Abstract
OBJECTIVES
To assess vector competence (infection, dissemination and transmission) of Culex pipiens quinquefasciatus for Florida (FL) West Nile virus (WNV) isolates.
METHODS
West Nile virus isolates (WN-FL-03: NY99 genotype; WN-FL-05-558, WN-FL-05-2186, WN-FL-05-510: WN02 genotype) collected from different regions of FL were used for vector competence experiments in Cx. p. quinquefasciatus from Alachua County and Indian River County in FL. Mosquitoes from both colonies were fed blood containing 7.9 ± 0.2 log10 plaque-forming units WNV/ml ± SE and incubated at 28 °C for 14 days. Vector competence, including rates of infection, dissemination, and transmission, was compared between colonies for WN-FL-03 using chi-squared. Virus titres in bodies, legs and saliva were compared using ANOVA. Daily measurements of in vitro replication of WNV isolates were evaluated in Vero cells so that a standardised virus dose for each isolate could be delivered to mosquitoes.
RESULTS
Infection and dissemination rates were high (≥95%) and not affected by isolate or colony (infection, P = 0.679; dissemination, P = 0.799). Transmission rates were low (≤20%), detected in one colony and affected by isolate (P = 0.008). Body and leg titres differed between isolates (body titre, P = 0.031; leg titre, P = 0.044) and colonies (body titre, P = 0.001; leg titre, P = 0.013) while saliva titre did not differ between isolates (P = 0.462).
CONCLUSIONS
Variation in vector competence of mosquito populations may be attributed, in part, to exposures to WNV with genetic differences leading to different rates of replication in mosquitoes. Evaluation of vector competence for different WNV isolates may help us understand vector–virus interactions and, hence, the role of vectors in complex virus transmission cycles in nature.
Keywords: Culex, Florida, vector competence, West Nile virus
Introduction
Since the introduction of the NY99 genotype (Eastern US clade) of West Nile virus (WNV; family Flaviviridae: genus Flavivirus) into North America in 1999, genetic variations have occurred in the virus (e.g. Vanlandingham et al. 2008; Chisenhall & Mores 2009; Anez et al. 2013). To visualise genetic changes that may constitute different genotypes, phylogenetic trees based on nucleotide sequences from structural or non-structural regions in genes are created using maximum likelihood and/or Bayesian interpretations of probability. The mean nucleotide substitution rate is often used to determine the consistency of changes over time (e.g. Anez et al. 2013). Some studies indicate that changes in the WNV genotype have occurred due to genetic drift (e.g. Anez et al. 2013), while others postulate a new strain was introduced in the early 2000s (Ebel et al. 2004). In 2002, the WN02 genotype (North American clade) was characterised, showing one amino acid substitution (i.e. valine-to-alanine mutation in the envelope protein at amino acid 159; Val-Ala-159) and 13 silent nucleotide mutations different from the NY99 genotype (Davis et al. 2005). Another study found two additional amino acid substitutions that are likely fixed in the WN02 and WN03 genotypes discussed below (Anez et al. 2013). Others showed the same dominant WN02 genotype in 44 WNV isolates collected in Harris County, Texas (TX), from 2002 to 2006 (Davis et al. 2007) that remained stable from 2002 to 2009 (McMullen et al. 2011). It is hypothesised that fixation of the WN02 mutation (Val-Ala-159) in the genotype present in TX was likely promulgated primarily by mosquito-related (e.g. timing – abundant mosquitoes transmitted the virus containing the mutation) rather than virus-related (e.g. fitness) factors (Davis et al. 2007). In nine of 17 WN02 isolates collected in Harris County, TX from 2002 to 2009, parts of the WN02 genotype reverted to the NY99 genotype (although the Val-Ala-159 mutation characteristic of WN02 remained fixed) (McMullen et al. 2011), showing the dynamics of these virus populations. The Southwestern WN03 (SW/WN03) genotype first detected in Arizona, Colorado and northern Mexico is expanding its geographical range (e.g. California, Illinois, New Mexico, New York, North Dakota and TX) and could be replacing WN02 (McMullen et al. 2011). Within the SW/WN03 genotype, phylogenetic analysis indicates five separate groups detailed by McMullen et al. (2011). Isolates of the SW/WN03 genotype collected from TX from 2005 to 2009 cluster with isolates from Arizona and Colorado, and further studies are needed to evaluate how these changes may affect vector competence (McMullen et al. 2011). Mann et al. (2013) showed co-circulation of WN02 and WN03 along the US-Mexico border from 2005 to 2010, although increased surveillance in northern Mexico is needed to fully evaluate transmission in this region.
