Abstract
For more than two decades, genetically engineered mouse models have been key to our mechanistic understanding of tumorigenesis and cancer progression. Recently, the massive quantity of data emerging from cancer genomics studies has demanded a corresponding increase in the efficiency and throughput of in vivo models for functional testing of putative cancer genes. Already a mainstay of cancer research, recent innovations in RNA interference (RNAi) technology have extended its utility for studying gene function and genetic interactions, enabling tissue-specific, inducible and reversible gene silencing in vivo. Concurrent advances in embryonic stem cell (ESC) culture and genome engineering have accelerated several steps of genetically engineered mouse model production and have facilitated the incorporation of RNAi technology into these models. Here, we review the current state of these technologies and examine how their integration has the potential to dramatically enhance the throughput and capabilities of animal models for cancer.
INTRODUCTION
Genetically engineered mouse models (GEMMs), have long served as invaluable tools for cancer biologists. The ability to infer gene function in vivo based on gain-of-function and loss-of-function phenotypes has led to major insights into the molecular mechanisms that drive tumor initiation, progression, and metastasis (Jonkers and Berns 2002; Francia et al. 2011). GEMMs have also proved to be essential in the preclinical setting, aiding in the development of new therapeutic agents and in characterization of drug resistance mechanisms (Sharpless and DePinho 2006; Singh et al. 2012). As our understanding of cancer has deepened, the technology used to model cancer has correspondingly become more sophisticated, employing the use of tissue-specific and/or -inducible promoters to express or ablate a gene of interest in a precise tissue or cellular subset. Although these GEMMs excel in their ability to closely recapitulate the genetics, signaling pathways, and histopathology of human cancers, they are hampered by lengthy and costly breeding requirements to generate sufficiently large experimental cohorts.
Now, a major challenge is to increase the speed and throughput capability of mouse model development in order to better accommodate the deluge of data emerging from large-scale oncogenomics efforts. Research consortia and individual laboratories are in the process of characterizing thousands of somatic mutations, epigenetic changes, and copy number variations in an extensive array of human tumors (Cancer Genome Atlas Research Network 2011) and cancer cell lines (Barretina et al. 2012). The identified alterations require functional validation to distinguish “driver” mutations, which confer a fitness advantage to the tumor, from “passenger” mutations, which occur because of a basal rate of mutation. Although in vitro experiments can be used to study the effect of a genetic modification on cell growth and signaling, cell culture conditions cannot fully recapitulate the endogenous tumor microenvironment and interactions with the immune system that can dictate tumor growth and response to therapy (Gilbert and Hemann 2010; Provenzano et al. 2012). Therefore, the ability of mouse models to accurately recapitulate these features of human cancer is critical for both basic science and preclinical applications.
A number of recent advances in RNA interference (RNAi) technology, embryonic stem (ES) cell culture, and genetic manipulation have led to the development of mouse models that can address these challenges. Over the last decade, RNAi has emerged as a key component of the molecular genetics toolbox available for both loss-of-function analyses and forward genetic screens. Continuing expansion of RNAi technology has enabled its use in conjunction with traditional GEMMs and mosaic models of cancer to provide reversible and regulatable systems for analyzing gene function in tumorigenesis in vivo. This article will discuss these methodologies and how they can be integrated as a flexible and powerful platform for modeling cancer in mice.
RNAi ENABLES REVERSIBLE REGULATION OF GENE EXPRESSION IN VIVO
RNAi operates through a highly conserved mechanism of sequence-specific post-transcriptional gene silencing triggered by the presence of double-stranded RNA (dsRNA). In animals, the RNAi program within somatic tissues is primarily regulated by microRNAs (miRNAs), small noncoding RNAs of 20–25 nucleotides in length transcribed by RNA polymerase II (Lee et al. 2004; Bartel 2009). Mature miRNAs are derived from a longer polyadenylated primary miRNA (pri-miRNA) transcript (Cai et al. 2004) through a series of cleavage steps. The Drosha/DGCR8 complex first cleaves the pri-miRNA in the nucleus to generate a stem loop pre-miRNA structure with a 2-nucleotide 3′ overhang that is exported to the cytoplasm (Lee et al. 2003; Denli et al. 2004; Gregory et al. 2004). The double-stranded pre-miRNA is then cleaved by the ribonuclease Dicer to produce the mature miRNA (Bernstein et al. 2001; Hutvágner et al. 2001; Hutvágner and Zamore 2002). The miRNA duplex is subsequently separated into single strands and the guide strand is loaded into the RNA-induced silencing complex (RISC). There, it pairs with complementary mRNAs and directs their degradation (Meister et al. 2004; Yekta et al. 2004) or translational silencing (Filipowicz et al. 2008). Other dsRNAs undergo analogous processing by Dicer to generate small interfering RNA (siRNA) effector duplexes that are also incorporated into RISC (Bernstein et al. 2001; Hammond et al. 2001).
