Abstract
Introduction
Lymph node transplantation is a promising surgical technique for the treatment of lymphedema. However, while initial clinical results have been largely promising, inconsistent responses have been reported in some cases. While the cause of this inconsistency remains unknown, it is likely that impaired lymphangiogenesis and spontaneous regeneration of lymphatic vessels in the transplanted lymph nodes may be a contributing factor suggesting that development of novel techniques to augment lymphangiogenesis may be clinically useful. The aim of this study was therefore to determine if sterile inflammatory reactions can serve as a physiologic means of augmenting lymphangiogenesis in transplanted lymph nodes using a murine model.
Methods
We used our previously reported model of lymph node transfer to study the effect of sterile inflammation on lymphatic regeneration. Mice were divided into 3 groups: Group 1 animals served as controls and underwent lymphadenectomy followed by immediate lymph node transplantation without inflammation. Group 2 animals (inflammation before transfer) were transplanted with lymph nodes harvested from donor animals in which a sterile inflammatory reaction was induced in the ipsilateral donor limb using complete Freund’s adjuvant and ovalbumin (CFA/OVA). Group 3 animals (inflammation after transfer) were transplanted with lymph nodes and then inflammation was induced in the ipsilateral limb using CFA/OVA. Lymphatic function, lymphangiogenesis, and lymph node histology were examined 28 days after transplant and compared with normal lymph node.
Results
Animals that had sterile inflammation after transplantation (group 3) had significantly improved lymphatic function (>2 fold increase) as assessed by lymphoscintigraphy, increased peri-nodal lymphangiogenesis, and functional lymphatics as compared with no-inflammation or inflammation before transplant groups (p<0.01). In addition, inflammation after transplantation was associated a more normal lymph node architecture, expansion of B cell zones, and decreased percentage of T cells as compared with the other experimental groups.
Conclusion
Sterile inflammation is a potent method of augmenting lymphatic function and lymphangiogenesis after lymph node transplantation and is associated with maintenance of lymph node architecture. Induction of inflammation after transplant is the most effective method and promotes maintenance of normal lymph node B and T cell architecture.
Keywords: Lymph node, transfer, lymphatic regeneration, lymphatic flow, lymphedema
Introduction
Lymphedema is a morbid disease that afflicts a significant number of patients who undergo lymph node dissection for cancer treatment. Although the disease course is highly variable, in most patients lymphedema is progressive and requires lifelong physical treatments such as massage and compression garments aiming to control swelling. These palliative treatments are helpful in many cases; however they are labor intensive, expensive, and a significant source of decreased quality of life for patients.(1) More importantly, physical treatments are not curative and most patients have progressive disease if treatments are halted.
Over the years, a number of surgical procedures have been developed by plastic surgeons in an effort to treat lymphedema. In recent years, the advent of supermicrosurgical techniques has spurred interest in physiologic procedures that aim to repair damaged lymphatic networks and restore lymphatic circulation. For example, several authors have reported their experience with lymph node transfers in which healthy lymph nodes are transferred to the lymphedematous extremity and re-vascularized using microvascular techniques. (2, 3) Lymphatic regeneration to the transferred lymph node occurs spontaneously and a few retrospective studies have reported excellent outcomes with these procedures.(2, 3) However, while these results are promising, others have anecdotally reported inconsistent and unpredictable outcomes with some patients showing excellent results while others demonstrating seemingly little to no benefit.
Although the precise cause of variable outcomes after lymph node transfers remains unknown, impaired or inadequate lymphangiogenesis to the transferred lymph nodes is likely to be a contributing factor. This hypothesis is supported by recent experimental studies in ovine, porcine, and murine models that have reported improved lymphatic regeneration if lymph node transfer is performed in conjunction with exogenous delivery of lymphangiogenic growth factors such as vascular endothelial growth factor-C (VEGF-C). (4-6) However, while this approach is intuitively attractive and seemingly has important clinical relevance, this approach has not gained widespread adoption in cancer survivors since these molecules (e.g. VEGF-C) are known to be important regulators of tumor growth and metastasis.(7-11) Thus, development of novel techniques that improve lymphangiogenesis after lymph node transfer without delivery of exogenous lymphangiogenic cytokines is a clinically relevant and worthwhile goal.
We recently developed a mouse model of lymph node transfer that has enabled us to analyze the cellular and molecular mechanisms that regulate lymphatic regeneration after this procedure.(12) Using a novel lymphatic reporter mouse, we have shown that lymphatic regeneration after lymph node transfer occurs spontaneously, results in reconnection of lymphatic vessels from the donor site to the afferent and efferent vessels surrounding the lymph node, and that this process is associated with endogenous expression of lymphangiogenic cytokines such as VEGF-C. In addition, in other studies we have shown that sterile local inflammatory reactions significantly increase lymphangiogenesis in tissues and draining lymph nodes.(13-16) Therefore, with this background in mind, we hypothesized that induction of sterile inflammation in conjunction with lymph node transfer would significantly increase lymphangiogenesis, increase spontaneous lymphatic reconnection between donor and recipient tissues, and result in improved lymphatic function as compared with lymph node transfer without inflammation. Further, we hypothesized that the timing of inflammation, either in the donor region prior to lymph node harvest or in the recipient location after transfer would have a significant effect on these outcomes. We report that induction of sterile inflammation after lymph node transfer markedly increases lymphangiogenesis around the lymph node, increases lymphatic drainage function, and maintains the normal cellular architecture of the transferred lymph node when compared to lymph node transfers without sterile inflammation or if inflammation was induced prior to transfer. Taken together, these data suggest that sterile inflammation may be a clinically useful means of improving lymphangiogenesis in transferred lymph nodes without the use of exogenous lymphangiogenic cytokine delivery.