Both the NY99 and WN02 genotypes produce high mortality in birds (primarily family Corvidae); however, in mosquitoes, WN02 replicates faster than NY99 (Moudy et al. 2007) at warmer temperatures (Kilpatrick et al. 2008). Hence, it is hypothesised that the WN02 genotype outcompeted NY99 by 2004 (Snapinn et al. 2007). At higher temperatures (44 °C), most California isolates (N = 3) from the WN02 genotype replicated faster in vertebrate cells, while replication in one isolate was inhibited, indicating temperature effects were not consistent across isolates (Andrade et al. 2011).
In 2012, 48 states in the United States experienced a total of 5245 WN cases, including 2663 cases of neuroinvasive disease (Nasci 2013), although the infecting virus genotype(s) related to these cases has not been reported. This was the highest number of neuroinvasive cases since 2003, with one-third of cases occurring in TX (Nasci 2013). Florida has experienced human WNV cases each year since 2001, although small numbers (<100 cases/year) have been observed since 2001 (CDC 2013). The largest number of human cases in FL was experienced in 2003 (N = 95), it then fell until 2010 (N = 12) when case numbers began to rise. In 2012 (N = 73), FL experienced more than twice as many cases as in 2011 (N = 24); however, the state ranked 17 in the number of human cases reported to the CDC in 2012 (CDC 2013). The relatively low numbers of human WNV cases in FL could be attributed to multiple factors including differential vector competence of local mosquitoes contributing to low virus transmission, potential vertebrate amplification hosts with cross-protective immunity due to previous infection with WNV or related flavivirus St. Louis encephalitis virus (SLEV) (280 sentinel chicken and 0 human SLE cases reported in FL 2003–2013), and effective mosquito control in high risk areas, thereby reducing populations of potential vectors.
A phylogenetic analysis of WNV isolates collected in 2003 (N = 1) and 2005 (N = 8) from different locations in Florida (Chisenhall & Mores 2009) showed that the 2003 isolate was similar to the NY99 genotype, while all 2005 isolates were similar to the WN02 genotype (Val-Ala-159). Phylogenetic analysis based on the envelope sequence showed that most 2005 isolates clustered with the WN02 genotype with one isolate having two additional (G to A) nucleotide substitutions at 2209 and 2233 (Chisenhall & Mores 2009). Analysis based on two non-structural protein (NS3/NS4A) sequences showed the 2005 isolates clustering with the WN02 genotype (Chisenhall & Mores 2009). The same study showed variation in nucleotide and amino acid site differences when FL isolates and reference sequences from GenBank were compared; however, most of these differences were not consistent between isolates so did not represent a pattern. The current study utilises the 2003 FL isolate and four of the eight 2005 FL isolates (Isolate numbers: 510, 558, 1102, 2186) shown in Table 1 of Chisenhall and Mores (2009).
Culex spp. are considered the primary vectors of WNV in North America. In the southeastern US, 64.6% of WNV-positive mosquito pools are Culex pipiens quinquefasciatus Say (Andreadis 2012). Consequently, we evaluated the extent to which FL Cx. p. quinquefasciatus differed in infection, dissemination and transmission for several WNV isolates collected in FL in 2003 and 2005 (Vitek et al. 2008; Chisenhall & Mores 2009).
Materials and methods
Mosquitoes and virus
Culex pipiens quinquefasciatus originating from Alachua County (generation F56) and Indian River County (generation F25) FL were reared at 28 °C and maintained under a 14:10 (light:dark) cycle (Richards et al. 2009) with 20% sucrose provided ad libitum.
