Abstract
Objective
To evaluate endometrial leukemia inhibitor factor (LIF) expression as a marker of endometrial receptivity in women with unexplained infertility (UI).
Design
Prospective case-control study.
Setting
University-associated infertility clinics.
Patient(s)
Women with UI for more than 1 year and healthy control women.
Intervention(s)
Endometrial biopsy.
Main Outcome Measure(s)
Time to pregnancy was compared between patients with UI who were evaluated for endometrial LIF protein as well as ανβ3 integrin expression. Endometrium was evaluated using immunohistochemistry (IHC) and messenger RNA by real time reverse transcriptase–polymerase chain reaction (PCR) (quantitative real-time reverse transcriptase–PCR) in samples from women with UI as well as healthy control women.
Result(s)
Leukemia inhibitor factor was expressed in epithelial cells in a cyclic fashion in controls, and overall expression in the secretory phase was similar between controls and women with UI, whereas ανβ3 integrin expression was reduced. However, using quantitative real-time PCR, LIF messenger RNA abundance was 4.4-fold lower in women with low levels of ανβ3 integrin expression compared with samples with normal integrins. By immunohistochemistry, ανβ3 integrin expression was always lacking when the histology was out of phase, whereas LIF expression was only negative in a subset of those samples. Reduced endometrial LIF expression was strongly associated with poor reproductive outcomes.
Conclusion(s)
Endometrial LIF expression peaks in the midsecretory phase and is reduced in some women with UI. The use of LIF in combination with ανβ3 integrin as biomarkers appears to be superior to integrin testing alone when evaluating endometrial receptivity, primarily because of its earlier pattern of expression during the secretory phase.
Keywords: Implantation, endometrium, leukemia inhibitory factor, LIF, endometriosis
Defects in endometrial receptivity that contribute to infertility have been linked to a wide array of disorders including endometriosis, polycystic ovary syndrome (PCOS), and tubal disease, all of which can negatively impact implantation (1–3). Testing for endometrial receptivity problems has become more common, including tests for integrins (ETEGRITYTEST.COM) and or cell cycle markers (endometrial function test, http://medicine.yale.edu/obgyn/kliman/infertility/eft/index.aspx). There has been increasing evidence to suggest that such defects in endometrial receptivity exist and due to inflammation, contributing to P resistance (4–6). In theory, any gene that depends on P for timely expression within the window of implantation might be altered, including implantation-specific proteins, such as N-acetylglucosamine-6-O-sulfotransferase, that regulates l-selectin ligand (4–6), or HOXA10, which directly regulates expression of many genes, including ανβ3 integrin (7) or estrogen receptors (ERs) that are normally down-regulated by P (8). Our laboratory has identified a number of dose-sensitive markers of endometrial P action in vivo, using an artificial menstrual cycle model in healthy women (9). One of the most sensitive markers of P action identified in this study was leukemia inhibitory factor (LIF).
Leukemia inhibitory factor was the first endometrial factor to be conclusively demonstrated as critical for both murine and primate embryo implantation (10–13). In a LIF null mouse, females were found to be infertile and exhibited a lack of embryo implantation, which could be rescued by administration of exogenous LIF. This profound defect in implantation was uterine specific, as successful implantation occurred after transplantation of LIF null embryos into normal host recipients (11). In a non-human primate model, injection of anti-LIF antibodies markedly reduces implantation rates (13).
Human endometrial LIF expression is cycle dependent with maximum expression during the period of receptivity to implantation (14–16) and secreted LIF protein is detectable in uterine flushings during this time period (17). Endometrial expression of LIF and its gp-130 receptor are up-regulated in response to hCG treatment (18) and by tumor necrosis factor α (TNF-α) and interleukin 1β (19). In addition, the LIF receptor is present on the blastocyst and placenta (14, 20) as well as on the glandular and luminal epithelium (15, 21). Finally, a significant reduction in LIF expression is associated with multiple human reproductive pathologies (22, 23).
The cell surface adhesion receptor ανβ3 integrin has been well characterized as a biomarker of endometrial receptivity (3). The β3 integrin subunit, like LIF, is expressed in the epithelial cells in the midsecretory phase of the menstrual cycle and reported to be absent in some women with unexplained infertility (UI) (24) and endometriosis (25). Expression of the ανβ3 integrin has been studied at the time of implantation in the endometrium and blastocyst in murine (26), rabbit (27), non-human primate, and human subjects (28–34). Whereas recent reports have linked these two important biomarkers (33, 35), investigation of their coexpression in women with infertility has been quite limited, although absence of both ανβ3 integrin and LIF has been independently associated with diminished pregnancy success in IVF (36–39).