Methods
Animals
All procedures were approved by the IACUC at Memorial Sloan-Kettering Cancer Center. Adult male C57/BL6 mice (10-12 weeks) were purchased from Jackson Labs (Bar Harbor, Maine), housed in temperature and light controlled environments, and fed a standard diet.
Induction of Sterile Inflammation and Lymph Node Transfer
We have previously shown that injection of complete Freund’s Adjuvant (CFA) and 2% ovalbumin (OVA; both from Sigma-Aldrich, St. Louis, MO) into the subcutaneous tissues of the forepaw reliably results in lymphangiogenesis in the draining axillary lymph node beginning 3 days after injection and peaking by days 7-14.(13-16) In addition, this treatment is well tolerated without significant systemic morbidity or toxicity. Immune adjuvants such as CFA are commonly used in vaccination since the local sterile inflammation that is produced in response to these agents acts to augment the potential for immunization.(17-19) This effect is mediated by multiple mechanisms including increasing the number of antigen presenting cells locally and augmentation of lymph node lymphangiogenesis. Lymph node lymphangiogenesis in response to sterile inflammation is regulated by changes in both pro- and anti-lymphangiogenic cytokine expression and is known to play an important physiological role in promoting migration of antigen presenting cells from the skin to the lymph node and enabling interaction of these cells with T and B cells within the node.(20-22) Thus, sterile inflammation in response to adjuvants such as CFA is a useful physiologic method of augmenting lymphangiogenesis.
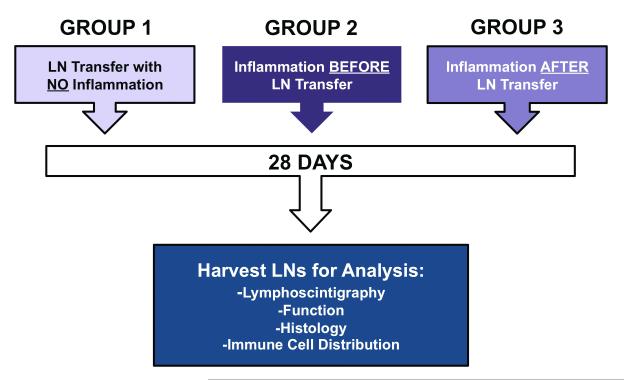
In order to test the hypothesis that the timing of CFA/OVA induced sterile inflammation can regulate lymphangiogenesis and lymphatic function after lymph node transfer, we divided mice into 3 groups (n=5-8/group); Figure 1). Group 1 animals (no inflammation) served as controls and had no CFA/OVA injection. In these animals, the right axillary and brachial lymph nodes and the axillary fat pads were surgically resected and simultaneously replaced by lymph nodes harvested from syngeneic donor animals. Group 2 animals (inflammation before transfer) tested the hypothesis that inflammation in the donor animal increases lymphatic repair. In this group, donor animals were vaccinated in the forepaw with 20 μl of a 1:1 mixture of CFA and 2% OVA and then 14 days later (i.e. at the peak of lymph node lymphangiogenesis), axillary lymph nodes were harvested and implanted in syngeneic mice in which the axillary/brachial lymph nodes had been resected. Finally, Group 3 animals (inflammation after transfer) tested the hypothesis that sterile inflammation after transplantation improves lymphangiogenesis and function. In these animals, brachial and axillary lymph nodes were excised as outlined above and replaced by lymph nodes harvested from donor animals. The ipsilateral forepaw was then injected with CFA/OVA 14 days after lymph node transplant. As an additional control, all animals underwent an incision of the left axillary area without axillary lymph node dissection (sham incision controls). Animals in all groups were sacrificed on post-transfer day 28 and analyzed as outlined below.
Figure 1.
Schematic diagram of experimental groups.
Analysis of Lymphatic Function
We used our previously described methods for lymphoscintigraphy to quantify interstitial fluid transport and lymph node uptake.(12) Briefly, 28 days following surgery, 50 μl of Technetium99 (99mTc) labeled sulfur colloid was injected into the distal forearms of experimental mice bilaterally and uptake of the radioactive colloid in the axillary lymph nodes in both axilla was assessed with an X-SPECT camera (Gamma Medica, Northridge, CA) for 90 minutes (n=4/group). Region-of-interest analysis was performed using ASIPro software (CTI Molecular Imaging, Knoxville, TN) and maximum nodal uptake was analyzed.