We used the following WNV isolates [2005 isolates were collected from pools of Culex nigripalpus and passaged once in African green monkey (Vero) cells]: (i) WN-FL-03 (Genbank accession number DQ983578), from a pool of Cx. nigripalpus collected in Indian River County, FL, in 2003, passaged five times in Vero cells and once in baby hamster kidney cells, (ii) WN-FL-05-558, collected in Manatee County, FL, in 2005, (iii) WN-FL-05-2186, collected in Indian River County, FL, in 2005, (iv) WN-FL-05-510, collected in Duval County, FL, in 2005 and (v) WN-FL-05-1102, collected in Duval County, FL, in 2005 (Vitek et al. 2008).
Growth curve in vitro
A preliminary time course experiment using the original field-collected Cx. nigripalpus pools (data not shown) determined the time point [i.e. between 1 and 2 days post-inoculation (dpi)] at which each isolate achieved approximately the same titre. Consequently, for the in vitro growth curve experiment shown here, samples of each WNV isolate with standardised titres (9.2 ± 0.1 log10 PFUeq WNV/ml previously frozen stock) were inoculated onto monolayers of Vero cells in two replicate 75-cm2 tissue-culture flasks at 35 °C and 5% CO2. Two negative control flasks were visually monitored for cytopathic effects (CPE). All flasks contained 12 ml of Medium 199 (with Earle’s salts, 10% foetal bovine serum, penicillin/streptomycin, mycostatin). Supernatant in WNV-infected flasks was sampled (0.1 ml) every 24 h at 1, 2, 3, 4, 5, 6 and 7 dpi. Samples were added to respective microcentrifuge tubes containing 0.9 ml of BA-1 diluent (standard buffer used in viral assays, see Lanciotti et al. 2000) and frozen at −80 °C until further analysis.
Mosquito infection
Twenty-four hours prior to feeding, 7–8 days old Cx. p. quinquefasciatus females were transferred to 1-l cardboard cages (Instawares, Kennesaw, GA) with mesh screening and provided water ad libitum in incubators at 28 °C. This temperature (28 °C) was chosen based on average daily temperatures obtained from the National Oceanic Administration Association (http://cdo.ncdc.noaa.gov/ulcd/ULCD) for an area of Florida with historically high WNV activity in sentinel chickens during arboviral early transmission phases. Cages were labelled according to virus treatment. Four WNV isolates (WN-FL-03, WN-FL-05-558, WN-FL-05-2186, and WN-FL-05-510) were used for vector competence experiments. Based on a preliminary in vitro time course experiment (data not shown) using the original field-collected Cx. nigripalpus pools, we inoculated Vero cells at staggered times for each isolate, took supernatant from each isolate at the time point determined to have approximately 9.2 log10 PFUeq/ml (different points in the growth curve but on the same calendar day) and used that supernatant to mix with blood for the in vivo experiment. Both mosquito colonies were exposed to WN-FL-03 while only the colony from Alachua County was exposed to the other three isolates. A 0.1 ml sample of each blood meal was added to 0.9 ml BA-1 diluent and stored at −80 °C until further processing to determine blood meal titre. Mosquitoes were allowed to feed on pledgets soaked in a mixture of virus and defibrinated bovine blood (warmed for 10 min at 35 °C) (Hemostat, Dixon, CA) for 30 min. We used one part virus supernatant and nine parts blood for each blood meal. Subsequent to feeding, mosquitoes were immobilised with cold and fully engorged mosquitoes were transferred to 1-l cardboard cages with mesh screening and placed in incubators at 28 °C. Mosquitoes were provided 20% sugar solution and water ad libitum and incubated for 14 days. Unfed or partially engorged mosquitoes were discarded.
Mosquito processing
At the end of the 14-day incubation period (IP), surviving mosquitoes were removed from cages and anesthetised with cold. For each mosquito, legs and wings were detached and legs transferred to an individual sample tube containing 1.0 ml BA-1 diluent with two 4.5-mm zinc-plated beads. Saliva was collected using standard conditions (Anderson et al. 2010b), that is live mosquitoes were allowed to salivate for 30–45 min into capillary tubes containing immersion oil. When the salivation period concluded, bodies and saliva were transferred to separate tubes labelled for each individual mosquito. All sample tubes contained 0.9 ml BA-1 diluent with two 4.5-mm zinc-plated beads.