Interestingly, treatment of mice with peritoneal fluid (PF) from women with endometriosis reduces expression of LIF and ανβ3 integrin, along with HOXA10 (26), likely due to an inflammatory milieu, as the one associated with endometriosis (40). These data are consistent with the finding that inflammatory components negatively impact endometrial receptivity by targeting specific protein expression. Although the factor(s) controlling the expression of LIF and ανβ3 integrin have not been firmly established, both biomarkers are upregulated by paracrine factors including heparin-binding epidermal growth factor (EGF) (41) and LIF has been shown to be strongly inhibited by interferon gamma (INF-γ) (40).
The purpose of this prospective case-control study of women with UI was to examine and compare the expression of LIF to the ανβ3 integrin during endometrial biopsies timed to the midsecretory phase. In healthy women expression of epithelial LIF is initiated earlier, in the secretory phase, than ανβ3 integrin, which is restricted to cycle day 20 and beyond (31). In samples that are histologically delayed, expression of integrin is always absent, masking the ability to use this biomarker to assess receptivity. In the present study, we compared LIF with integrin testing to determine whether this earlier expression of LIF would help adjudicate the presence or absence of endometrial receptivity defects, when ανβ3 integrin was lacking due to delayed endometrial histology. In addition, we compared the time to pregnancy in women who were missing integrins compared with women who were lacking LIF expression.
Materials and Methods
Study Population and Endometrial Biopsy
All tissues were obtained in accordance with the Committee for the Protection of Human Subjects at the University of North Carolina and Greenville Hospital System under approved Institutional Review Board protocols. We recruited ovulatory women with healthy male partners with at least 1 year of UI for inclusion in this study. Each signed an informed consent for an Institutional Review Board-approved protocol (GHS #00015759) to obtain a urinary LH-timed endometrial biopsy. To be included, each woman was required to have regular cyclic menses (25–32 days apart), partners with normal sperm parameters according to the World Health Organization, and at least one patent fallopian tube. Women with PCOS or known uterine fibroids were excluded. A total of 66 women were initially recruited, but after exclusion, 55 were included. Exclusion included the discovery of fibroids, male factor infertility, ovulatory dysfunction, or lack of adequate tissue for analysis.
As controls, 20 paid volunteer female subjects were recruited who regular cyclic menses using a separate Institutional Review Board protocol at UNC (05-1757). None of these subjects had signs or symptoms of endometriosis or a history of infertility and all were in good health. There was no attempt to match controls to the cases in terms of age, body mass index (BMI), or gravidity. All subjects underwent an endometrial biopsy, timed to the midsecretory phase using urinary LH testing. Additional endometrial biopsies were obtained from the proliferative phase in healthy volunteers to verify the cycle dependency of LIF expression.
Endometrial biopsies were performed using a pipelle suction curettage on LH + 7–10; cycle day 21–24 in all subjects. The menstrual cycle stage was determined by a single pathologist (D.P.S.) using the dating criteria of Noyes et al. (42). Portions of endometrial biopsies were snap frozen in liquid nitrogen in the clinic and transported to the laboratory where they were stored at −80°C until further use, whereas the remainder of the samples was placed in 10% buffered formalin for paraffin embedding and sectioning.
Immunohistochemistry
Immunohistochemistry was performed on sections of endometrium and stained for LIF as well as the ανβ3 integrin. For LIF immunostaining, primary antibody (N-18; Santa Cruz) was serially diluted in a solution of phosphate-buffered saline (PBS) containing 1% normal goat serum and 0.1% sodium azide to optimize the appropriate concentrations to achieve maximum sensitivity and specificity. Monoclonal antibodies to the β3 integrin subunit (SSA6; provided by Sepal, Inc.) were used at concentrations determined by limiting dilution on cryopreserved sections. After initial incubation in blocking solution (4% normal goat serum for 30 minutes at room temperature), primary antibody was applied and further incubated at 4°C overnight. Tissue sections were deparaffinized in xylene and rehydrated then incubated with primary antibody at 4°C overnight. Negative control sections were treated with nonimmune serum diluted in the same manner. The semiquantitative assessment of expression was made using the HSCORE (0–4), by a single blinded observer (B.A.L.) and calculated using the following equation: HSCORE = Σ Pi (i + 1)/100, where i is the intensity of staining with a value of 1, 2, or 3, (weak, moderate or strong, respectively) and Pi is the percentage of stained epithelial cells for each intensity, varying from 0–100%. The use of HSCORE has previously been validated as a semiquantitative assay for immunohistochemical staining (43).
