Abstract
Background
While fat grafting can address many soft tissue deficits, results remain inconsistent. In this study, we compared physical properties of fat following injection using an automated, low shear device or the modified Coleman technique.
Methods
Lipoaspirate was obtained from nine patients and processed for injection using either a modified Coleman technique or with an automated, low shear device. Fat was passed through a 2 mm cannula and compared to minimally processed fat. A rheometer was used to measure the storage modulus and shear rate at which tissues began to lose their solid-like properties. Viscosity was also measured and gross properties of treatment groups were qualitatively evaluated with a glass slide test.
Results
Fat injected through an automated, low shear device closely matched physical properties of minimally processed fat. The storage modulus (G′) of fat for the device group was greater than the modified Coleman group and the onset of breakdown was delayed. Similarly, viscosity measurement of fat from the automated device closely matched minimally processed fat and was greater than the modified Coleman group.
Conclusions
The physical properties of lipoaspirate processed using an automated, low shear device with a 2 mm cannula preserved the intactness of fat more than the modified Coleman technique. Our rheological data demonstrate less damage using an automated device compared to modified Coleman technique and potentially support its use for improved fat graft integrity.
Keywords: fat grafting, fat transfer, tissue regeneration, shear, rheology, storage modulus, loss modulus, viscoelastic properties
Introduction
Since its original description in 1893 by Neuber, plastic surgeons have struggled to elucidate the optimal method for fat grafting (1, 2). Its ever growing popularity stems from its potential as an ideal soft tissue filler for treating cosmetic, traumatic, and reconstructive deficits (2, 3). Nonetheless, fat grafting remains a mercurial technique, and the onus persists to define an optimal method for harvesting, processing, and injecting fat that produces the most effective and consistent results (2–4).
The current problem with fat grafting centers about its lack of reproducibility and inconsistent results, with fat resorption shown to be anywhere from 20 to 90% (2, 3, 5). Much has previously been published on fat harvesting and processing, however, comparatively little evidence exists to inform surgeons on fat reinjection. There is no study that we are aware of that evaluates the biomechanical properties of fat after processing and injection with mainstream techniques such as the modified Coleman procedure (3, 6). We know that fat plays an important role in load transfers during body movements and exercise, but most of the mechanical studies of fat have centered around breast and gluteal tissue, and not isolated fat (6).
The significant effect that physical biomechanics exert on fat has been firmly established. We know that size can matter, given previous work that has shown larger cannulas cause less shear. Shear stress arises when a force acts parallel to a surface, rather than perpendicular to it. For example, rubbing your hands together induces a shear stress, while pressing your palms into each other does not. For fat injections, shear stress is exerted on the fat as it is pushed through tubing, such as a syringe barrel or cannula. In one study, a large 2.5 mm cannula used for injection significantly improved adipocyte viability when compared to a smaller 1.6 mm one; however, other studies have shown no significant differences following injection with 14-, 16-, and 20-gauge needles (2, 7, 8). Previous work also suggests that using needles for injection may be more harmful to fat than using cannulas because of potentially greater shear stress induced (5). Lee et al., corroborating the work of Shiffman and Mirrafati, demonstrated that fat is highly resistant to both negative and positive pressure when evaluated by weight and histology, and static pressure significantly damages fat only at extreme levels (9, 10). Shear stress, on the other hand, has been shown to be harmful to fat, with higher flower rates inducing more shear stress (9). Flow rates on the order of 0.5 to 1 cc/sec have thus been recommended to optimize fat viability (9). Importantly fat, as a viscoelastic material, undergoes shear-thinning which simply means that as shear stress increases, material viscosity decreases (11). Increasing strains on dynamic testing have been shown to break apart fat causing it to become less firm (11).
