Abstract
Purpose
Local transdermal therapy to the breast may achieve effective target-organ drug delivery, while diminishing systemic effects. We conducted a randomized, double-blind, placebo-controlled phase II trial comparing transdermal 4-hydroxytamoxifen gel (4-OHT) to oral tamoxifen (oral-T) in women with ductal carcinoma in-situ (DCIS).
Methods
27pre and postmenopausal women were randomized to 4-OHT (4mg/day) or oral-T (20mg/day) for 6-10 weeks before surgery. Plasma, nipple aspirate fluid, and breast adipose tissue concentrations of tamoxifen and its major metabolites were determined by liquid chromatography-tandem mass spectrometry. The primary endpoint was Ki67 labeling in DCIS lesions, measured by immunohistochemistry. In plasma, insulin-like growth factor-1 (IGF-1), sex hormone-binding globulin (SHBG), and coagulation protein concentrations were determined.
Results
Post-therapy Ki-67 decreased by 3.4% in the 4-OHT and 5.1% in the oral-T group (p < 0.03 in both, between-group p=0. 99). Mean plasma 4-OHT was 0.2 and 1.1 ng/mL in 4-OHT and oral groups, respectively (p=0.0003), while mean breast adipose tissue concentrations of 4-OHT were 5.8 ng/g in the 4-OHT group and 5.4 ng/g in the oral group (p=0.88). There were significant increases in plasma SHBG, Factor VIII and von Willebrand factor and a significant decrease in plasma IGF-1 with oral-T, but not with 4-OHT. The incidence of hot flashes was similar in both groups.
Conclusions
The anti-proliferative effect of 4-OHT gel applied to breast skin was similar to that of oral-T, but effects on endocrine and coagulation parameters were reduced. These findings support the further evaluation of local transdermal therapy for DCIS and breast cancer prevention.
Keywords: breast cancer prevention, tamoxifen, 4-OHT, endoxifen, ductal carcinoma in-situ
Introduction
Mammary ductal carcinoma in-situ (DCIS) accounts for 20% of new breast cancers [1], with 57,000 new cases diagnosed in the US in 2011[2]. Although disease-specific survival rates approach 98% [3], the risk for the development of subsequent invasive breast cancer may reach 30% following local therapy [4], so that DCIS patients are advised to undertake systemic therapy in the form of oral tamoxifen (oral-T) in order to further reduce the risk of new (local) breast events.
Despite the success of tamoxifen in reducing recurrence risk of estrogen receptor (ER) positive DCIS and that of new breast primaries [5.6], its systemic effects have led to generally low acceptance in the DCIS and prevention setting [4-8]. These relate to estrogen agonist activity on the endometrium and the activation of coagulation pathways, leading to an increased risk of uterine events and thromboembolism [9]. Hot flashes and vaginal symptoms are an additional barrier to acceptance [7,10]. Thus a particular challenge for primary and secondary breast cancer prevention efforts is to devise an efficacious and non-toxic intervention which is likely to be widely accepted by women who will benefit from it.
One possible solution is transdermal delivery of active drugs through the skin envelope of the breast to achieve high local concentrations with low systemic exposure, exploiting the embryological origins of the breast as a skin appendage (a modified eccrine gland) with a well-developed internal lymphatic circulation [11]. Results from previous studies show that drugs applied to the breast skin are selectively concentrated in the breast [12,13], whereas drugs applied to the skin of other regions of the body penetrate the skin into the vascular system and are distributed systemically. Thus transdermal drug application to the breast skin can be considered as local transdermal therapy (LTT), a concept which is further reviewed elsewhere [14]. In previous studies, 4-hydroxytamoxifen (4-OHT) gel was applied to the breast skin in settings ranging from 2-3 weeks of pre-operative treatment in postmenopausal women with invasive cancer to treatment of mastalgia in premenopausal women for up to one year [12,13,15]. Since LTT is most suited to women with DCIS or those at high risk, we performed a pre-surgical randomized trial of LTT in women with DCIS, testing 4-OHT gel against oral-T. Here we report results from 26 evaluable subjects who completed the study prior to its closure due to expiration of the shelf-life of the 4-OHT gel, with no additional drug available.
Participants and Methods
Study design
Between November 2009 and March 2012, pre and postmenopausal women(age range 45-86) with a diagnosis of ER positive DCIS, (as defined in ASCO/CAP guidelines [16]) were recruited at Northwestern University and Washington University to a randomized, double-blind, placebo-controlled trial of LTT with 4-OHT gel versus oral-T during the window between diagnostic core needle biopsy and surgical excision (NCT00952731 or N01-CN-35157). Women at risk for thromboembolic disease were excluded, as were those with a history of exogenous hormone use within the past month, and tamoxifen or raloxifene use within the past two years. Randomization was stratified by menopausal status, and enrollment site. Initially, the FDA required exclusions for grade 3 and comedo-type DCIS, mammographic DCIS size of > 5cm, and palpable lesions; these were removed following enrollment of nine subjects in the first year.
Study medication
4-OHT gel (Besins Healthcare BHR Pharma, LLC) was formulated as 0.2%(w/v) gel containing 200 mg of 4-OHT(E:Z=1:1) in 100 mL of hydroalcoholic, fast-drying gel supplied in a metered-dose container that dispensed 1.0 mL of gel (2 mg of 4-OHT or placebo) with each pump. Oral-T (20mg) and placebo capsules were supplied by NCI, Division of Cancer Prevention: (Z)-tamoxifen tablets in opaque gelatin capsules filled with microcystalline cellulose powder.
Study Procedures
All participants provided informed consent. Baseline assessments included a history and physical, explanation of gel application, completion of the Breast Cancer Prevention Trial Eight Symptom Scale (BESS) questionnaire [17], collection of a venous blood sample, and collection of nipple aspirate fluid (NAF) from NAF yielders [18] . Following randomization, study drug was shipped to participants: the gel group received 4-OHT gel (4 mg daily, 2 mg to each breast) and oral placebo; the oral group received tamoxifen capsules (20 mg daily) and placebo gel. Treatment began within 5 days post-randomization and ended on the day prior to surgical resection. Participants were instructed to apply the gel to the entire skin envelope of each breast each morning, after a shower. Duration of therapy was 6-10 weeks. Compliance was assessed through participant diaries, counts of returned pills and of returned gel canisters. Participants who took at least 80% of the prescribed dose were considered compliant.