Histology
In order to identify functional lymphatic vessels in transferred lymph nodes, we injected the distal forearms of all animals with 10 μl ferritin from equine spleen (Sigma) 60 minutes prior to sacrifice. This technique enables us to identify functional lymphatics since the iron III component of ferritin is transported selectively by the lymphatic vessels and can be visualized with Prussian blue staining after fixation (see below) using 5% potassium ferrocyanide and 10% hydrochloric acid solution. (23) (12, 24) Functional lymphatics visualized using this technique stain blue and are easily identified. Images were captured using a Mirax slide scanner (Carl Zeiss).
After sacrifice, the transferred and sham-operated control lymph nodes were quickly harvested together with the perinodal fat, briefly fixed in 2% paraformaldehyde/0.2% gluteraldehyde, embedded in paraffin, and sectioned at a thickness of 5 μm. For immunohistochemistry, sections were permeabilized in Triton-X, blocked in appropriate secondary serum and stained with monoclonal or polyclonal antibodies (see below) using our previously published methods.(25)
To determine how sterile inflammation altered infiltration of lymphatic vessels into the transferred lymph nodes, we performed immunohistochemical localization of lymphatic vessel endothelial hyaluronan (LYVE-1) in the transferred lymph nodes and perinodal fat using a monoclonal anti-LYVE-1 antibody (R&D Systems, Minneapolis, MN).(12) LYVE-1 is a specific marker of lymphatic endothelial cells and enables us to visualize lymphatic vessels. The number of LYVE-1+ vessels/high powered field (40X magnification; 3 high powered fields per animal) was quantified in each group (n=4-6/group) in the perinodal fat by 2-blinded reviewers.
We have previously shown that T and B cells survive in the lymph node after lymph node transfer in the mouse model. (12) However, in our previous study we noted that although these cells survived, the follicular structure and T/B cell distribution patterns of the transferred lymph nodes were abnormal. Therefore, to test the hypothesis that sterile inflammation can regulate lymph node architecture after lymph node transfer, we localized T and B cells using primary antibodies against CD3 (stains all T cells; Abcam, Cambridge, MA) and B220 (stains all B cells; R&D, Minneapolis, MN) using our previously published methods.(25) Staining was visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB; Dako, Carpinteria, CA) (19). Percentage of T and B cells as a function of total lymph node area was quantified using Metamorph software (Molecular Devices Corp., Sunnyvale, CA).
Statistical Analysis
All data are presented as mean ± standard deviation with p<0.05 considered significant. A minimum of 5-8 animals per group was used for all experiments. Comparison of two groups was performed using Student’s t-test and comparison of multiple groups was performed using ANOVA with Tukey-Cramer post hoc tests to compare all values.
Results
Sterile inflammation increases interstitial flow and lymph node uptake after lymph node transfer
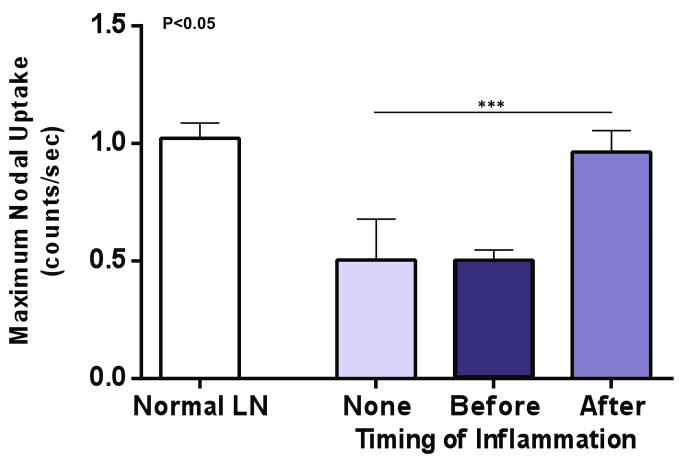
We have previously shown that lymphoscintigraphy with 99mTc labeled colloid is a sensitive method that can be used to analyze lymphatic flow and lymph node uptake.(12) Consistent with our previous study, we found that lymph node transfer without inflammation resulted in some recovery of lymphatic function and lymph node uptake of 99mTc 28 days after transfer (Figure 2). However, analysis of animals in which inflammation was induced in the lymph node after transfer, demonstrated that this approach was the most effective means of restoring interstitial flow and lymph node uptake resulting a peak nodal uptake of 99mTc that was essentially the same as a control non-operated lymph node. Although this increase was not statistically significant as compared with the no-inflammation group, the increase in nodal uptake was significantly higher than that observed in the inflammation before transfer group.
Figure 2. Sterile inflammation increases interstitial flow and lymph node uptake after lymph node transfer.
99mTc colloid lymphoscintigraphy in the various experimental groups demonstrating increased uptake of 99mTc if sterile inflammation is induced after lymph node transfer versus lymph node transfers without inflammation or induction of inflammation prior to transfer.