LIF and β3 Integrin Subunit Messenger RNA Expression by Quantitative Real-Time Reverse Transcriptase–polymerase Chain Reaction
To evaluate and compare LIF expression with integrin expression, we first examined messenger RNA (mRNA) derived from endometrium from healthy controls during the proliferative or midsecretory phase using quantitative real-time reverse transcriptase–polymerase chain reaction (PCR), performed an MX3000 real-time thermocycler (Stratagene) with the conditions listed here. We also studied the mRNA expression pattern during the midsecretory phase in women with UI for comparison with control subjects. Total RNA was extracted from frozen endometrial biopsies using Trizol Reagent (Ambion) according to the manufacturer's suggested conditions. The RNA quantification was performed using RiboGreen (Invitrogen) with a ribosomal RNA standard curve. First-strand complementary DNA (cDNA) was synthesized from 1,000 ng of total RNA using AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies). A no-template control was used as a negative control. In the no-template control the primer, probe, and master mix were included without cDNA. Quantitative real-time PCR was performed using primers specific for LIF and β3 integrin subunit. Relative quantitation was obtained using the delta-delta Ct method with peptidylprolyl isomerase A (PPIA, cyclophilin A) as a constitutive housekeeping control gene. The total reaction volume for all real-time PCR experiments was 20 μL contained cDNA from 90 ng total RNA, 1 μL 20 × TaqMan Mix of primer and probe (Applied Biosystems), 10 μL 2 × Brilliant II QPCR Master Mix (Agilent Technologies). Reactions were performed in 96-well plates on a Stratagene MX3000 device. Thermal cycler conditions were one cycle at 50°C for 5 minutes, and one cycle at 95°C for 10 minutes, followed by 40 cycles of 95°C for 25 seconds, 60°C for 1 minute. The PCR primers and fluorogenic probes included Hs04194521-s1 (PPIA), HS01001469 (ITGb3; β3 integrin subunit), and Hs01055668-m1 (LIF) (Applied Biosystems). These probe-primer sets are designed across an exon–exon junction, and therefore, are expected to provide signal only from mRNA of these genes and not from similar sequence. The PPIA was chosen because previous work suggested that PPIA exhibits little variation across the menstrual cycle (Steven L. Young, unpublished data). The reverse transcriptase–PCR data were grouped by cycle phase and analyzed by one-way analysis of variance (ANOVA) using Tukey's multiple comparison test for post hoc analysis.
Statistical Comparisons of Clinical Outcomes
The demographic data and HSCOREs for LIF and the β3 integrin subunit were compared by Student's t-test using 95% confidence (P<.05) for significance (Table 1). Fisher's exact test was used for comparisons of categorical data. Multiple logistic regression was used to compare age, BMI, LIF, and β3 integrin subunit expression with pregnancy outcomes. Time to pregnancy and pregnancy outcomes were compared between UI cases based on LIF and β3 integrin subunit staining (HSCORE) using Kaplan Meier survival analysis and Prism statistical software in monitored cycles (GraphPad). Monitored cycles included natural cycles, and ovulation induction with oral or gonadotropin therapies, and included IVF and frozen embryo transfer. Cycles that occurred after laparoscopy were excluded.
Table 1.
Characteristics for subjects with unexplained infertility (UI) and healthy controls.
| Characteristic | Controls (n = 20) | UI (n = 55) | P value |
|---|---|---|---|
| Age (y) | 25.7 (5.6) | 33.1 (4.0) | <.001 |
| Gravidity | 0.61 (1.1) | 0.4 (0.6) | NS |
| HSCORE | |||
| LIF | 1.88 (1.1) | 2.04 (1.5) | NS |
| β3 | 1.96 (1.3) | 0.96 (1.3) | <.01 |
Note: LIF = leukemia inhibitor factor; NS = not significant.
Results
LIF and β3 Integrin Expression Patterns
In the population studied, healthy controls were younger than the patients with UI (P<.001; Table 1). The overall mean LIF HSCORE was not different between patients and control subjects, whereas the average HSCORE for the β3 integrin subunit was reduced in patients with UI (P<.01). Endometrial LIF protein by immunostaining was positive in most (17/20; 85%) samples from healthy controls (example of positive immunostaining shown in Fig. 1A), but was reduced (HSCORE < 1) in 19/55 (65%) of the endometrial samples from women with UI (example shown in Fig. 1B). This difference did not achieve predetermined statistical significance (P=.14). Positive immunostaining was present predominantly in the epithelial glands and lumen, with distinct immunostaining on the uterodome (pinopode) projections on the luminal surface (Fig. 1A, inset).
Figure 1.
