Abstract
Gold nanoparticles (AuNPs) are important components for biomedical applications. AuNPs have been widely employed for diagnostics, and have seen increasing use in the area of therapeutics. In this mini-review, we present fabrication strategies for AuNPs and highlight a selection of recent applications of these materials in bionanotechnology.
1. Introduction
Gold nanoparticles (AuNPs) have been widely employed in bionanotechnology based on their unique properties and multiple surface functionalities. The ease of AuNP functionalization provides a versatile platform for nanobiological assemblies with oligonucleotides,1 antibodies,2 and proteins.3 Bioconjugates of AuNPs have also become promising candidates in the design of novel biomaterials for the investigation of biological systems.4
The versatility of AuNPs has provided useful materials for a range of biomedical applications (Scheme 1). In diagnostics, the binding event between the analytes and the AuNPs can alter the physicochemical properties of AuNPs such as surface plasmon resonance, conductivity, and redox behavior, leading to detectable signals.5 AuNPs also serve as practical platforms for therapeutic agents, with their high surface area allowing a dense presentation of multifunctional moieties (e.g., drugs6 and targeting agents7). In this review, we provide a brief overview of the synthesis, properties, and conjugation strategies of spherical AuNPs as well as highlight a few of their recent applications in bionanotechnology.
Scheme 1.
Applications of AuNPs in bionanotechnology.
2. Synthesis of gold nanoparticles
A wide array of solution based approaches has been developed in the past few decades to control as the size,8 shape,9 and surface functionality.10 Turkevich et al. developed a synthetic method for creating AuNPs in 1951 by treating hydrogen tetrachloroaurate (HAuCl4) with citric acid in boiling water, where the citrate acts as both reducing and stabilizing agent (Scheme 2B).11 Frens further refined this method by changing the gold-to-citrate ratio to control particle size.12 This protocol has been widely employed to prepare dilute solutions of moderately stable spherical AuNPs with diameters of 10 to 20 nm, though larger AuNPs (e.g., 100 nm) can also be prepared. These citrate-stabilized AuNPs can undergo irreversible aggregation during functionalization process with thiolate ligands. Several strategies have been developed to conquer this problem including using a surfactant, Tween 20, prior to the modification to prevent aggregation (Scheme 2B), 13 or using thioctic acid as an intermediate via a two-step functionalization. 14 However, the requirement for high dilution makes large scale production challenging.
Scheme 2.
(A) Two-phase synthesis of AuNPs by reduction of HAuCl4 in presence of alkanethiols as the stabilizing ligands and NaBH4 as reducing agent. Place-exchange reaction for alkanethiol-protected AuNPs can then be performed with functionalized thiols. (B) Citrate-stabilized AuNPs were prepared with HAuCl4 solution under reflux conditions where citrate acts as both the stabilizing ligand and reducing agent. The ligand exchange of functionalized thiols for citrate-stabilized AuNPs was achieved by using Tween 20 as an intermediate.
Brust and Schriffin achieved a breakthrough in AuNP synthesis in 1994 by creating organic soluble alkanethiol-stabilized AuNPs through a biphasic reduction protocol using tetraoctylammonium bromide (TOAB) as the phase transfer reagent and sodium borohydride (NaBH4) as the reducing agent (Scheme 2A).15 This methodology produces low dispersity AuNPs from 1.5 to 5 nm by varying the reaction conditions such as gold-to-thiol ratio, reduction rate, and reaction temperature.16 These alkanethiol-protected AuNPs possess higher stability when compared to most other AuNPs due to the synergic effect of the strong thiol-gold interactions and van der Waals attractions between the neighboring ligands.17 These nanoparticles can be thoroughly dried and redispersed in solution without any aggregation making them excellent precursors for further functionalization.
3. Properties of gold nanoparticles
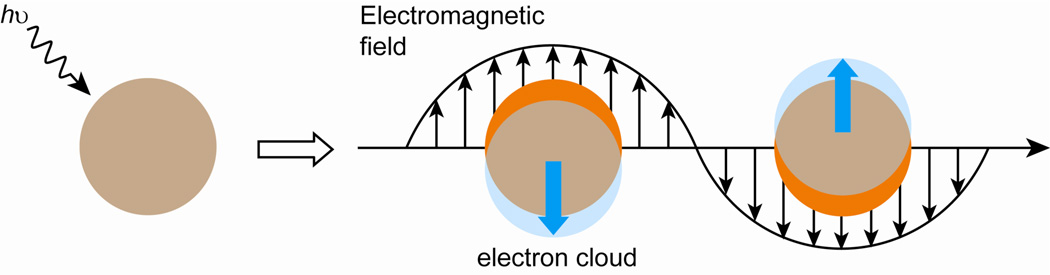
Spherical AuNPs possess useful attributes such as size- and shape-related optoelectronic properties,18 large surface-to-volume ratio, excellent biocompatibility, and low toxicity.19 These properties make AuNPs an important tool in bionanotechnology (Table 1). Important physical properties of AuNPs include surface plasmon resonance (SPR) and the ability to quench fluorescence. Spherical AuNPs exhibit a range of colors (e.g., brown, orange, red and purple) in aqueous solution as the core size increases from 1 to 100 nm, and generally show a size-relative absorption peak from 500 to 550 nm.20 This absorption band arises from the collective oscillation of the conduction electrons due to the resonant excitation by the incident photons (Figure 1) which is called a “surface plasmon band”.21 However, this band is absent in both small nanoparticles (d < 2 nm) and the bulk material. This phenomenon is influenced not only by size, but also by shape, solvent, surface ligand, core charge, temperature and is even sensitive to the proximity of other nanoparticles.22 The aggregation of nanoparticles results in significant red-shifting of SPR frequency, broadening of surface plasmon band and changing the solution color from red to blue due to the interparticle plasmon coupling.23
Table 1.
Properties of AuNPs and their area of application.
| Properties | Application area |
|---|---|
| Redox activity | Electronic devices28 and electrochemical sensing29 |
| Surface-enhanced Raman scattering (SERS) | Imaging30 and sensing31 |
| Surface plasmon resonance (SPR) | Colorimetric sensing32 and photothermal therapy33 |
| Fluorescence quenching | Sensor fabrication34 and materials science35 |
Fig. 1.
Schematic representation of the oscillation of conduction electrons across the nanoparticle in the electromagnetic field of the incident light.
The superb quenching ability of AuNPs to proximal fluorophores comes through the deactivation pathway based on the good overlap between the emission spectrum of excited fluorophores and the surface plasmon band of the AuNPs.24 This fluorescence resonance energy transfer (FRET) phenomenon is observed even in the presence of 1 nm AuNPs due to the fact that radiative and nonradiative decay rates of fluorescent molecules are both distinctly affected by the nanoparticles.25 AuNPs also can act as electron acceptors to quench fluorophores in the photoinduced electron transfer (PET) process.26 This PET process is modulated by charging/discharging the gold core which can be utilized in sensor fabrication.27
4. Conjugation strategies for gold nanoparticles
The labile capping ligands on AuNPs (citrates, thiols, or other adsorbed ligands) can be displaced by thiols through a place ligand exchange reaction to synthesize mixed monolayer-protected AuNPs (Scheme 2A).36 In this method, external thiols displace the existing ligands of AuNPs in an equilibrium process. The loading efficiency onto AuNPs surface is controlled through the reaction time and the feed ratio of the functional ligands. Furthermore, introducing two or more functional ligands during the place exchange reaction can provide mixed monolayer-protected AuNPs for synergistic applications.
Place ligand exchange allows the secondary tethering of organic molecules or biomolecules to the surface of AuNPs (Scheme 3).37 Non-covalent conjugation is a simpler way for molecules to bind to AuNPs, as they can attach via different interactions such as through specific binding affinity,38 electrostatic interactions, 39 and hydrophobic interactions.40 These non-covalent interactions are widely utilized in delivery and sensing areas due to their ease of release and reversible nature. Alternatively, covalent conjugation of molecules to AuNPs stabilizes the conjugates, which is more useful when stable constructs are required, e.g. for imaging. Two major strategies for covalent conjugation are direct attachment of the thiolate molecule to the AuNPs surface and covalent bond formation (e.g., amine-carboxylate coupling41 and click reaction42 ). Particularly, these covalent conjugations are usually done between free molecule and the pre-grafted thiolate ligands on the AuNP surface.
Scheme 3.
Conjugation strategies of AuNPs through covalent and non-covalent conjugation.