This study represents a companion to our other findings looking at the in vitro and in vivo differences between fat injected using the modified Coleman procedure or an automated, low shear device (Adipose Tissue Injector, ATI). In those studies, we showed significantly higher fat viability in vitro and significantly more fat retention in vivo when using the device as compared to modified Coleman technique. In order to investigate what might explain those findings, in this study we focused on the processing and injection aspects of fat transfer, with analysis on changes to material properties of fat. Specifically, we studied the viscoelastic properties of fat after processing and injecting with the modified Coleman technique versus using the automated device. In both cases, the injected fat underwent laminar flow due to the low flow rate and the small diameters of the components tested, such as syringes, cannulas, and ATI device tubing. Our results underscore the importance of minimally traumatic fat transfer that, when respected, will maximize integrity of grafted fat. Automation provides for a higher level of control in fat grafting and represents an evolution in the process of fat transfer by offering a high-throughput method for processing and injecting fat in a way that may minimize mechanical damage.
Methods
Fat Harvesting and Processing
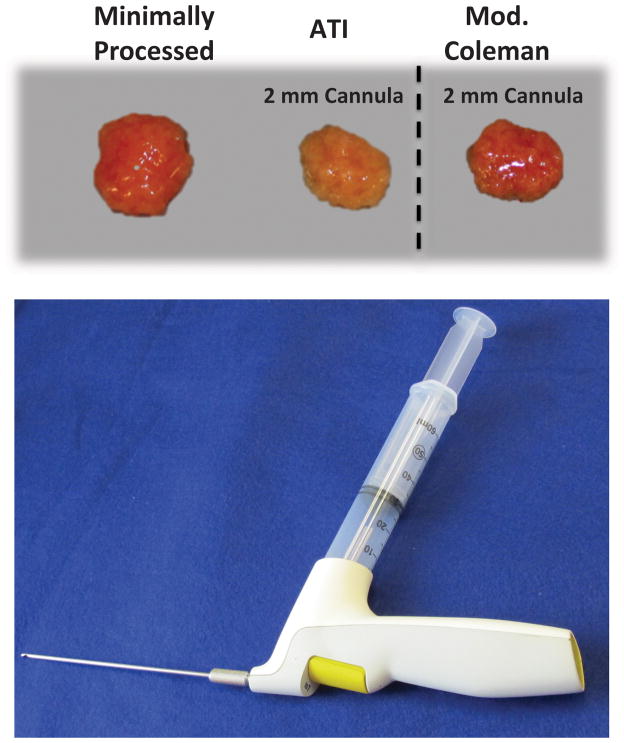
Lipoaspirate and abdominoplasty samples were obtained from nine healthy donors after acquiring informed consent, in accordance with Stanford University Institutional Review Board guidelines. All lipoaspirate samples were obtained by suction assisted liposuction. Both the ATI device and modified Coleman method were applied to each patient’s fat allowing for direct comparison and elimination of differences resulting from harvest method or surgeon preferences. Fat samples were allowed to separate by gravity sedimentation, and then the liquid oil portion was decanted and the blood was suctioned off. Lipoaspirate was then separated into three groups. For the minimally processed group, fat was transferred directly into a 50 cc conical tube using a large caliber 25 cc tip on a serological pipette. For the remaining two groups, fat was transferred in the same manner into a 60 cc syringe. For the ATI device group, the 60 cc syringe was attached to the injector with a 2 mm cannula. For the modified Coleman group, a three way Luer-Lock stop-cock was used to transfer the fat sequentially from the 60 cc syringe to a 10 cc syringe to a 1 cc syringe, and then the fat was also injected through a 2 mm cannula (Figure 1A). Finally, in addition to the three prior groups, abdominoplasty fat was obtained and sectioned with scissors into approximately 6 cm by 6 cm by 2 cm pieces and placed in culture dishes. Fat was then transferred immediately for rheological testing.
Figure 1.
Processing of Fat. A) Photograph of processed fat from three groups including minimally processed, ATI with 2 mm cannula, and modified Coleman with 2 mm cannula. B) Photograph of Adipose Tissue Injector with 60 cc reservoir syringe and 2 mm cannula attached.
