Abstract
Physical activity can prevent and/or attenuate atherosclerosis, a disease clearly linked to inflammation. Paradoxically, even brief exercise induces a stress response and increases inflammatory cells like monocytes in the circulation. We hypothesized that exercise would regulate the expression of genes, gene pathways, and microRNAs in monocytes in a way that could limit pro-inflammatory function and drive monocytes to prevent, rather than contribute to, atherosclerosis. Twelve healthy men (22-30 yr old) performed ten 2-min bouts of cycle ergometer exercise at a constant work equivalent to an average of 82% of maximum O2 consumption interspersed with 1-min rest. Blood was drawn before and immediately after the exercise. Monocytes were isolated from peripheral blood mononuclear cells. Flow cytometry was used to identify monocyte subtypes. We used Affymetrix U133+2.0 arrays for gene expression and Agilent Human miRNA V2 Microarray for miRNAs. A stringent statistical approach (FDR < 0.05) was used to determine that exercise significantly altered the expression of 894 annotated genes and 19 miRNAs. We found distinct gene alterations that were likely to direct monocytes in an anti-inflammatory, anti-atherogenic pathway, including the downregulation of monocyte TNF, TLR4, and CD36 genes and the upregulation of EREG and CXCR4. Exercise significantly altered a number of microRNAs that likely influence monocytes involvement in vascular health. Exercise leads to a novel genomic profile of circulating monocytes, which appears to promote cardiovascular health despite the overall stress response.
Keywords: Genomics, Epigenetics, Gene Array, Leukocytes, Immune System, Exercise Immunology
1. Introduction
It is increasingly recognized that physical activity can (at appropriate levels of duration and intensity) prevent and/or attenuate atherosclerosis (Ahmed, Blaha, Nasir, Rivera, and Blumenthal, 2012), a disease now recognized to be associated with chronic inflammation (Woollard, 2013). Paradoxically, even brief bouts of physical activity substantially perturb cellular homeostasis and dramatically increase the number of inflammatory cells in the circulation, simulating stress responses in many ways that parallel the classical “danger” pattern of immune and inflammatory activation (Pradeu and Cooper, 2012). Precisely how exercise can stimulate immune and inflammatory processes and simultaneously attenuate cardiovascular disease remains unknown. In this study, we reasoned that brief exercise would activate many pro-inflammatory genes in circulating monocytes [inflammatory cells that play a key role in atherosclerosis (Woollard, 2013)] and their regulatory elements, microRNAs [short (20-24 nt) noncoding RNAs that are involved in post-transcriptional regulation of gene expression by affecting both the stability and translation of mRNAs]. However, we hypothesized that exercise would alter the expression of genes, gene pathways, and microRNAs in the circulating monocytes in a way that could limit pro-inflammatory function and drive monocytes to prevent, rather than exacerbate, atherosclerosis.
There is increasing focus on understanding the mechanisms that could attenuate activation of monocytes and prevent them from invading endothelium, contributing to plaque formation. Recent research demonstrates the substantial genomic, epigenetic, and functional heterogeneity of monocytes in cardiovascular disease (Zawada, Rogacev, Schirmer, Sester, Bohm, Fliser, and Heine, 2012), but very little is known about the impact of brief exercise expression and regulation of genes in circulating monocytes. Consequently, our studies provided us with the unique opportunity to compare the impact of brief exercise on monocyte gene expression and regulation with observations made by other workers in humans with atherosclerosis and vascular disease. Moreover, based on flow cytometry analysis, there is the evolving recognition of distinct monocyte subtypes within the circulation and that these monocyte subtypes might influence the pathophysiology of cardiovascular disease (Wong, Yeap, Tai, Ong, Dang, and Wong, 2012). Exercise acutely increases the number of circulating monocytes and initial investigations suggest that the monocyte subtypes are also affected (Shantsila, Tapp, Wrigley, Montoro-Garcia, Ghattas, Jaipersad, and Lip, 2012). What is not known is whether brief exercise alters gene and microRNA expression in circulating monocytes and whether these changes could potentially play a mechanistic role in the pathogenesis of atherosclerosis.
2. Methods
2.1. Participants
Twelve healthy young men (22–30 y/o) participated in this study (Table 1). Elite athletes, individuals who participated vigorously in competitive sports, and potential volunteers with a history of any chronic medical conditions or medication use were excluded from participation. The Institutional Review Board at the University of California Irvine approved the study and written informed consent was obtained from all participants upon enrollment.
Table 1. Anthropometric characteristics and exercise responses of the 12 subjects*.
| Age (years) | 26 ± 0.6 |
| Height (cm) | 179.6 ± 2.6 |
| Body Mass (kg) | 84.5 ± 3.7 |
| BMI (kg/m2) | 26 ± 0.8 |
| Peak VO2 (ml/kg/min) | 50.1 ± 2 |
| Average VO2 during the constant work rate as percent of peak VO2 | 81.7±2.2 |
| Lymphocytes (% change from before to after exercise, p=1.5E-06) | 169±12 |
| Monocytes (% change from before to after exercise, p=6.4E-04) | 91.4±14 |
| Plasma Lactate Before Exercise (mmol/l) | 1.2±0.07 |
| Plasma Lactate After Exercise (mmol/l, p= 6.8E-09 ) | 10.8±0.5 |
Values are means ± SE, BMI (body mass index); Peak V˙O2 (peak oxygen uptake)
2.2. Anthropometric Measurements
Standard calibrated scales and stadiometers were used to determine height and body mass.
2.3. Measurement of Fitness
Each volunteer performed a ramp-type progressive cycle ergometer exercise test using the SensorMedics metabolic system (Ergoline 800S, Yorba Linda, CA). After sitting comfortably without pedaling (“resting”) on the cycle ergometer for 3 min and 1 min of unloaded pedaling, the work rate (WR) was incremented at 20-30 watts/min to the limit of the subject's tolerance. Participants were vigorously encouraged during the high-intensity phases of the exercise protocol. Gas exchange was measured breath-by-breath and peak V˙O2 was calculated using standard methods.
2.4. Exercise Protocol
At least 48 hours, but not exceeding ten days following the completion of the ramp test, each volunteer performed an intermittent-exercise protocol. This exercise regimen was designed to more closely mimic naturally occurring patterns of physical activity and has been used extensively over the years in our laboratory. The protocol consists of ten 2-min bouts of constant work rate cycle ergometry, with a 1-min rest interval between each bout. The participants were instructed not to perform any type of vigorous physical activity 48 hours prior to the exercise challenge. The work rate was individualized for each subject and on average was equivalent to 82% of the participants' peak V˙O2.
2.5. Blood Sampling and Analysis
Participants arrived at the laboratory between 7:30-8:30 AM after an overnight fast. An indwelling catheter was inserted into the antecubital vein. A baseline sample was taken 30 min after the placement of the catheter and before the onset of exercise. We waited 30 min to ensure that measurable physiological parameters of stress (e.g., heart rate and blood pressure) were at baseline levels. The volunteers then completed the intermittent exercise protocol and additional blood samples were obtained immediately after exercise. Complete blood counts for white blood cell analysis were obtained by standard methods from the clinical hematology laboratory. Plasma lactate was measured before and after exercise using YSI 2300 STAT Plus™ Glucose & Lactate Analyzer.