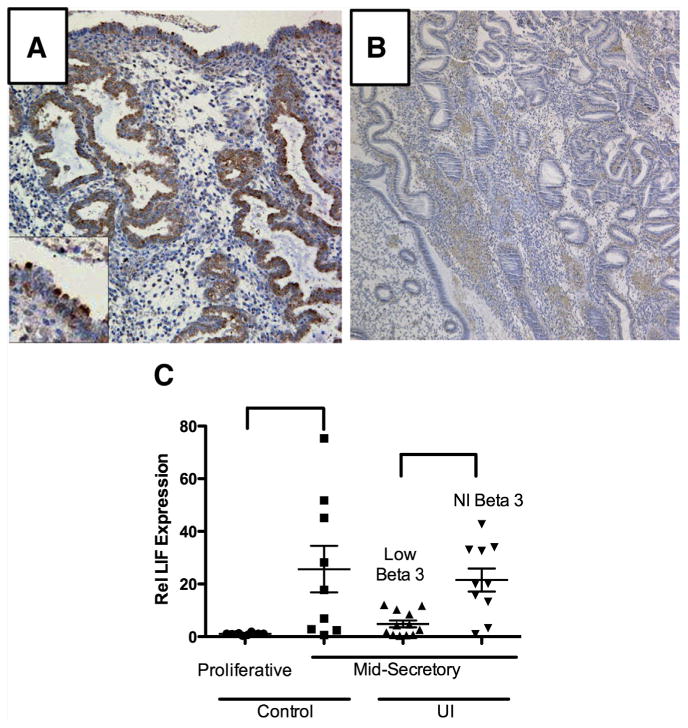
Expression of leukemia inhibitor factor (LIF) in controls and subjects with unexplained infertility (UI). (A) Leukemia inhibitor factor-positive immunostaining showing luminal staining (inset) on the uterine projections. This pattern was seen more often in controls compared with patients with UI. (B) An example of negative LIF immunostaining in a woman with UI. (C) Using quantitative real-time polymerase chain reaction (PCR) for LIF expression, the cycle dependence of LIF is seen in healthy controls from the proliferative and midsecretory phase (left side; P=.03). The LIF expression was also compared in the midsecretory phase of women with UI and known endometriosis. Those women with low integrin expression also exhibited depressed LIF expression, whereas those with normal integrin (NI) expression had higher levels of LIF messenger RNA expression (right side; P=.004).
The reverse transcriptase–PCR results for integrin expression in the UI group were arbitrarily divided into two groups: those with normal integrin expression and those with low integrin expression. We compared LIF mRNA expression with β3 integrin subunit expression in women with UI and control subjects. As expected endometrial LIF was low in the proliferative phase and increased 24-fold in the midsecretory phase (P=.004). In UI patients low LIF expression was largely confined to the group of women who also had low β3 integrin subunit mRNA expression (Fig. 1C). In subjects with low β3 integrin subunit expression there was a 4.4-fold reduction in LIF mRNA abundance (P=.03) compared with the group with normal ανβ3 integrin expression.
Using multivariant logistic regression analysis, with pregnancy as the dependent variable and adjusting for age, β3 integrin subunit, and LIF HSCOREs, LIF was the only variable to provide an association with pregnancy (P=.014). We examined the time to pregnancy in women based on either LIF or β3 integrin subunit expression in women undergoing monitored cycles. Monitored cycles were conducted by clinicians based on patient preferences and were not included in the conduct of the present study. Cycle treatments including oral ovulatory agents, injectable gonadotropins, and IVF and frozen embryo transfer and the proportions of each were similar between women with normal or abnormal LIF expression (Table 2). In monitored cycles, women with normal integrin expression were found to have a significant advantage with a reduced time to pregnancy compared with those with absent integrin expression (P<.01). Still, many women who lacked ανβ3 integrin expression conceived successfully (Fig. 2A), possibly due to the high proportion with histologic delay. When we compared LIF immunohistochemistry results to β3 integrin subunit expression, women with absent integrins, but positive LIF staining, exhibited a significantly higher pregnancy rate (PR) than women who lacked both LIF and ανβ3 integrin expression. Women with UI, low LIF, and low integrin expression rarely conceived within the 12-month time period of observation (Fig. 2B). Two conceptions occurred in this group—one in the month of biopsy and the other after 12 months of monitored cycles.
Table 2.
Monitored cycles in subjects with unexplained infertility (UI) by leukemia inhibitor factor (LIF) results.
| Type of cycle | LIF negative (n = 72 cycle) |
LIF positive (n = 132) |
|---|---|---|
| Natural | 2 (2.7) | 10 (7.6) |
| Oral agents (clomiphene citrate [CC] or letrozole) | 44 (61) | 72 (55.4) |
| Sequential (oral plus gonadotropins) | 16 (22.2) | 33 (25.0) |
| Superovulation | 4 (5.5) | 6 (4.5) |
| IVF/frozen embryo transfer | 6 (8.3) | 11 (8.3) |
Note: P values are not significant.