5. Applications in bionanotechnology
5.1. Sensing
AuNPs are readily conjugated with recognition moieties such as antibodies or oligonucleotides for the detection of target biomolecules,43 allowing in vitro detection and diagnostics applications for diseases such as cancer.44 As an example, AuNPs play a critical role in the “bio-barcode assay”,45 an ultrasensitive method for detecting target proteins and nucleic acids. The principle of the “bio-barcode assay” utilizes AuNPs conjugated with both barcode oligonucleotides and target-specific antibodies, and magnetic microparticles (MMPs) functionalized with monoclonal antibodies for the target moiety. These complexes produce a sandwich complex upon detection of the target molecule that releases a large amount of barcode oligonucleotides, providing both identification and quantification of the target (Scheme 4). As an example of the sensitivity of this method, Mirkin et al. have demonstrated the detection of prostate specific antigen (PSA) using this methodology with a limit of detection of 330 fg/mL.46
Scheme 4.
AuNP-based bio-barcode detection strategy. Reprinted with permission from Ref. 46.
Aptamer-conjugated AuNPs that combine the selectivity and affinity of aptamers with the spectroscopic properties of AuNPs were utilized to detect small molecule47 and cancer cells.48 Zeng et al. have demonstrated an aptamer-nanoparticle strip biosensor (ANSB) system for the detection of Ramos (lymphoma) cells (Figure 2).49 Under optimal conditions, the ANSB showed a detection limit of 4000 Ramos cells using visual detection and 800 Ramos cells with a portable strip reader.
Fig. 2.
(A) Schematic illustration of detecting Ramos cells on ANSB. Ramos cells are captured on the test zone through specific aptamer-cell interaction, while excess aptamer-conjugated AuNPs are captured on the control zone through aptamer-DNA hybridization. (B) Typical photo images (top) and corresponding responses (bottom) of ANSB with samples containing different amounts of Ramos cells (target cells) and CLL cells (control cells). From left to right: 0 Ramos cells; 8 × 104 CCL cells; 8 × 104 Ramos cells; 8 × 104 CLL cells and 8 × 104 Ramos cells. C = Control zone; T = Test zone. The large signal in the control zone is due to excess aptamer-conjugated AuNPs. Adapted with permission from Ref. 49.
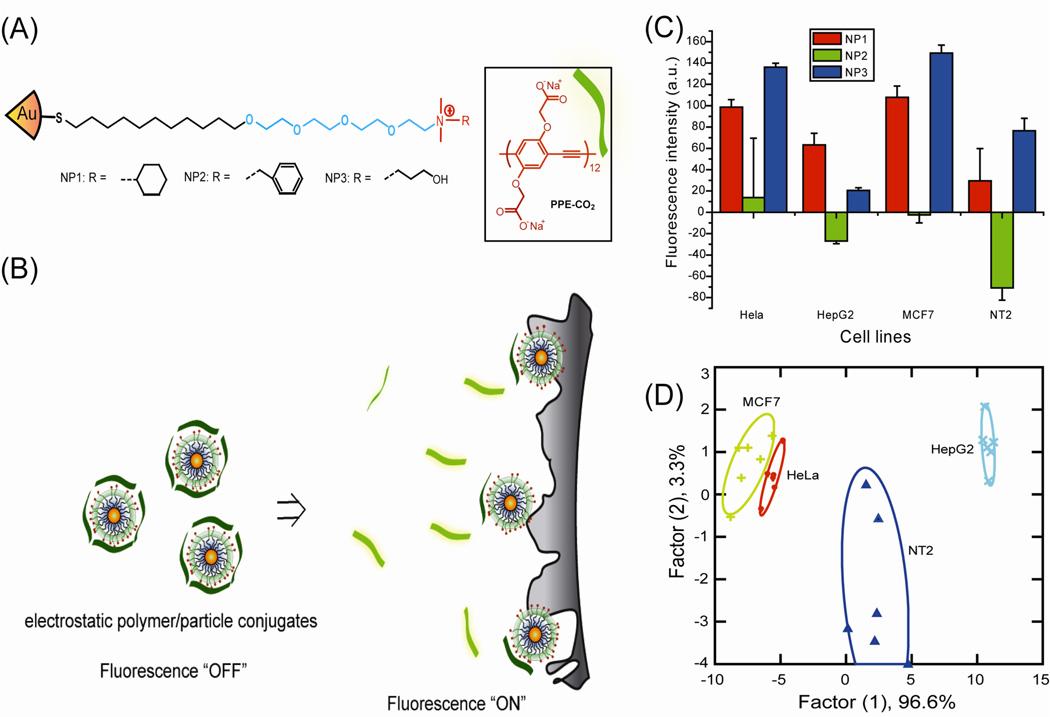
A new “chemical nose” methodology using non-covalent conjugates of AuNP and fluorophore was introduced by Rotello and co-workers to provide high sensitivity sensing of biomolecular targets.50 AuNP-fluorophore conjugates provide an alternative bio-detection method to “lock and key” specific recognition based approaches, using an array of selective receptors to generate a pattern that is able to recognize analytes. The initial sensor system was composed of quaternary ammonium-functionalized AuNPs with PPE (poly(para-phenyleneethynylene)) where the PPE serves as a fluorescence transduction element that can be quenched by cationic AuNPs.51 Competitive binding of analyte can disrupt the PPE from the complex, resulting in the recovered fluorescence from PPE and also producing a readable signal. This method was able to differentiate 12 different species/strains of bacteria with 95% accuracy.51(b) Moreover, this strategy was used to differentiate normal, cancerous and metastatic cells in a rapid and accurate assay (Figure 3).51(a) Additionally, GFP (green fluorescent protein) replaced the polymer transducer to provide higher sensitivity (5000 cells relative to the previous 20,000) in mammalian cancer cells sensing.52
Fig. 3.
(A) Molecular structures of the cationic AuNPs and the fluorescent polymer (PPECO2). (B) Displacement of quenched PPECO2 by cell with concomitant restoration of fluorescence. (C) Fluorescence change for 4 different cancer cell lines using AuNP-PPECO2 conjugates. (D) Canonical score plot for the two factors of simplified fluorescence response patterns obtained with AuNP-PPECO2 conjugates arrays against different mammalian cell types. The canonical scores were calculated by LDA (linear discriminant analysis). for the identification of 4 cell lines. Reprinted with permission from Ref. 51(a).
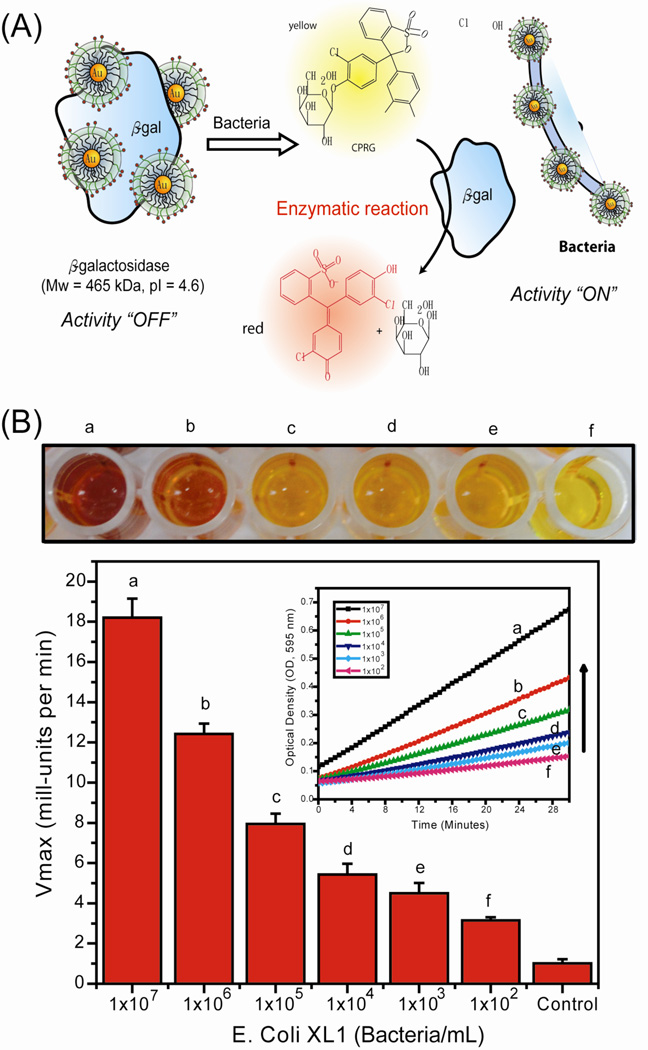
Recently, the array-based sensing strategy was adapted to an enzyme-amplified array sensing (EAAS) approach, where the sensitivity is amplified through enzymatic catalysis.53 The system works by having the analyte protein competitively bind with the AuNP, releasing the β-galactosidase (β-Gal) and restoring its activity. The cleavage of the substrate provides an enzyme-amplified fluorescent readout of the binding event, allowing the identification of proteins even in desalted human urine. A similar approach was used for the construction of a colorimetric enzyme-nanoparticle conjugate system by using chlorophenol red β-D-galactopyranoside (CPRG), a chromogenic substrate, for the detection of bacteria (Figure 3).54 Bacteria sensing was achieved at concentrations of 1 × 102 bacteria/mL in solution and 1 × 104 bacteria/mL in a field-friendly test strip format.