2.6. Flow Cytometry
Surface antigen-specific fluorescent-conjugated monoclonal antibodies (MAbs), anti-CD14 fluorescein isothiocyanate (FITC) - (clone HCD14) and anti-CD16 phycoerythrin (PE)-(clone 3G8) and their respective isotype controls (Biolegend, San Diego, CA) were used to identify monocyte subpopulations: classical (CD14++/CD16−), nonclassical (CD16++/CD14+) and intermediate (CD16+/CD14++) monocytes.
An Accuri-C6 flow cytometer (Becton Dickinson) was used for data acquisition. At least 100,000 events were collected from each sample. Flow cytometry data analysis was performed using Accuri-C6 Plus software (Becton Dickinson). Monocytes were gated based on their FSC/SSC characteristics, using this gate, classical cells were; identified as (CD14++/CD16−), nonclassical as (CD16++/CD14+) and intermediate as (CD16+/CD14++). We used a gating approach recently published by Ziegler-Heitbrock and Hofer (Ziegler-Heitbrock and Hofer, 2013) to analyze the data.
2.7. Monocyte Isolation
The duration from blood draw to stabilization of RNA never exceeded 150 min. Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-treated blood using OptiPrep® Density Gradient Medium (SIGMA). Monocytes were isolated from PBMC using a negative magnetic cell separation methods (Miltenyi Biotec Monocyte Isolation Kit II Human and the autoMACS® Pro Separator). The Monocyte Isolation Kit was developed to isolate monocytes from human PBMCs with minimum perturbation. Non-monocytes, i.e., T cells, NK cells, B cells, dendritic cells and basophils, are indirectly magnetically labeled using a cocktail of biotin-conjugated antibodies against CD3, CD7, CD16, CD19, CD56, CD123 and Glycophorin A, and Anti-Biotin MicroBeads. Our monocyte isolation procedure consistently yielded high purity monocytes with an average of 93.2 ±1.4% purity in the before exercise samples and 92.3±1.2% purity in the after exercise samples as shown by flow cytometry (stained with CD14-FITC).
2.8. RNA Extraction
Total RNA for gene and microRNA analysis was extracted from isolated monocytes using TRIzol® (Gibco BRL Life Technologies, Rockville, MD). For gene expression study only, RNA was purified using Qiagen-RNeasy Mini Kit. RNA pellets were resuspended in diethyl pyrocarbonate-treated water. RNA integrity was assessed (prior to beginning target processing) using Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Palo Alto, CA, USA). We only analyzed samples with RNA Integrity Number (RNI)≥9.1).
2.9. Gene Expression Microarrays
Microarray processing was performed as recommended by the manufacturer and is available in the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). Briefly, first-strand cDNA is synthesized from 250ng of isolated total RNA for each one of the pre and post exercise samples. After making the complementary second strand, the double-stranded cDNA is used to generate biotin-tagged cRNA from an in- vitro transcription (IVT) using T7 RNA polymerase. Ten μg of fragmented target cRNA was hybridized on an Affymetrix U133 plus 2 arrays. Arrays were scanned using GeneChip® Scanner 3000 7G and Command Console Software v 3.2.3. to produce *.CEL intensity files.
2.10. microRNA Microarrays
One hundred ng of total RNA from each sample was labeled with the fluorescent dye Cyanine 3-pCp (Cy3) using the microRNA Labeling Reagent and Hybridization Kit (Agilent Technologies, Palo Alto, CA) following the manufacturer's protocol. Cy3-labeled RNA from each sample was hybridized to Agilent Human microRNA Version 2 Microarray. The hybridized array was then washed and scanned according to Agilent specifications and data was extracted from the scanned image using Feature Extraction version 10.2 (Agilent Technologies).
2.11. Data Analysis
The results were analyzed using GeneSpring GX 12.1 Software (Agilent Technologies, Inc).
2.11.1. Gene Expression
Raw data was normalized using GC-RMA. Only probe sets that reached a signal value ≥50 in at least 50% of the values in any one out of the two conditions were included in the analysis. Overall, 17,566 of 54,675 probe sets represented on the array met these criteria. The microarray cell files and GC-RMA normalized data have been deposited in the GEO database, series accession number http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51837.
Traditional Student's paired t-test was first applied to each probe set and fold change (FC, increase or decrease) >1.2 and Multiple Testing Correction (False Discovery Rate, FDR<0.05) (Benjamini-Hochberg) procedure was carried out.
The final list of significantly changed probe sets was then additionally analyzed using the functional annotation tools provided by DAVID, the Database for Annotation, Visualization and Integrated Discovery to classify the genes into pathways using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Only pathways with Expression Analysis Systematic Explorer (EASE) score ≤ 0.038 are presented in this analysis. EASE score is a modified Fisher exact P value in the DAVID system used for gene-enrichment analysis. EASE score P value = 0 represents perfect enrichment. P value ≤ 0.05 is considered as gene enrichment in a specific annotation category (http://david.abcc.ncifcrf.gov/helps/functional_annotation.html#summary).
2.11.2. microRNA Microarrays
All raw signal values lower than 1 were adjusted to 1 and normalized using percentile shift (90th percentile). Only entities that had a present or marginal flag and passed the 20 percentile filtration in at least 100% of values in any one out of the two conditions were selected for further analysis. Overall, 168 out of 961 entities represented on the array met these criteria. The microRNA data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51837.
Traditional Student's paired t-test was first applied to each probe set and fold change (FC, increase or decrease) ≥1.2 and false discovery rate (FDR<0.05) (Benjamini-Hochberg) procedure was carried out.
The final list of significantly changed microRNAs was then additionally analyzed using TargetScan database release 5.1 (provided by GeneSpring) http://www.targetscan.org/. We looked for the predicted target genes for each microRNA (context percentile=50, conserved database). Briefly, TargetScan predicts gene targets of microRNAs by searching for the presence of conserved 8mer and 7mer sites that match the seed region (positions 2-7 of a mature microRNA) of each microRNA.
2.11.3. An “Intersecting” Analysis of microRNA and Gene Expression Level
We have recently published our overall approach for an intersecting analysis of the impact of exercise on mRNA and microRNA (Radom-Aizik, Zaldivar, Jr., Oliver, Galassetti, and Cooper, 2010). Briefly, we first computationally identified all predicted target genes that contained sequences corresponding to the nucleotide sequences of the monocyte microRNAs that had been altered by exercise. From that set of genes we identified those genes that we found to have actually been altered by exercise (as noted, 1236 probe sets). We then performed pathway analysis in this overlapping gene set. We present only pathways with EASE score≤0.05.
2.11.4. Gene and microRNA Expression Analysis by Quantitative PCR (RT-PCR)
For confirmation of gene expression microarray findings, TaqMan assays were carried out on eleven genes: 8 genes (DUSP5, FAS, MAPK7, CXCR4, HSPA1A, HSPA8, NR4A1, TNF) are related to the MAPK signaling pathway which is involved in regulation of many cellular events, including cell proliferation, transformation, differentiation and apoptosis, and to three genes (AREG, HBEGF, EREG) linked to the growth factors family.
For confirmation of microRNA microarray expression findings, TaqMan assays were carried on three microRNAs that were found to have possible connection to atherosclerosis; miRs 23b and 130a had lower expression, and miR29b had higher expression following exercise.
The RT-PCR analysis was performed with the Applied Biosystems 7900HT PCR System by using TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression probes (Applied Biosystems) (FAS: assay ID, Hs00907759_m1; MAPK7: assay ID, Hs00611114_g1; HSPA1A: assay ID, Hs00359163_s1; HSPA8: assay ID, Hs00852842_gH; CXCR4: assay ID, Hs00976734_m1; DUSP5: assay ID, Hs00244839_m1, NR4A1: assay ID, Hs00374230_m1; TNF; assay ID, Hs00174128_m1, AREG: assay ID, Hs 00155832_m1; EREG: assay ID, Hs 00914313_m1, and HBEGF: assay ID, Hs 00961131_m1). Actin beta was used as an endogenous control.