Adipose Tissue Injector
The ATI device is an easy to hold, sterile, single use battery powered device designed and produced by the TauTona group (Menlo Park, CA). Its outlet port is a Luer-Lock design made to fit standard commercially available syringes and cannulas. Its inlet port similarly is a Luer-Lock designed to accept and stably hold a 60 cc syringe. Pressing the large yellow button at the back of the device sets the device in “slow” mode. In this mode, each press of the trigger delivers fat in precise 400 μL aliquots, and fat is simultaneously drawn from the reservoir syringe into the device to refill its internal pump. Holding the trigger delivers continuous fixed 400 μl aliquots of lipoaspirate at a controlled flow rate of 0.5 cc per second until the trigger is released. Flow rate of delivery on the ATI may be increased to 1 cc per second by pressing the large yellow back button a second time; however, this was not used in this study (Figure 1B).
Dynamic Oscillatory Rheology Testing
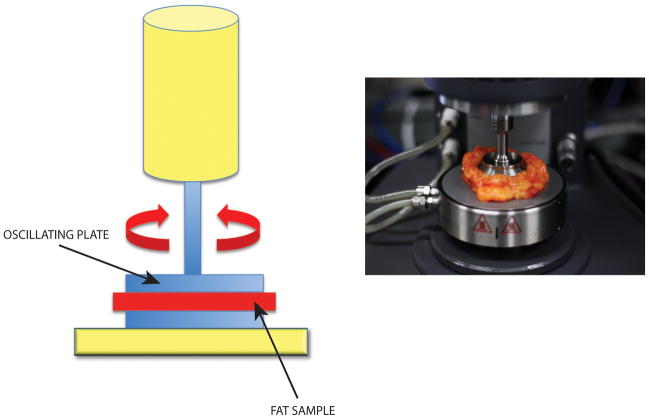
Experiments were performed on an ARG2 rheometer (TA Instruments, New Castle, DE) with a parallel plate geometry (40 mm diameter). The rheometer stage was held at 37°C and all samples were allowed to equilibrate for five minutes prior to the start of testing. Abdominoplasty samples were warmed to 37°C in an incubator for a minimum of 15 minutes prior to testing. The gap size was set based on the thickness of the sample. Lipoaspirate samples were characterized with a gap size of 1 mm. Samples were analyzed with rate sweeps going from 0.1 to 100 s−1 and oscillatory stress of 0.2 Pa (n ≥ 3 per patient per sample type, three patients tested) (Figure 2A and B). Storage modulus (G′) values, which quantify the elastic-like stiffness of a material, were reported at 1 s−1. Loss modulus (G″), which measures the viscous-like component of a material, was reported by taking a five-point moving average of values. The rate at which G″ increased by 20% compared to its previous value was defined as the breakdown point at which material behaved less solid-like and more liquid-like.
Figure 2.
Rheological Testing. A) Schematic of rheometer with sample (red) placed between blue parallel oscillating plates. B) Photograph of pre-trimmed abdominoplasty fat specimen in rheometer.
Linear Shear Rheology Testing
For linear shear testing, the same ARG2 rheometer was utilized, and the viscosity of the samples was characterized under constant linear shear rates at low and high rates of 5 s−1 and 500 s−1. First, samples were subjected to 120 seconds of low rate shear, followed immediately by 60 seconds of high rate shear, then finally back to low rate shear for another 120 seconds.
Glass Slide Testing
A small aliquot of each sample was placed on a flat glass sheet. The glass sheet was then quickly turned up to 60 degrees elevation and lipoaspirate samples were observed with continuous picture capture using a Cannon Rebel digital SLR.
Statistical Analysis
Means and standard deviations were calculated from numerical data. Bar graphs represent means and one standard deviation is depicted by error bars. A one-way ANOVA for multiple group comparison was used for statistical analysis. A *p-value < 0.05 was considered statistically significant.