Experimental RNAi takes advantage of this pathway by introducing exogenous dsRNAs designed to complement (and thus silence) genes of interest. Such engineered RNAi triggers can be introduced at several stages of the pathway (Fig. 1): Short synthetic siRNA duplexes that mimic the products of Dicer cleavage and can directly incorporate into RISC, long dsRNAs that require processing by Dicer, and short hairpin RNAs (shRNAs) that resemble pre-miRNAs and also undergo Dicer-mediated cleavage. Historically, long dsRNAs were first used to knock down genes in vivo in Caenorhabditis elegans (Fire et al. 1998) and Drosophila melanogaster (Kennerdell and Carthew 1998). These organisms proved highly amenable to loss-of-function analysis using RNAi in vivo, at least in part because of the relative ease of delivering dsRNAs.
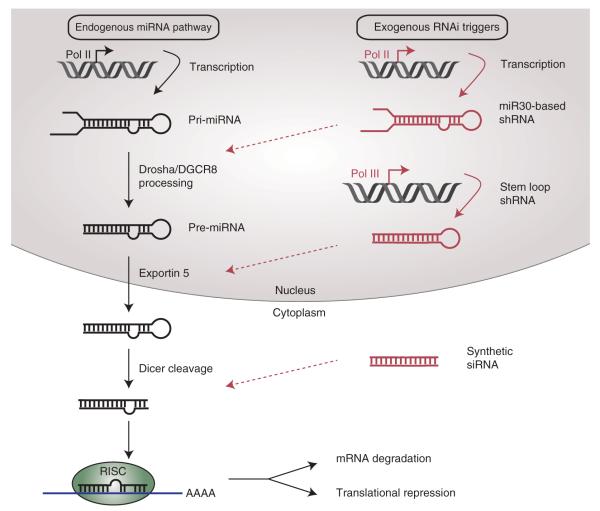
FIGURE 1.
MicroRNA biogenesis and introduction of exogenous RNAi triggers. Endogenous miRNAs are usually transcribed by RNA Pol II to produce primary miRNA transcripts (pri-miRNA). Pri-miRNAs are processed in the nucleus by the Drosha/DGCR8 complex to produce precursor miRNAs (pre-miRNAs) that are subsequently exported to the cytoplasm (left panel). Cytoplasmic pre-miRNAs undergo further cleavage by Dicer, yielding a 22-nucleotide double-stranded RNA duplex that is incorporated into the RISC complex to mediate mRNA silencing. Exogenous RNAi triggers (right panel, in red) can mimic each of these stages: miR30-based shRNAs are incorporated into the pathway at the Drosha/DGCR8 processing step, stem loop shRNAs resemble pre-miRNAs and are exported to the cytoplasm, and synthetic siRNA duplexes mimic Dicer cleavage products.
RNAi SYSTEMS FOR MAMMALIAN CELLS
In both C. elegans and Drosophila, the combination of RNAi with genetic mutations enables the investigation of complex genetic interactions and the description of signaling networks. However, mammalian systems present distinct challenges in achieving effective RNAi that have necessitated the development of a variety of specialized tools. In mammalian cells, transfection of dsRNA above 30 base pairs in length induces an antiviral interferon-mediated response that inhibits protein synthesis (Gil and Esteban 2000), necessitating the use of shorter dsRNA species. Synthetic siRNAs circumvent this obstacle and act as potent induces of gene silencing in mammalian cells (Elbashir et al. 2001), but their transient effects make them less suitable for long-term studies in proliferating cells. Additionally, siRNAs are prone to eliciting off-target effects when transfected at concentrations sufficient for knockdown (Persengiev et al. 2004). shRNAs consisting of a guide strand and an antisense complementary strand separated by a spacer (or loop) region can be stably introduced into mammalian cells by plasmid transfection (Brummelkamp et al. 2002a; Paddison et al. 2002) or transduction with retroviral vectors that incorporate into the host genome (Brummelkamp et al. 2002b). The shRNA can then be transcribed from an RNA Pol III promoter within the vector, such as U6 or H1, to generate a 50–70-nucleotide hairpin structure. The hairpin product is then recognized by Dicer and cleaved into siRNAs that incorporate into RISC. RNA Pol III promoters have been widely used because of their efficient transcription in many cell types, well-defined start sites and ability to generate nonpolyadenylated transcripts (Brummelkamp et al. 2002a). Stem-loop shRNAs can also be expressed in an inducible fashion using the tetracycline repressor system in conjunction with modified tet-responsive U6 and H1 promoters (Czauderna et al. 2003; Matsukura et al. 2003).