For microRNA expression, we used Assays-on-Demand microRNA probes (Applied Biosystems) (miR-23b: assay ID, 000400, miR-29b; assay ID, 000413, and miR 130a; assay ID, 000454). RNU44 was used as an endogenous control.
2.11.5. Physiological Data
The physiological data are presented as mean and standard error (SE). Two-sided paired t-test was applied for testing changes from before to after the exercise and the significance level was set at 0.05.
3. Results
3.1. Anthropometric and Physiological Characteristics
The anthropometric and physiological characteristics of the 12 subjects appear in Table 1.
3.2. Monocyte and Monocyte Subtype Response to Exercise
As shown in Table 1, the number of monocytes detected by CBC was significantly and substantially elevated at peak exercise (p<6.4E-04). As previously shown (Simpson, McFarlin, McSporran, Spielmann, Hartaigh, and Guy, 2009;Steppich, Dayyani, Gruber, Lorenz, Mack, and Ziegler-Heitbrock, 2000), classical and non-classical monocyte number increased after exercise however, the non-classical cell numbers increased in relatively greater numbers causing the classical subtype to have a lowered proportion. (Figure).
Figure 1.
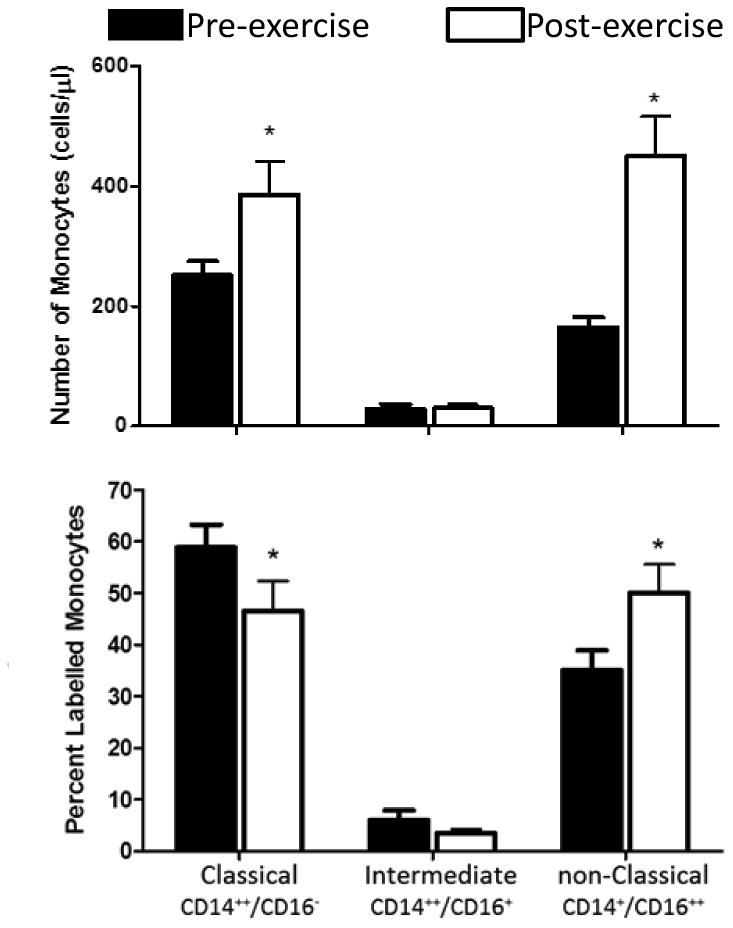
Effect of exercise (pre vs. post) on circulating monocyte subtypes (classical, CD14++/CD16-; intermediate, CD14++/CD16+; non classical, CD14+/CD16++). Top panel shows the absolute numbers. Bottom panel shows the percent changes. The brief exercise bout increased both the classical and non-classical absolute number of monocyte subtype *P<0.01, before versus after exercise. This finding corroborates initial results of a number of investigators (see text).
3.3. Monocyte Gene Expression in Response to Exercise
Our intermittent exercise protocol altered the expression level of 1236 probe sets (894 annotated genes) in the population of monocytes in the circulating blood. Seven Kegg pathways were enriched with genes whose expression was altered by exercise (EASE<0.038) (Table 2).
Table 2. Seven gene-expression Kegg pathways altered by exercise in monocytes.
| KEGG Pathway | Number of Genes | EASE Score |
|---|---|---|
| Systemic lupus erythematosus | 18 | 4.40E-06 |
| MAPK signaling pathway | 24 | 5.10E-03 |
| RNA degradation | 8 | 2.00E-02 |
| Pathogenic Escherichia coli infection | 8 | 2.00E-02 |
| Apoptosis | 10 | 2.50E-02 |
| Natural killer cell mediated cytotoxicity | 13 | 2.80E-02 |
| Neurotrophin signaling pathway | 12 | 3.80E-02 |
3.4. The Effect of Exercise on Monocyte microRNA Expression
The intermittent exercise protocol altered the expression level of 19 microRNAs (14 had higher expression) with potential target genes. In Table 3 we present the 19 microRNAs and their possible link to atherosclerosis.
Table 3. microRNAs in monocytes significantly affected by exercise and their possible connection to atherosclerosis.
| microRNA | Fold Change | p-value FDR | Possible link to atherosclerosis |
|---|---|---|---|
| miR-199a-3p | ↓1.5 | 0.044 | Unknown |
| miR-130a | ↓1.5 | 0.044 | Involved in angiogenesis in endothelial progenitor cells (Zhang, Kandic, and Kutryk, 2011). |
| miR-151-5p | ↓1.4 | 0.044 | Unknown |
| miR-221 | ↓1.3 | 0.042 | Involved in vascular remodeling (Wei, Schober, and Weber, 2013); regulation of monocytes into dendritic cells (Lu, Huang, Zhang, Roensch, Cao, Nakayama, Blazar, Zeng, and Zhou, 2011). |
| miR-23b | ↓1.3 | 0.044 | Plays an atheroprotective role in shear stress vascular remodeling (Neth, Nazari-Jahantigh, Schober, and Weber, 2013). |
| miR-29b | ↑1.9 | 0.044 | Plays a key role in the mechanisms through which LDLs alter vascular smooth muscle function (Chen, Wang, Hu, Chang, Liao, Dai, and Juo, 2011); significantly upregulated in atherosclerotic aortic aneurysm tissue (Kin, Miyagawa, Fukushima, Shirakawa, Torikai, Shimamura, Daimon, Kawahara, Kuratani, and Sawa, 2012). |
| miR-29c | ↑1.5 | 0.043 | Unknown |
| miR-1305 | ↑1.5 | 0.028 | Unknown |
| miR-362-3p | ↑1.4 | 0.044 | Downregulated more than twofold in both brain and blood following experimental injury to the cerebral vasculature (Liu, Tian, Ander, Xu, Stamova, Zhan, Turner, Jickling, and Sharp, 2010). |
| miR-660 | ↑1.4 | 0.028 | Increase the efficiency of ex vivo platelet generation (Emmrich, Henke, Hegermann, Ochs, Reinhardt, and Klusmann, 2012). |
| miR-324-3p | ↑1.4 | 0.035 | Unknown |
| miR-1202 | ↑1.3 | 0.044 | Unknown |
| miR-140-5p | ↑1.3 | 0.028 | Circulating levels are elevated in severely obese individuals (Ortega, Mercader, Catalan, Moreno-Navarrete, Pueyo, Sabater, Gomez-Ambrosi, Anglada, Fernandez-Formoso, Ricart, Fruhbeck, and Fernandez-Real, 2013). |
| miR-532-5p | ↑1.3 | 0.028 | Circulating levels are elevated in severely obese individuals (Ortega et al., 2013). |
| miR-362-5p | ↑1.3 | 0.028 | Unknown |
| miR-30e | ↑1.3 | 0.044 | Substantially downregulated in animal model of atherosclerotic lesions (Han, Wang, Qu, Sun, Li, Jiang, and Luo, 2013). |
| miR-532-3p | ↑1.3 | 0.044 | Unknown |
| miR-15a | ↑1.3 | 0.030 | Involved in blood brain barrier disruption in animal models of vascular injury (Rink and Khanna, 2011). |
| miR-338-3p | ↑1.3 | 0.033 | Unknown |
3.5. Intersecting Analysis of microRNA and Gene Expression Level
Intersecting analysis of gene and microRNA expression showed evidence of possible microRNAs regulation of 3 pathways (Table 4) that have a link to atherosclerosis. In Table 4 we present 3 microRNAs and we show: 1) microRNAs that were affected by exercise; 2) the number of potentially targeted genes for each affected microRNA (in silico analysis); and 3) from the genes identified in #2, we show those genes that we found also to have actually been affected by exercise. Kegg pathway analysis of these sets of genes showed three significant pathways: jak-STAT signaling pathway, endocytosis, and the p53 signaling pathway.