5.2. Therapeutics
The transport of therapeutic agents to the cells by AuNPs is a critical process in biomedical treatment. Several research groups have used functionalized AuNPs to investigate the interactions with cell membrane to improve delivery efficiency.55 For example, Stellacci et al. have demonstrated that surface ligand arrangement on AuNPs can regulate cell membrane penetration. 56 AuNPs functionalized with an ordered arrangement of amphiphilic molecules were able to penetrate the cell membrane while AuNPs coated with a random arrangement of these same molecules were trapped in vesicular bodies.
AuNP therapeutics can be delivered into cells through either passive or active targeting mechanisms. Passive targeting is based on the enhanced permeability and retention (EPR) effect where the AuNPs will accumulate within the tumor via its irregular vasculature, allowing larger particles to pass through the endothelim.57 Active-targeting relies on a surface functional ligand explicitly designed for the target analyte to provide specificity and selectivity.58 Effective targeting and delivery strategies using AuNPs have been developed for therapeutic applications including photothermal therapy,59 genetic regulation,60 and drug treatment.61
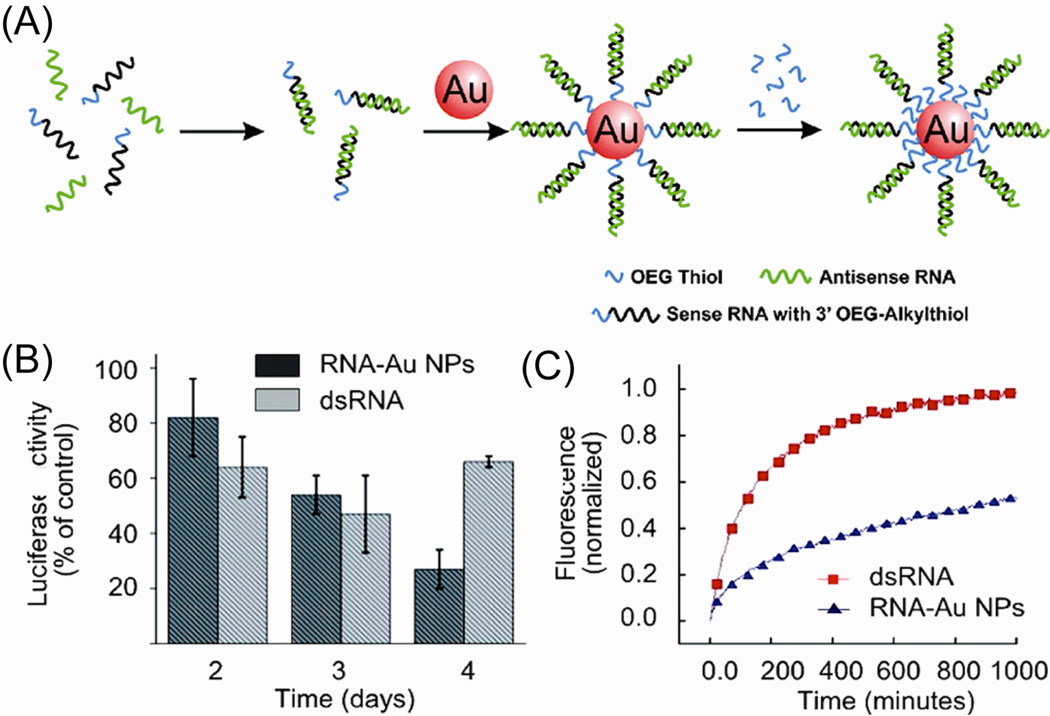
In one arena, AuNPs have been exploited as attractive scaffolds for the creation of transfection agents in gene therapy to cure cancer and genetic disorders. Mirkin et al. have reported the use of AuNP-oligonucleotide complexes as intracellular gene regulation agents for controlling protein expression in cells.62 RNA-AuNP conjugates were used to knockdown luciferase expression (Figure 5),63 showing the conjugates have a half-life six times longer than that of free dsRNA and demonstrating a high gene knockdown capability in cell models. Rotello et al. also have demonstrated that cationic AuNPs, featuring cationic amino acid-based side chains, can be used for DNA transfection. Lysine-based motif coating AuNPs provided effective non-toxic transfection vectors for DNA delivery that were up to 28 times more effective than polylysine.64
Fig. 5.
(A) Preparation of polyvalent RNA-AuNP conjugates. (B) Knockdown of luciferase expression over 4 days. (C) Stability of RNA-AuNP conjugates, showing the comparison of the stability of dsRNA (red) and RNA-AuNP conjugates (blue) in 10% serum. Reprinted with permission from Ref. 64.
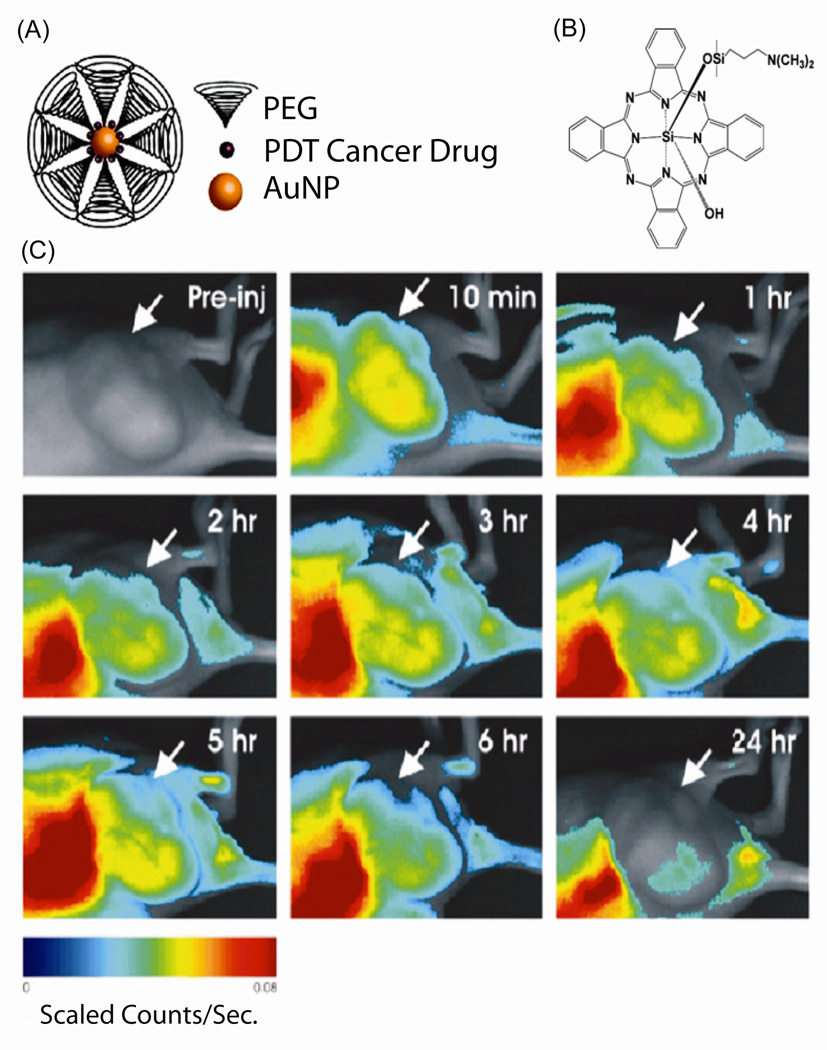
Loading of drugs onto AuNP can be performed through either non-covalent interactions or covalent conjugation. Drug encapsulation with AuNPs has been demonstrated through the use of hydrophobic65 or hydrophilic66 pockets generated by the monolayer. In a recent example, Burda et al. utilized polyethylene glycol (PEG) coated AuNPs to provide an amphiphilic environment to capture the hydrophobic silicon phthalocyanine 4 (Pc 4), a photodynamic therapy (PDT) drug (Figure 6A and 6B).67 They found that the drug release mechanism was through passive accumulation, and the non-covalent Pc 4-AuNP conjugates released their drugs quickly and penetrated deeply into tumors within hours (Figure 6C).
Fig. 6.
(A) PEG functionalized AuNPs with loading of PDT cancer drug. (B) Chemical structure of the PDT drug Pc 4. (C) In vivo fluorescence imaging of AuNP-Pc 4 conjugates injected mouse at various time points within 24 hr. Arrows indicate the tumor location. Reprinted with permission from Ref. 68.