Virus detection
Samples were homogenised at 25 Hz for 3 min (Tissue-Lyser; Qiagen, Inc., Valencia, CA) and centrifuged at 4 °C and 3148 g for 4 min. Nucleic acids were extracted using the MagNA Pure LC System and Total Nucleic Acid Isolation Kit (Roche, Mannheim, Germany). The amount of viral RNA [plaque-forming unit equivalents (PFUeq)] in each sample (i.e. blood meal, body, legs, saliva) was determined using the LightCycler® 480 system (Roche) and Superscript III One-Step Quantitative RT-PCR kit (Invitrogen, Carlsbad, CA) for quantitative realtime TaqMan RT-PCR (qRT-PCR). We used an established protocol relating a standard curve to plaque assay quantification of WNV (Richards et al. 2007).
The infection rate was the percentage of all mosquitoes tested having infected bodies. The dissemination rate was the percentage of mosquitoes with infected bodies that also had infected legs. The transmission rate was the percentage of mosquitoes with infected legs that also had infected saliva.
Statistical analysis
Virus growth during the 7 days IP in vitro was visualised using scatterplots (Figure 1). Analysis of variance (ANOVA, PROC GLM) was used to evaluate differences in titres between WNV isolates over time. If significant differences were observed, then a Duncan test was used to determine differences in the means. Chi-square tests were used to test for significant differences (P < 0.05) in infection, dissemination and transmission rates between virus isolates (SAS Institute, Cary, NC). When sample sizes were <5, Fisher’s exact test (P < 0.05) was used instead of chi-square. Titres of bodies, legs, and saliva were log-transformed [log (x + 1)] prior to using ANOVA to determine significant differences (P < 0.05) in titres between isolates and colonies. When significant differences were observed, Duncan tests were used to determine which means were significantly different (P < 0.05).
Figure 1.
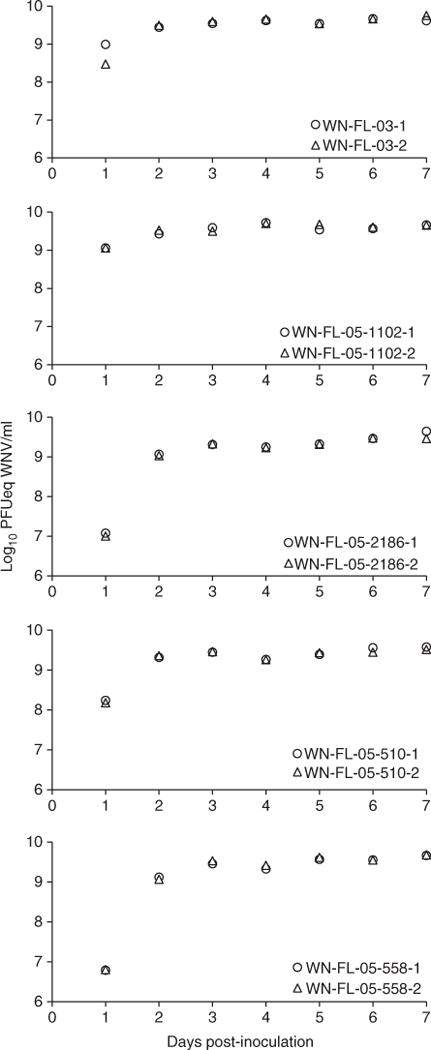
Growth curves of West Nile virus (WNV) in Vero cells (N = 2 replicates per time point indicated by different symbols) incubated at 35 °C after inoculation with WNV isolates (WN-FL-03, WN-FL-05-1102, WN-FL-05-2186, WN-FL-05-510, WN-FL-05-558).