Results
Effects of Processing on Storage/Loss Modulus of Fat
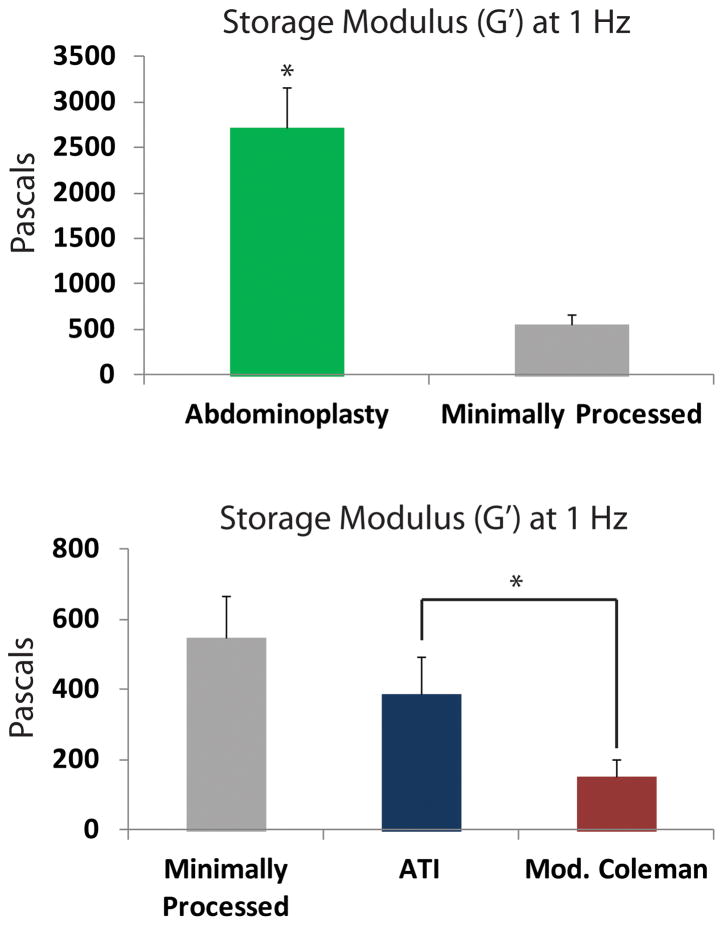
The storage modulus (G′), reflecting stiffness of fat, was measured to see how mechanical properties were affected by injection techniques. As expected, the storage modulus of unprocessed fat obtained directly from an abdominoplasty specimen was significantly greater than minimally processed fat obtained through lipoaspiration (*p < 0.05) (Figure 3A). Importantly, G′ of the ATI device group most closely approximated the minimally processed group (p > 0.05) and was significantly higher than the modified Coleman group (*p < 0.05) (Figure 3B). The higher storage modulus from the ATI device group suggests that the fat remained more intact during processing and injection.
Figure 3.
Storage Modulus Testing. A) Storage modulus (G′) of abdominoplasty fat (green bar) was significantly greater than minimally processed fat (gray bar) (*p < 0.05). B) Storage modulus of fat from ATI (blue) was significantly greater than fat from modified Coleman (red) (*p < 0.05).
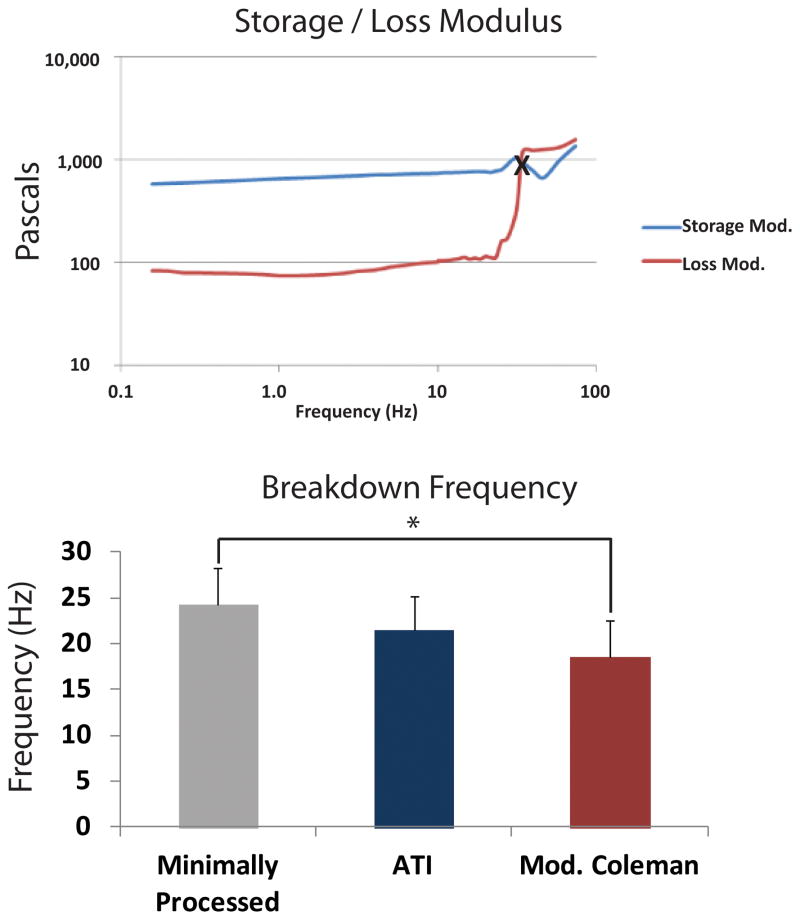
To further evaluate intactness of fat with the ATI device or modified Coleman technique, we measured the onset of sample breakdown. In particular, we determined the breakdown rate where the loss modulus (G″) was noted to increase by 20% over previous values. This value closely approximates the crossover point when a sample begins to behave less like a solid and more like a liquid. Therefore, higher rates for this point correspond to increased structural integrity of fat. A representative plot of minimally processed fat showing the storage and loss modulus as a function of rate is shown in Figure 4A. As expected, minimally processed fat had the highest rate for onset of breakdown, meaning it was able to withstand the highest shear rate before it began to break apart and behave more liquid-like (Figure 4B). Interestingly, breakdown rate for fat with the Adipose Tissue Injector was not statistically different from minimally processed lipoaspirate (p > 0.05) (Figure 4B). In contrast, fat from the modified Coleman technique had a lower breakdown rate, which was statistically less than minimally processed fat (*p < 0.05) (Figure 4B).