Sterile inflammation after lymph node transfer improves lymphatic function
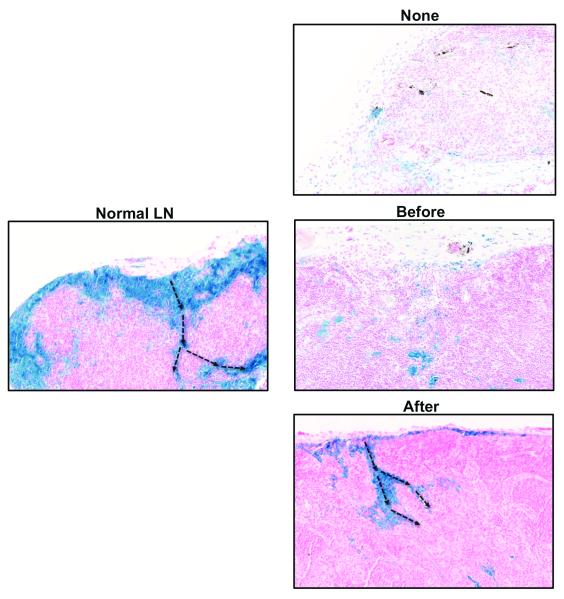
Prussian blue staining of lymph nodes in the various animal groups confirmed our lymphoscintographic findings and demonstrated increased numbers of functional lymphatic vessels in group 3 animals (i.e. inflammation after lymph node transfer). In these animals, functional lymphatic vessels could easily be seen entering the lymph node capsule and extending along the hilum (Figure 3). In contrast, in animals that had no inflammation, or in which inflammation was induced prior to transfer, we found fewer and more disorganized lymphatic channels suggesting that functional regeneration in these animals had not been completed by the 28-day time point.
Figure 3. Sterile inflammation after lymph node transfer improves lymphatic function.
Representative histological images (20x magnification) of ferritin staining in lymph nodes harvested from animals in various experimental groups (blue stain). Note subcapsular pattern of staining and drainage into medullary area in normal lymph node and animals in which inflammation was induced after lymph node transfer (black arrows show direction of flow). Note relative lack of staining in lymph nodes without inflammation or in those in which inflammation was induced prior to tissue transfer.
Inflammation after lymph node transfer increases peri-nodal lymphangiogenesis
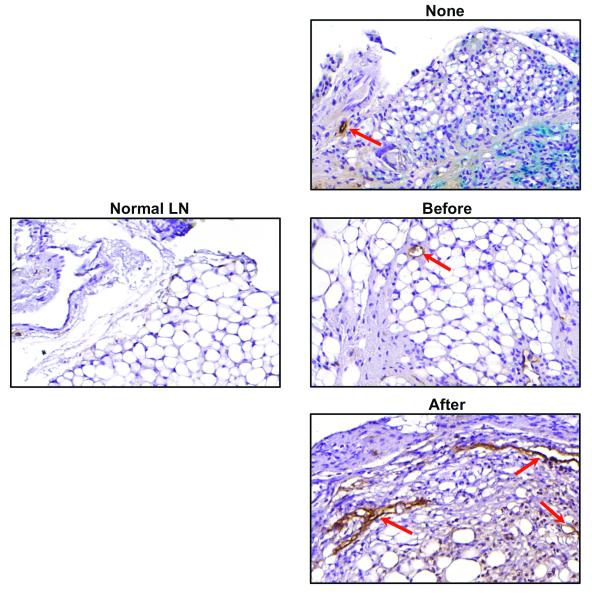
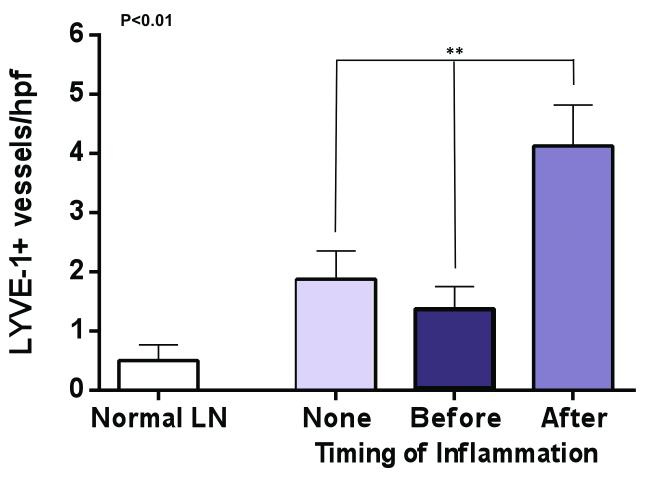
Consistent with out previous study, we found that lymph node transfer was associated with lymphangiogenesis in the perinodal fat as compared with a normal lymph node (Figure 4A, B). Our analysis demonstrated only sparse numbers of LYVE-1+ vessels in the perinodal fat of a normal lymph node consistent with previous studies demonstrating little evidence of lymphangiogenesis at a quiescence state.(21) In contrast, animals treated with lymph node transplant without inflammation had a more than 3-fold increase in the number of LYVE-1+ vessels surrounding their lymph node (p<0.05). Interestingly, inflammation before lymph node transplant appeared to decrease the number of LYVE-1+ vessels in this area as compared with the no inflammation group, however, this difference was not statistically significant. More importantly, we found that inflammation after transfer was associated with an even more robust increase in lymphangiogenesis as compared with lymph node transfers performed without inflammation (2.2 fold increase in LYVE-1+ vessel counts) or compared with normal lymph nodes (8 fold increase). Taken together, these findings suggest that sterile inflammation after lymph node transfer can more than double lymphangiogenesis around the lymph node as compared to lymph node transfers performed without sterile inflammation.
