diet that is high in fruits and vegetables has attracted much interest because of an apparent protective effect against cardiovascular disease (26, 27) further studies are needed to expand these investigations to sarcopenia (28), decline in physical performance, and progression to disability among older adults.
Acknowledgments
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5-0002, and NIA Grant R01 AG027012. This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Author Contributions: Fulvio Lauretani and Richard Semba: concept and design, analysis and interpretation of data, preparation of manuscript. Stefania Bandinelli: acquisition of subjects and data, analysis and interpretation of data. Vittoria Giacomini, Anna Maria Corsi and Margaret Dayhoff-Brannigan: concept and design, interpretation of data, preparation of manuscript.
Jack Guralnik and Luigi Ferrucci: concept and design, acquisition of subjects and data, analysis and interpretation of data, preparation of manuscript.
Sponsors’ Role: None.
Financial Disclosure: The authors received no financial support in relation to this manuscript and declare that they have no conflict of interest to disclose concerning this manuscript.
References
- 1.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 4.Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2006 Dec 6; doi: 10.1016/j.abb.2006.11.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba RD, Blaum C, Guralnik JM, Totin D, Ricks MO, Fried LP. Low carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15:482–87. doi: 10.1007/BF03327377. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–94. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 8.Houston DK, Stevens J, Cai J, Haines PS. Dairy, fruit and vegetables intakes and functional limitations and disability in a biracial cohort: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2005;81:515–22. doi: 10.1093/ajcn.81.2.515. [DOI] [PubMed] [Google Scholar]
- 9.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C: National Academy Press; 2000. [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 11.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26 (suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publication No. 95-4009. [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Bandinelli S, Benvenuti E, Del Lungo I, Baccini M, Benvenuti F, Di Iorio A, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging. 1999;11:287–93. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 16.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–53. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- 17.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 18.van Lettow M, Harries AD, Kumwenda JJ, Zijlstra EE, Clark TD, Taha TE, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4:61. doi: 10.1186/1471-2334-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh AP, Miller ME, Saikin AM, Rejeski WJ, Hu N, Lauretani F, et al. Lower Extremity Strength and Power Are Associated With 400-Meter Walk Time in Older Adults: The InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–1193. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morley JE. Anorexia and weight loss in older persons. J Gerontol A Biol Sci Med Sci. 2003;58:131–7. doi: 10.1093/gerona/58.2.m131. [DOI] [PubMed] [Google Scholar]
- 21.Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc. 2000;32:1576–81. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Morillas-Ruiz JM, Villegas Garcia JA, Lopez FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr. 2006;25:444–53. doi: 10.1016/j.clnu.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Margaritis I, Palazzetti S, Rousseau AS, Richard MJ, Favier A. Antioxidant supplementation and tapering exercise improve exercise-induced antioxidant response. J Am Coll Nutr. 2003;22:147–56. doi: 10.1080/07315724.2003.10719288. [DOI] [PubMed] [Google Scholar]
- 24.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 25.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 26.de Lorgeril M, Salen P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006;9:118–23. doi: 10.1079/phn2005933. [DOI] [PubMed] [Google Scholar]
- 27.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 28.Weindruch R. Interventions based on the possibility that oxidative stress contributes to sarcopenia. J Gerontol A Biol Sci Med Sci. 1995;50:157–61. doi: 10.1093/gerona/50a.special_issue.157. [DOI] [PubMed] [Google Scholar]