Figure 4.
Loss Modulus Testing. A) Representative chart of storage modulus (G′) (blue line) and loss modulus (G″) (red line) from minimally processed fat. Note the shear rate at the crossover point between the G′ and G″ data, marked by “X”, where the material behaves less solid-like and more liquid-like. B) Breakdown rate for fat from each group. The ATI group (blue bar) was similar to minimally processed fat (gray bar). Breakdown rate for modified Coleman (red bar) was significantly less than minimally processed fat (*p < 0.05).
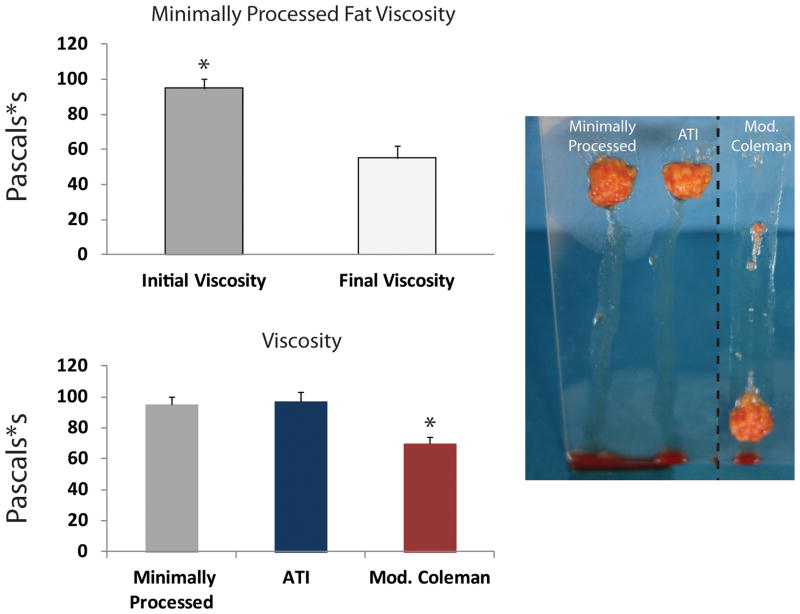
Effects of Processing on Viscosity of Fat
Shear-thinning materials demonstrate a decrease in viscosity with increasing shear rates. Some shear-thinning materials can “self-heal”, meaning that they fully recover their original viscosity after being exposed to high shear rates. To determine if fat possesses shear-thinning and self-healing properties, minimally processed lipoaspirate was subjected to constant linear shear stress at rates of 5 s−1 and 500 s−1. Viscosity was first measured at a low rate, then measured during a high rate, and finally measured again at a low rate. As shown in Figure 5A, minimally processed fat was found to have significantly higher original viscosity compared to the final viscosity after exposure to high shear rates. This demonstrates that exposing fat to high shear rates will result in irreversible damage, and the mechanical integrity of the fat cannot fully recover from this damage. Having thus confirmed lipoaspirate to be a shear-thinning but not self-healing material, viscosity was then measured for fat processed through the ATI device or modified Coleman technique. Interestingly, at a low rate, the viscosity of fat processed with the ATI device was similar to minimally processed fat (Figure 5B). Importantly, this was significantly greater than the measured viscosity for fat processed with the modified Coleman technique (*p < 0.05).