Assessments similar to those performed at baseline were repeated on the day prior to, or on the morning of surgery. During surgery, breast adipose tissue from the surgical sample was snap frozen and stored at −80° C for measurement of tamoxifen and metabolites. The samples were obtained from a location adjacent to the DCIS lesion to provide uniformity between participants undergoing breast conservation and mastectomy. The paraffin block of the core and excision samples were acquired by the recruiting institution and 10 sections from each specimen were submitted to the NU Pathology Core Facility. The sections were cut in batches (with pre- and post-treatment samples in the same batch), shipped cold, and processed for immunohistochemistry within four weeks.
The BESS Questionnaire was repeated at day 15 and at the end of treatment (1 day before surgery or day of surgery), and the post-surgical visit (approximately 7-14 days after surgery). In an independent assessment at the same time points, adverse events were coded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Study endpoints
The primary efficacy endpoint of this study was to demonstrate that daily application of 4-OHT gel to the breasts results in a reduction in the Ki-67 labeling index (LI) of DCIS lesions, similar to that seen with oral-T, comparing the diagnostic core biopsy to the surgical excision sample. Secondary endpoints were 1) to compare concentrations of tamoxifen and its metabolites [4-OHT, endoxifen, N-desmethyl tamoxifen (NDT)] in breast tissue, plasma and NAF obtained on the day of surgery; 2) to assess changes in known tamoxifen-modulated pathways in the breast (COX-2 and maspin protein expression, [19,20]) and plasma (SHBG, IGF-1 [14]) . Side effect endpoints were 1) the incidence of hot flashes at baseline and before surgery; 2) changes in coagulation related proteins in women on the gel and the oral arms from baseline to immediately before surgery.
Ki-67, COX-2 and Maspin expression
immunohistochemical (IHC) assessment of these markers was performed on paraffin embedded sections of the core and excision specimens, using standard IHC techniques and MCF7, HCT116 and H292 cells as controls. For maspin we used primary mouse monoclonal antibody (Clone-G167-70,BD Pharmingen, San Jose, California), dilution 1:200; for COX-2, primary mouse monoclonal antibody (Clone-CX-294, Dako , Denmark), dilution 1:100-; and for Ki67, primary mouse monoclonal antibody (Clone-MIB-1, Dako, Denmark), dilution 1:100- antigen. Dako Envision Plus system HRP labeled polymer for 20 min at 37°C was used as the detection system. Scoring was performed on DCIS lesions only, with manual counting of positively stained DCIS cells. The Ki67 LI was assessed on an average of 300 DCIS cells at 40X magnification. The H score system (Score range: 0-300) was used for COX-2 and maspin markers by a single observer who was blinded to treatment status, with random verification of 20% of slides by a pathologist (PK) [21].
Plasma and breast tissue concentration measurement of tamoxifen and its metabolites
(Z)-tamoxifen, (Z)-NDT, (E) and (Z)-4-OHT, and (Z)-endoxifen were measured by liquid chromatography-tandem mass spectrometry with a turbo ion spray interface operating in positive mode (API 3000; AB SCIEX, Foster City, CA). Briefly, 100 μL of plasma was mixed with 200 μL of acetonitrile containing 1 ng each of the deuterated analogs of the analytes (TRC, Toronto, Canada), centrifuged at 4°C and 7000 RPM for 10 minutes, and supernatant diluted with 200 μL of water before analysis. For analysis of NAF, samples were collected in a capillary tube and diluted with 200 μL of phosphate buffered saline; 100 μL of the diluted NAF sample was used for analysis. Breast adipose tissue samples, 25 mg, were minced and treated with 125 μL of a 1 mg/mL arsenic solution in 2% nitric acid (Inorganic Ventures, Christiansburg, VA) and extracted as described above. Chromatographic separation was achieved with a Kinetex PFP 2.6μ column, 50×2.1 mm (Phenomenex, Torrance, CA). The mobile phase was A: 0.1% formic acid in water (v/v) and B: 0.1% formic acid in acetonitrile (v/v). The flow rate was 0.3 ml/min at 25 °C. Retention times for (Z)-tamoxifen, (Z)-NDT, (Z)-4-OHT, (E)-4-OHT and (Z)-endoxifen were 7.3, 6.8, 5.1, 4.7 and 4.5 min, respectively. Total run time was 13 min. Acquisition was performed in multiple reaction monitoring mode using m/z 372.2 → 72.1, 388.2 → 72.1, 374.2 → 72.1 and 358.2 → 72.1 at low resolution for tamoxifen, 4-OHT, endoxifen and NDT, respectively. In three participants, matched samples were not available: breast adipose tissue was not collected in two, and the plasma sample was missing in one.
Since the fraction of E and Z isoforms of 4-OHT was of particular interest, we used an additional validated method to study plasma concentrations of these metabolites in a different laboratory (Eurofins Medinet, Chantilly, VA): Plasma from blood samples collected in lithium-heparin tubes was frozen at −20°C, shipped in batches on dry ice to the Eurofins Medinet central laboratory; LC-MS/MS was used for the simultaneous determination of (E) 4-OHT and (Z) 4-OHT, with a lower limit of quantitation (LOQ) of 10 pg/mL and upper LOQ of 10,000 pg/mL. Eurofins Medinet developed and validated the method for BHR Pharma, in accordance with the FDA Guidance on Bioanalytical Method Validation [22].
Circulating Marker assessment
Plasma samples collected with anticoagulant K3-EDTA were used for human IGF-1 and SHBG assays, and coagulation protein assays (factor VIII, factor IX, von Willebrand factor, and protein S)[23]. The human IGF-1 and SHBG assays were performed with Quantikine Enzyme-linked immunosorbent assay (ELISA) Kits (R&D Systems, Cat# DG100 for IGF-1, and Cat# DSHBG0 for SHBG assay). The lower limit of detection was 56 pg/ml, and 5 pmol/L; %CV values were 4.3 and 5.6 for IGF-1, and SHBG assay, respectively. Factor VIII, and factor IX were determined with VisuLize™ antigen ELISA kits (Affinity Biologicals Inc.). von Willebrand factor was measured with immune-turbidimetric assay (Diagnostica Stago Inc.Cat#00518) by STA® analyzer, and total protein S was assayed with an ELISA kit (REAADS® Inc.)