Alternatively, synthetic RNAi trigger sequences can be inserted within the context of an endogenous miRNA backbone (Zeng et al. 2002, 2005), with miR30 being the most common. This configuration is more efficiently processed via the miRNA biogenesis pathway and produces up to 12-fold more mature siRNA species than traditional stem-loop shRNAs (Silva et al. 2005). Indeed, miR30-based shRNAs present at single copy in the genome can efficiently suppress their target mRNAs (Dickins et al. 2005). This property makes miR30-based shRNAs particularly suitable for in vivo use, where the shRNA is expressed as a transgene (as discussed below), and reduces the likelihood of off-target effects. Importantly, as endogenous miRNAs are transcribed by RNA Pol II (Lee et al. 2004), expression of these miR30-based shRNAs can be driven from any Pol II promoter of choice, thereby allowing for tissue-specific gene silencing (Dickins et al. 2005; Stegmeier et al. 2005). The ability to utilize Pol II promoters also enables integration of RNAi with the widely used tetracycline-inducible Tet-on/Tet-off systems (Gossen et al. 1995). Thus, the miR30-based shRNA system provides a flexible approach for inducible and reversible suppression of genes for in vitro and in vivo applications. Both miR30-based and traditional stem-loop shRNA constructs can also be multiplexed by engineering vectors carrying two or more shRNAs in tandem (Cheng et al. 2009; Chicas et al. 2010). Coordinate silencing of gene pairs can serve as an important tool to assess the cooperation between genes involved in tumorigenesis, to dissect the functions of redundant genes, or to identify targets for combination therapies.
The adoption of RNAi to analyze gene function in mammalian systems has been further facilitated by a number of technological advances, including genome-wide shRNA libraries for mouse and human genes. Such libraries are available for stem-loop (Paddison et al. 2004) and miR30-based shRNAs (Silva et al. 2005) in retroviral vectors. Additionally, libraries constructed in lentiviral vectors (Stegmeier et al. 2005; Moffat et al. 2006) enable transduction of nondividing cells and tissues. shRNAs can also be easily subcloned from library plasmids to any other vector of choice, including tetracycline-inducible constructs (Chang et al. 2006).
In addition to existing libraries, researchers can rapidly generate custom shRNA libraries using on-chip oligonucleotide synthesis followed by PCR cloning into recipient vectors (Paddison et al. 2004). This approach can be particularly useful for producing smaller pathway-specific shRNA libraries, comprising a greater number of shRNAs per gene.
MODELING CANCER IN VIVO USING TRANSPLANT-BASED GEMMs AND RNAi
The use of RNAi to silence gene expression in cell transplant–based models of cancer is a natural extension of its application in cell culture. These models involve the transplantation of stem, progenitor, or tumor cells from a tissue of interest into a recipient host. The cells can be derived from a wild-type or genetically engineered tumor-prone donor and may be manipulated ex vivo prior to transplantation. Orthotopic transplantation into syngeneic recipient mice can be used to model interactions between the tumor cells, tissue environment, and immune system. Such models have been developed for breast (Evers et al. 2010), liver (Zender et al. 2006), pancreas (Agbunag and Bar-Sagi 2004), and the hematopoietic system (Hemann et al. 2003).