Figure 5.
Viscosity measurements. A) Original viscosity of minimally processed fat at a low shear rate (5 s−1) was significantly greater than the final viscosity after exposure to a high shear rate (500 s−1), showing that exposure to high shear rates results in irreversible damage. B) Final viscosity of fat processed with ATI (blue bar) was similar to minimally processed fat (gray bar), both of which were significantly greater than modified Coleman (red bar) (*p < 0.05). C) Qualitative glass slide testing showed less streaking from minimally processed fat and Adipose Tissue Injector compared to modified Coleman group.
Qualitative Glass Slide Evaluation of Viscosity
We found that our viscosity measurements were matched by a qualitative evaluation of fat intactness using a glass slide test. Minimally processed fat, fat processed using the ATI device, and fat processed using the modified Coleman technique were placed on the end of a glass slide. Once the slide was elevated to 60 degrees, fat specimens began sliding, leaving streaks of oil and fat particles behind (Figure 5C). Minimally processed fat and fat processed with the ATI device both slid the least. In comparison, fat processed with the modified Coleman technique resulted in far greater streaking, consistent with decreased fat integrity.
Discussion
With its abundance and biocompatibility, fat is considered a potentially ideal soft tissue filler; however, its use has been hampered by many unanswered questions. In a recent discussion of a study by Lee et al., Rubin highlighted several important variables which merit further study including the response of fat to shear stress, the impact of more “clinically relevant cannulas”, and a search “to find a balance for fat grafting technique that sits between rapid fire lipoaspirate injection and impossibly slow (or at least impractical) injection rates” (9, 12). Apropos to these concerns, we have previously evaluated the capability of the ATI device to enhance fat transfer viability, showing significantly greater fat viability and in vivo retention compared to the modified Coleman technique. This present study served to demonstrate the material properties of fat with each of these techniques and offers insight to why the ATI device is more “fat friendly”.
In order to determine how injection techniques affected lipoaspirate samples, we used rheology to characterize the physical properties of fat. Rheology is the study of material flow and is particularly useful for characterizing “soft” materials, which display properties somewhere between elastic solids and viscous liquids. Elastic solids are defined by their instantaneous deformation to an applied force and instantaneous return to their original geometry when that force is removed. Meanwhile, viscous materials undergo a time-dependent deformation. Soft, or “viscoelastic,” materials display both instantaneous and time-dependent responses to applied forces. The properties of viscoelastic materials can also change depending on how quickly a stress is applied to them. For example, Silly Putty® can bounce like a rubber ball when it is dropped, but it will also flatten under its own weight when left sitting on a surface for an extended period of time. The bouncing corresponds to elastic behavior on a short time scale, while the flattening corresponds to viscous flow on a long time scale. Along with fat, a myriad of other biological tissues are viscoelastic, including cartilage, ligaments, tendons, and muscle (13–15).