Statistical Design and Analysis
The study was powered to detect a 50% reduction in Ki67 LI from baseline to post-therapy, with the hypothesis that change would be similar in the two groups. Therefore, if the mean relative decrease in the 4-OHT group was at least 30%, this would be considered equivalent to a relative decrease of up to 50% in the tamoxifen group. With alpha=5% and beta=20%, the planned sample size was 112 women, expecting that 90 would be evaluable for the primary endpoint of Ki67 LI. The study was halted early, but our assumptions regarding relative variability in the data and relative change from baseline have held for the main variable. In particular, the baseline means for Ki67 are 8.3% and 6.7% in the oral and in the 4-OHT groups respectively, while the corresponding SDs are 5.2% and 5.6%. This gives the coefficients of variation of 5.2/8.3= 0.63 which is exactly what we assumed for the oral group and 5.6/6.7=0.84 which is around 30% larger than what we assumed for the 4-OHT group.
For continuous variables in the immunochemistry, drug concentration, and blood coagulation data, means and standard deviations are reported; the significance of changes between baseline and post-treatment within groups were evaluated with the paired t-test, and differences between treatment groups assessed using the unpaired t-test. For categorized demographic data, we examined the association of these variables with treatment group via Fisher’s exact test. For the analysis of quality of life, the 33 symptoms in the BESS Questionnaire were divided into eight clusters as described by Cella et. al. [17]. The mean score within each cluster was used to evaluate significance of changes from baseline to post-treatment within groups as well as the differences between treatment groups using the Wilcoxon signed-rank test.
Results
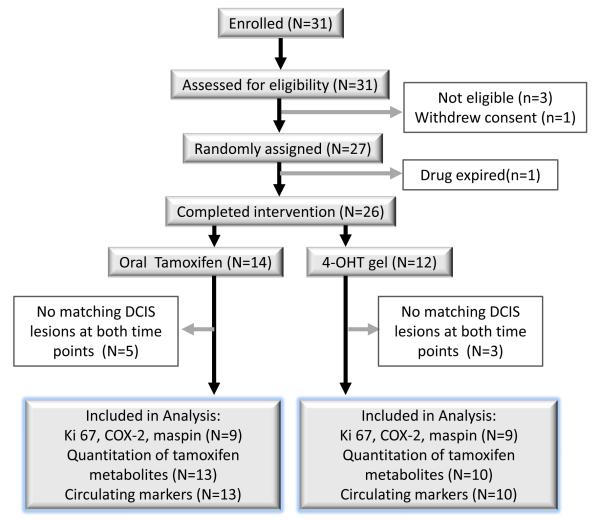
A total of 31 subjects were enrolled over 29 months (November 2009 to July 2011), at which point the shelf-life of the drug expired and the study was closed. Three participants were ineligible (two with ER negative DCIS, and one with high creatinine); one participant withdrew consent before randomization. Of 27 randomized participants, one was withdrawn from the study due to lack of drug supply. A total of 26 subjects completed the study, 14 in the oral-T group and 12 in the topical 4-OHT gel group (see Figure 1). The range of therapy duration was 6-10 weeks and the median time on treatment was 6 weeks (see Table 1).
Figure 1.
CONSORT diagram (participant flow diagram)
Table 1.
Participant characteristics at baseline1, DCIS size from surgical specimen, and the duration of treatment according to treatment groups
| Oral-T (20mg/day) | 4-OHT gel (4mg/day) |
P | |
|---|---|---|---|
| No. of participants | N=14 | N=12 | |
| Age, years (IQR)* | 54 (50, 61) | 60 (52, 65) | 0.29 |
| Menopausal Status | |||
| Pre | 3 (21.4%) | 4 (33.3%) | 0.67 |
| Post | 11 (78.6%) | 8 (66.7%) | |
| Race | |||
| Caucasian | 6 (42.9%) | 7 (58.3%) | 0.70 |
| Non-Caucasian | 8 (57.1%) | 5 (41.7%) | |
| DCIS grade | |||
| 1 | 3 (21.4%) | 1 (8.3%) | 0.69 |
| 2 | 10 (71.4%) | 9 (75.0%) | |
| 3 | 1 (7.1%) | 2 (16.7%) | |
| DCIS size at surgery, cm (IQR)* | 1.9 (0.6, 2.4) | 0.58 (0.4,1.36) | 0.23 |
| %ER expression(IQR)* | 80% (67,95) | 85% (67, 100) | 0.86 |
| %PR expression(IQR)* | 67% (26,90) | 67% (33,75) | 0.84 |
| Days of treatment(IQR)* | 44 (42,47) | 46 (45,48) | 0.29 |
| 40-59 days | 12 (85.7%) | 10 (83.3%) | |
| 60-69 days | 2 (14.3%) | 2 (16.7%) |
A total of 27 participants were randomized, but 26 participants completed the intervention.
Values are reported in median with interquartile range(IQR)
Baseline Participant Characteristics
Participant demographics and clinical characteristics according to treatment groups were not significantly different (see Table 1). In particular, there were no significant differences in DCIS grade, lesion size, ER and PR expression, age or menopausal status.
Tissue markers
We were not able to obtain matched core and excision samples from two participants. Another six participants were excluded from analysis of IHC endpoints because the DCIS lesion had been exhausted in the baseline sample in one, and there was insufficient DCIS remaining in the excision specimen in five additional participants. Thus, of 26 women completing the study, matched DCIS lesions from baseline and post-treatment specimens were not available on eight, yielding a total of 18 subjects who were evaluable for IHC markers (9 in the tamoxifen group, and 9 in the 4-OHT gel group). The changes in Ki-67 LI in DCIS lesions, according to the treatment group, are summarized in Table 2.The mean Ki-67 LI after the treatment decreased significantly from baseline in both treatment groups (mean reduction 5.1% in tamoxifen group, p= 0.008 and 3.4% in the topical 4-OHT group, p= 0.03. This mean reduction in the two groups was statistically similar (p= 0.99).
Table 2.
Ki67, COX-2, and maspin changes according to the treatment groups
| Oral-T (20mg/day) (N=9) | 4-OHT gel(4mg/day) (N=9) |
||||
|---|---|---|---|---|---|
| Mean ± SD | P* | Mean ± SD | P* | P† | |
| Ki-67 | |||||
| baseline | 8.3 ± 5.2 | 6.7 ± 5.6 | |||
| post-treatment | 3.2 ± 2.3 | 3.2 ± 2.6 | |||
| Changes from baseline | −5.1 ± 5.5 | 0.008 | −3.4 ± 5.0 | 0.03 | 0.99 |
| COX-2 | |||||
| baseline | 67.2 ± 72.4 | 53.9 ± 55.7 | |||
| post-treatment | 78.9 ± 63.5 | 35.7 ± 28.9 | |||
| Changes from baseline | 11.7 ± 109.8 | 0.46 | −18.2 ± 32.2 | 0.44 | 0.19 |
| Maspin | |||||
| baseline | 107 ± 56 | 120 ± 97 | |||
| post-treatment | 142 ± 69 | 162 ± 37 | |||
| Changes from baseline | 35 ± 71 | 0.43 | 41 ± 115 | 0.23 | 0.38 |
Abbreviation: DCIS= Ductal carcinoma in situ; Cyclooxygenase-2 =COX-2.