Drugs covalently conjugated to AuNPs can be released by glutathione (GSH) displacement68 or through cleavable linkers.69 Rotello et al. have demonstrated GSH-mediated release using AuNPs featured a mixed monolayer composed of cationic ligands and fluorogenic ligands. The cationic surface of the nanoparticles facilitated their penetration through cell membranes and the payload release was triggered by intracellular GSH.70 Kotov et al. applied the GSH-mediated release strategy using 6-mercaptopurine-9-b-D-ribofuranoside functionalized AuNPs to enhance the anti-proliferative effect against K-562 leukemia cells compared to the free drug.70 Recently, Forbes and Rotello have applied the GSH-mediated approach to investigate the movement of AuNPs carrying either fluorescein or doxorubicin molecules in a tumor model.71 The results indicate that cationic AuNPs may be more effective in delivering payloads to the majority of tumor cells while anionic AuNPs are able to deliver drug deep into tissues. Alternatively, Rotello et al. have used a light-controlled external release strategy to deliver the anticancer drug 5-fluorouracil into cells using AuNPs featured a mixed monolayer of zwitterionic and photocleavable ligands on the surface.72 Other strategies for delivering covalently attached drugs using AuNPs include the reduction of disulfide bonds73 and pH-mediated release74.
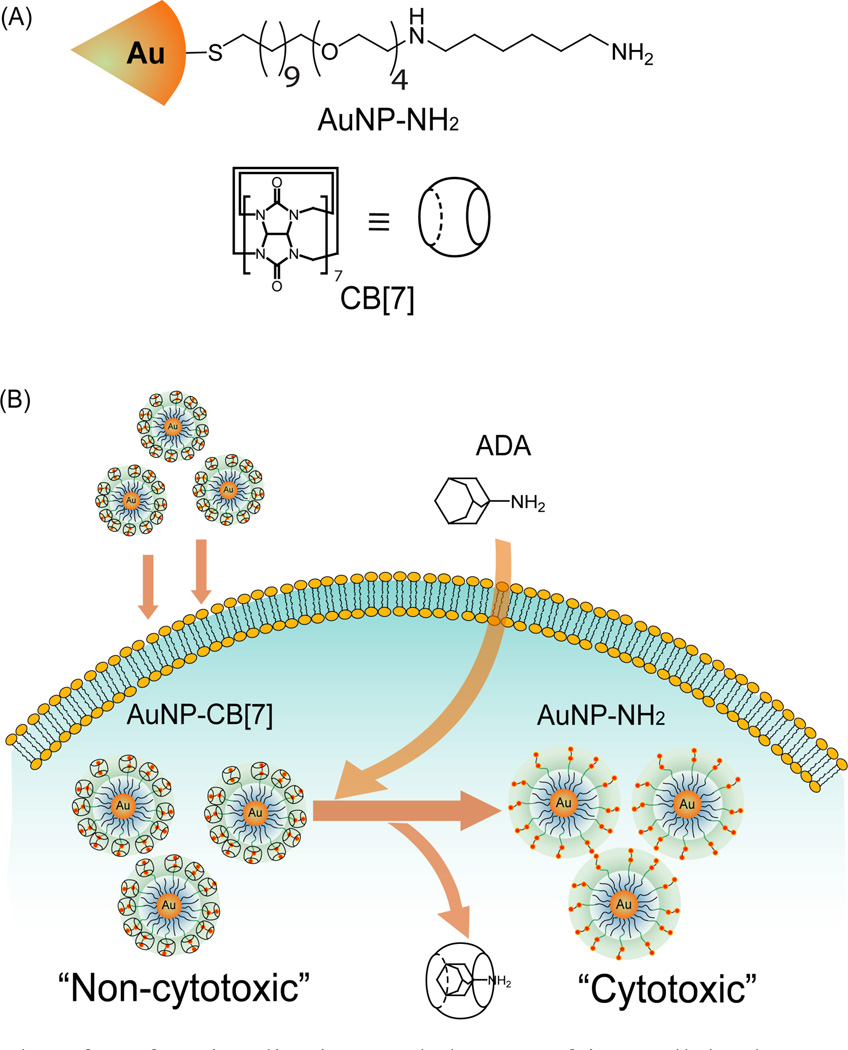
Using AuNPs as therapeutic moieties in their own right is another potential approach for medical treatment. For instance, Feldheim et al. have synthesized a mixed thiol monolayer-coated AuNPs for bacterial growth inhibition.75 Muhkherjee et al. have utilized naked AuNPs for treatment studies of multiple myeloma, a plasma cell disorder.76 AuNPs have been shown to inhibit the proliferation of multiple myeloma cells through cell-cycle arrest in the G1 phase via the up-regulation of cell-cycle proteins p21 and p27. Recently, Rotello et al. demonstrated a host-guest system to mediate the activation of a therapeutic diaminohexane-terminated AuNP (AuNP-NH2) and modulate its cytotoxicity.77 The results show that the threading of cucurbit[7]uril (CB[7]) onto a particle surface reduces the high toxicity of the AuNP-NH2 through sequestration of the particle in the endosomes. When treated with 1-adamantylamine (ADA), the CB[7] is displaced from the nanoparticle surface, releasing the toxic particle from the endosome and killing the cancer cell (Scheme 5).
Scheme 5.
AuNP and surface functionalization, and the use of intracellular host-guest complexation to trigger nanoparticle cytotoxicity. (A) Structure of AuNP–NH2 and CB[7] (B) Activation of AuNP–NH2– CB[7] cytotoxicity by dethreading of CB[7] from the nanoparticle surface by ADA. Reprinted with permission from Ref. 78.
5.3. Imaging
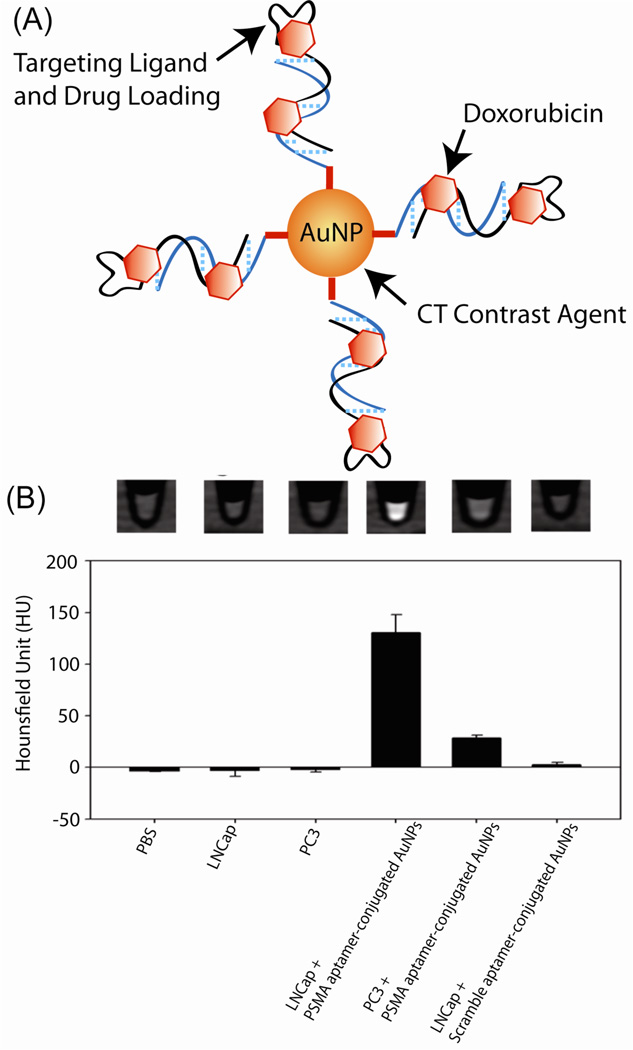
The versatile optical and electronic properties of AuNPs have been employed for cell imaging using various techniques, including computed tomography (CT),78 dark-field light scattering, 79 optical coherence tomography (OCT), 80 photothermal heterodyne imaging technique81 and Raman spectroscopy.82 For example, AuNPs serve as a contrast agents for CT imaging based on the higher atomic number and electron density of gold (79 and 19.32 g/cm3) as compared to the currently used iodine (53 and 4.9 g/cm3). Hainfeld et al. have demonstrated the feasibility of AuNPs to enhance the in vivo vascular contrast in CT imaging,83 and Kopelman et al. further designed immuno-targeted AuNPs to selectively target tumor specific antigens.84 Recently, Jon et al. used a prostate specific membrane antigen (PSMA) aptamer-conjugated AuNPs (PSMA-AuNPs) to establish a molecular CT image for the specific imaging of prostate cancer cells (Figure 7).85 These results showed that PSMA-AuNPs had a 4-fold greater CT intensity for a targeted LNCaP cell than that of a nontargeted PC3 cell. PSMA aptamer-conjugated AuNPs loaded with the anti-cancer drug doxorubicin were significantly more potent against targeted LNCaP cells than against nontargeted PC3 cells.