Results
In vitro virus growth kinetics
We conducted in vitro assays to determine the extent to which virus growth of FL isolates (isolates of NY99 and WN02 genotypes characterised in Chisenhall & Mores 2009) differed over time and primarily to generate virus stocks of approximately equal titre to blood feed mosquitoes in vector competence assays. Tissue-culture flasks were inoculated with a standardised virus dose (9.2 ± 0.1 log10 PFUeq WNV/ml) of each isolate. No CPE were observed in the uninfected flasks during the experiment. Figure 1 shows the time course of WNV growth for different isolates. Means of viral titres were compared between isolates over time (Table 1). Means of viral titres for the entire 7 days sampling time period were significantly different between isolates. There was a significant difference in mean viral titres of isolates at different days post-infection (Table 1); however, when separate analyses were carried out for each time point, significant (all P < 0.05) titre differences were observed between isolates for every time point except 7 dpi (F = 1.59, df = 4, 5; P = 0.308). Viral titres of isolates changed significantly over the course of the experiment (isolate × dpi interaction) (Table 1). Viral titres of WN-FL-03 were significantly higher (P < 0.05) than other isolates each time point from 1 to 6 dpi except for WN-FL-05-558 that was highest at 5 dpi.
Table 1.
Analysis of variance showing differences in the mean titres (log10 PFUeq WNV/ml) of virus supernatant between isolates in vitro at different dpi (sampled every day for 7 days)
| Variable | df (numerator, denominator) | F | P |
|---|---|---|---|
| Isolate | 4, 34 | 120.68 | <0.0001 |
| Dpi | 6, 34 | 526.88 | <0.0001 |
| Isolate × dpi | 24, 34 | 33.77 | <0.0001 |
dpi, days post-inoculation; WNV, West Nile virus.
Virus titre of blood meal and effects of virus isolate on vector competence
Mosquitoes were fed blood meals containing 7.9 ± 0.2 log10 PFUeq WNV/ml (one part virus supernatant: nine parts blood). Titres of WNV for each isolate were determined in in vitro assays described previously; hence, no significant variation was observed in blood meal titres between isolates (F = 0.32, df = 3, 4; P = 0.812). Infection, dissemination and transmission rates are shown in Table 2. Infection and dissemination rates were high (≥95%) and not significantly affected by isolate (infection rate: X2 = 1.52, df = 3, P = 0.679; dissemination rate: X2 = 1.04, df = 3, P = 0.791) or colony (infection rate: X2 =1.03, df=1, P = 0.311; dissemination rate: X2 = 0.065, df = 1, P = 0.799). Transmission was observed in mosquitoes fed WN-FL-05-558 and WN-FL-05-510 and rates were significantly different between isolates (transmission rate: Fisher’s exact P = 0.008). Significant differences were observed in WNV titres of body and leg tissues between isolates and colonies, but not saliva (Table 3, Figure 2).
Table 2.
The rates of infection (% with WNV-positive bodies), dissemination (% infected with WNV-positive legs) and transmission (% disseminated with WNV-positive saliva) for Culex pipiens quinquefasciatus orally infected with different WNV isolates and incubated at 28 °C for 14 days
| WNV Isolate | WNV Genotype | Mosquito colony | Number of tested | Number of infected (%) | Number of disseminated (%) | Number of transmitted (%) |
|---|---|---|---|---|---|---|
| WN-FL-03 | NY-99 | Alachua | 20 | 19 (95) | 19 (100) | 0 (0) |
| WN-FL-03 | NY-99 | Indian River | 20 | 20 (100) | 19 (95) | 0 (0) |
| WN-FL-05-558 | WN02 | Alachua | 20 | 20 (100) | 20 (100) | 4 (20) |
| WN-FL-05-2186 | WN02 | Alachua | 20 | 20 (100) | 19 (95) | 0 (0) |
| WN-FL-05-510 | WN02 | Alachua | 20 | 20 (100) | 19 (95) | 1 (5) |
WNV, West Nile virus.
Table 3.
Analysis of variance showing differences in the mean titres (log10 PFUeq WNV/ml) of WNV in bodies, legs and saliva between Culex pipiens quinquefasciatus colonies (Alachua and Indian River Counties) fed different virus isolates (WN-FL-03, WN-FL-05-558, WN-FL-05-2186 and WN-FL-05-510) and incubated at 28 °C for 14 days
| Variable | df (numerator, denominator) | F | P |
|---|---|---|---|
| Body titre | |||
| Virus isolate | 3, 94 | 3.04 | 0.031 |
| Mosquito colony | 1, 94 | 11.44 | 0.001 |
| Leg titre | |||
| Virus isolate | 3, 91 | 2.81 | 0.044 |
| Mosquito colony | 1, 91 | 6.47 | 0.013 |
| Saliva titre | |||
| Virus isolate | 1, 3 | 0.71 | 0.462 |
| Mosquito colony | – | – | – |
WNV, West Nile virus.