Table 4. Gene pathways likely to have been influenced by the effect of exercise on monocyte microRNAs.
| microRNA | FC# | Number of “microRNA-susceptible” genes affected by exercise | Pathways* | Link to atherosclerosis |
|---|---|---|---|---|
| miR-30e | ↑1.3 | 65 | Jak-STAT signaling | JAK/STAT regulates the initiation and progression of atherosclerosis and the remodeling in response to injury. Under inflammatory, atherogenic, and immune stimulation, JAK-STAT is activated in monocytes (Ortiz-Munoz, Martin-Ventura, Hernandez-Vargas, Mallavia, Lopez-Parra, Lopez-Franco, Munoz-Garcia, Fernandez-Vizarra, Ortega, Egido, and Gomez-Guerrero, 2009). |
| miR-23b | ↓1.3 | 54 | p53 signaling | Oxidative stress induced by OxLDL increases the intracellular level and the DNA binding activity of p53; 2) The induction of apoptosis by OxLDL in macrophages is associated with enhanced expression of p53; p53 knockout mice show increased aortic plaque formation (Maziere and Maziere, 2009). |
| hsa-miR-130a | ↓1.5 | 39 | Endocytosis | Monocyte endocytosis of oxidized LDLs is an essential mechanism of atherosclerotic plaque formation (Gratchev, Sobenin, Orekhov, and Kzhyshkowska, 2012). |
FC (fold change in response to exercise);
KEGG pathways, EASE≤0.05
3.6. RT-PCR Verification of Specific Genes and microRNAs
We used TaqMan assays to verify the expression level changes from before to after exercise of 11 genes (Supplemental Table S1) and three microRNAs. The three microRNAs included: miR- 29b (FC=1.9, p=0.007), which was upregulated by exercise; and miR-23b (FC=1.3, p=0.015) and miR-130a (FC=1.5, p=0.03), which were downregulated. TaqMan assays results (FC, and significance) matched well with the microarray results for both gene expression and the microRNAs.
4. Discussion
This is the first study, we believe, to demonstrate substantial changes in gene and microRNA expression in circulating monocytes in healthy individuals following brief exercise, a profound physiological perturbation that occurs in daily life. Exercise led to altered expression of genes and microRNAs that could attenuate pathological activation of monocytes. Thus, our studies uniquely suggest that some of the anti-atherogenic effects of exercise may be mediated through the acute effects of exercise on modulation of gene and microRNA expression in circulating monocytes.
4.1. Impact of Brief Exercise on Monocyte Gene Expression
An examination of gene responses in the current study sheds new light on possible specific anti-atherogenic monocyte mechanisms associated with brief exercise that occur despite the simultaneous exercise activation of stress and inflammatory responses. Exercise is a challenge to cellular homeostasis and elicits a stress response. Indeed, while the predominant health effects of exercise are beneficial, exercise can impair health [e.g., exercise induced anaphylaxis and asthma] when there is an imbalance between the pro- and anti-inflammatory responses to exercise. We did find that many pro-inflammatory genes in monocytes were upregulated by exercise, similar to earlier observations we have made in PBMCs in general, neutrophils, and NK cells (Radom-Aizik, Zaldivar, Haddad, and Cooper, 2013). The increase in the number of circulating monocytes is in and of itself a hallmark of an acute inflammatory response. In addition, when we examined the 25 genes whose expression was most upregulated by exercise (ranging from 19.6 to 2.3 fold increase) we found from literature searches that 13 of the genes have been associated with pro-inflammatory activation. Among them, for example, DUSP2 worsens inflammatory diseases like arthritis (Wei, Jiao, Postlethwaite, Stuart, Wang, Sun, and Gu, 2013), HSP70 promotes atherosclerosis (Yadav, Kumar, and Jha, 2013), and PDE4B plays a role in autoimmune inflammatory diseases like systemic lupus erythematosus (Yougbare, Boire, Roy, Lugnier, and Rouseau, 2013). Six of the 25 genes were clearly associated with anti-inflammatory functions, and the remaining six had not been linked previously with either pro- or antiinflammatory activity.
But when we compared monocyte and/or PBMC genes that are known by previous investigators to be upregulated in patients with peripheral vascular disease with exercise changes, some intriguing differences in regulation were observed (Table 5). Noteworthy among the differential gene expression was the downregulation by exercise of CD36, known to be upregulated in patients with vascular disease. In the case of CD36, a possible protective effect of exercise would be a reduction in the ability of monocytes to scavenge oxidized LDL and, in so doing, contribute to the formation of atherosclerotic plaques.
Table 5.
Differential exercise regulation of monocyte genes previously associated with inflammation and/or coronary artery disease (CAD). All data from human subjects. Data from CAD patients is derived from previous studies. The exercise data is from the present study in healthy people.