Figure 4. Inflammation after lymph node transfer increases peri-nodal lymphangiogenesis.
A. Representative photomicrographs (20x magnification) of LYVE-1 immunohistochemistry (red arrows) in normal lymph node as well as in lymph nodes transferred with no inflammation, or inflammation induced before or after transfer.
B. Quantification of LYVE-1+ vessels in perilymphatic fat of lymph nodes in the various experimental groups. Note significant increase in animals from the inflammation after transfer group.
Inflammation after lymph node transfer preserves B cell follicular architecture
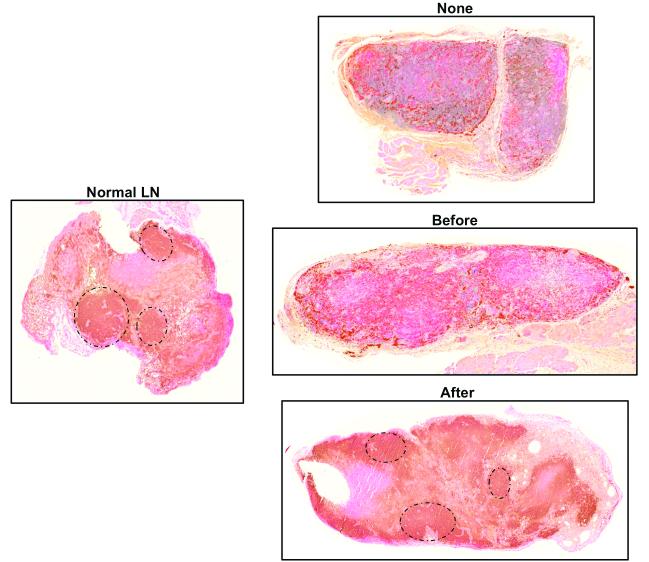
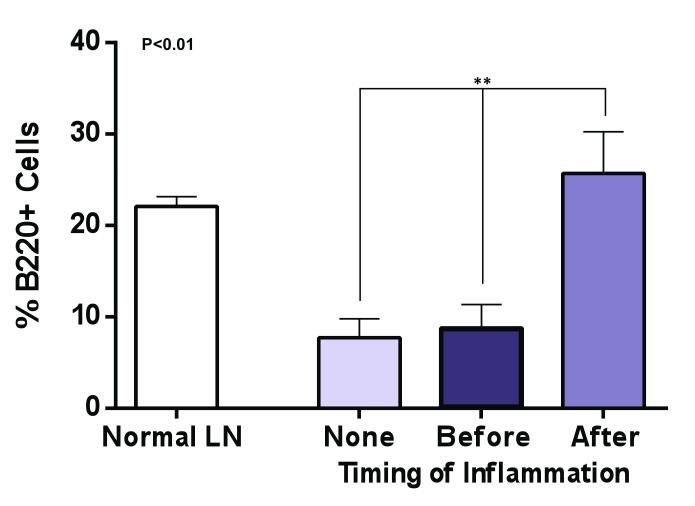
In our previous study on lymph node transfers in a murine model, we found that although the B and T cell populations of the lymph node survived lymph node transfer, the architecture of the transplanted lymph node was not normal with loss of B cell follicle structure and localization of T cells in the cortical regions.(12) Because the B cell follicle structure is a critical regulator of adaptive immune functions (i.e. vaccination, memory responses), this finding suggested that the transplanted lymph nodes were not normal in their immunologic responses. Our findings in the current study were consistent with this observation (Figure 5A) again demonstrating loss of follicular patterning and T cell expansion in the no inflammation transfer group. Likewise, we found that lymph nodes that had inflammation before transfer also had similar abnormal architecture although these nodes were noticeably larger than the no-inflammation transfer group. In contrast, we were surprised to find that transferred lymph nodes harvested from the inflammation-after group not only had more robust staining for B cells but also a follicular pattern in their distribution similar to the normal lymph node suggesting that these transferred lymph nodes had more normal immunological function.
Figure 5. Inflammation after lymph node transfer preserves B cell follicular architecture.
A. Representative photomicrographs (5x magnification) of lymph nodes from various groups stained for B220, a B cell marker. Note follicular pattern of B cell clusters in normal lymph node and in lymph node harvested from animals in which inflammation was induced after transfer (black circles).