Ki-67 LI was represented in %; COX-2 and maspin in H-score.
Paired t-test for changes from baseline within a treatment group.
Unpaired t- test for changes from baseline between treatment groups.
COX-2 and maspin changes by treatment are shown in Table 2. There were no significant differences between baseline and post-treatment in COX-2 or maspin expression within each treatment group. Also, no differences were noted between treatment groups with respect to the baseline-to-post-treatment changes in these biomarkers (p=0.19 for COX-2; p=0.38 for maspin).
Plasma and Tissue Concentrations of tamoxifen and its metabolites
There were a total of 23 participants (13 treated with tamoxifen and 10 with 4-OHT gel) whose plasma and breast adipose tissue samples, matched pre- and post-treatment, were available for quantitation of tamoxifen and its metabolites (NDT, 4-OHT, and endoxifen). The mean plasma and tissue concentration of each analyte is reported in Table 3. Detectable levels of tamoxifen, NDT and endoxifen were found only in the oral-T group, with mean values being substantially higher in tissue than in plasma. In contrast, (Z) 4-OHT was detectable in the tissue of both oral and gel groups, at equivalent concentrations (5.4 ng/g and 5.8 ng/g, respectively, p=0.88), while plasma concentrations were markedly different (1.1 ng/mL in the oral-T group and 0.2 ng/mL in the 4-OHT gel group, p=0.0003 (see Table 3). (E) 4-OHT was present in breast adipose tissue of the gel group at a concentration of 5.2 ± 10.0 ng/g. Data on plasma (Z) 4-OHT concentrations from both laboratories were very similar, but the (E) 4-OHT assay was more sensitive in the Eurofins laboaratory, and (E) 4-OHT was detectable in plasma of both gel and oral groups, at levels that were considerably lower than those of (Z)4-OHT (see Table 3).
Table 3.
Concentrations of tamoxifen and its metabolites in breast tissue(ng/g) and plasma(ng/mL)
| Breast adipose tissue | Plasma | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Analytes | Tamoxifen (20mg/day) |
4-OHT gel (4mg/day) |
Tamoxifen (20mg/day) |
4-OHT gel (4mg/day) |
||
| (N= 13) | (N=10) | P* | (N= 13) | (N=10) | P* | |
| (Z) Tamoxifen | 2959± 1035 | BQL | 90 ± 45 | BQL | ||
| (Z) NDT | 492 ± 192 | BQL | 149 ± 57 | BQL | ||
| (E) 4-OHT | BQL | 5.2 ± 10.0 | BQL | BQL | ||
| (Z) 4-OHT | 5.4 ± 2.8 | 5.8 ± 9.3 | 0.88 | 1.1 ± 0.7 | 0.2 ± 0.2 | 0.0003 |
| (Z) Endoxifen | 8.0 ± 6.8 | BQL | 5.9 ± 3.2 | BQL | ||
|
| ||||||
| Independent validation in Eurofins laboratory | ||||||
|
| ||||||
| (E) 4-OHT | 0.010±0.006 | 0.056±0.072 | 0.06 | |||
| (Z) 4-OHT | 1.488±0.771 | 0.261±0.284 | <0.0001 | |||
Abbreviation: NDT= N-desmethyltamoxifen; 4-OHT=4-hydroxytomoxifen; Endoxifen= N-desmethyl 4-hydroxytamoxifen; IQR=interquartile range; BQL= below the lowest limit of quantification (LLOQ).
All the concentrations were reported as means ± standard deviation. Tissue concentration was represented in ng/g (LLOQ = 3ng/g). (E) isomers of NDT, and Endoxifen were BQL. Plasma concentration was represented in ng/mL (LLOQ = 20ng/mL for tamoxifen and NDT; 1ng/mL for 4-OHT and Endoxifen). (E) isomers of NDT, 4-OHT, and Endoxifen were BQL.
LLOQ of plasma concentration measured by Eurofins laboratory was 10 pg/mL for both E and Z isomers.
Unpaired t-test test between oral and topical treatment groups
Overall, we found that 4-OHT was detectable in breast tissue for 9 of 10 participants in the 4-OHT gel group for whom samples were available; and in plasma for 5 participants. We observed a direct correlation between plasma and tissue concentration of (Z) 4-OHT in the oral-T group (Spearman correlation coefficient =0.79, p=0.0007); however there was no such correlation in the 4-OHT gel group(Spearman correlation coefficient=0.24, p=0.48). We then looked at individuals with the highest (Z) 4-OHT tissue concentrations (≥ 1.5- fold of the mean); in the 4-OHT gel treated group, there were two such participants, with 14.9 ng/g and 33.2 ng/g of (Z) 4-OHT in breast. However their plasma (Z) 4-OHT was undetectable for one participant, and 0.22 ng/mL for the other, compared to the mean (0.2 ng/mL).
Drug concentrations in NAF
We obtained post-treatment NAF (3 μL to 40 μL) from the contralateral breast of six participants, and were able to detect tamoxifen or its metabolites in NAF samples of four participants, two in the tamoxifen and two in the 4-OHT gel group. In the oral-T group, NAF concentrations of tamoxifen and NDT were higher than the plasma concentrations (participant A: 320 vs. 94.3 ng/mL for tamoxifen, and 406 vs. 133 ng/mL for NDT; participant B: 182 vs.160 ng/mL for tamoxifen, and 355 vs. 222 ng/mL for NDT) whereas 4-OHT and endoxifen were undetectable. In the 4-OHT gel group, tamoxifen, NDT, and endoxifen were undetectable, but both isomers of 4-OHT were detected in similar amounts, with (Z) 4-OHT concentration > 40 fold higher than the plasma concentration (participant C: 16.7 vs. 0.42 ng/mL; participant D: 25.1 ng/mL vs. BQL. Of the two NAF samples with undetectable tamoxifen or metabolites, one subject received oral-T, had detectable plasma tamoxifen (54 ng/mL) and NDT (136 ng/mL), but unfortunately no breast tissue sample was collected for drug quantitation at surgery. The other subject with undetectable tamoxifen or metabolites in NAF received active 4-OHT gel, and had a high breast adipose tissue 4-OHT level of 33.2 ng/g for (Z) 4-OHT.