| Gene | CAD | Exercise | Possible Mechanisms |
|---|---|---|---|
| CD36 | ↑ | ↓ | Facilitates scavenging of modified LDL and activates inflammatory pathways (Febbraio and Silverstein, 2007). |
| TLR4 | ↑ | ↓ | Pathogenesis and destabilization of atherosclerotic plaques (den Dekker, Cheng, Pasterkamp, and Duckers, 2010). |
| VCAN | ↑ | ↓ | Versican involved in advanced lesions of atherosclerosis, at the borders of lipid-filled necrotic cores as well as at the plaque-thrombus interface (Wight and Merrilees, 2004). |
| CDKN1A | ↑ | ↓ | Regulates AMPK control of cell proliferation in vascular disease (Ferri, 2012). |
| DNAJB6 | ↑ | ↓ | Controls HSP function, known to be a key immune modulator in atherosclerotic plaques (Lu and Kakkar, 2010). |
| FAM198B | ↑ | ↓ | Unknown |
| HIST1H2BG | ↑ | ↓ | Histone modification is a critical component of a transcriptional cascade regulating SMC proliferation and might play a pivotal role in the development of proliferative vascular diseases (Findeisen, Gizard, Zhao, Qing, Heywood, Jones, Cohn, and Bruemmer, 2011). |
| PGRMC1 | ↑ | ↓ | Regulator of progesterone signaling, progesterone antagonizes the vasoprotective effects of estrogen (Wassmann, Wassmann, and Nickenig, 2005). |
| PLDN | ↓ | ↑ | Unknown |
| PRKAR1A | ↓ | ↑ | Regulator of intracellular cAMP. cAMP influences vascular endothelium, the production of reactive oxygen species, the recruitment of circulating monocytes to the artery wall and their differentiation into macrophages-foam cells (Fantidis, 2010). |
Similarly, we found a reduction in monocyte TLR4 gene expression following brief exercise, and TLR4 gene expression was elevated in patients with peripheral vascular disease. Interestingly, Oliveira and Gleeson (Oliveira and Gleeson, 2010) used flow cytometry to demonstrate that 1.5 hr of continuous heavy exercise led to a reduction in TLR4 surface receptor using flow cytometry. When the TLR4 receptor is engaged, it activates innate immune cells and is particularly responsive to lipopolysaccharide. In murine models, TLR4 antagonist inhibited vascular inflammation and atherogenesis (Lu, Zhang, Li, Jin, and Huang, 2013), and in human monocytes, Estruch and coworkers (Estruch, Bancells, Beloki, Sanchez-Quesada, Ordonez-Llanos, and Benitez, 2013) discovered that TLR4-mediated inflammatory activation was induced by LDL. Simpson and coworkers (Simpson et al., 2009) showed that an acute bout of aerobic exercise causes changes in TLR expression within specific blood monocyte subpopulations and suggested that the mechanism might be occurring at the cellular level, a speculation that is consistent with our observed changes in monocyte gene expression. The reduced expression of the TLR4 gene in monocytes with exercise, if sustained, could act to limit the participation of monocytes in the inflammatory milieu of the nascent atherosclerotic plaque. The reduced expression of the TLR4 gene in monocytes with exercise, if sustained, could act to limit the participation of monocytes in the inflammatory milieu of the nascent atherosclerotic plaque.
As shown in Tables 6 and 7, even a perfunctory investigation of the monocyte genes that were most substantially up- and downregulated by exercise (≥ 1.9 fold-change) identified intriguing possible protective mechanisms. For example, the upregulation of vascular growth factor genes such as HBEGF and EREG could be construed to prepare the circulating monocytes to play a role in vascular repair. Indeed, endothelial dysfunction and loss of endothelial integrity lead to progression of atherosclerotic vascular disease and the timely repair of endothelial injuries is essential in atheroclerosis prevention. Monocytes aid vascular repair and promote angiogenesis through a variety of mechanisms ranging from secretion of vascular growth factors in a paracrine manner to their acting as pluripotential cells and integrating themselves into the endothelium (Favre, Terborg, and Horrevoets, 2013).
Table 6. Examples of monocyte genes that were upregulated by exercise (fold change ≥ 2.0) and likely involved in vascular health.
| Gene | Name | FC | Possible link to vascular health |
|---|---|---|---|
| AREG | amphiregulin | 19.3 | Previously shown to be produced by monocytes (Bles, Di, Boeynaems, and Communi, 2010;Mograbi, Rochet, Imbert, Bourget, Bocciardi, Emiliozzi, and Rossi, 1997); can stimulate vascular smooth muscle growth (Kyotani, Ota, Itaya-Hironaka, Yamauchi, Sakuramoto-Tsuchida, Zhao, Ozawa, Nagayama, Ito, Takasawa, Kimura, Uno, and Yoshizumi, 2013). |
| HBEGF | heparin-binding EGF-like growth factor | 7.8 | Can play a beneficial role in vascular remodeling. (Zhang, Sunnarborg, McNaughton, Johns, Lee, and Faber, 2008). |
| EREG | epiregulin | 6.8 | Stimulates vascular repair in experimental myocardial infarct (Nguyen, Maltais, Perrault, Tanguay, Tardif, Stevens, Borie, Harel, Mansour, and Noiseux, 2010). |
| NR4A2 | nuclear receptor subfamily 4, group A, member 2 | 5.4 | Emerging insights suggest that NR4A mediates regulation of inflammatory gene expression in macrophages (Zhao and Bruemmer, 2009). |
| PDE4B | phosphodiesterase 4B, cAMP-specific | 4.9 | Involved in Ca+2 alterations in HUVEC cells that are associated with endothelial responsiveness (Campos-Toimil, Keravis, Orallo, Takeda, and Lugnier, 2008). |
| HSPA1A | heat shock 70kDa protein 1A | 4.8 | Modulates VEGF control of vascular remodeling (Bruns, Yuldasheva, Latham, Bao, Pellet-Many, Frankel, Stephen, Howell, Wheatcroft, Kearney, Zachary, and Ponnambalam, 2012). |
| SOCS3 | suppressor of cytokine signaling 3 | 3.7 | Decreasing SOCS3 causes increased resistin expression in monocytes, a proatherogenic mechanism (Gan, Pirvulescu, Stan, Simion, Calin, Manduteanu, and Butoi, 2013). |
| CXCR4 | chemokine (C-X-C motif) receptor 4 | 2.7 | CXCR4+ appear to be reparative monocytes in myocardial injury (Shantsila, Tapp, Wrigley, Montoro-Garcia, and Lip, 2013) |
| LDLR | low density lipoprotein receptor | 2.2 | Accumulating evidence that lipoprotein scavenger receptors in macrophages and monocytes play functional roles in prevention and pathophysiology of atherosclerosis (Kzhyshkowska, Neyen, and Gordon, 2012) |
| PTGS2 | prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | 2.1 | Pleiotropic modulators of monocyte inflammation (Ricciotti and FitzGerald, 2011) |
| THBD | thrombomodulin | 2.0 | Involved in endothelial-like and angiogenic properties in monocyte-derived immature dendritic cells (Berger, Dyugovskaya, Polyakov, and Lavie, 2012). |
Table 7.
Examples of monocyte genes that were downregulated by exercise (fold change ≥ 1.9) and likely involved in vascular health.