Fig. 7.
(A) Schematic of the method for preparing doxorubicin-loaded aptamer-conjugated AuNPs. (B) CT images and (b) HU values of PBS, LNCaP, PC3 cells, and LNCaP and PC3 cells treated with PSMA aptamer conjugated AuNPs (5 nM) or scramble aptamer-conjugated AuNPs (5 nM) for 6 h. Adapted with permission from Ref. 86.
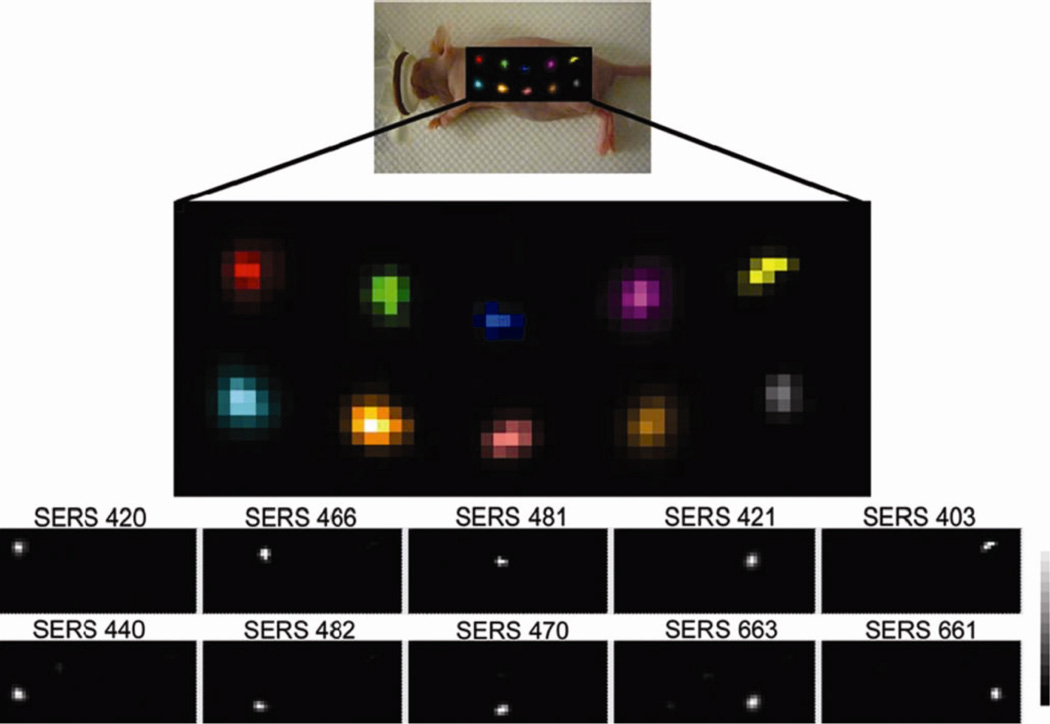
AuNPs were also used to prepare surface-enhanced Raman scattering (SERS) nanoparticles for small-animal Raman imaging.86 Using AuNPs with a silica coating and a Raman-active molecular layer, Gambhir et al. have demonstrated the ability to separate the spectral fingerprints of 10 different types of SERS nanoparticles in a living mouse and the colocalization of five different SERS nanoparticles within deep tissues after intravenous injection (Figure 8).87
Fig. 8.
Evaluation of 10 different multiplexed SERS nanoparticles in vivo. Raman map of 10 different SERS particles injected in a nude mouse. Reprinted with permission from Ref. 88.
Conclusions and Outlook
AuNPs have multiple attributes that make them potent tools for the use in bionanotechnology. The wide range of surface functionality and bioconjugates coupled with the outstanding physical properties of AuNPs make these systems valuable for imaging applications. Moreover, the creation of highly sensitive and selective diagnostic system for target analytes can be achieved by engineering their surface monolayer. AuNP-based delivery vectors have also shown promise in therapeutics with their high surface loading of drug and gene as well as the controllable release of the payloads. Taken together, AuNPs are incredibly versatile materials for next-generation biomedical applications.
Fig. 4.
(A) Schematic illustration of the method for EAAS strategy. (B) Limitation of detection of E. coli using the β-Gal-AuNP nanocomposite. At the top, microplate wells show the color change upon variation of the bacteria concentration. Kinetic absorbance responses upon addition of different bacteria concentrations are shown. Reprinted with permission from Ref. 54.
Acknowledgments
The research was supported by the National Science Foundation (NSF) Center for Hierarchical Manufacturing at the University of Massachusetts (NSEC, DMI-0531171), the NIH (GM077173) and NSF IGERT (DGE-0504485 to BC).
References
- 1.(a) Zhang T, Chen P, Sun Y, Xing Y, Yang Y, Dong Y, Xu L, Yang Z, Liu D. Chem. Commun. 2011;47:5774–5776. doi: 10.1039/c1cc11337b. [DOI] [PubMed] [Google Scholar]; (b) Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z, Du Q, Xing J, Zhao Y, Wang PC, Dong A, Liang X-J. ACS Nano. 2010;4:5505–5511. doi: 10.1021/nn101638u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Park S, Hamad-Schifferli K. ACS Nano. 2010;4:2555–2560. doi: 10.1021/nn100362m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Mukherjee P, Bhattacharya R, Bone N, Lee YK, Patra CR, Wang S, Lu L, Secreto C, Banerjee PC, Yaszemski MJ, Kay NE, Mukhopadhyay D. J. Nanobiotech. 2007;5:4. doi: 10.1186/1477-3155-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Eck W, Craig G, Sigdel A, Ritter G, Old LJ, Tang L, Brennan MF, Allen PJ, Mason MD. ACS Nano. 2008;2:2263–2272. doi: 10.1021/nn800429d. [DOI] [PubMed] [Google Scholar]; (c) El-Sayed IH, Huang XH, El-Sayed MA. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 3.(a) Calzolai L, Franchini F, Gilliland D, Rossi F. Nano Lett. 2010;10:3101–3105. doi: 10.1021/nl101746v. [DOI] [PubMed] [Google Scholar]; (b) You C-C, Arvizo RR, Rotello VM. Chem. Commun. 2006;42:2905–2907. doi: 10.1039/b605508g. [DOI] [PubMed] [Google Scholar]; (c) Aubin-Tam ME, Hamad-Schifferli K. Langmuir. 2005;21:12080–12084. doi: 10.1021/la052102e. [DOI] [PubMed] [Google Scholar]
- 4.(a) Moyano DF, Rotello VM. Langmuir. 2011 doi: 10.1021/la2004535. Article ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jamison JA, 1, Bryant EL, Kadali SB, Wong MS, Colvin VL, Matthews KS, Calabretta MK. J. Nanoparticle Research. 2011;13:625–636. [Google Scholar]; (c) You CC, Chompoosor A, Rotello VM. Nano Today. 2007;2:34–43. [Google Scholar]
- 5.(a) Uehara N. Anal. Sci. 2010;26:1219–1228. doi: 10.2116/analsci.26.1219. [DOI] [PubMed] [Google Scholar]; (b) Wang Z, Ma L. Coord. Chem. Rev. 2009;253:1607–1618. [Google Scholar]; (b) Radwan SH, Azzazy HME. Expert. Rev.Mol. Diagn. 2009;9:511–524. doi: 10.1586/erm.09.33. [DOI] [PubMed] [Google Scholar]; (c) Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Anal. Bioanal. Chem. 2008;391:943–950. doi: 10.1007/s00216-007-1768-z. [DOI] [PubMed] [Google Scholar]
- 6.(a) Brown SD, Nativo P, Smith J-A, Stirling D, Edwards PR, Venugopal B, J.Flint D, Plumb JA, Graham D, Wheate NJ. J. Am. Chem. Soc. 2010;132:4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alexander CM, Maye MM, Dabrowiak JC. Chem. Commun. 2011;47:3418–3420. doi: 10.1039/c0cc04916f. [DOI] [PubMed] [Google Scholar]; (c) Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. Langmuir. 2010;26:2248–2255. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]; (d) Duncan B, Kim C, Rotello VM. J. Control. Release. 2010;148:122–127. doi: 10.1016/j.jconrel.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Khan JA, Kudgus RA, Szabolcs A, Dutta S, Wang E, Cao S, Curran GL, Shah V, Curley S, Mukhopadhyay D, Robertson JD, Bhattacharya R, Mukherjee P. Plos One. 2011;6:e20347. doi: 10.1371/journal.pone.0020347. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, Muders M, Wang S, Buhrow SA, Safgren SL, Yaszemski MJ, Reid JM, Ames MM, Mukherjee P, Mukbopadhyay D. Cancer Research. 2008;68:1970–1978. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 8.(a) Sardar R, Shumaker-Parry JS. J. Am. Chem. Soc. 2011;133:8179–8190. doi: 10.1021/ja107934h. [DOI] [PubMed] [Google Scholar]; (b) Hussain I, Graham S, Wang ZX, Tan B, Sherrington DC, Rannard SP, Cooper AI, Brust M. J. Am. Chem. Soc. 2005;127:16398–16399. doi: 10.1021/ja055321v. [DOI] [PubMed] [Google Scholar]; (c) Jana NR, Gearheart L, Murphy CJ. Langmuir. 2001;17:6782–6786. [Google Scholar]
- 9.Grzelczak M, Perez-Juste J, Mulvaney P, Liz-Marzan LM. Chem. Soc. Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wilton-Ely JDET. Dalton Trans. 2008:25–29. doi: 10.1039/b714144k. [DOI] [PubMed] [Google Scholar]; (b) Roux S, Garcia B, Bridot JL, Salome M, Marquette C, Lemelle L, Gillet P, Blum L, Perriat P, Tillement O. Langmuir. 2005;21:2526–2536. doi: 10.1021/la048082i. [DOI] [PubMed] [Google Scholar]; (c) Ackerson CJ, Jadzinsky PD, Kornberg RD. J. Am. Chem. Soc. 2005;127:6550–6551. doi: 10.1021/ja046114i. [DOI] [PubMed] [Google Scholar]; (d) Daniel MC, Astruc D. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 11.Turkevich J, Stevenson PC, Hillier J. Discuss. Faraday Soc. 1951;11:55–75. [Google Scholar]