Tamoxifen responsive-circulating markers
Mean baseline IGF-1, SHBG, vWF, factor VIII, factor IX, and total protein S levels are shown in Table 4. Overall, these markers of systemic hormonal effects were induced in the tamoxifen group, but not in the 4-OHT group. Specifically, median SHBG levels increased significantly following tamoxifen therapy (p= 0.002), but not with 4-OHT gel therapy (p=0.67). Mean vWF and factor VIII levels increased significantly with oral-T therapy (p= 0.02 and p=0.03, respectively) but not with 4-OHT gel therapy (p=0.88, and p=0.17, respectively). The mean levels of factor IX and total protein S did not change for either treatment group. Finally, mean IGF-1 levels were significantly lower than baseline in the oral-T group (p= 0.003), but not in the 4-OHT gel group. However, between-group comparisons of these treatment-related changes did not reach statistical significance.
Table 4.
Changes in circulating markers according to the treatments
| Tamoxifen (20mg/day) (N=13) |
4-OHT gel (4mg/day) (N=10) |
||||
|---|---|---|---|---|---|
| Mean ± SD | P* | Mean ± SD | P* | P† | |
| IGF-1 (ng/mL) | |||||
| baseline | 59.0 ± 11.4 | 63.7 ± 8.6 | |||
| post-treatment | 50.3 ± 9.7 | 58.5 ± 6.6 | |||
| Changes from baseline | −8.7 ± 8.3 | 0.003 | −5.2 ± 9.5 | 0.12 | 0.35 |
| SHBG (ng/mL) | |||||
| baseline | 98.4 ± 45.0 | 89.4 ± 70.2 | |||
| post-treatment | 143.9 ± 69.0 | 99.7 ± 76.0 | |||
| Changes from baseline | 45.5 ± 40.2 | 0.002 | 10.3 ± 74.4 | 0.67 | 0.20 |
| %vWF | |||||
| baseline | 167.4 ± 89.2 | 179.9 ± 68.3 | |||
| post-treatment | 218.6 ± 134.6 | 177.3 ± 65.3 | |||
| Changes from baseline | 51.2 ± 71.0 | 0.02 | −2.6 ± 52.3 | 0.88 | 0.06 |
| %Factor VIII | |||||
| baseline | 157.1 ± 47.5 | 158.4 ± 23.4 | |||
| post-treatment | 168.7 ± 51.6 | 167.1 ± 24.5 | |||
| Changes from baseline | 11.6 ± 17.3 | 0.03 | 8.7 ± 18.5 | 0.17 | 0.70 |
| %Factor IX | |||||
| baseline | 86.6 ± 8.8 | 86.7 ± 7.0 | |||
| post-treatment | 87.0 ± 12.2 | 81.1 ± 12.7 | |||
| Changes from baseline | 0.4 ± 10.2 | 0.89 | −5.6 ± 13.6 | 0.22 | 0.24 |
| %Total Protein S | |||||
| baseline | 94.3 ± 8.9 | 97.5 ± 7.0 | |||
| post-treatment | 91.6 ± 12.2 | 95.9 ± 9.2 | |||
| Changes from baseline | −2.7 ± 9.3 | 0.32 | −1.6 ± 6.5 | 0.46 | 0.76 |
Abbreviation: IGF-1 = insulin-like growth factor-1; SHBG= sex hormone-binding globulin; vWF= von Willebrand factor.
Paired t-test between baseline and post-treatment value within a treatment group
Unpaired t- test for changes from baseline between treatment groups.
Quality of Life Assessment
Quality of life parameters assessed by BESS questionnaire are summarized in Table 5. At baseline, the mean scores for all clusters were similar for the two treatment groups, with the exception of the vaginal symptom cluster which was marginally higher in the 4-OHT gel group (0.14 compared to 0.00, p=0.052) Following treatment, the mean score for vasomotor symptoms (hot flashes, night sweats, and cold sweats) increased slightly compared to baseline in both groups (oral-T p=0.06 and 4-OHT gel group p=0.13), but, these changes were not significantly different between the two treatment groups (p = 0.83). The gastrointestinal symptom cluster score was somewhat higher in the oral-T group at baseline, and although the within-group change was not significant in either oral or transdermal groups, the between group comparison did reach statistical significance (p=0.049). There were no other between-group differences in the change of symptom severity from baseline to post-treatment. In addition, in our collection of CTCAE data, no serious adverse events were reported in this study.
Table 5.
Summary of BESS Quality of Life Assessmentby symptom clusters according to the treatments
| Symptom Cluster | Tamoxifen(20mg/day) (N=14) |
4-OHT gel(4mg/day) (N=12) |
|||
|---|---|---|---|---|---|
| Mean ± SD | P* | Mean ± SD | P* | P† | |
| Cognitive | |||||
| baseline | 0.62 ± 0.61 | 0.61 ± 1.11 | 0.54 | ||
| post-treatment | 0.71 ± 1.18 | 0.69 ± 1.20 | |||
| Changes from baseline | 0.10 ± 0.86 | 0.99 | 0.08 ± 0.47 | 0.81 | 0.64 |
| Body pain | |||||
| baseline | 0.76 ± 1.12 | 0.56 ± 0.94 | 0.49 | ||
| post-treatment | 1.19 ± 1.11 | 0.58 ± 0.74 | |||
| Changes from baseline | 0.43 ± 0.92 | 0.11 | 0.03 ± 0.61 | 0.78 | 0.27 |
| Vasomotor | |||||
| baseline | 0.33 ± 0.45 | 0.19 ± 0.41 | 0.35 | ||
| post-treatment | 0.88 ± 1.26 | 0.53 ± 0.73 | |||
| Changes from baseline | 0.55 ± 1.05 | 0.06 | 0.33 ± 0.64 | 0.13 | 0.83 |
| Gastrointestinal | |||||
| baseline | 0.12 ± 0.31 | 0.00 ± 0.00 | 0.18 | ||
| post-treatment | 0.02 ± 0.09 | 0.06 ± 0.13 | |||
| Changes from baseline | −0.10 ± 0.24 | 0.50 | 0.06 ± 0.13 | 0.50 | 0.049 |
| Sexual problems | |||||
| baseline | 0.32 ± 0.72 | 0.42 ± 0.67 | 0.55 | ||
| post-treatment | 0.11 ± 0.40 | 0.25 ± 0.5 | |||
| Changes from baseline | −0.21 ± 0.54 | 0.25 | −0.17 ± 0.81 | 0.53 | 0.48 |
| Bladder | |||||
| baseline | 0.14 ± 0.36 | 0.17 ± 0.39 | 0.87 | ||
| post-treatment | 0.25 ± 0.33 | 0.25 ± 0.40 | |||
| Changes from baseline | 0.11 ± 0.49 | 0.59 | 0.08 ± 0.19 | 0.50 | 0.67 |
| Body image | |||||
| baseline | 0.68 ± 0.77 | 0.63 ± 0.83 | 0.55 | ||
| post-treatment | 0.64 ± 0.89 | 0.75 ± 1.06 | |||
| Changes from baseline | −0.04 ± 0.87 | 0.92 | 0.13± 0.53 | 0.75 | 0.26 |
| Vaginal | |||||
| baseline | 0.00 ± 0.00 | 0.14 ± 0.26 | 0.052 | ||
| post-treatment | 0.07 ± 0.19 | 0.14 ± 0.33 | |||
| Changes from baseline | 0.07 ± 0.19 | 0.50 | 0.00 ± 0.14 | 0.75 | 0.08 |
Wilcoxon signed-rank tests were used for the changes from baseline within a treatment group.