Our group first illustrated the integration of RNAi technology with transplant-based cancer models by demonstrating that knockdown of p53 accelerates tumorigenesis in an Eμ-Myc-driven model of lymphoma (Hemann et al. 2003). To do so, we transduced hematopoietic progenitor cells from Eμ-Myc mice with retroviral vectors expressing a series of stem-loop shRNAs targeting p53. We found that upon transplantation into mice, the degree of acceleration of lymphomagenesis correlated with the level of p53 knockdown. This study indicated that in vitro transduction and RNAi silencing could subsequently be used to probe cancer phenotypes in vivo. Numerous comparable studies have since been performed in a variety of cell types and cancer models (Ma et al. 2012). Although this approach is applicable to xenografts using human cancer cell lines, the use of cells derived from murine tissue allows for experiments to be conducted in a defined genetic background. Additionally, murine cells can be transplanted orthotopically into syngeneic recipients, thereby permitting analysis of tumor cell interactions with an intact immune system (Chang et al. 2012).
Early iterations of RNAi (both in vitro and in vivo) represented a major advance in loss-of-function genetics, though they had their limitations. In particular, the limited potency of first-generation RNA Pol III–driven shRNAs often required a high copy number to achieve efficient knockdown (Stegmeier et al. 2005). This is not ideal, as high shRNA expression has been associated with toxicity due to saturation of the endogenous miRNA machinery (Grimm et al. 2006). Additionally, despite high infection efficiencies, retroviral shRNAs were frequently subject to epigenetic silencing (Lund et al. 1996). The miR30-based shRNAs driven by RNA Pol II promoters overcame several of these limitations. In particular, the ability of miR30-based shRNAs to enter the miRNA pathway at an early stage and to suppress targets at single copy reduces their impact on the endogenous miRNA machinery (Premsrirut et al. 2011). Furthermore, a fluorescent reporter can be inserted between the Pol II promoter and the miR30 shRNA (Zuber et al. 2010). As the fluorescent protein and the shRNA are transcribed together, the fluorescence acts as a faithful readout of shRNA expression rather than vector integration, thereby allowing one to sort for cells specifically expressing the shRNA of interest. This is particularly useful in conjunction with tetracycline-inducible systems, in which vector integration may not correlate perfectly with shRNA expression (Zuber et al. 2010).
shRNA potency has been further improved through better understanding of miRNA biogenesis and thermodynamics, leading to improvements in RNAi design and prediction algorithms (Matveeva et al. 2010). A complementary approach for identifying potent shRNAs allows for functional identification of effective shRNAs using a large-scale fluorescence-based sensor assay (Fellmann et al. 2011). These advances have made it possible to achieve selective and potent gene knockdown in a wide variety of cell types, using sufficiently low shRNA levels to limit off-target effects (Premsrirut et al. 2011).
miR30-based shRNAs are now being used to analyze cancer phenotypes in vivo in both constitutive and inducible systems. For instance, three recent studies from our group and others have identified Brd4, Myb, and the Polycomb repressive complex as essential for the maintenance of AML driven by the MLL-AF9 oncoprotein and NrasG12D (Zuber et al. 2011a; Zuber et al. 2011b; Shi et al. 2012). In these studies, leukemia cells expressing the reverse tetracycline transactivator rtTA3 were transduced with miR30-based shRNAs in a tetracycline-inducible vector (Zuber et al. 2010) and retransplanted into secondary recipients. After disease establishment, treatment with the tetracycline analog doxycycline induced shRNA expression and thus gene knockdown. Conversely, mice transplanted with liver progenitor cells harboring a tetracycline-regulated shRNA against p53 develop liver carcinomas that involute upon p53 restoration via Dox withdrawal (Xue et al. 2007). The induction of shRNA expression after tumorigenesis enabled the role of the above genes in disease maintenance to be specifically addressed and controlled for variability in initial engraftment. The inducible shRNA system can also be applied to questions of oncogene addiction by observing the effect of “turning off” the expression of the oncogene after tumor establishment. Identification of oncogenes required for the maintenance of a particular cancer type are important because this knowledge is critical to informing potential drug targets (Luo et al. 2009).
The mosaic transplant–based system provides an additional strategy to measure the impact of gene knockdown on relative cell proliferation using in vivo competition assays. In these assays, knockdown cells are labeled with GFP and are cotransplanted in a defined ratio with uninfected cells or cells expressing a control shRNA and red fluorescent protein (RFP). The relative proportion of GFP-expressing cells can thus be monitored over time using flow cytometry and serves as a readout for competitive fitness of the test cell population (Zuber et al. 2010).