Figure 2.
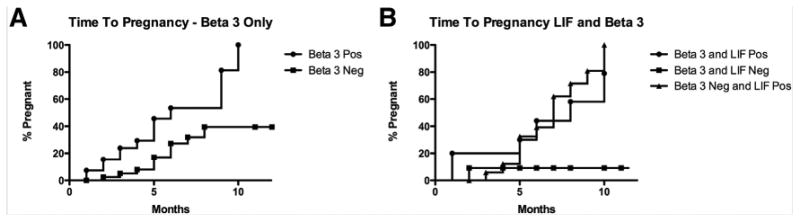
(A) Time to pregnancy using Kaplan Meier survival analysis based on β3 integrin subunit protein expression alone was significantly different between those women who did or did not express this biomarker by immunohistochemistry (P<.01). (B) When leukemia inhibitor factor (LIF) expression was included along with the β3 integrin subunit, those women who expressed LIF were similar to those who expressed the β3 integrin subunit, but very few subjects successfully conceived (2) when both biomarkers were reduced (P=.02). One subject conceived in the month of biopsy sampling and another woman, in an unmonitored cycle 12 months after the biopsy.
Discussion
Numerous proteins have been shown to be biomarkers for the assessment of endometrial receptivity based on their temporal and spatial relation to the attachment phase of the embryo to the endometrium or through the use of mouse gene knockout models (44). The use of ανβ3 integrin for determination of endometrial receptivity is a well-established test for endometrial receptivity (http://Etegritytest.com) but its use is limited by relative poor performance of most anti-integrin antibodies in formalin-fixed tissues (45), as well as the uniform absence of staining in biopsies exhibiting subnuclear vacuoles consistent with histologic delay (31, 46). In the present study, we report for the first time that LIF and ανβ3 integrin expression patterns are related, with simultaneous loss of both biomarkers in a subset of women with UI. We also showed that reduced LIF expression was more specific to poor reproductive outcomes and has an advantage versus integrin expression alone, because it is expressed earlier in the cycle compared with the abrupt initiation of ανβ3 integrin expression on cycle day 20 (LH + 6). We found that histologically “in phase” samples that were missing ανβ3 integrin usually lacked LIF expression, whereas “out of phase” samples lacking ανβ3 integrin expression usually expressed LIF normally. In those patients where LIF was missing (in cases with delayed histology), reproductive outcomes appear to be compromised, similar to cases where ανβ3 integrin expression was lacking in “in phase” histology (type II defects). These data suggest that the presence of normal LIF expression in cases where β3 integrin subunit is absent due to histologic delay, is reassuring regarding endometrial receptivity. When absent, LIF provides a clear advantage versus integrin testing alone, as ανβ3 integrin expression is always negative and lacks any predictive value for pregnancy outcome. As a well-established endometrial biomarker critical for implantation in rodents as well as primates (10–12, 47–50), LIF may have advantages versus integrin testing alone for the assessment of endometrial receptivity.
In this prospective case-controlled comparison of UI with control subjects, endometrial LIF expression was elevated only during the secretory phase compared with the proliferative phase, consistent with previous reports (15). We and other investigators have reported reduced LIF expression in the endometrium of women with infertility (17, 22, 51–53) and such defects in LIF expression have been previously associated with endometriosis and adenomyosis (54–57) and hydrosalpinx (33), similar to reports on integrin expression (3). These similarities in patterns of expression may reflect common regulatory controls such as HOXA10 (7). The synchronous loss of both LIF and ανβ3 integrin expression argues for a common cause in their dysfunctional expression. Future studies are needed to determine what those factors are that lead to this particular type of endometrial dysfunction.
Reduced endometrial LIF expression in women with UI may be associated with an endometrial P resistance, abrogating the anti-inflammatory actions of P (58, 59). Endometrial LIF is positively regulated by estrogen (E) and P, heparin-binding EGF-like growth factor (41, 60), and hCG (61), whereas ανβ3 integrin is negatively impacted by E (8), but stimulated by heparin-binding EGF (reference value). Increased local production of E by aromatase expression has been linked to inflammatory changes associated with endometriosis (62, 63). In unpublished studies, we find that E does not inhibit LIF expression, as it does ανβ3 integrin expression in endometrial epithelium. This may account for the early onset of LIF expression during the secretory phase. Endometrial LIF expression is indirectly regulated by multiple factors including cytokines, including stimulation by interleukin-1, TNF-α, platelet-derived growth factor, EGF, and transforming growth factor β (TGF-β), as well as down-regulation by INF-γ (40). An inhibitory role of INF-γ on LIF was recently reported in diabetic NOD mice, a murine model of impaired implantation and fertility (50). The INF-γ is elevated in the serum and PF of women with endometriosis (64, 65) and produced by endometriotic monocytes (66). The INF-γ induces other proinflammatory cytokines associated with endometriosis and poor reproductive performance, such as interleukin 6 (67–69), and is a possible mechanism by which LIF expression is decreased in eutopic endometrium of women with endometriosis. Inflammatory cytokines have only been studied in the context of the uterine ανβ3 integrin by intraperitoneal (IP) injection of PF from women with endometriosis and reduced fertility. In those studies we reported a coordinated reduction in not only ανβ3 integrin expression but also LIF and HOXA10 in the uteri of injected female mice (26).