B. Quantification of B cells (percentage of B cells as a function of lymph node area). Note significant increase in B cells in inflammation after transfer group as compared with no inflammation or inflammation before transfer groups.
Quantification of B cell populations in the transferred lymph nodes also suggested that inflammation-after transfer may be more efficacious in restoring immune function as compared with no inflammation or inflammation-before transfer because we found a more than 2-fold increase in the number of B cells in this group (Figure 5B). In fact, the percentage of the lymph node comprised of B cells in the inflammation-after transfer was nearly identical to a normal lymph node. This finding is important not only for the potential immunologic function of the transferred lymph node but may also provide a rationale for increased lymphangiogenesis in this group since B cells are a known major source of VEGF-A and VEGF-C in lymph node lymphangiogenesis.(21, 22)
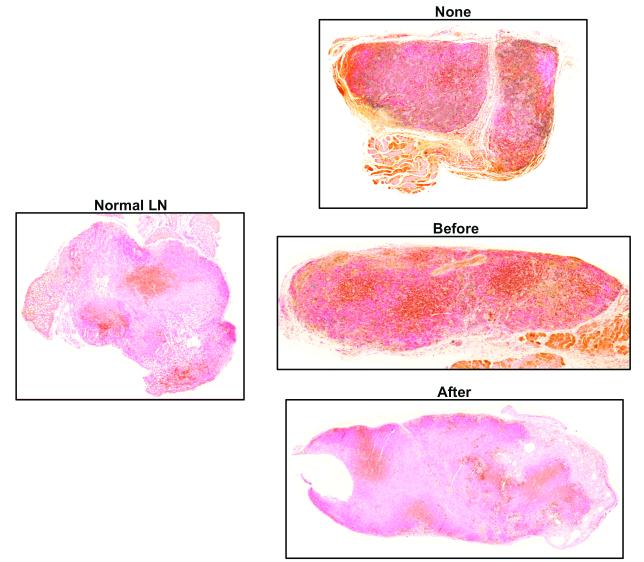
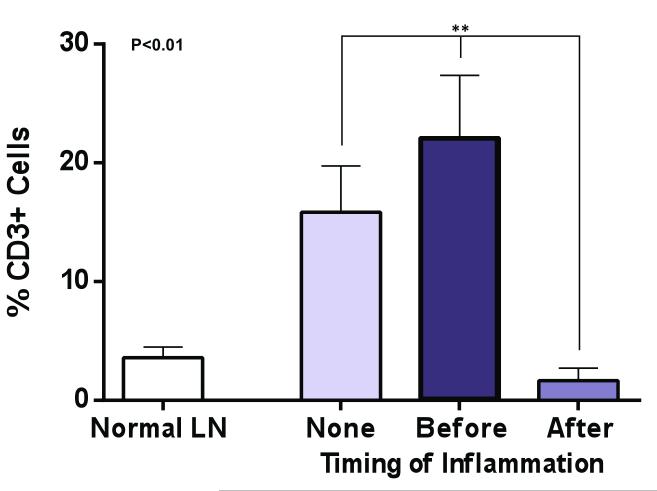
Sterile inflammation after lymph node transfer attenuates T cell infiltration
Consistent with our previous report, histological analysis of lymph nodes transferred without inflammation or inflammation-before transfer demonstrated a scattered pattern of T cell staining (Figure 6A).(12) In contrast to a normal lymph node, we found that in these animals T cells were distributed throughout the lymph node rather than cortical regions. In addition, there appeared to be a relative increase in staining for CD3 cells as compared with normal lymph nodes. Indeed, quantification of T cell regions in the no inflammation or inflammation-before transfer groups revealed a significant increase in T cell area in these groups as compared to a normal lymph node (3-5 fold increase; p<0.01; Figure 6B). In contrast, we found that sterile inflammation-after lymph node transplantation was associated with a more scattered pattern of T cell distribution in the transplanted lymph node. Moreover, the intensity of staining and percentage of the lymph node comprised of T cells was markedly decreased as compared to no inflammation or inflammation before transplant groups (Figure 6B). These patterns resembled the normal lymph node architecture and provide further support for the hypothesis that inflammation after transport is associated with improved lymph node function. In addition, these findings are important since previous studies have shown that T cells are inhibitors of lymphangiogenesis.(14, 21) Thus, decreased T cell areas in lymph nodes harvested from animals with sterile inflammation after transfer may contribute to the increased lymphangiogenesis and function we observed in this group.
Figure 6. Sterile inflammation after lymph node transfer attenuates T cell infiltration.
A. Representative photomicrographs (5x magnification) of lymph nodes from various groups stained for CD3, a pan T cell marker. Note relative paucity and distribution of lymph node T cells in normal and inflammation after groups. In contrast, note relative increase staining for T cells in no inflammation or inflammation before transfer groups.
B. Quantification of T cells (percentage of T cells as a function of lymph node area). Note significant decrease in T cells in inflammation after transfer group as compared with no inflammation or inflammation before transfer groups.