| Gene | Name | FC | Possible link to vascular health |
|---|---|---|---|
| EGR2 | early growth response 2 | 4.4 | Recent data suggest that factors like leptin upregulate EGR2, hastening the differentiation of monocytes to macrophages and possibly contributing to vascular impairment in diseases like diabetes (Jaedicke, Roythorne, Padget, Todryk, Preshaw, and Taylor, 2013). |
| TNF | tumor necrosis factor | 3.6 | TNF is increased in atherosclerosis and monocyte production of this cytokine is profoundly stimulated in the plaque milieu (Yehuda, Szuchman-Sapir, Khatib, Musa, and Tamir, 2011). |
| CD36 | CD36 molecule (thrombospondin receptor) | 2.8 | As noted, this scavenger receptor is upregulated in monocytes with coronary artery disease |
| CXCL5 | chemokine (C-X-C motif) ligand 5 | 2.7 | Platelet-derived CXCL5 attracts leukocytes and further activates other platelets (Gear and Camerini, 2003). |
| PDK4 | pyruvate dehydrogenase kinase, isozyme 4 | 2.6 | This gene is upregulated in experimental models of myocardial infarct (Marketou, Kintsurashvili, Papanicolaou, Lucero, Gavras, and Gavras, 2010). |
| CTTN | cortactin | 2.3 | Gene that produces cortactin, involved in endothelial integrity (Maharjan, Kim, Agrawal, Choi, Kim, Kim, Suh, and Kwon, 2013). |
| PDZK1IP1 | PDZK1 interacting protein 1 | 2.2 | May be involved in cholesterol metabolism (Silver, Wang, and Vogel, 2003). |
| CDC42EP3 | CDC42 effector protein (Rho GTPase binding) 3 | 2.1 | Downregulation of CDC42EP3 would be expected to result in impairment of monocyte phagocytosis (Maffei, Bulgarelli, Fiorcari, Bertoncelli, Martinelli, Guarnotta, Castelli, Deaglio, Debbia, De, Bonacorsi, Zucchini, Narni, Tripodo, Luppi, Cossarizza, and Marasca, 2013) |
| HDAC9 | histone deacetylase 9 | 2.1 | HDAC9 mRNA expression is increased in carotid atherosclerotic plaques and HDAC9 genetic variant has been associated with increased ischemic stroke risk (Markus et al., 2013) |
| PF4V1 | platelet factor 4 variant 1 | 1.9 | Produced by monocytes and appears to be atherogenic;iIts downregulation in exercise may be protective (Vandercappellen, Van, and Struyf, 2011) |
Conversely, as shown in Table 7, the down-regulation of certain key monocyte genes by exercise might also play a role in atherosclerosis prevention or attenuation. Reduction in the TNF gene expression is particularly remarkable as this inflammatory cytokine is clearly associated with monocytes and atherosclerosis. Experimentally, exposure of monocytes to human atherosclerotic plaque extract led to a substantial increase in TNF gene expression. In our study, the effect of exercise was a down-regulation of the gene. We also found down-regulation of HDAC9, one of the histone deacetylases. There is accumulating and exciting data showing the role that DNA acetylation and deacetylation can play in a variety of related inflammatory diseases. Markus and coworkers (Markus, Makela, Bevan, Raitoharju, Oksala, Bis, O'Donnell, Hainsworth, and Lehtimaki, 2013) just showed that HDAC9 mRNA expression is increased in carotid atherosclerotic plaques and that an HDAC9 genetic variant is associated with increased ischemic stroke risk. Our observation of a downregulation of HDAC9 gene expression in monocytes suggests a novel mechanism through which exercise exerts an anti-inflammatory effect that could prevent or attenuate atherosclerosis.
4.2. Monocyte Subtype Changes in Response to Exercise
The concentration of monocytes doubled in the circulation as a result of the brief exercise protocol (Table 1), reflecting the well described acute effect of physical activity on leukocytes in general and monocytes in particular. Using flow cytometry we classified subtypes of circulating monocytes. The emerging paradigm identifies classical (CD14++CD16‒), intermediate (CD14++CD16‒), and nonclassical (CD14+CD16++) subsets (Wong et al., 2012) Intriguingly, Berg and coworkers (Berg, Ljungcrantz, Andersson, Bryngelsson, Hedblad, Fredrikson, Nilsson, and Bjorkbacka, 2012) found in a retrospective study that elevated levels of classical monocytes were associated with the incidence of ischemic cardiovascular events. Although some workers suggest that the classical monocytes represent a more pro-inflammatory population of cells, and, consequently, are more likely to promote rather than attenuate atherosclerosis, additional studies are needed to determine the prognostic value of monocyte subtypes as an indicator frank cardiovascular disease or disease risk (Mehta and Reilly, 2012).
We found a significant reduction in the proportion of classical monocytes accompanied by a significant increase in nonclassical monocytes after exercise (Figure 1). This finding is consistent with studies done previously in other laboratories (Heimbeck, Hofer, Eder, Wright, Frankenberger, Marei, Boghdadi, Scherberich, and Ziegler-Heitbrock, 2010). Less is known about possible changes in gene expression that might reflect differences in CD14 and CD16 monocyte surface markers. Wong and coworkers (Wong, Tai, Wong, Han, Sem, Yeap, Kourilsky, and Wong, 2011) recently found distinct patterns of gene expression associated with the subtypes.
4.3. microRNAs, Pathway Analysis, and Intersecting Analysis
Evidence is rapidly accumulating demonstrating the pivotal role played by microRNAs in inflammation and atherosclerosis (Zernecke, 2012). We found a significant effect of brief exercise in 19 monocyte microRNAs (Table 3). The general understanding of how specific microRNAs are linked to disease mechanisms is at a very early stage, and our data are among the first to demonstrate that these microRNAs can be affected by exercise in monocytes. Nonetheless, in ten of the exercise-affected microRNAs, we used the existing literature and were able to identify possible pathophysiological links to vascular disease. For example, mir-30e, which is downregulated in an animal model of atherosclerotic lesions (Han et al., 2013), was significantly increased by brief exercise in monocytes. Moreover, whole blood-derived mir-30e was significantly increased in asthmatic subjects who were exposed to diesel exhaust, suggesting a generally pro-inflammatory role for this microRNA (Yamamoto, Singh, Sava, Pui, Tebbutt, and Carlsten, 2013). We found that another microRNA, mir-221, was downregulated by exercise. This microRNA may hasten the maturation of monocytes into inflammatory dendritic cells (Lu et al., 2011) and contributes to hyper-proliferation and IL-6 release in airway smooth muscle cells derived from patients with severe asthma (Perry, Baker, Gibeon, Adcock, and Chung, 2013), again suggesting that the exercise-associated downregulation of this microRNA may be beneficial by limiting pro-inflammatory responses in monocytes otherwise activated by exercise.
In previous studies, we developed an approach to determining the interaction between those microRNAs influenced by exercise and the potential genes and gene pathways that the affected microRNAs could regulate (Radom-Aizik et al., 2013). From this analysis, three pathways emerged: Jak-STAT, p53 Signaling, and Endocytosis. The ways in which these three pathways in monocytes might be linked to atherosclerosis are outlined in Table 4.
Three alternative mechanisms might explain the robust effect of exercise on monocyte gene and microRNAs expression: the first, a direct effect of exercise within the three sub populations of circulating monocytes; the second, an indirect effect, a shift in the proportion of the three sub population of monocytes and the third also an indirect effect, the mobilization into the circulation of monocytes that were expressing genes differently in their marginal pools than those monocytes already in the circulating blood. In human studies, it would be unfeasible to sample gene and microRNAs expression of marginal pools of monocytes. Nonetheless, our data do permit us to draw some inferences concerning possible mechanisms.
Consider first, for example, the gene with the highest increase in expression--amphiregulin (AREG). If the fold change in AREG occurred solely because of the addition of marginal monocytes to the circulating pool and there was no direct effect of exercise on AREG gene expression in the circulation, then, given the doubling of the monocyte count in the circulation following exercise, the 19.3-fold increase that we observed for this gene could occur, only if the monocytes that entered the circulation during exercise were expressing AREG at levels approximately 40-fold greater than the circulating monocytes. The decrease in expression for another gene, early growth response 2 (EGR2) which had a 4.4 fold decrease, is even harder to explain solely on the basis of shifting monocytes into the circulating pool. If, in the extreme case, the marginal monocytes had no detectable expression of this gene, the lowest possible post exercise ratio would be 50%, 2-fold decrease. Even if we take into consideration the change in subpopulations of monocytes the large fold change in some of the genes cannot be explained. Thus, it is reasonable to speculate from the current data that exercise has some effect on gene expression even in the circulating population of monocytes.