The strengths of the study include the prospective collection of endometrial biopsies in women with UI. In addition, we had expert assistance from a gynecological pathologist (D.P.S.) and an experienced reader of HSCOREs (B.A.L.). Weaknesses include the nonuniform treatment protocols of women after the biopsy was performed. Because many women with UI and their physicians choose to do some form of ovulation induction other than IVF, we examined the time to pregnancy is this “real life” exposure to different types of monitored cycles. The types of medications used did not differ between groups, therefore we believe that this did not alter the outcomes or the conclusions of the study.
In conclusion, ανβ3 integrin expression has been used as a biological marker of endometrial receptivity but is limited by the lack of expression in samples with histologic delay. The LIF and ανβ3 integrin expression occur during the window of implantation in healthy controls and the absence of both biomarkers is associated with poor reproductive outcomes. Like the ανβ3 integrin, LIF expression was reduced in a subset of women with UI, but the presence of LIF was reassuring when present in samples exhibiting histologic delay. A reduction in normal expression of secretory phase endometrial LIF predicted poor reproductive outcomes and may reflect an inflammatory basis for infertility. More research is required to identify the factor(s) involved in implantation defects and how to best treat such problems before undergoing expensive or invasive therapies for infertility.
Acknowledgments
B.A.L. reports a grant from National Institutes of Health-National Institute of Child Health and Human Development (HD067721).
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54-HD35041-12 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by National Institute of Child Health and Human Development/National Institutes of Health R01 HD067721 (S.L.Y. and B.A.L.).
Footnotes
J.M.F. has nothing to disclose. K.J.H. has nothing to disclose. L.Y. has nothing to disclose. D.P.S. has nothing to disclose. S.L.Y. has nothing to disclose.
References
- 1.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 3.Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Sem Reprod Med. 2007;25:461–75. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Sem Reprod Med. 2010;28:51–8. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 5.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Fazelabas AT. Progesterone resistance in a baboon model of endometriosis. Sem Reprod Endocrinol. 2010;28:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- 7.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol. 2002;16:571–9. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 8.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol. 2006;4(Suppl 1):S9. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young SL, Lessey BA, Balthazar U, Zaino RJ, Jin JP, Savaris RF, et al. Relationship between progesterone dose, endometrial histology and gene expression using an in vivo leuteal phase defect model. Reprod Sci. 2011;18:273A. [Google Scholar]
- 10.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991;88:11408–12. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 12.Terakawa J, Wakitani S, Sugiyama M, Inoue N, Ohmori Y, Kiso Y, et al. Embryo implantation is blocked by intraperitoneal injection with anti-LIF antibody in mice. J Reprod Dev. 2011;57:700–7. doi: 10.1262/jrd.11-048h. [DOI] [PubMed] [Google Scholar]
- 13.Yue ZP, Yang ZM, Wei P, Li SJ, Wang HB, Tan JH, et al. Leukemia inhibitory factor, leukemia inhibitory factor receptor, and glycoprotein 130 in rhesus monkey uterus during menstrual cycle and early pregnancy. Biol Reprod. 2000;63:508. doi: 10.1095/biolreprod63.2.508. [DOI] [PubMed] [Google Scholar]
- 14.Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. Leukaemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fert. 1994;101:421–6. doi: 10.1530/jrf.0.1010421. [DOI] [PubMed] [Google Scholar]
- 15.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–20. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogiagis D, Salamonsen LA, Sandeman RM, Squires TJ, Butt AR, Fry RC. Effect of immunisation against leukaemia inhibitory factor on the establishment of pregnancy in sheep. Reprod Nutr Dev. 1997;37:459–68. doi: 10.1051/rnd:19970407. [DOI] [PubMed] [Google Scholar]
- 17.Laird SM, Tuckerman EM, Dalton CF, Dunphy BC, Li TC, Zhang X. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum Reprod. 1997;12:569–74. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- 18.Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, et al. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148:618–26. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- 19.Laird SM, Tuckerman EM, Cork BA, Li TC. Expression of nuclear factor kappa B in human endometrium; role in the control of interleukin 6 and leukaemia inhibitory factor production. Mol Hum Reprod. 2000;6:34–40. doi: 10.1093/molehr/6.1.34. [DOI] [PubMed] [Google Scholar]