This approach has also been used in conjunction with shRNA libraries to perform screening in vivo. Our group recently performed a screen to identify tumor suppressor genes in lymphoma using the Eμ-Myc lymphoma model (Scuoppo et al. 2012). To do so, we designed a custom retroviral shRNA library targeting genes that are recurrently deleted in non-Hodgkins lymphoma, and transduced the library in pools into hematopoietic stem and progenitor cells. Upon adoptive transplant into syngeneic recipients, shRNAs targeting functional tumor suppressors accelerated tumorigenesis in an analogous manner to p53 knockdown (Hemann et al. 2003). These shRNAs were PCR-amplified from the resulting tumors and identified by sequencing. Our group has previously used a similar strategy to identify tumor suppressors in liver cancer (Zender et al. 2008).
Sabatini and colleagues recently employed a negative selection screening strategy in in vivo using a lentiviral stem loop shRNA library targeting metabolic genes (Possemato et al. 2011). Negative selection screens, such as these, are designed to identify synthetic lethal interactions or genes required for the maintenance of cancer growth; cells expressing an shRNA against a positive regulator of cell growth thus become depleted from the population. Although their study utilized a human breast cancer xenograft model, similar strategies should be feasible with cells derived from mouse tumors, provided that a sufficient number of cells engraft to maintain representation of the initial library pool.
DIRECT INTRODUCTION OF RNAi VECTORS IN VIVO
Direct delivery of shRNA expression vectors in vivo provides a powerful alternative to achieve stable gene knockdown, particularly in tissues that are not amenable to transplantation. By combining the ease of retroviral production and delivery with existing GEMMs, it is possible to create and study a broad range of genotypic configurations for assessing their impact on development and disease.
One approach for viral delivery of shRNAs uses the replication-competent avian sarcoma leukosis virus (ASLV) system. shRNAs can be inserted into the RCAS (replication-competent avian sarcoma) viral vector, which can only infect cells engineered to express the tumor virus receptor A (TVA) (Bromberg-White et al. 2004; Lewis et al. 2001). Therefore, transgenic expression of TVA under a tissue-specific or Cre-activatable promoter can be used to achieve tissue-specific transduction and transgene expression in vivo (Lewis et al. 2003). The RCAS/TVA system has been used to manipulate gene expression in models of glioma (Holmen 2005),liver(Lewis et al. 2005),breast(Du et al. 2006),and pancreatic cancer (Seidler et al. 2008) via injection of virus-producing cells directly into the tissue of interest. In the pancreatic cancer model, Seidler and colleagues demonstrated that in vivo knockdown of p53 by intrapancreatic injection of virus-producing cells could accelerate the progression of KrasG12D-driven pancreatic intra-epithelial neoplasias to invasive ductal adenocarcinoma. This approach can be applied in principle to any other cancer model for which there are available tissue-specific promoters and accessible routes of viral delivery. Although ASLV only infects dividing cells, ASLV-pseudotyped lentiviral vectors can be used to infect nondividing tissues in vivo as well (Siwko et al. 2008).
Lentiviral vectors can be used directly to deliver shRNAs to tissues accessible by injection. For instance, localized injection of lentiviral shRNA vectors has been used to silence gene expression in the mouse brain (Van den Haute et al. 2003), spinal cord (Raoul et al. 2005), and airway (Wang et al. 2012). Although these models have been used primarily to study noncancer pathologies, the same approach can be applied to study cancer, for instance, by inducing the knockdown of tumor-suppressor genes on a tumor-susceptible background. Conversely, lentiviral delivery of Cre recombinase has been employed to generate mosaic models of glioma (Marumoto et al. 2009) and lung cancer (DuPage et al. 2009) driven by Cre-activatable alleles of Hras, Kras, and/or p53. In such models, inclusion of shRNAs in the lentiviral vector can allow researchers to assess whether the depletion of a gene accelerates or inhibits tumorigenesis.
In addition to postnatal delivery, lentiviruses harboring shRNAs can be delivered during embryonic development, which can be useful for tissues that lose accessibility to viral delivery postnatally. The epidermis, in which the proliferative basal layer becomes inaccessible because of stratification, can be efficiently transduced in this manner by intra-amniotic microinjection before the onset of stratification (Beronja et al. 2010). Using this method, lentiviral constructs carrying shRNAs, Cre, and fluorescent reporters can be introduced into the epidermal progenitors and continue to be expressed throughout adulthood. Additionally, coinjection of multiple viral species enables assessment of the effect of single-gene and combined knockdowns on growth and tumorigenesis in the context of an otherwise wild-type tissue. Of note, the approach can be scaled up for in vivo screening of shRNA libraries to identify genes affecting different phenotypes, including tumorigenesis.