Figure 2.
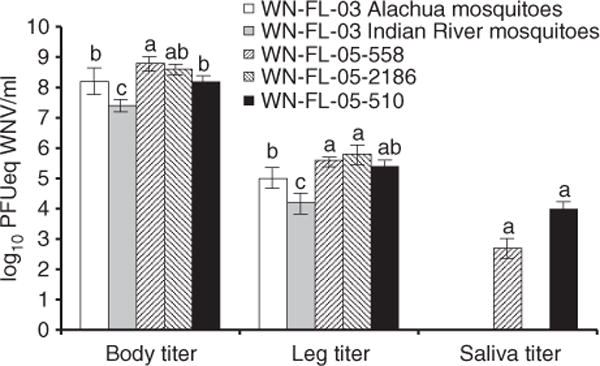
Titres (log10 PFUeq WNV/ml) ± standard error of WNV in tissues of Culex pipiens quinquefasciatus orally infected with different WNV isolates (WN-FL-03, WN-FL-05-2186, WN-FL-05-510 and WN-FL-05-558) and incubated at 28 °C for 14 days. Treatment groups with the same letter in the same group are not significantly different by means comparison. WNV, West Nile virus.
Discussion
Patterns of observed virus titre differences between isolates (i.e. infectivity) differed between in vitro and in vivo experiments, although we did not monitor the time course of mosquito infection, so caution is advised when comparing the results of these separate experiments. Our finding that the WN-FL-03 (NY99 genotype) isolate replicated to a higher degree at most time points in vitro did not lead to greater vector competence in Cx. p. quinquefasciatus after a 14-days IP at 28 °C. Another study using the same WN-FL-03 isolate (fed lower dose of 6.7 ± 0.1 log10 PFUeq WNV/ml) used here showed a range of 86–88% infection and 77–79% dissemination in FL mosquitoes (earlier generations of the same Cx. p. quinquefasciatus colonies used here from Alachua and Indian River Counties that were 7 days old at time of blood feeding) at 28 °C (Richards et al. 2010). In the current study, Cx. p. quinquefasciatus showed no barriers to midgut infection or escape of virus from the midgut to legs (≥95% infection and dissemination) for any WNV isolate (fed 7.9 ± 0.2 log10 PFUeq WNV/ml). It follows that the higher dose used here as compared to other studies that show infection and dissemination rates affected by virus dose in artificially delivered blood meals (e.g. Richards et al. 2007, 2010; Anderson et al. 2010a, b) would result in higher infection and dissemination rates. Viral titres of bodies and legs were influenced by isolates and mosquito colonies used here. Isolates of the WN02 genotype (WN-FL-05 isolates) exhibited higher titres in mosquito bodies and legs than the one isolate of the NY99 genotype (WN-FL-03) used here. This may indicate a higher level of virus replication in mosquito tissues infected with the WN02 genotype compared with the NY99 genotype, although only one isolate of the NY99 genotype was tested here and this may not be representative of other isolates of this genotype. However, high titres in bodies and legs did not lead to higher transmission rates, as WN-FL-05-2186 had similar body and leg titres but showed no transmission in this study. Our results support other reports of WN02 replicating more efficiently than NY99 in Culex pipiens pipiens and Culex tarsalis (Ebel et al. 2004; Moudy et al. 2007; Kilpatrick et al. 2008). This trend is not always the case as no vector competence differences were observed in Cx. tarsalis infected with each of these two genotypes; however, different WNV isolates were used in this case (Anderson et al. 2012) and may further demonstrate the complexities of vector-virus interactions leading to virus transmission in the laboratory and nature. Based on the variation observed here and in other studies examining Culex–WNV interactions (e.g. Richards et al. 2010), we expect that testing additional isolates of either the WN02 or NY99 genotype would show differences in virus replication in mosquito tissues and that these dynamics would change with different mosquito populations.