For our rheological studies, samples were pressed between a stage and a flat (parallel) plate that rotates and shears the sample. Based on how fast the plate rotates and how much rotational force is applied to the system, the physical properties of the sample are determined. With oscillatory testing, where the plate rotates back and forth at either a set rate or amplitude, viscoelastic mechanical properties of the sample can be measured. This includes the storage modulus, denoted G′, which measures the immediate response of a viscoelastic material to an applied stress (force/area). G′ quantifies the elastic-like stiffness of the material, and a greater G′ is associated with more sample stiffness. In contrast, the loss modulus, denoted G″, is a measure of the time-dependent response of the material to the applied stress and quantifies how much energy is lost. A rapid increase in G″ corresponds to a breakdown event where a material begins to lose its solid-like character and behaves more liquid-like. For example, ketchup in a bottle behaves like a solid, but after application of a force such as hitting the bottom of the bottle or stirring with a knife (when G″ increases sharply), it begins to have more like a liquid and flow. Thus, greater values for G′ and G″ are both indirect measures for intactness of fat.
Another property measured in this study was viscosity, which is essentially how “thick” the sample feels when you try to deform it. Of note, some materials may have interesting deviations in their viscosity and demonstrate the phenomenon of shear-thinning. When a material such as fat displays shear-thinning, its viscosity will decrease as it is strained (deformed) more rapidly. Ketchup is also a shear-thinning material. It has a very high viscosity at low shear rates, which is why it will not easily pour out of a bottle when the bottle is simply turned upside down. However, if you increase the strain rate of the ketchup by squeezing the bottle or tapping it on the base, its viscosity will decrease and it will flow much more easily.
By measuring material properties of fat, we observed that the ATI device was more “fat friendly” than the modified Coleman technique. Properties of fat processed using the ATI device more closely matched those of minimally processed lipoaspirate. This was reflected in a significantly greater storage modulus for the ATI device relative to the modified Coleman technique. A greater breakdown rate was also observed for the ATI compared to the modified Coleman group. Finally, viscosity measurements for both minimally processed fat and fat from the ATI device were significantly greater than the modified Coleman group. This was punctuated by our qualitative glass slide test where minimally processed and the ATI groups had far less streaking than the modified Coleman group.
From these data, we demonstrate that by understanding the mechanical properties of fat, strategies may be developed which better preserve fat. Given this principle, it is instructive to carefully examine each element of injection techniques to ensure that the lipoaspirate suffers the least trauma possible. Minimizing trauma to fat grafts by gentle handling of lipoaspirate and curtailing any unnecessary manipulation are critical cornerstones of optimal fat transfer technique. Automated devices, such as the ATI device, offer a novel and simple method to process and perform lipotransfer that may better respect the physical properties of fat and takes the guesswork out of an otherwise capricious technique. Ultimately, the device assists the surgeon by ensuring improved handling (i.e. low shear) of fat to optimize retention and clinical outcomes.
Acknowledgments
The authors thank Prof. Sarah Heilshorn, Department of Materials Science & Engineering, Stanford University, for use of rheometry equipment and helpful discussions.
Footnotes
Data presented in this manuscript have not been previously presented at any meeting
Author Contributions
D.A.A., J.R., M.T.C., A.P.A., G.C.G., D.C.W., and M.T.L. designed the study. D.A.A., J.R., M.T.C., A.P.A., K.P., A.Mc.A., K.S.Y., E.R.Z., R.T., C.D., and L.G. performed the study. D.A.A., J.R., A.P.A., M.T.C., A.M., G.G.W., and M.S.H. analyzed the results. D.A.A., J.R., A.P.A., G.C.G., M.T.L., and D.C.W. wrote the manuscript. B.D. and J.R.R. helped design the TauTona Device and provided the engineering expertise. L.G. provided the lipoaspirate used for the study material.
Financial Disclosure and Products Page
G.C.G. was supported by NIH grants R01 AG-25016, R01 DK-074095, and 1R01 HL104236-01. D.C.W. was supported by the ACS Franklin H. Martin Faculty Research Fellowship, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award. M.T.L. was supported by NIH grants R01 DE021683-01, RC2 DE020771, the Oak Foundation, and Hagey Laboratory for Pediatric Regenerative Medicine.