- 12.Frens G. Nature: Phys. Sci. 1973;241:20–22. [Google Scholar]
- 13.Aslan K, Perez-Luna VH. Langmuir. 2002;18:6059–6065. [Google Scholar]
- 14.Lin SY, Tsai YT, Chen CC, Lin CM, Chen CH. J. Phys. Chem. B. 2004;108:2134–2139. [Google Scholar]
- 15.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. J. Chem. Soc. Chem.Commun. 1994;7:801–802. [Google Scholar]
- 16.Hostetler MJ, Wingate JE, Zhong CJ, Harris JE, Vachet RW, Clark MR, Londono JD, Green SJ, Stokes JJ, Wignall GD, Glish GL, Porter MD, D.Evans N, Murray RW. Langmuir. 1998;14:17–30. [Google Scholar]
- 17.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 18.(a) Sau TK, Rogach AL, Jaeckel F, Klar TA, Feldmann J. Adv. Mater. 2011;22:1805–1825. doi: 10.1002/adma.200902557. [DOI] [PubMed] [Google Scholar]; (b) Hu M, Chen J, Li Z-Y, Au L, Hartland GV, Li X, Marquez M, Xia Y. Chem. Soc. Rev. 2006;35:1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 19.(a) Khlebtsov N, Dykman L. Chem. Soc. Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]; (b) Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 20.(a) Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]; (b) Eustis S, El-Sayed MA. Chem. Soc. Rev. 2006;35:209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]; (c) Link S, El-Sayed MA. J. Phys. Chem. B. 1999;103:4212–4217. [Google Scholar]
- 21.(a) Mie G. Ann. Phys. 1908;25:377–445. [Google Scholar]; (b) Templeton AC, Pietron JJ, Murray RW, Mulvaney P. J. Phys. Chem. B. 2000;104:564–570. [Google Scholar]
- 22.(a) Toderas F, Baia M, Maniu D, Astilean S. J. Optoelectron. Adv. Mater. 2008;10:2282–2284. [Google Scholar]; (b) Srivastava S, Frankamp BL, Rotello VM. Chem. Mat. 2005;17:487–490. [Google Scholar]; (c) Kelly KL, Coronado E, Zhao LL, Schatz GC. J. Phys. Chem. B. 2003;107:668–677. [Google Scholar]; (d) Link S, El-Sayed MA. Int. Rev. Phys. Chem. 2000;19:409–453. [Google Scholar]
- 23.Su KH, Wei QH, Zhang X, Mock JJ, Smith DR, Schultz S. Nano Lett. 2003;3:1087–1090. [Google Scholar]
- 24.(a) Pons T, Medintz IL, Sapsford KE, Higashiya S, Grimes AF, English DS, Mattoussi H. Nano Lett. 2007;7:3157–3164. doi: 10.1021/nl071729+. [DOI] [PubMed] [Google Scholar]; (b) Sapsford KE, Berti L, Medintz IL. Angew. Chem. Int. Ed. 2006;45:4562–4589. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]; (c) Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS. J. Am. Chem. Soc. 2005;127:3270–3271. doi: 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]; (d) Gersten J, Nitzan A. J. Chem. Phys. 1981;75:1139–1152. [Google Scholar]
- 25.(a) Bigioni TP, Whetten RL, Dag O. J. Phys. Chem. B. 2000;104:6983–6986. [Google Scholar]; (b) Mohamed MB, Volkov V, Link S, El-Sayed MA. Chem. Phys. Lett. 2000;317:517–523. [Google Scholar]; (c) Dulkeith E, Ringler M, Klar TA, Feldmann J, Javier AM, Parak WJ. Nano Lett. 2005;5:585–589. doi: 10.1021/nl0480969. [DOI] [PubMed] [Google Scholar]
- 26.Thomas KG, Kamat PV. Acc. Chem. Res. 2003;36:888–898. doi: 10.1021/ar030030h. [DOI] [PubMed] [Google Scholar]
- 27.Kamat PV, Barazzouk S, Hotchandani adn S. Angew. Chem. Int. Ed. 2002;41:2764–2767. doi: 10.1002/1521-3773(20020802)41:15<2764::AID-ANIE2764>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.(a) Lee S, Yoon SM, Shin HJ, Joo WJ, Yi DK, Choi JY, Amarnath U, Paik CA. Nanotechnology. 2008;19:075606–075611. doi: 10.1088/0957-4484/19/7/075606. [DOI] [PubMed] [Google Scholar]; (b) Prakash A, Ouyang J, Lin J-L, Yang Y. J. Appl. Phys. 2006;100:054309–054313. [Google Scholar]
- 29.(a) Kumar SS, Kwak K, Lee D. Anal. Chem. 2011;83:3244–3247. doi: 10.1021/ac200384w. [DOI] [PubMed] [Google Scholar]; (b) Guo S, Wang E. Anal. Chim. Acta. 2007;598:181–192. doi: 10.1016/j.aca.2007.07.054. [DOI] [PubMed] [Google Scholar]; (c) Tseng RJ, Huang JX, Ouyang J, Kaner RB, Yang Y. Nano Lett. 2005;5:1077–1080. doi: 10.1021/nl050587l. [DOI] [PubMed] [Google Scholar]; (d) Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I. Science. 2003;299:1877–1881. doi: 10.1126/science.1080664. [DOI] [PubMed] [Google Scholar]
- 30.(a) Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samanta A, Maiti KK, Soh K-S, Liao X, Vendrell M, Dinish US, Yun S-W, Bhuvaneswari R, Kim H, Rautela S, Chung J, Olivo M, Chang Y-T. Angew. Chem. Int. Edit. 2011;50:6089–6092. doi: 10.1002/anie.201007841. [DOI] [PubMed] [Google Scholar]; (c) Porter MD, Lipert RJ, Siperko LM, Wang G, Narayanana R. Chem. Soc. Rev. 2008;37:1001–1011. doi: 10.1039/b708461g. [DOI] [PubMed] [Google Scholar]
- 31.(a) Lou T, Wang Y, Li J, Peng H, Xiong H, Chen L. Anal. Bioanal. Chem. 2011;401:333–338. doi: 10.1007/s00216-011-5067-3. [DOI] [PubMed] [Google Scholar]; (b) Thuy NTB, Yokogawa R, Yoshimura Y, Fujimoto K, Koyano M, Maenosono S. Analyst. 2010;135:595–602. doi: 10.1039/b919969a. [DOI] [PubMed] [Google Scholar]; (c) Dasary SSR, Singh AK, Senapati D, Yu H, Ray PC. J. Am. Chem. Soc. 2009;131:13806–13812. doi: 10.1021/ja905134d. [DOI] [PubMed] [Google Scholar]
- 32.(a) Li X, Wang J, Sun L, Wang Z. Chem. Commun. 2010;46:988–990. doi: 10.1039/b920135a. [DOI] [PubMed] [Google Scholar]; (b) Kim Y-R, Mahajan RK, Kim JS, Kim H. ACS Appl. Mater. Interfaces. 2010;2:292–295. doi: 10.1021/am9006963. [DOI] [PubMed] [Google Scholar]; (c) Mao X, Ma Y, Zhang A, Zhang L, Zeng L, Liu G. Anal. Chem. 2009;81:1660–1668. doi: 10.1021/ac8024653. [DOI] [PubMed] [Google Scholar]; (d) Jiang Y, Zhao H, Lin Y, Zhu N, Ma Y, Mao L. Angew. Chem. Int. Edit. 2010;49:4800–4804. doi: 10.1002/anie.201001057. [DOI] [PubMed] [Google Scholar]; (e) Zhang J, Wang L, Zhang H, Boey F, Song S, Fan C. Small. 2009;6:201–204. doi: 10.1002/smll.200901012. [DOI] [PubMed] [Google Scholar]; (f) Liu G, Mao X, Phillips JA, Xu H, Tan W, Zeng L. Anal. Chem. 2009;81:10013–10018. doi: 10.1021/ac901889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(a) Van de Broek B, Devoogdt N, D’Hollander A, Gijs H-L, Jans K, Lagae L, Muyldermans S, Maes G, Borghs G. ACS Nano. 2011;5:4319–4328. doi: 10.1021/nn1023363. [DOI] [PubMed] [Google Scholar]; (b) Huang X, Kang B, Qian W, Mackey MA, Chen PC, Oyelere AK, El-Sayed IH, El-Sayed MA. J. Biomed. Opt. 2011;15:058002. doi: 10.1117/1.3486538. [DOI] [PubMed] [Google Scholar]
- 34.(a) Swierczewska M, Lee S, Chen X. Phys. Chem. Chem. Phys. 2011;13:9929–9941. doi: 10.1039/c0cp02967j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS. J. Am. Chem. Soc. 2005;127:3270–3271. doi: 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]; (c) Maxwell DJ, Taylor JR, Nie S. J. Am. Chem. Soc. 2002;124:9606–9612. doi: 10.1021/ja025814p. [DOI] [PubMed] [Google Scholar]; (d) Dubertret B, Calame M, Libchaber AJ. Nat. Biotechnol. 2001;19:365–370. doi: 10.1038/86762. [DOI] [PubMed] [Google Scholar]