Others have shown genetic (i.e. nucleotide substitution) differences between the isolates tested here from different regions in FL (Chisenhall & Mores 2009). Hence, even within FL, genetic diversity exists between WNV populations and may impact epidemiologic cycles if these differences influence vector-virus interactions. Others report that RNA viruses, such as WNV, naturally exist as genetically diverse, but related quasispecies such that selective pressures can affect genetic variance (Jerzak et al. 2005; Anez et al. 2013). Higher WNV (NY99 genotype) diversity has been shown in mosquitoes compared with birds, possibly related to the longer duration of infection in mosquitoes (Jerzak et al. 2005), although a contrary relationship has been shown for dengue virus (serotype three), human hosts and mosquito vectors (Lin et al. 2004). Invertebrate and vertebrate evaluations of WNV isolates in FL and other regions should continue to interpret fluctuations in the occurrence of human cases. The complexity of biological and environmental conditions affecting vector–virus interactions will undoubtedly continue to promote genetic changes in WNV (Brault 2009). Knowledge of these interactions is important for determining risk and targeting control to the most dangerous mosquito populations.
It has been proposed that more diverse WNV populations exhibit higher fitness in mosquitoes because invertebrate immune response mechanisms such as RNA interference may be less effective against co-circulating genotypes (Brackney et al. 2009, Fitzpatrick et al. 2010). The WN02 genotype appears to have replaced the NY99 genotype in North America, although no conclusive evidence has been presented yet showing how this occurred. It is possible that a greater diversity and ability of mosquitoes to transmit the WN02 compared with NY99 genotype has contributed to its success (e.g. Ebel et al. 2004; Moudy et al. 2007). A recent phylogenetic analysis compared 363 WNV sequences in GenBank to 32 sequences from WNV samples collected across the US from 1999 to 2011 (Anez et al. 2013). The same study concluded that genetic variability in WNV (including all known circulating genotypes) increased from 2000 to 2002 and continued to increase in diversity (with a brief dip in diversity from 2002 to 2003) until stabilising in approximately 2005. Experiments studying competitive co-infections of different WNV genotypes in vertebrate and invertebrate hosts are needed to evaluate this potential route of displacement. When assessing risk for WNV and other emerging arboviruses, virus adaptation to increase survival and replication in vector hosts will continue to play a major role in epidemiology (Kilpatrick 2011). The dynamics of WNV genetics and the increase in WNV neuroinvasive cases in recent years coupled with the reduction in funding for arboviral surveillance and mosquito control highlights the need to remain watchful of this invasive pathogen.
Acknowledgments
This research was supported by the Florida Department of Agriculture and Consumer Services Grant 012018, and National Institutes of Health Grant AI-42164. We thank Christopher Vitek for collecting the WNV isolates and two anonymous reviewers for their helpful comments that improved the manuscript.