Discussion
In the current study we have shown that sterile inflammation, particularly if induced after lymph node transplantation has been performed, is a potent means of increasing lymphatic function, lymphangiogenesis, and functional recovery of lymphatic vessels in the transplanted lymph node. These findings suggest that relatively simple physiologic interventions may have an important clinical utility in microvascular lymph node transplantation. More importantly, our study is the first to show that lymphangiogenesis after lymph node transplantation can be augmented without the addition or delivery of exogenous lymphangiogenic cytokines such as VEGF-C or VEGF-A. This is critically important in cancer survivors (i.e. the vast majority of patients who undergo lymph node transplantation) since lymphangiogenic cytokines are known to be critical regulators of tumor growth and metastasis.
Recent experimental studies have shown that lymphangiogenesis is regulated by a coordinated expression of both pro- and anti-lymphangiogenic pathways.(14, 21) For example, it is now widely known that lymphangiogenic growth factors such as VEGF-A, VEGF-C, fibroblast growth factor, and others promote lymphangiogenesis in a number of physiologic settings by promoting lymphatic endothelial cell proliferation, differentiation, and function. More recently, our lab and others have shown that anti-lymphangiogenic pathways can also influence this process.(14, 25) For example, we have shown that transforming growth factor beta-1 and interferon gamma can both potently inhibit lymphangiogenesis even when VEGF-C or VEGF-A expression is elevated.(14, 25) Thus, promoting changes in the balance of pro- and anti-lymphangiogenic mechanisms is a physiologically relevant means of regulating lymphangiogenesis. This is important because inhibition of anti-lymphangiogenic mechanisms (i.e. “removing the brake”) have not been associated with worsening tumor biology or metastatic potential.
In the current study we found that increased lymphangiogenesis resulting from inflammation after lymph node transplantation correlated with expansion of lymph node B cell populations and diminished numbers of T cells. This finding is important since B cells are known to be a major source of lymphangiogenic cytokines in inflammatory lymph node lymphangiogenesis, and T cells play an opposite role by inhibiting lymphangiogenesis.(22) This finding was in contrast to the opposite pattern of B and T cell distribution (i.e. decreased B cells/increased T cells) in the no-inflammation and inflammation-before transfer groups. Thus, it is possible that the increased lymphangiogenesis we noted in the inflammation after transfer group was a result of the combination of increased lymphangiogenic forces (i.e. B cells) and decreased anti-lymphangiogenic pathways (i.e. T cells).
Immune adjuvants such as CFA/OVA are commonly used to induce sterile inflammatory reactions to improve vaccination. Although CFA/OVA is not used commonly in humans for this purpose, our lab has extensive experience with this protocol in mice and used the current experiment as a proof-of-principal that sterile inflammation can induce increased lymphangiogenesis after lymph node transfer. It is clear that additional studies will be needed to analyze the effects of other immune adjuvants and to translate the efficacy of these treatments in clinical cases.
An interesting observation in our experiment was the architectural changes in the transplanted lymph nodes depending on the timing of sterile inflammation. We found if lymph nodes were transplanted without inflammation or if inflammation was induced in donor animals prior to transplantation, then the follicular pattern of B cells as well as the cortical distribution of T cells was lost. In contrast, induction of inflammation after transfer was associated with a normal follicular pattern of B cells and a primarily cortical distribution of T cells. This finding is important since the architectural arrangement of the lymph node is known to play a critical role in the regulation of immune response suggesting that inflammation after transplantation is superior in transfers performed without inflammation or inflammation prior to transfer. Future studies will test the immunologic function of transferred lymph nodes under a variety of experimental conditions.
While the precise mechanisms that regulate the more normal pattern of B and T cell distribution in the lymph nodes of mice induced to have inflammation after transfer remain unknown, one likely mechanism is increased flow of interstitial fluid to the lymph node from the periphery. This hypothesis is based on previous studies demonstrating that interstitial fluid flow in the lymph node coordinates the distribution of B and T cells in the lymph node by regulating gradients of chemokine C-C motif ligand 21 (CCL21) expression.(26, 27) Thus, we hypothesize that inflammation following lymph node transfer increased lymphangiogenesis and regeneration of functional lymphatics by altering the balance between pro- and anti-lymphangiogenic forces and that this response increased the flow of interstitial fluid from the periphery to the transferred lymph node. The increased flow in the lymph node, in turn, leads to preservation of the lymphatic vascular network, normal patterns of chemokine expression, and maintenance of lymph node structure. Future studies will aim to analyze the effect of interstitial fluid flow on transplanted lymph node architecture/biology.