In summary, we hypothesized that exercise would lead to alteration in the gene expression profile pattern of circulating monocytes. Despite elements of an overall “stress/danger” response characteristic of inflammation (i.e., a substantial increase in the numbers of monocytes in the circulation and activation of many pro-inflammatory genes), we found distinct gene alterations that were likely to direct monocytes in an antiinflammatory, anti-atherogenic pathway. Salient among these were the downregulation of monocyte TNF, TLR4, and CD36 genes and the upregulation of EREG and CXCR4. We also found that exercise significantly altered a number of microRNAs in monocytes, and that these microRNAs may play a role in the regulation of monocyte gene pathways that could play a role in vascular health. We cannot yet determine whether the altered gene expression by brief exercise in circulating monocytes is a direct effect of exercise or is caused by monocyte trafficking. Nor do we know whether brief exercise leads to long-term changes in monocytes. Nonetheless, our data provide novel directions and the generation of new hypotheses that target specific pathways by which exercise could benefit cardiovascular health through effects on monocytes, cells intimately tied to both the pathophysiology and prevention of atherosclerosis.
Supplementary Material
Acknowledgments
We thank Ms. Cherryl Nugas for assisting in the genomic analysis, Ms. Georgia Bachman for assisting in monocyte isolation and Mr. Scott Graf for performing the exercise challenges.
Funding Sources: This work was supported in part by National Institutes of Health grants P01HD-048721, the UCI Institute for Clinical and Translational Science (CTSA grant) UL1 TR000153, and the UC Irvine Pediatric Systems Biology Research Fund.
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shlomit Radom-Aizik, Pediatric Exercise and Genomics Research Center, Department of Pediatrics, UC Irvine School of Medicine.
Frank P. Zaldivar, Jr, Pediatric Exercise and Genomics Research Center, Department of Pediatrics, UC Irvine School of Medicine.
Fadia Haddad, Pediatric Exercise and Genomics Research Center, Department of Pediatrics, UC Irvine School of Medicine.
Dan M. Cooper, Pediatric Exercise and Genomics Research Center, Department of Pediatrics, UC Irvine School of Medicine
References
- Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109:288–295. doi: 10.1016/j.amjcard.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Bjorkbacka H. Elevated CD14++ Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- Berger S, Dyugovskaya L, Polyakov A, Lavie L. Short-term fibronectin treatment induces endothelial-like and angiogenic properties in monocyte-derived immature dendritic cells: involvement of intracellular VEGF and MAPK regulation. Eur J Cell Biol. 2012;91:640–653. doi: 10.1016/j.ejcb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Bles N, Di PL, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116:3219–3226. doi: 10.1182/blood-2010-01-265611. [DOI] [PubMed] [Google Scholar]
- Bruns AF, Yuldasheva N, Latham AM, Bao L, Pellet-Many C, Frankel P, Stephen SL, Howell GJ, Wheatcroft SB, Kearney MT, Zachary IC, Ponnambalam S. A heat-shock protein axis regulates VEGFR2 proteolysis, blood vessel development and repair. PLoS ONE. 2012;7:e48539. doi: 10.1371/journal.pone.0048539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154:82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Wang YS, Hu CY, Chang WC, Liao YC, Dai CY, Juo SH. OxLDL up-regulates microRNA-29b, leading to epigenetic modifications of MMP-2/MMP-9 genes: a novel mechanism for cardiovascular diseases. FASEB J. 2011;25:1718–1728. doi: 10.1096/fj.10-174904. [DOI] [PubMed] [Google Scholar]
- den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209:314–320. doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- Emmrich S, Henke K, Hegermann J, Ochs M, Reinhardt D, Klusmann JH. miRNAs can increase the efficiency of ex vivo platelet generation. Ann Hematol. 2012;91:1673–1684. doi: 10.1007/s00277-012-1517-z. [DOI] [PubMed] [Google Scholar]
- Estruch M, Bancells C, Beloki L, Sanchez-Quesada JL, Ordonez-Llanos J, Benitez S. CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis. 2013;229:356–362. doi: 10.1016/j.atherosclerosis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Fantidis P. The role of intracellular 3′5′-cyclic adenosine monophosphate (cAMP) in atherosclerosis. Curr Vasc Pharmacol. 2010;8:464–472. doi: 10.2174/157016110791330843. [DOI] [PubMed] [Google Scholar]
- Favre J, Terborg N, Horrevoets AJ. The diverse identity of angiogenic monocytes. Eur J Clin Invest. 2013;43:100–107. doi: 10.1111/eci.12009. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri N. AMP-activated protein kinase and the control of smooth muscle cell hyperproliferation in vascular disease. Vascul Pharmacol. 2012;56:9–13. doi: 10.1016/j.vph.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Findeisen HM, Gizard F, Zhao Y, Qing H, Heywood EB, Jones KL, Cohn D, Bruemmer D. Epigenetic regulation of vascular smooth muscle cell proliferation and neointima formation by histone deacetylase inhibition. Arterioscler Thromb Vasc Biol. 2011;31:851–860. doi: 10.1161/ATVBAHA.110.221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan AM, Pirvulescu MM, Stan D, Simion V, Calin M, Manduteanu I, Butoi E. Monocytes and smooth muscle cells cross-talk activates STAT3 and induces resistin and reactive oxygen species and production. J Cell Biochem. 2013;114:2273–2283. doi: 10.1002/jcb.24571. [DOI] [PubMed] [Google Scholar]
- Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. 2012;217:476–482. doi: 10.1016/j.imbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Han H, Wang YH, Qu GJ, Sun TT, Li FQ, Jiang W, Luo SS. Differentiated miRNA expression and validation of signaling pathways in apoE gene knockout mice by cross-verification microarray platform. Exp Mol Med. 2013;45:e13. doi: 10.1038/emm.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbeck I, Hofer TP, Eder C, Wright AK, Frankenberger M, Marei A, Boghdadi G, Scherberich J, Ziegler-Heitbrock L. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytometry A. 2010;77:823–830. doi: 10.1002/cyto.a.20942. [DOI] [PubMed] [Google Scholar]
- Jaedicke KM, Roythorne A, Padget K, Todryk S, Preshaw PM, Taylor JJ. Leptin up-regulates TLR2 in human monocytes. J Leukoc Biol. 2013;93:561–571. doi: 10.1189/jlb.1211606. [DOI] [PubMed] [Google Scholar]
- Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T, Sawa Y. Tissue- and plasma-specific MicroRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745. doi: 10.1161/JAHA.112.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyotani Y, Ota H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Zhao J, Ozawa K, Nagayama K, Ito S, Takasawa S, Kimura H, Uno M, Yoshizumi M. Intermittent hypoxia induces the proliferation of rat vascular smooth muscle cell with the increases in epidermal growth factor family and erbB2 receptor. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Huang X, Zhang X, Roensch K, Cao Q, Nakayama KI, Blazar BR, Zeng Y, Zhou X. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kakkar V. The role of heat shock protein (HSP) in atherosclerosis: Pathophysiology and clinical opportunities. Curr Med Chem. 2010;17:957–973. doi: 10.2174/092986710790820688. [DOI] [PubMed] [Google Scholar]
- Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol. 2013;216:61–71. doi: 10.1530/JOE-12-0338. [DOI] [PubMed] [Google Scholar]