Although lentiviral transduction is not tissue-specific in and of itself, tissue specificity is generally dictated by the injection site and can be reinforced by inclusion of a tissue-specific promoter in the viral vector, injection of a tetracycline-inducible construct onto a tissue-specific rtTA background, or by the physical barriers of the injection site. In addition to viral vectors, shRNAs can also be expressed in vivo using transposon-based delivery systems such as sleeping beauty (SB) (Heggestad et al. 2004; Fletcher 2010). In this approach, a plasmid containing a gene of interest or shRNA flanked by inverted repeats is codelivered with an additional plasmid encoding the transposase. The transient expression of the transposase then promotes insertion of the cargo into the genome. The plasmids are frequently delivered by hydrodynamic injection, which can be used to target the liver, muscle, and kidney (Suda and Liu 2007).
The above-mentioned applications of RNAi technology in mosaic models of cancer provide a flexible and powerful tool for manipulating gene expression in vivo. The described approaches enable rapid assessment of candidate cancer genes arising from large-scale tumor-sequencing studies and/or in vitro RNAi screens in an immunocompetent in vivo setting, often in a multiplexed fashion.
RNAi IN GERMLINE GEMMs
RNAi can also be integrated with transgenic and/or ES cell technology to produce germline RNAi mouse models. In principle, such models possess advantages that are complementary to both the traditional conditional GEMMs and the mosaic models described above. First, shRNAs repress gene expression in trans, requiring only one allele for knockdown. This can greatly simplify breeding, especially for mouse models that require the perturbation of multiple genes. Second, because RNAi does not alter the endogenous genomic locus, it enables reversible gene silencing, which is not readily accomplished using standard conditional gene deletion approaches. Finally, germline RNAi GEMMs can be used to achieve knockdown in tissues for which there are no tractable delivery routes or transplant models that accurately reflect the histopathology of human cancer.
RNAi transgenic mice have been produced using several different methods. Initial proof-of-principle studies employed stem-loop shRNAs driven by ubiquitous RNA Pol III promoters, and produced mice via pronuclear injection (Hasuwa et al. 2002), ES cell targeting followed by tetraploid complementation (Carmell et al. 2003; Kunath et al. 2003), and transduction of fertilized eggs with lentiviral shRNA vectors (Tiscornia et al. 2003). Although these studies demonstrated sufficient knockdown, constitutive shRNA expression from a ubiquitous promoter is not an ideal system to model cancers that normally arise in a nonmutated tissue context. Subsequent models introduced tissue specificity by engineering shRNA constructs that required Cre-mediated excision of a spacer to activate or abrogate shRNA expression (Ventura et al. 2004; Coumoul et al. 2005). Breeding these RNAi transgenic mice onto a strain with tissue-specific Cre expression then confers tissue specificity to the shRNA.
The adaptation of the Pol II-driven miR30-based shRNA system for transgenic models added an additional layer of flexibility by enabling the coupling of shRNA expression to tissue-specific (Rao et al. 2006; Tinkle et al. 2008) and tetracycline-inducible promoters (Dickins et al. 2007). Our group tested the utility of the inducible system by generating transgenic mice carrying a tetracycline-inducible shRNA against p53. Double transgenic mice carrying the p53 shRNA as well as a tetracycline transactivator (tTA) under a liver or B-cell-specific promoter exhibited p53 depletion that could be reversed upon treatment with doxycycline. The p53 depletion also accelerated lymphomagenesis in the presence of a Eμ-Myc transgene, and restoration of p53 by Dox treatment induced tumor regression, thereby highlighting the dependence of the cancer on p53 disruption.