Wilcoxon rank-sum tests were used for baseline, and the changes from baseline between treatment groups.
Discussion
Local transdermal therapy (LTT) to the breast for prevention of in-breast recurrence of DCIS and occurrence of new primary tumors is a promising approach with the potential of significantly reducing side effects through reduced systemic exposure. We report the first study of this approach in women with DCIS, comparing a proven breast cancer prevention agent (tamoxifen) given orally, and one of its active metabolites (4-hydroxytamoxifen) given transdermally to the breast for at least 6 weeks. Although we did not reach our target accrual, we report results on the crucial issue of drug concentration in blood and plasma, and preliminary data on biomarkers of efficacy (Ki67 labeling in DCIS tissue) and systemic exposure (plasma levels of IGF-1, SHBG, and coagulation proteins).
Our primary endpoint was Ki-67 LI, which is the best validated and most widely accepted endpoint for window-of-opportunity studies of systemic agents for breast cancer [24]. Encouragingly, although the power of our study to ‘prove’ equivalence between groups is limited since only 28% of the subjects were accrued, our assumptions regarding variability in Ki67-LI and relative change from baseline have held, in that the baseline means for Ki67-LI in the two groups and the corresponding SDs and coefficients of variation are very close to the assumptions used for the statistical plan. The drop in Ki67-LI was larger than anticipated in both groups: 61% rather than 50% in the oral group, and 52% rather than 30% in the 4-OHT group, consistent with the projected ‘effect size’. Our findings are strengthened by an earlier study of postmenopausal women with ER positive invasive cancers, where 2-3 weeks of treatment with up to 2mg of 4–OHT gel (1 mg per breast) was compared to oral-T prior to cancer resection; cell proliferation decreased to a similar degree with oral and transdermal therapy, and 4-OHT plasma concentrations were significantly lower in the transdermal group [13]. In contrast, we included pre and postmenopausal women, used a higher daily dose of 4-OHT (4 mg) and a longer treatment interval of 6-10 weeks to allow an assessment of vasomotor symptoms.
We assessed COX-2 and maspin labeling of DCIS lesions because of previous evidence that their expression is modulated by tamoxifen[25][19] Although significant modulation in COX-2 and maspin expression was not seen in either group, these potential markers of DCIS biology remain of interest [20,26].
Our results support the hypothesis that effective breast concentrations can be achieved with low systemic exposure; the breast adipose tissue concentrations of (Z) 4-OHT were equivalent in the oral and LTT groups (over 5 ng/mg tissue in both groups). Our results compare favorably with the previous study where median 4-OHT concentration in non-tumor breast tissue was 2.0 ng/g in the oral-T group and 0.8 ng/g in the 4-OHT gel group (2 mg daily) [13]. In contrast, the mean plasma level of 4-OHT was more than five-fold lower in the 4-OHT gel group than in the tamoxifen group using two independent methods in different laboratories. Although data on NAF concentrations were available on only four women, these too support the main finding of high mammary concentrations of 4-OHT achieved with LTT, and are of interest because they imply a within-breast distribution of the drug that allows high concentrations to appear in nipple fluid. Thus our pharmacokinetic results compare favorably with previous reports and suggest that LTT for breast cancer prevention and for DCIS therapy using 4-OHT gel should be effective; a reduction in long term side effects remains to be demonstrated; nevertheless, the data are encouraging and support the design of future studies.
We measured 4-OHT isomers since they differ in anti-estrogenic activity, with the (Z) isomer of 4-OHT and endoxifen being the major biologically active forms [27-29]. The pure (Z) form is difficult to stabilize in the manufacturing process, and 4-OHT gel contains equal amounts of both isomers. We found that the concentrations of 4-OHT isomers were similar in breast adipose tissue in the oral and gel groups, whereas plasma concentrations were significantly lower in the gel group, with no evidence of isomerization (Z →E) (Table 3). This contrasts with the result from an earlier topical study using 3H labeled (Z) 4-OHT to the breasts [30], where the authors reported a progressive (Z →E) isomeriza on in breast tissue samples collected from 12 h to day 7. Other topical 4-OHT gel studies reported total concentration of 4-OHT rather than concentration of each isomer [12,13,15]. In agreement with previous studies [12,13,15], we did not see any further metabolic transformation of 4-OHT to endoxifen in the breast adipose tissue following topical 4-OHT gel administration.
Recently, endoxifen has attracted attention based on its greater abundance relative to 4-OHT in women on oral-T [31,32], and a report that endoxifen causes proteosomic degradation of ERα and may have more selective anti-estrogenic effects [33]. We found marginally higher concentrations of endoxifen than 4-OHT in the breast adipose tissue of the oral-T group, and it is possible that the combined presence of endoxifen and 4-OHT implies better efficacy. However, it remains reassuring that the magnitude of the post-therapy Ki67 decrease was similar in the oral and gel groups. Endoxifen and 4-OHT have equal binding affinity for the ER [34,35], and in vitro transdermal permeability [36]; future studies using a gel formulation of endoxifen would therefore be of interest.
Tamoxifen has been reported to affect plasma levels of IGF-1 and SHBG [37-39], providing a measure of the pharmacologic action of tamoxifen upon the hormone axis. Previous reports document a decrease in plasma IGF-I levels with tamoxifen therapy [37] and an increase in serum SHBG related to the estrogenic effect on the liver [38,39], which is dose-dependent [40]. We observed significant decreases in IGF-1 and increases in SHBG in the tamoxifen group, but not in the 4-OHT gel group, supporting the notion that systemic effects of 4-OHT gel are small, if any. However, given the small sample sizes, the magnitude of the change was not statistically different between groups.