- 20.Kojima K, Kanzaki H, Iwai M, Hatayama H, Fujimoto M, Narukawa S, et al. Expression of leukaemia inhibitory factor (LIF) receptor in human placenta: a possible role for LIF in the growth and differentiation of trophoblasts. Hum Reprod. 1995;10:1907–11. doi: 10.1093/oxfordjournals.humrep.a136205. [DOI] [PubMed] [Google Scholar]
- 21.Aghajanova L, Stavreus-Evers A, Nikas Y, Hovatta O, Landgren BM. Coexpression of pinopodes and leukemia inhibitory factor, as well as its receptor, in human endometrium. Fertil Steril. 2003;79(Suppl 1):808–14. doi: 10.1016/s0015-0282(02)04830-6. [DOI] [PubMed] [Google Scholar]
- 22.Aghajanova L, Altmae S, Bjuresten K, Hovatta O, Landgren BM, Stavreus-Evers A. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril. 2009;91:2602–10. doi: 10.1016/j.fertnstert.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Paiva P, Menkhorst E, Salamonsen L, Dimitriadis E. Leukemia inhibitory factor and interleukin-11: critical regulators in the establishment of pregnancy. Cytokine Growth Factor Rev. 2009;20:319–28. doi: 10.1016/j.cytogfr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535–42. [PubMed] [Google Scholar]
- 25.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 26.Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril. 2000;74:41–8. doi: 10.1016/s0015-0282(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 27.Illera MJ, Cullinan E, Gui Y, Yuan L, Beyler SA, Lessey BA. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biol Reprod. 2000;62:1285–90. doi: 10.1095/biolreprod62.5.1285. [DOI] [PubMed] [Google Scholar]
- 28.Fazleabas AT, Bell SC, Fleming S, Sun J, Lessey BA. Distribution of integrins and the extracellular matrix proteins in the baboon endometrium during the menstrual cycle and early pregnancy. Biol Reprod. 1997;56:348–56. doi: 10.1095/biolreprod56.2.348. [DOI] [PubMed] [Google Scholar]
- 29.Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10:1571–8. doi: 10.1093/humrep/10.6.1571. [DOI] [PubMed] [Google Scholar]
- 30.Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Investig. 1992;90:188–95. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994;62:497–506. [PubMed] [Google Scholar]
- 32.Aplin JD, Spanswick C, Behzad F, Kimber SJ, Vicovac L. Integrins β5, β3, αv are apically distributed in endometrial epithelium. Mol Hum Reprod. 1996;2:527–34. doi: 10.1093/molehr/2.7.527. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Xu BF, Chen QJ, Sun XX. Effects of hydrosalpinx on pinopodes, leukaemia inhibitory factor, integrin beta3 and MUC1 expression in the peri-implantation endometrium. Eur J Obstet Gynecol Reprod Biol. 2010;151:171–5. doi: 10.1016/j.ejogrb.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12:1393–8. doi: 10.1093/humrep/12.7.1393. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, Chang C, Liu Z, Chen LM, Chen Q. Treatment with low-dose aspirin increased the level LIF and integrin beta3 expression in mice during the implantation window. Placenta. 2010;31:1101–5. doi: 10.1016/j.placenta.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, 3rd, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27:881–8. doi: 10.1093/humrep/der452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledee-Bataille N, Lapree-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17:213–8. doi: 10.1093/humrep/17.1.213. [DOI] [PubMed] [Google Scholar]
- 38.Serafini PC, Silva ID, Smith GD, Motta EL, Rocha AM, Baracat EC. Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod Biol Endocrinol. 2009;7:30. doi: 10.1186/1477-7827-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serafini P, Rocha AM, Osorio CT, da Silva I, Motta EL, Baracat EC. Endometrial leukemia inhibitory factor as a predictor of pregnancy after in vitro fertilization. Intern J Gynaecol Obstet. 2008;102:23–7. doi: 10.1016/j.ijgo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Arici A, Engin O, Attar E, Olive DL. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in human endometrium. J Clin Endocrinol Metab. 1995;80:1908–15. doi: 10.1210/jcem.80.6.7775640. [DOI] [PubMed] [Google Scholar]
- 41.Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–55. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- 42.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertility and Sterility. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 43.Budwit-Novotny DA, McCarty KS, Sr, Cox EB, Soper JR, Mutch DG, Creasman WT, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–25. [PubMed] [Google Scholar]