RAPID SITE-SPECIFIC TRANSGENE INSERTION USING RECOMBINASE-MEDIATED CASSETTE EXCHANGE
The RNAi transgenic models described above, like most transgenics, are generated by random insertion of the DNA construct into the mouse genome. Although this approach has been used quite successfully in generating mouse lines with sufficient levels of knockdown, the random integrations can lead to variability in transgene copy number and expression level, resulting in possible phenotypic differences in independently generated lines. As a result, a large number of founder animals must be screened in order to determine the appropriate expression levels. In principle, shRNA constructs can be inserted into a specific locus as “knock-in” alleles. However, knock-in and knockout alleles are engineered using homologous recombination with large targeting vectors, an inefficient and laborious process that is difficult to scale up for increased throughput within an individual laboratory. Targeting vectors must be designed with unique homology arms for each locus, and ES cells derived from one mouse strain might not be efficiently targeted with vectors designed for another. Furthermore, homologous recombination itself is often inefficient, requiring the screening of a large number of colonies for correct integration.
Some of the limitations of ES cell targeting and transgene insertion can be overcome using recombinase-mediated cassette exchange (RMCE), a strategy that enables efficient insertion of transgenic elements into specific sites in the genome (Seibler et al. 1998). The technique (Fig. 2A), first developed by Bode and colleagues, is a two-step process that employs the FLP recombinase/FRT recognition site system (O’Gorman et al. 1991; Schlake and Bode 1994; Seibler et al. 1998). First, a homing site consisting of a selectable marker flanked by heterotypic FRT recognition sites is inserted into the cell line via homologous recombination at a designated genomic locus. When a circular donor vector harboring the same FRT sites is introduced in conjunction with a construct expressing the FLP recombinase, the regions between the FRT sites are exchanged, leading to insertion of the donor transgene at the homing locus.
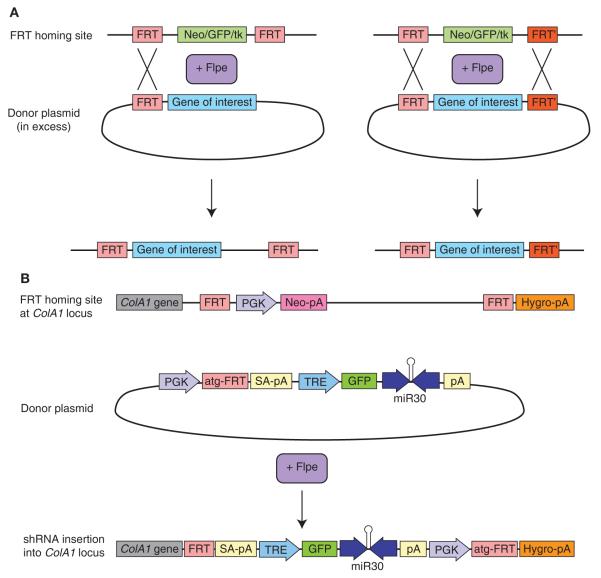
FIGURE 2.
Single-copy shRNA introduction via recombinase-mediated cassette exchange (RMCE). (A) RMCE involves the exchange of DNA sequences flanked by recombinase recognition sites between donor and recipient DNA sources. Donor vectors may contain one FRT site for insertion of the complete plasmid or two heterotypic FRT sites for insertion of the intervening plasmid segment. Cotransfection of FLP recombinase with an excess of donor plasmid harboring the gene of interest flanked by FRT recognition sites results in gene insertion into a homing cassette containing the same FRT sites. (B) System for inserting a single-copy shRNA into a defined genomic locus (based on RMCE system of Beard et al. 2006). ES cells are targeted with a FRT homing cassette at the ColA1 locus. The cassette contains an FRT-flanked neomycin resistance gene as well as a hygromycin resistance gene that is missing the ATG start codon. The ES cell line also carries an M2-rtTA, introduced into the Rosa26 locus by homologous recombination to enable doxycyclineinducible gene expression. The donor “FLP-in” vector contains a PGK promoter and an ATG codon followed immediately by an FRT site. The vector also includes a minimal tetracycline-responsive CMV (TRE) promoter, followed by the gene of interest or shRNA. A splice acceptor and polyadenylation site upstream of the TRE promoter minimizes readthrough from the ColA1 promoter. Coelectroporation of the FLP-in vector and a CAGGS promoter–driven FLP result in incorporation of the entire vector between the FRT sites in the ColA1 homing cassette. The PGK promoter and start codon are positioned adjacent to and in frame with the hygromycin resistance gene. Correctly integrated clones can therefore be selected with hygromycin treatment.