J.R.R. is the Vice President of Research and Development and Chief Operating Officer at TauTona Group. G.C.G and M.T.L. have equity in the TauTona Group. M.T.L. co-authored this manuscript while on sabbatical from Stanford University.
M.T.C., K.J.P., D.A.A., J.R., A.Mc.A., K.S.Y., E.R.Z., R.T., C.D., M.S.H., G.G.W., A.P.A., A.M., B.D., L.G., and D.C.W., have no financial interest or commercial association with any of the subject matter or products mentioned in our manuscript.
References
- 1.Coleman WP., 3rd The history of liposuction and fat transplantation in America. Dermatologic clinics. 1999;17:723–727. v. doi: 10.1016/s0733-8635(05)70121-2. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman MR, Bradley JP, Dickinson B, et al. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plastic and reconstructive surgery. 2007;119:323–331. doi: 10.1097/01.prs.0000244903.51440.8c. [DOI] [PubMed] [Google Scholar]
- 3.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plastic and reconstructive surgery. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 4.Bucky LP, Percec I. The science of autologous fat grafting: views on current and future approaches to neoadipogenesis. Aesthetic surgery journal/the American Society for Aesthetic Plastic surgery. 2008;28:313–321. doi: 10.1016/j.asj.2008.02.004. quiz 322-314. [DOI] [PubMed] [Google Scholar]
- 5.Smith P, Adams WP, Jr, Lipschitz AH, et al. Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plastic and reconstructive surgery. 2006;117:1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. [DOI] [PubMed] [Google Scholar]
- 6.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology. 2008;45:677–688. [PubMed] [Google Scholar]
- 7.Ozsoy Z, Kul Z, Bilir A. The role of cannula diameter in improved adipocyte viability: a quantitative analysis. Aesthetic surgery journal/the American Society for Aesthetic Plastic surgery. 2006;26:287–289. doi: 10.1016/j.asj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham JC, Lee JH, Medina MA, 3rd, McCormack MC, Randolph MA, Austen WG., Jr The impact of liposuction cannula size on adipocyte viability. Annals of plastic surgery. 2012;69:479–481. doi: 10.1097/SAP.0b013e31824a459f. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG., Jr The effect of pressure and shear on autologous fat grafting. Plastic and reconstructive surgery. 2013;131:1125–1136. doi: 10.1097/PRS.0b013e3182879f4a. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2001;27:819–826. doi: 10.1046/j.1524-4725.2001.01062.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel PN, Smith CK, Patrick CW., Jr Rheological and recovery properties of poly(ethylene glycol) diacrylate hydrogels and human adipose tissue. Journal of biomedical materials research Part A. 2005;73:313–319. doi: 10.1002/jbm.a.30291. [DOI] [PubMed] [Google Scholar]
- 12.Kokai L, Rubin JP. Discussion: the effect of pressure and shear on autologous fat grafting. Plastic and reconstructive surgery. 2013;131:1137–1138. doi: 10.1097/PRS.0b013e3182899124. [DOI] [PubMed] [Google Scholar]
- 13.Pearson B, Espino DM. Effect of hydration on the frequency-dependent viscoelastic properties of articular cartilage. Proc Inst Mech Eng H. 2013 doi: 10.1177/0954411913501294. [DOI] [PubMed] [Google Scholar]
- 14.Davis FM, De Vita R. A nonlinear constitutive model for stress relaxation in ligaments and tendons. Ann Biomed Eng. 2012;40:2541–2550. doi: 10.1007/s10439-012-0596-2. [DOI] [PubMed] [Google Scholar]
- 15.Gras LL, Mitton D, Viot P, Laporte S. Viscoelastic properties of the human sternocleidomastoideus muscle of aged women in relaxation. J Mech Behav Biomed Mater. 2013;27:77–83. doi: 10.1016/j.jmbbm.2013.06.010. [DOI] [PubMed] [Google Scholar]