- 35.(a) Liang TC, Lin HC. J. Mater. Chem. 2009;19:4753–4763. [Google Scholar]; (b) Imahoriand H, Fukuzumi S. Adv. Mater. 2001;13:1197–1199. [Google Scholar]
- 36.(a) Templeton AC, Wuelfing WP, Murray RW. Acc. Chem. Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]; (b) Hong R, Fernandez JM, Nakade H, Arvizo RR, Emrick T, Rotello VM. Chem. Commum. 2006;42:2347–2349. doi: 10.1039/b603988j. [DOI] [PubMed] [Google Scholar]
- 37.(a) Levy R, Thanh NTK, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, Brust M, Fernig DG. J. Am. Chem. Soc. 2004;126:10076–10084. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]; (b) Woehrle GH, O Brown L, Hutchison JE. J. Am. Chem. Soc. 2005;127:2172–2183. doi: 10.1021/ja0457718. [DOI] [PubMed] [Google Scholar]; (c) Rucareanu S, Gandubert VJ, Lennox RB. Chem. Mater. 2006;18:4674–4680. [Google Scholar]
- 38.(a) Jiang XZ, Housni A, Gody G, Boullanger P, Charreyre MT, Delair T, Narain R. Bioconjugate Chem. 2010;21:521–530. doi: 10.1021/bc900431p. [DOI] [PubMed] [Google Scholar]; (b) Zheng M, Huang XY. J Am. Chem. Soc. 2004;126:12047–12054. doi: 10.1021/ja047029d. [DOI] [PubMed] [Google Scholar]
- 39.(a) Ghosh P, Yang X, Arvizo R, Zhu ZJ, Mo Z, Rotello VM. J. Am. Chem.Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samanta B, Yang XC, Ofir Y, Park MH, Patra D, Agasti S, Miranda OR, Mo ZH, Rotello VM. Angew. Chem. Int. Ed. 2009;48:5341–5344. doi: 10.1002/anie.200901590. [DOI] [PubMed] [Google Scholar]
- 40.Cardenas M, Barauskas J, Schillen K, Brennan JL, Brust M, Nylander T. Langmuir. 2006;22:3294–3299. doi: 10.1021/la0530438. [DOI] [PubMed] [Google Scholar]
- 41.Drechsler U, Fischer NO, Frankamp BL, Rotello VM. Adv. Mater. 2004;16:271–274. [Google Scholar]
- 42.(a) Li XR, Guo J, Asong J, Wolfert MA, Boons GJ. J. Am. Chem. Soc. 2011;133:11147–11153. doi: 10.1021/ja2012164. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang MX, Huang BH, Sun XY, Pang DW. Langmuir. 2010;26:10171–10176. doi: 10.1021/la100315u. [DOI] [PubMed] [Google Scholar]; (c) Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S, Vind J, Svendsen A, Nolte RJM, Rowan AE, Brust M. Bioconjugate Chem. 2006;17:1373–1375. doi: 10.1021/bc0601018. [DOI] [PubMed] [Google Scholar]
- 43.(a) Huo Q, Colon J, Cordero A, Bogdanovic J, Baker CH, Goodison S, Pensky MY. J. Nanobiotech. 2011;9:20. doi: 10.1186/1477-3155-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bogdanovic J, Colon J, Baker C, Huo Q. Anal. Biochem. 2010;405:96–102. doi: 10.1016/j.ab.2010.06.008. [DOI] [PubMed] [Google Scholar]; (c) Jiang Y, Zhao H, Lin Y, Zhu N, Ma Y, Mao L. Angew. Chem. Int. Edit. 2010;49:4800–4804. doi: 10.1002/anie.201001057. [DOI] [PubMed] [Google Scholar]; (d) Woo J-R, Lim D-K, Nam J-M. Small. 2010;7:648–655. doi: 10.1002/smll.201002080. [DOI] [PubMed] [Google Scholar]
- 44.(a) Fang S-B, Tseng WY, Lee H-C, Tsai C-K, Huang J-T, Hou S-Y. J.Microbial. Meth. 2009;77:225–228. doi: 10.1016/j.mimet.2009.02.008. [DOI] [PubMed] [Google Scholar]; (b) Laderman EI, Whitworth E, Dumaual E, Jones M, Hudak A, Hogrefe W, Carney J, Groen J. Clin. Vaccine Immunol. 2008;15:159–163. doi: 10.1128/CVI.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.(a) Nam JM, Park SJ, Mirkin CA. J. Am. Chem. Soc. 2002;124:3820–3821. doi: 10.1021/ja0178766. [DOI] [PubMed] [Google Scholar]; (b) Nam JM, Thaxton CS, Mirkin CA. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]; (c) Nam JM, Stoeva SI, Mirkin CA. J. Am. Chem. Soc. 2004;126:5932–5933. doi: 10.1021/ja049384+. [DOI] [PubMed] [Google Scholar]; (d) Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Thaxton CS, Hill HD, Georganopoulou DG, Stoeva SI, Mirkin CA. Anal. Chem. 2005;77:8174–8178. doi: 10.1021/ac0514265. [DOI] [PubMed] [Google Scholar]; (f) Hill HD, Mirkin CA. Nat. Protocols. 2007;1:324–336. doi: 10.1038/nprot.2006.51. [DOI] [PubMed] [Google Scholar]; (g) Nam J-M, Jang K-J, Groves JT. Nat. Protocols. 2007;2:1438–1444. doi: 10.1038/nprot.2007.201. [DOI] [PubMed] [Google Scholar]
- 46.Thaxton CS, Elghanian R, Thomas AD, Stoeva SI, Lee J-S, Smith ND, J.Schaeffer A, Klocker H, Horninger W, Bartsch G, Mirkin CA. Proc. Natl. Acad. Sci.U.S.A. 2009;106:18437–18442. doi: 10.1073/pnas.0904719106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Wang L, Zhang H, Boey F, Song S, Fan C. Small. 2010;6:201–204. doi: 10.1002/smll.200901012. [DOI] [PubMed] [Google Scholar]
- 48.Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W. Anal. Chem. 2008;80:1067–1072. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Mao X, Phillips JA, Xu H, Tan W, Zeng L. Anal. Chem. 2009;81:10013–10018. doi: 10.1021/ac901889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.(a) Bunz UHF, Rotello VM. Angew. Chem. Int. Edit. 2010;49:3268–3279. doi: 10.1002/anie.200906928. [DOI] [PubMed] [Google Scholar]; (b) Miranda OR, Creran B, Rotello VM. Curr. Opin. Chem. Bio. 2010;14:728–736. doi: 10.1016/j.cbpa.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) De M, Rana S, Akpinar H, Miranda OR, Arvizo RR, Bunz UHF, Rotello VM. Nat. Chem. 2009;1:461–465. doi: 10.1038/nchem.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.(a) Bajaj A, Miranda OR, Kim I-B, Phillips RL, Jerry DJ, Bunz UHF, Rotello VM. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10912–10916. doi: 10.1073/pnas.0900975106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phillips RL, Miranda OR, You C-C, Rotello VM, Bunz UHF. Angew. Chem. Int. Edit. 2008;47:2590–2594. doi: 10.1002/anie.200703369. [DOI] [PubMed] [Google Scholar]
- 52.Bajaj A, Rana S, Miranda OR, Yawe JC, Jerry DJ, Bunz UHF, M.Rotello V. Chem. Sci. 2010;1:134–138. [Google Scholar]