Similarly, the use of oral-T has been associated with changes in coagulation proteins such as von Willebrand factor (vWF), factor VIII, factor IX, and total protein S [12,40,41]. We found that the post-treatment levels of factor VIII and vWF were significantly increased post-therapy in the tamoxifen but not in the 4-OHT gel group. Thus, the avoidance of first-pass metabolism of tamoxifen in the liver potentially avoids changes in the clotting cascade that contribute to the pro-thrombotic effects of SERMS [42-44], a clear advantage.
Another significant adverse effect of SERM therapy relates to the induction of hot flashes, in both pre and postmenopausal women. The very low plasma concentrations observed following transdermal application of 4-OHT raises the possibility that hot flashes will be reduced by LTT with active tamoxifen metabolites [12,15,45]. However, the lowest plasma level of tamoxifen metabolite exposure to cause hot flashes has not been defined, and it is possible that even low-level exposure may be sufficient to cause hot flashes. We did not observe a significant effect on hot flash frequency following a minimum of six weeks of therapy, although our data is clearly limited by small numbers.
Finally, population variation in the efficiency of tamoxifen metabolism, related to polymorphisms in the CYP2D6 and other genes [46] may adversely affect efficacy of orally administered tamoxifen. LTT with an active tamoxifen metabolite circumvents the need for prodrug activation, potentially avoiding one source of low bioavailability of active [31,32] . Our finding supports this notion since topical gel application of 4-OHT achieved the similar breast concentration of 4-OHT compared to oral administration of tamoxifen.
An important question with transdermal delivery to the breast pertains to whether this is local therapy (higher concentrations in the breast than elsewhere) or systemic therapy (with similar concentrations throughout the body). Previous work has shown that 4-OHT applied to the breast skin results in 10-fold higher breast tumor levels than when it is applied to the arm or shoulder [12]. Although this differential accumulation was attributed to the binding of 4-OHT to ERs present in the breast, receptor binding alone is insufficient to explain 4-OHT retention at the levels observed [47,48]. A more likely explanation relates to the embryological origin of the breast as a skin appendage (i.e. a modified eccrine gland), so that the breast parenchyma and its skin envelope are a single unit with a well-developed internal lymphatic circulation [11], further evidenced by that fact that the skin and parenchyma of the breast drain to the same sentinel lymph nodes [49,50]. If breast retention of locally applied drugs is an anatomic rather than physiologic phenomenon, it predicts that other drugs applied to the skin of the breast should also concentrate in the parenchyma to a greater degree than can be expected based on transdermal systemic delivery through the circulation. Thus LTT may be applicable to a variety of agents as long as they are effective prevention agents and show sufficient dermal permeation.
Our trial was slow to accrue, particularly in the beginning. Few window-of-opportunity trials have been performed in women with DCIS, and despite the consensus among physicians that a surgical delay of six weeks was not risky, the majority of eligible subjects were unwilling to experience this delay. Furthermore, initially restrictive eligibility criteria, designed to minimize the likelihood that participants with a core biopsy showing DCIS had undiagnosed invasive disease, greatly decreased the pool of available subjects, and resulted in the recruitment of women with very small DCIS lesions, leading to an attrition of almost 30% in the assessment of biomarkers in matched pre- and post-therapy lesions. Ultimately, slow accrual led to expiration of the study agent, and a decision by the manufacturer to not produce additional supplies. In future studies, it is clear that enrollment criteria should be as open as possible.
In summary, our data support the notion that local transdermal drug delivery to the breast will achieve sufficient drug concentrations to be effective, with low systemic exposure. This concept deserves further testing with 4-OHT, and is likely to be applicable to other lipophilic drugs with low molecular weight.
Translational Relevance.
Women at high risk for breast cancer and those with ductal carcinoma in-situ (DCIS) are reluctant to accept oral (ie systemic) therapy with tamoxifen. This is a major barrier to the implementation of pharmacologic prevention strategies. A possible solution is local transdermal therapy (LTT), applying active drug metabolites to the breast skin. One such candidate is 4-hydroxytamoxifen (4-OHT), a potent anti-estrogenic metabolite of tamoxifen. We conducted a randomized, double-blind, presurgical phase II trial comparing transdermal 4-OHT gel to oral tamoxifen (oral-T) in women with DCIS. We observed equivalent anti-proliferative effect of transdermal 4-OHT gel and oral-T, but systemic effects on endocrine and coagulation parameters were reduced with transdermal delivery. Furthermore, plasma concentrations of 4-OHT in the gel group were 1/5th those in the oral group, but breast adipose tissue concentrations were similar. These findings support the further evaluation of LTT for DCIS therapy and breast cancer prevention.