References
- Anderson SL, Richards SL, Tabachnick WJ, Smartt CT. The effects of West Nile virus dose on temporal progression of vector competence in Culex pipiens quinquefasciatus Say (Diptera: Culicidae) Journal of the American Mosquito Control Association. 2010a;26:103–107. doi: 10.2987/09-5926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SL, Richards SL, Smartt CT. A simple method for examining arbovirus transmission in mosquitoes. Journal of the American Mosquito Control Association. 2010b;26:108–111. doi: 10.2987/09-5935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JF, Main AJ, Cheng G, Ferrandino FJ, Fikrig E. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. American Journal of Tropical Medicine and Hygiene. 2012;86:134–139. doi: 10.4269/ajtmh.2012.11-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade CC, Maharaj PD, Reisen WK, Brault AC. North American West Nile virus genotype isolates demonstrate differential replicative capacities in response to temperature. Journal of General Virology. 2011;92:2523–2533. doi: 10.1099/vir.0.032318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. Journal of the American Mosquito Control Association. 2012;28:137–151. doi: 10.2987/8756-971X-28.4s.137. [DOI] [PubMed] [Google Scholar]
- Anez G, Grinev A, Chancey C, et al. Evolutionary dynamics of West Nile virus in the United States, 1999–2011: phylogeny, selection pressure and evolutionary time-scale analysis. PLoS Neglected Tropical Diseases. 2013;7:e2245. doi: 10.1371/journal.pntd.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney DE, Beane JE, Ebel GD. RNAi targeting of West Nile virus in mosquito midguts promotes virus diversification. PLoS Pathogens. 2009;5:e1000502. doi: 10.1371/journal.ppat.1000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Veterinary Research. 2009;40:43. doi: 10.1051/vetres/2009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. ArboNET Maps. 2013 http://www.cdc.gov/ncidod/dvbid/westnile/usgs_frame.html (accessed 28 May 2013)
- Chisenhall DM, Mores CN. Diversification of West Nile virus in a subtropical region. Virology Journal. 2009;6:106. doi: 10.1186/1743-422X-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Davis CT, Li L, May FJ, et al. Genetic stasis of dominant West Nile virus genotype, Houston, Texas. Emerging Infectious Diseases. 2007;13:601–604. doi: 10.3201/eid1304.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. American Journal of Tropical Medicine and Hygiene. 2004;71:493–500. [PubMed] [Google Scholar]
- Fitzpatrick KA, Deardorff ER, Pesko K, et al. Population variation of West Nile virus confers a host-specific fitness benefit in mosquitoes. Virology. 2010;404:89–95. doi: 10.1016/j.virol.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzak G, Bernard KA, Kramer LD, Ebel GD. Genetic variation in West Nile virus from naturally infected mosquitoes and birds suggests quasispecies structure and strong purifying selection. Journal of General Virology. 2005;86:2175–2183. doi: 10.1099/vir.0.81015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. Globalization, land use, and the invasion of West Nile virus. Science. 2011;334:323–327. doi: 10.1126/science.1201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. Journal of Clinical Microbiology. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SR, Hsieh SC, Yueh YY, et al. Study of sequence variation of dengue type 3 virus in naturally infected mosquitoes and human hosts: Implications for transmission and evolution. Journal of Virology. 2004;78:12717–12721. doi: 10.1128/JVI.78.22.12717-12721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BR, McMullen AR, Guzman H, Tesh RB, Barrett ADT. Dynamic transmission of West Nile virus across the United States-Mexican border. Virology. 2013;436:75–80. doi: 10.1016/j.virol.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen AR, May FJ, Li L, et al. Evolution of new genotype of West Nile virus in North America. Emerging Infectious Diseases. 2011;17:785–793. doi: 10.3201/eid1705.101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy RM, Meola MA, Morein LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. American Journal of Tropical Medicine and Hygiene. 2007;77:365–370. [PubMed] [Google Scholar]
- Nasci RS. Monitoring and controlling West Nile virus: are your prevention practices in place? Journal of Environmental Health. 2013;13(75):42–43. [PubMed] [Google Scholar]
- Richards SL, Mores CN, Lord CC, Tabachnick WJ. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector-Borne and Zoonotic Disease. 2007;7:629–636. doi: 10.1089/vbz.2007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. American Journal of Tropical Medicine and Hygiene. 2009;81:264–272. [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Lord CC, Pesko KA, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for West Nile virus. American Journal of Tropical Medicine and Hygiene. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapinn KW, Holmes EC, Young DS, Bernard KA, Kramer LD, Ebel GD. Declining growth rate of West Nile virus in North America. Journal of Virology. 2007;81:2531–2534. doi: 10.1128/JVI.02169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandingham DL, McGee CE, Klinger KA, Galbraith SE, Barrett ADT, Higgs S. Short report: comparison of oral infectious dose of West Nile virus isolates representing three distinct genotypes in Culex quinquefasciatus. American Journal of Tropical Medicine and Hygiene. 2008;79:951–954. [PMC free article] [PubMed] [Google Scholar]
- Vitek CJ, Richards SL, Mores CN, Day JF, Lord CC. Arbovirus transmission by Culex nigripalpus in Florida, 2005. Journal of Medical Entomology. 2008;45:483–493. doi: 10.1603/0022-2585(2008)45[483:atbcni]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