- 44.Lessey BA. Assessment of endometrial receptivity. Fertil Steril. 2011;96:522–9. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- 45.Lessey BA, Castelbaum AJ. Integrins and endometriosis: fact or artefact? Hum Reprod. 1998;13:3578. doi: 10.1093/oxfordjournals.humrep.a019683. [DOI] [PubMed] [Google Scholar]
- 46.Creus M, Balasch J, Ordi J, Febregues F, Casamitjana R, Quinto L, et al. Integrin expression in normal and out-of-phase endometria. Hum Reprod. 1998;13:3460–8. doi: 10.1093/humrep/13.12.3460. [DOI] [PubMed] [Google Scholar]
- 47.Dimitriadis E, Menkhorst E, Salamonsen LA, Paiva P. Review: LIF and IL11 in trophoblast-endometrial interactions during the establishment of pregnancy. Placenta. 2010;31(Suppl):S99–104. doi: 10.1016/j.placenta.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Intern J Dev Biol. 2010;54:313–22. doi: 10.1387/ijdb.082772ed. [DOI] [PubMed] [Google Scholar]
- 49.Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One. 2011;6:e25288. doi: 10.1371/journal.pone.0025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albaghdadi AJ, Kan FW. Endometrial receptivity defects and impaired implantation in diabetic NOD mice. Biol Reprod. 2012;87:30. doi: 10.1095/biolreprod.112.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am J Reprod Immunol. 1998;39:137–43. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 52.Tawfeek MA, Eid MA, Hasan AM, Mostafa M, El-Serogy HA. Assessment of leukemia inhibitory factor and glycoprotein 130 expression in endometrium and uterine flushing: a possible diagnostic tool for impaired fertility. BMC Women Health. 2012;12:10. doi: 10.1186/1472-6874-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai HD, Chang CC, Hsieh YY, Lo HY. Leukemia inhibitory factor expression in different endometrial locations between fertile and infertile women throughout different menstrual phases. J Assist Reprod Genet. 2000;17:415–8. doi: 10.1023/A:1009457016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Y, Sun X, Yang X, Zhang J, Xue Q, Cai B, et al. Leukemia inhibitory factor is dysregulated in the endometrium and uterine flushing fluid of patients with adenomyosis during implantation window. Fertil Steril. 2010;94:85–9. doi: 10.1016/j.fertnstert.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Hum Reprod. 2006;21:3054–8. doi: 10.1093/humrep/del225. [DOI] [PubMed] [Google Scholar]
- 56.Mikolajczyk M, Skrzypczak J, Szymanowski K, Wirstlein P. The assessment of LIF in uterine flushing—a possible new diagnostic tool in states of impaired fertility. Reprod Biol. 2003;3:259–70. [PubMed] [Google Scholar]
- 57.Dimitriadis E, Stoikos C, Stafford-Bell M, Clark I, Paiva P, Kovacs G, et al. Interleukin-11, IL-11 receptoralpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. J Reprod Immunol. 2006;69:53–64. doi: 10.1016/j.jri.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 59.Lea RG, Sandra O. Immunoendocrine aspects of endometrial function and implantation. Reproduction. 2007;134:389–404. doi: 10.1530/REP-07-0167. [DOI] [PubMed] [Google Scholar]
- 60.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–61. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 61.Perrier d'Hauterive S, Charlet-Renard C, Berndt S, Dubois M, Munaut C, Goffin F, et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum Reprod. 2004;19:2633–43. doi: 10.1093/humrep/deh450. [DOI] [PubMed] [Google Scholar]
- 62.Hirata T, Osuga Y, Takamura M, Saito A, Hasegawa A, Koga K, et al. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertil Steril. 2011;96:113–7. doi: 10.1016/j.fertnstert.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 63.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Othman Eel D, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137:240–6. doi: 10.1016/j.ejogrb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Podgaec S, Abrao MS, Dias JA, Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–9. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 66.Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol. 2002;47:251–5. doi: 10.1034/j.1600-0897.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 67.Akoum A, Lemay A, Paradis I, Rheault N, Maheux R. Secretion of interleukin-6 by human endometriotic cells and regulation by proinflammatory cytokines and sex steroids. Hum Reprod. 1996;11:2269. doi: 10.1093/oxfordjournals.humrep.a019088. [DOI] [PubMed] [Google Scholar]
- 68.Von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6:627–34. doi: 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- 69.Velasco I, Acien P, Campos A, Acien MI, Ruiz-Macia E. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol. 2010;84:199–205. doi: 10.1016/j.jri.2009.11.004. [DOI] [PubMed] [Google Scholar]