An important point about our mouse model is that in these experiments lymph nodes are transferred as avascular grafts rather than vascularized transfers as performed clinically. However, unlike human lymph nodes, murine nodes are small and revascularize spontaneously even without inflammation. This is important and makes the mouse model useful as a highly effective and rapid screening tool to identify interventions that improve lymphangiogenesis after lymph node transfer. In addition, the mouse model is substantially less expensive than large animal models and analysis is simplified by the availability of molecular reagents in mice. Using this model, therefore, we can identify promising putative interventions in mice and use more targeted approaches in large animals thereby decreasing costs and animal needs. For example, translation of this study to large animals will decrease the number of experimental groups substantially since it is clear that post transfer inflammation is more efficacious than pre transfer. However, as with any model, our findings will need to be confirmed and analyzed using large animal models and well designed clinical trials.
In conclusion, we have shown for the first time that sterile inflammatory reactions elicited after lymph node transplantation can potently improve lymphangiogenesis and recovery of lymphatic function without the need for exogenous delivery of lymphangiogenic cytokines. More importantly, we have found that this recovery correlates with normal lymph node B and T cell architecture suggesting that this approach also preserves immune function.
Acknowledgments
Funding: NIHR01 HL111130-02 awarded to BJM.
Footnotes
Disclosures: None of the authors have any conflicts of interest or disclosures.
References
- 1.Ahmed RL, Prizment A, Lazovich D, et al. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26:5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013;131:1286–1298. doi: 10.1097/PRS.0b013e31828bd3b3. [DOI] [PubMed] [Google Scholar]
- 3.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saaristo AM, Niemi TS, Viitanen TP, et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg. 2012;255:468–473. doi: 10.1097/SLA.0b013e3182426757. [DOI] [PubMed] [Google Scholar]
- 5.Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 6.Baker A, Kim H, Semple JL, et al. Experimental assessment of pro-lymphangiogenic growth factors in the treatment of post-surgical lymphedema following lymphadenectomy. Breast Cancer Res. 2010;12:R70. doi: 10.1186/bcr2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpanen T, Egeblad M, Karkkainen MJ, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 8.Kondo K, Kaneko T, Baba M, et al. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biological & pharmaceutical bulletin. 2007;30:633–637. doi: 10.1248/bpb.30.633. [DOI] [PubMed] [Google Scholar]
- 9.Kazama S, Watanabe T, Kanazawa T, et al. Vascular endothelial growth factor-C (VEGF-C) is a more specific risk factor for lymph node metastasis than VEGF-D in submucosal colorectal cancer. Hepatogastroenterology. 2007;54:71–76. [PubMed] [Google Scholar]
- 10.Feng Y, Wang W, Hu J, et al. Expression of VEGF-C and VEGF-D as significant markers for assessment of lymphangiogenesis and lymph node metastasis in non-small cell lung cancer. Anat Rec (Hoboken) 2010;293:802–812. doi: 10.1002/ar.21096. [DOI] [PubMed] [Google Scholar]
- 11.Boone B, Blokx W, De Bacquer D, et al. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch. 2008;453:257–265. doi: 10.1007/s00428-008-0641-6. [DOI] [PubMed] [Google Scholar]
- 12.Aschen S, Farias-Eisner G, Cuzzone DA, et al. Lymph Node Transplantation Results in Spontaneous Lymphatic Reconnection and Restoration of Lymphatic Flow. Plast Reconstr Surg. 2013 doi: 10.1097/01.prs.0000436840.69752.7e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zampell JC, Avraham T, Yoder N, et al. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am J Physiol Cell Physiol. 2012;302:C392–404. doi: 10.1152/ajpcell.00306.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zampell JC, Yan A, Avraham T, et al. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol. 2011;300:C1107–1121. doi: 10.1152/ajpcell.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zampell JC, Yan A, Elhadad S, et al. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One. 2012;7:e49940. doi: 10.1371/journal.pone.0049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng CL, Ohki K, Hossain A, et al. Complete Freund’s adjuvant promotes the increases of IFN-gamma and nitric oxide in suppressing chronic arthritis induced by pristane. Inflammation. 2003;27:247–255. doi: 10.1023/a:1025092615584. [DOI] [PubMed] [Google Scholar]
- 18.Richens ER, Tungekar MF, Behbehani K. Complete Freund’s adjuvant has a differential amplification action on the induction of diabetes by streptozotocin in various murine strains: CFA amplifies STZ in murine diabetes. Pathology. 1987;19:351–357. doi: 10.3109/00313028709103882. [DOI] [PubMed] [Google Scholar]
- 19.Pierce JC. Mechanism of the lymph node enlarging effect of Freund’s adjuvant. Surg Forum. 1968;19:95–97. [PubMed] [Google Scholar]
- 20.Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 21.Kataru RP, Kim H, Jang C, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Webster B, Ekland EH, Agle LM, et al. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isaka N, Padera TP, Hagendoorn J, et al. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64:4400–4404. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 24.Leu AJ, Berk DA, Lymboussaki A, et al. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 25.Avraham T, Daluvoy S, Zampell J, et al. Blockade of Transforming Growth Factor-{beta}1 Accelerates Lymphatic Regeneration during Wound Repair. Am J Pathol. 2010;177:3202–3214. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miteva DO, Rutkowski JM, Dixon JB, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomei AA, Siegert S, Britschgi MR, et al. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]