- Maffei R, Bulgarelli J, Fiorcari S, Bertoncelli L, Martinelli S, Guarnotta C, Castelli I, Deaglio S, Debbia G, De BS, Bonacorsi G, Zucchini P, Narni F, Tripodo C, Luppi M, Cossarizza A, Marasca R. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica. 2013;98:1115–1123. doi: 10.3324/haematol.2012.073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan S, Kim K, Agrawal V, Choi HJ, Kim NJ, Kim YM, Suh YG, Kwon YG. Sac-1004, a novel vascular leakage blocker, enhances endothelial barrier through the cAMP/Rac/cortactin pathway. Biochem Biophys Res Commun. 2013;435:420–427. doi: 10.1016/j.bbrc.2013.04.104. [DOI] [PubMed] [Google Scholar]
- Marketou M, Kintsurashvili E, Papanicolaou KN, Lucero HA, Gavras I, Gavras H. Cardioprotective effects of a selective B(2) receptor agonist of bradykinin post-acute myocardial infarct. Am J Hypertens. 2010;23:562–568. doi: 10.1038/ajh.2010.20. [DOI] [PubMed] [Google Scholar]
- Markus HS, Makela KM, Bevan S, Raitoharju E, Oksala N, Bis JC, O'Donnell C, Hainsworth A, Lehtimaki T. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke. 2013;44:1220–1225. doi: 10.1161/STROKEAHA.111.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziere C, Maziere JC. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic Biol Med. 2009;46:127–137. doi: 10.1016/j.freeradbiomed.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Reilly MP. Monocyte mayhem: do subtypes modulate distinct atherosclerosis phenotypes? Circ Cardiovasc Genet. 2012;5:7–9. doi: 10.1161/CIRCGENETICS.111.962647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mograbi B, Rochet N, Imbert V, Bourget I, Bocciardi R, Emiliozzi C, Rossi B. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur Cytokine Netw. 1997;8:73–81. [PubMed] [Google Scholar]
- Neth P, Nazari-Jahantigh M, Schober A, Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 2013;99:294–303. doi: 10.1093/cvr/cvt096. [DOI] [PubMed] [Google Scholar]
- Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res. 2010;3:547–558. doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Gleeson M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur. J Appl. Physiol. 2010;109:251–257. doi: 10.1007/s00421-009-1350-9. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Mercader JM, Catalan V, Moreno-Navarrete JM, Pueyo N, Sabater M, Gomez-Ambrosi J, Anglada R, Fernandez-Formoso JA, Ricart W, Fruhbeck G, Fernandez-Real JM. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- Ortiz-Munoz G, Martin-Ventura JL, Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O, Munoz-Garcia B, Fernandez-Vizarra P, Ortega L, Egido J, Gomez-Guerrero C. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:525–531. doi: 10.1161/ATVBAHA.108.173781. [DOI] [PubMed] [Google Scholar]
- Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway Smooth Muscle Hyperproliferation is Regulated by microRNA-221 in Severe Asthma. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:287. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radom-Aizik S, Zaldivar F, Jr, Oliver S, Galassetti P, Cooper DM. Evidence for microRNA Involvement in Exercise-Associated Neutrophil Gene Expression Changes. JAP IN PRESS. 2010 doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radom-Aizik S, Zaldivar FP, Haddad F, Cooper DM. Impact of Brief Exercise on Peripheral Blood Nk Cell Gene and Microrna Expression in Young Adults. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.01341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantsila E, Tapp LD, Wrigley BJ, Montoro-Garcia S, Ghattas A, Jaipersad A, Lip GY. The effects of exercise and diurnal variation on monocyte subsets and monocyte-platelet aggregates. Eur J Clin Invest. 2012;42:832–839. doi: 10.1111/j.1365-2362.2012.02656.x. [DOI] [PubMed] [Google Scholar]
- Shantsila E, Tapp LD, Wrigley BJ, Montoro-Garcia S, Lip GY. CXCR4 positive and angiogenic monocytes in myocardial infarction. Thromb Haemost. 2013;109:255–262. doi: 10.1160/TH12-06-0395. [DOI] [PubMed] [Google Scholar]
- Silver DL, Wang N, Vogel S. Identification of small PDZK1-associated protein, DD96/MAP17, as a regulator of PDZK1 and plasma high density lipoprotein levels. J Biol Chem. 2003;278:28528–28532. doi: 10.1074/jbc.M304109200. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, McFarlin BK, McSporran C, Spielmann G, Hartaigh B, Guy K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun. 2009;23:232–239. doi: 10.1016/j.bbi.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–C586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Vandercappellen J, Van DJ, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011;22:1–18. doi: 10.1016/j.cytogfr.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Wassmann S, Nickenig G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ Res. 2005;97:1046–1054. doi: 10.1161/01.RES.0000188212.57180.55. [DOI] [PubMed] [Google Scholar]
- Wei W, Jiao Y, Postlethwaite A, Stuart JM, Wang Y, Sun D, Gu W. Dual-specificity phosphatases 2: surprising positive effect at the molecular level and a potential biomarker of diseases. Genes Immun. 2013;14:1–6. doi: 10.1038/gene.2012.54. [DOI] [PubMed] [Google Scholar]
- Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304:H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- Wong KL, Tai JJY, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- Woollard KJ. Immunological aspects of atherosclerosis. Clin Sci (Lond) 2013;125:221–235. doi: 10.1042/CS20120576. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Kumar V, Jha V. Heat Shock Proteins 60 and 70 Specific Proinflammatory and Cytotoxic Response of CD4(+)CD28(null) Cells in Chronic Kidney Disease. Mediators Inflamm. 2013;2013:384807. doi: 10.1155/2013/384807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Singh A, Sava F, Pui M, Tebbutt SJ, Carlsten C. MicroRNA expression in response to controlled exposure to diesel exhaust: attenuation by the antioxidant N-acetylcysteine in a randomized crossover study. Environ Health Perspect. 2013;121:670–675. doi: 10.1289/ehp.1205963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda H, Szuchman-Sapir A, Khatib S, Musa R, Tamir S. Human atherosclerotic plaque lipid extract promotes expression of proinflammatory factors in human monocytes and macrophage-like cells. Atherosclerosis. 2011;218:339–343. doi: 10.1016/j.atherosclerosis.2011.07.120. [DOI] [PubMed] [Google Scholar]
- Yougbare I, Boire G, Roy M, Lugnier C, Rouseau E. NCS 613 exhibits antiinflammatory effects on PBMCs from lupus patients by inhibiting p38 MAPK and NF-kappaB signalling pathways while reducing proinflammatory cytokine production. Can J Physiol Pharmacol. 2013;91:353–361. doi: 10.1139/cjpp-2012-0233. [DOI] [PubMed] [Google Scholar]
- Zawada AM, Rogacev KS, Schirmer SH, Sester M, Bohm M, Fliser D, Heine GH. Monocyte heterogeneity in human cardiovascular disease. Immunobiology. 2012;217:1273–1284. doi: 10.1016/j.imbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Zernecke A. MicroRNAs in the regulation of immune cell functions - implications for atherosclerotic vascular disease. Thromb Haemost. 2012;107:626–633. doi: 10.1160/TH11-08-0603. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sunnarborg SW, McNaughton KK, Johns TG, Lee DC, Faber JE. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circ Res. 2008;102:1275–1285. doi: 10.1161/CIRCRESAHA.108.171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bruemmer D. NR4A Orphan Nuclear Receptors in Cardiovascular Biology. Drug Discov Today Dis Mech. 2009;6:e43–e48. doi: 10.1016/j.ddmec.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