Acknowledgments
Grant Support: This work was supported by the National Institutes of Health [contract# N01-CN-35157] and BHR Pharma, LLC. The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Reference List
- 1.Weaver DL, Rosenberg RD, Barlow WE, Ichikawa L, Carney PA, Kerlikowske K, et al. Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer. 2006;106:732–742. doi: 10.1002/cncr.21652. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, Desantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014 doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 4.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100:1800–1806. doi: 10.1002/cncr.20205. [DOI] [PubMed] [Google Scholar]
- 6.Yen TW, Hunt KK, Mirza NQ, Thomas ES, Singletary SE, Babiera GV, et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100:942–949. doi: 10.1002/cncr.20085. [DOI] [PubMed] [Google Scholar]
- 7.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8:580–585. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 8.Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103:1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 10.Day R, Ganz PA, Costantino J, Cronin WM, Wickerham DL, Fisher B. Health-Related Quality of Life and Tamoxifen in Breast Cancer Prevention: A Report From the National Adjuvant Breast and Bowel Project P-1 Study. J Surg Oncol 9 A.D. 17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 11.Ackerman AB, Kessler G, Gyorfi T, Tsou HC, Gottlieb GJ. Contrary view: the breast is not an organ per se, but a distinctive region of skin and subcutaneous tissue. Am J Dermatopathol. 2007;29:211–218. doi: 10.1097/DAD.0b013e3180325d6b. [DOI] [PubMed] [Google Scholar]
- 12.Pujol H, Girault J, Rouanet P, Fournier S, Grenier J, Simony J, et al. Phase I study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissue. Cancer Chemother Pharmacol. 1995;36:493–498. doi: 10.1007/BF00685799. [DOI] [PubMed] [Google Scholar]
- 13.Rouanet P, Linares-Cruz G, Dravet F, Poujol S, Gourgou S, Simony-Lafontaine J, et al. Neoadjuvant percutaneous 4-hydroxytamoxifen decreases breast tumoral cell proliferation: a prospective controlled randomized study comparing three doses of 4-hydroxytamoxifen gel to oral tamoxifen. J Clin Oncol. 2005;23:2980–2987. doi: 10.1200/JCO.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 14.Lazzeroni M, Serrano D, Dunn BK, Heckman-Stoddard BM, Lee O, Khan S, et al. Oral low dose and topical tamoxifen for breast cancer prevention: modern approaches for an old drug. Breast Cancer Res. 2012;14:214. doi: 10.1186/bcr3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansel R, Goyal A, Nestour EL, Masini-Eteve V, O’Connell K. A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389–397. doi: 10.1007/s10549-007-9507-x. [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Land SR, Chang CH, Day R, Costantino JP, Wolmark N, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–526. doi: 10.1007/s10549-007-9682-9. [DOI] [PubMed] [Google Scholar]
- 18.Chatterton RT, Jr., Khan SA, Heinz R, Ivancic D, Lee O. Patterns of sex steroid hormones in nipple aspirate fluid during the menstrual cycle and after menopause in relation to serum concentrations. Cancer Epidemiol Biomarkers Prev. 2010;19:275–279. doi: 10.1158/1055-9965.EPI-09-0381. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Shi HY, Nawaz Z, Zhang M. Tamoxifen induces the expression of maspin through estrogen receptor-alpha. Cancer Lett. 2004;209:55–65. doi: 10.1016/j.canlet.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–429. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynes MW, Konopa K, Singh S, Reyna-Asuncion B, Ranger-Moore J, Sternau A, et al. Thymidylate synthase protein expression by IHC and gene copy number by SISH correlate and show great variability in non-small cell lung cancer. J Thorac Oncol. 2012;7:982–992. doi: 10.1097/JTO.0b013e31824fe95a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDER Guidance for the Industry. Bioanalytical Method Validation. U.S.Department of Health and Human Services,Food and Drug Administration,Center for Drug Evaluation and Research and Center for Veterinary Medicine (CVM) 2001. Ref Type: Report.
- 23.Green D, McMahon B, Foiles N, Tian L. Measurement of hemostatic factors in EDTA plasma. Am J Clin Pathol. 2008;130:811–815. doi: 10.1309/AJCPRU5QLKLQ0OMS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsett M, Smith I, Robertson J, Robison L, Pinhel I, Johnson L, et al. Endocrine therapy, new biologicals, and new study designs for presurgical studies in breast cancer. J Natl Cancer Inst Monogr. 2011;2011:120–123. doi: 10.1093/jncimonographs/lgr034. [DOI] [PubMed] [Google Scholar]
- 25.Barker S, Malouitre SD, Glover HR, Puddefoot JR, Vinson GP. Comparison of effects of 4-hydroxy tamoxifen and trilostane on oestrogen-regulated gene expression in MCF-7 cells: up-regulation of oestrogen receptor beta. J Steroid Biochem Mol Biol. 2006;100:141–151. doi: 10.1016/j.jsbmb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Umekita Y, Yoshida H. Expression of maspin is up-regulated during the progression of mammary ductal carcinoma. Histopathology. 2003;42:541–545. doi: 10.1046/j.1365-2559.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 27.Allen KE, Clark ER, Jordan VC. Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol. 1980;71:83–91. doi: 10.1111/j.1476-5381.1980.tb10912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981;256:859–868. [PubMed] [Google Scholar]
- 29.Robertson DW, Katzenellenbogen JA, Long DJ, Rorke EA, Katzenellenbogen BS. Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem. 1982;16:1–13. doi: 10.1016/0022-4731(82)90137-6. [DOI] [PubMed] [Google Scholar]
- 30.Mauvais-Javis P, Baudot N, Castaigne D, Banzet P, Kuttenn F. trans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breast. Cancer Res. 1986;46:1521–1525. [PubMed] [Google Scholar]
- 31.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 32.Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 34.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyltamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 35.Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–512. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- 36.Lee O, Ivancic D, Chatterton RT, Rademaker A, Khan SA. In vitro human skin permeation of endoxifen: potential for local transdermal therapy for primary prevention and carcinoma in situ of the breast. Breast Cancer:Targets and Therapy. 2011;3:61–70. doi: 10.2147/BCTT.S20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonning PE, Lien EA, Lundgren S, Kvinnsland S. Clinical pharmacokinetics of endocrine agents used in advanced breast cancer. Clin Pharmacokinet. 1992;22:327–358. doi: 10.2165/00003088-199222050-00002. [DOI] [PubMed] [Google Scholar]
- 38.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, et al. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res. 2004;10:2336–2343. doi: 10.1158/1078-0432.ccr-03-0538. [DOI] [PubMed] [Google Scholar]
- 39.Ellmen J, Hakulinen P, Partanen A, Hayes DF. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat. 2003;82:103–111. doi: 10.1023/B:BREA.0000003957.54851.11. [DOI] [PubMed] [Google Scholar]
- 40.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 41.Cosman F, Baz-Hecht M, Cushman M, Vardy MD, Cruz JD, Nieves JW, et al. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res. 2005;116:1–13. doi: 10.1016/j.thromres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 43.Cosman F, Baz-Hecht M, Cushman M, Vardy MD, Cruz JD, Nieves JW, et al. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res. 2005;116:1–13. doi: 10.1016/j.thromres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 45.Mauvais-Javis P, Baudot N, Castaigne D, Banzet P, Kuttenn F. trans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breast. Cancer Research. 1986;46(3):1521–5. [PubMed] [Google Scholar]
- 46.Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk [see comments] J Natl Cancer Inst. 1998;90:37–42. doi: 10.1093/jnci/90.1.37. [DOI] [PubMed] [Google Scholar]
- 48.Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson NSB, Gazet JC, et al. Estrogen and progesterone receptors in the normal female breast. Cancer Res. 1991;51:1817–1822. [PubMed] [Google Scholar]
- 49.Povoski SP, Olsen JO, Young DC, Clarke J, Burak WE, Walker MJ, et al. Prospective Randomized trial comparing intradermal, intraparenchymal, and subareolar injection routes for sentinel lymph node mapping and biopsy in breast cancer. Ann Surg Oncol. 2006;13:10–11. doi: 10.1245/s10434-006-9022-z. [DOI] [PubMed] [Google Scholar]
- 50.Klimberg VS, Rubio IT, Henry R, Cowan C, Colvert M, Korourian S. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg. 1999;229:860–864. doi: 10.1097/00000658-199906000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]