- 53.Miranda OR, Chen H-T, You C-C, Mortenson DE, Yang X-C, Bunzand VM, Rotello UHF. J. Am. Chem. Soc. 2010;132:5285–5289. doi: 10.1021/ja1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miranda OR, Li X, Garcia-Gonzalez L, Zhu Z-J, Yan B, Bunz UHF, M.Rotello V. J. Am. Chem. Soc. 2011;133:9650–9653. doi: 10.1021/ja2021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.(a) Arvizo RR, Miranda OR, Thompson MA, Pabelick CM, Bhattacharya R, Robertson JD, Rotello VM, Prakash YS, Mukherjee P. Nano Lett. 2010;10:2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cho EC, Zhang Q, Xia Y. Nat. Nanotech. 2011;6:385–391. doi: 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Verma A, Stellacci F. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 56.(a) Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine andF, Stellacci DJ. Nat. Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chou LYT, Ming K, Chan WCW. Chem. Soc. Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 57.(a) Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. Nanoscale Res. Lett. 2011;6:283–293. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 58.(a) Li X, Zhou HY, Yang L, Du GQ, Pai-Panandiker AS, Huang XF, Yan B. Biomaterials. 2011;32:2540–2545. doi: 10.1016/j.biomaterials.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiao PF, Zhou HY, Chen LX, Yan B. Curr. Med. Chem. 2011;18:2086–2102. doi: 10.2174/092986711795656199. [DOI] [PubMed] [Google Scholar]; (c) Choi CHJ, Alabi CA, Webster P, Davis ME. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.(a) Van de Broek B, Devoogdt N, D’Hollander A, Gijs H-L, Jans K, Lagae L, Muyldermans S, Maes G, Borghs G. ACS Nano. 2011;5:4319–4328. doi: 10.1021/nn1023363. [DOI] [PubMed] [Google Scholar]; (b) Huang X, Kang B, Qian W, Mackey MA, Chen PC, Oyelere AK, El-Sayed IH, El-Sayed MA. J. Biomed.l Optics. 2011;15:058002. doi: 10.1117/1.3486538. [DOI] [PubMed] [Google Scholar]
- 60.(a) McMahon KM, Mutharasan RK, Tripathy S, Veliceasa D, Bobeica M, Shumaker DK, Luthi AJ, Helfand BT, Ardehali H, Mirkin CA, Volpert O, Thaxton CS. Nano Lett. 2011;11:1208–1214. doi: 10.1021/nl1041947. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen AM, Taratula O, Wei D, Yen H-I, Thomas T, Thomas TJ, Minko T, He H. ACS Nano. 2010;4:3679–3688. doi: 10.1021/nn901796n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hu C, Peng Q, Chen F, Zhong Z, Zhuo R. Bioconjugate Chem. 2010;21:836–843. doi: 10.1021/bc900374d. [DOI] [PubMed] [Google Scholar]; (d) Ahmed M, Deng Z, Narain R. ACS Appl. Mater. Interfaces. 2009;1:1980–1987. doi: 10.1021/am900357x. [DOI] [PubMed] [Google Scholar]; (e) Rhim W-K, Kim J-S, Nam J-M. Small. 2008;4:1651–1655. doi: 10.1002/smll.200800628. [DOI] [PubMed] [Google Scholar]
- 61.(a) Brown SD, Nativo P, Smith JA, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ. J. Am. Chem. Soc. 2010;132:4678–4684. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. Langmuir. 2009;26:2248–2255. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]
- 62.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 63.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. J. Am.Chem. Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh PS, Kim C-K, Han G, Forbes NS, Rotello VM. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim CK, Ghosh P, Pagliuca C, Zhu Z-J, Menichetti S, Rotello VM. J. Am.Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei BW, Burda C. J. Am.Chem. Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng Y, Meyers JD, Broome A-M, Kenney ME, Basilion JP, Burda C. J.Am. Chem. Soc. 2011;133:2583–2591. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong R, Han G, Kim B, Forbes NS, Rotello VM. J. Am. Chem.Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 69.Nakanishi J, Nakayama H, Shimizu T, Ishida H, Kikuchi Y, Yamaguchi K, Horiike Y. J. Am. Chem. Soc. 2009;131:3822–3823. doi: 10.1021/ja809236a. [DOI] [PubMed] [Google Scholar]
- 70.Podsiadlo P, Sinani VA, Bahng JH, Kam NWS, Lee J, Kotov NA. Langmuir. 2008;24:568–574. doi: 10.1021/la702782k. [DOI] [PubMed] [Google Scholar]
- 71.Kim B, Han G, Toley BJ, Kim C-K, Rotello VM, Forbes NS. Nat. Nanotech. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agasti SS, Chompoosor A, You C-C, Ghosh P, Kim CK, Rotello VM. J.Am. Chem. Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saito G, Swanson JA, Lee KD. Adv. Drug Deliv. Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 74.(a) Wang F, Wang YC, Dou S, Xiong MH, Sun TM, Wang J. ACS Nano. 2011;5:3679–3692. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]; (b) Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong SQ. Biomaterials. 2009;30:6065–6075. doi: 10.1016/j.biomaterials.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 75.(a) Bresee J, Maier KE, Boncella AE, Melander C, Feldheim DL. Small. 2011;7:2027–2031. doi: 10.1002/smll.201100420. [DOI] [PubMed] [Google Scholar]; (b) Bresee J, Maier KE, Melander C, Feldheim DL. Chem. Commun. 2010;46:7516–7518. doi: 10.1039/c0cc02663h. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharya R, Patra CR, Verma R, Kumar S, Greipp PR, Mukherjee P. Adv. Mater. 2007;19:711–716. [Google Scholar]
- 77.Kim C, Agasti SS, Zhu Z, Isaacs L, Rotello VM. Nat. Chem. 2010;2:962–966. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.(a) Wang H, Zheng LF, Peng C, Guo R, Shen MW, Shi XY, Zhang GX. Biomaterials. 2011;32:2979–2988. doi: 10.1016/j.biomaterials.2011.01.001. [DOI] [PubMed] [Google Scholar]; (b) Aydogan B, Li J, Rajh T, Chaudhary A, Chmura SJ, Pelizzari C, Wietholt C, Kurtoglu M, Redmond P. Mol. Imaging Biol. 2010;12:463–467. doi: 10.1007/s11307-010-0299-8. [DOI] [PubMed] [Google Scholar]
- 79.Qian W, Huang X, Kang B, El-Sayed MA. J. Biomed. Opt. 2010;15:058002. doi: 10.1117/1.3486538. [DOI] [PubMed] [Google Scholar]
- 80.(a) Kim CS, Wilder-Smith P, Ahn Y-C, Liaw L-HL, Chen Z, Kwon YJ. J.Biomed. Opt. 2009;14:034008. doi: 10.1117/1.3130323. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zagaynova EV, Shirmanova MV, Kirillin MY, Khlebtsov BN, Orlova AG, Balalaeva IV, Sirotkina MA, Bugrova ML, Agrba PD, Kamensky VA. Phys. Med. Biol. 2008;53:4995–5009. doi: 10.1088/0031-9155/53/18/010. [DOI] [PubMed] [Google Scholar]
- 81.(a) Leduc C, Jung JM, Carney RR, Stellacci F, Lounis B. ACS Nano. 2011;5:2587–2592. doi: 10.1021/nn1023285. [DOI] [PubMed] [Google Scholar]; (b) Berciaud S, Cognet L, Blab GA, Lounis B. Phys. Rev. Lett. 2004;93:257402. doi: 10.1103/PhysRevLett.93.257402. [DOI] [PubMed] [Google Scholar]
- 82.(a) Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, J.Natan M, Gambhir SS. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5844–5849. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alric C, Taleb J, Le Duc G, Mandon C, Billotey C, Le Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P, Roux S, Tillement O. J. Am. Chem.Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 84.Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Nano Lett. 2008;8:4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim D, Jeong YY, Jon S. ACS Nano. 2010;4:3689–3696. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 86.Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. Nat. Biotech. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 87.Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natanand SS, Gambhir MJ. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]