Flp/FRT homing cassettes have been targeted to the Rosa26 (Seibler et al. 2007) and ColA1 (Beard et al. 2006) loci in ES cells. The ColA1-targeted ES cell line, known as KH2, carries additional features that increase the flexibility of the system, including rtTA expression to enable tetracycline-inducible gene expression. Coelectroporation of the “FLP-in” vector (containing a TRE promoter–driven gene or shRNA of interest) and a CAGGS promoter–driven FLP result in incorporation of the entire FLP-in vector between the FRT sites in the ColA1 homing cassette (Fig. 2B). Using this method, 80% of hygromycin-resistant colonies show a single correct integration of the transgene (Beard et al. 2006).
This powerful system thus allows for the insertion of a transgene at single copy at a defined location in the genome. The inserted transgenic elements can be shRNAs, other noncoding RNAs, cDNAs encoding known gain-of-function oncogenic mutants, candidate oncogenes arising from large-scale screening or sequencing efforts, or fluorescent/luminescent reporters for in vivo imaging. We and others have shown that insertion of either stem-loop (Hitz et al. 2007; Seibler et al. 2007) or miR30-based shRNAs (Premsrirut et al. 2011) into the Rosa26 or ColA1 locus, respectively, can achieve efficient knockdown in vivo. To do so, we modified the RMCE donor vector developed by Beard and colleagues (Beard et al. 2006) to include a cDNA encoding GFP, followed by a miR30-based shRNA cassette downstream of the tetracycline inducible promoter. As the GFP is cotranscribed with the shRNA, it serves as a fluorescent readout of shRNA level.
As an early test of the system, we produced mice harboring shRNAs targeting the adenomatous polyposis coli (APC) tumor suppressor, and examined the consequences of reversible suppression of APC at different stages of development or tumorigenesis (Premsrirut et al. 2011). When mice engineered to express the APC shRNA were crossed to a Rosa26-rtTA line, the resulting double-transgenic offspring developed T-cell acute lymphoblastic leukemia upon APC depletion by Dox treatment. Dox withdrawal in transplanted leukemias induced tumor regression, indicating a role for APC in the disease maintenance. Our laboratory has published a detailed protocol for generating shRNA-expressing mice by this method (Dow et al. 2012). The ease and efficiency of RMCE makes this approach amenable to higher-throughput GEMM production, because parental ES cells may be targeted with several vectors in parallel and banked for future blastocyst injections.
The FLP/FRT-based RMCE system is also amenable to multiplexing using combinations of mutant FRT sites that do not cross-react (Turan et al. 2010, 2011). For instance, non-cross-reacting pairs of heterotypic mutant FRT sites can be inserted as homing cassettes into two distinct genomic loci. Two donor plasmids (each harboring FRT sites specific to one of the target loci) can then be inserted in parallel into their respective loci by cotransfection of FLP. Although not yet achieved, this site-specific, single-copy insertion method could in principle be used to reproducibly express two candidate oncogenes in order to assess their cooperation in tumorigenesis. Integration of this approach with the tissue-specific and tetracycline-inducible promoters, as well as the Cre/Lox system, can further enhance the flexibility of this system for modeling cancer in mice. In addition to FLP/FRT, RMCE methods are also available for other recombinase and integrase systems (Oumard et al. 2006).
ACCELERATING MOUSE MODEL PRODUCTION USING NONGERMLINE GEMMs
Conditional knockouts, knock-ins, and other germline GEMMs have become the gold standard for modeling spontaneous tumorigenesis events within the autochthonous tissue environment and in the context of a functional immune system. Indeed, preclinical studies indicate that GEMMs for K-ras-driven cancers of the lung and pancreas can accurately predict therapeutic response in patients (Singh et al. 2010). The major limitations of germline GEMMs are the substantial time, labor, and cost requirements associated with generating sufficiently large experimental cohorts. The production of traditional GEMMs involves the targeting of ES cells by homologous recombination, followed by blastocyst injection, and assessment of founder animals for germline transmission (Jonkers and Berns 2002). For loss-of-function studies, animals must often be bred to homozygosity; conditional GEMMs that include tissue-specific or inducible gene deletion require further intercrossing of multiple alleles to generate the experimental genotype. Depending on the number of alleles, this process can take 1–2 yr per mouse model. Additionally, if homozygous deletion of a gene results in lethality, colonies must be maintained as heterozygotes. In this case, only a fraction of offspring derived from a breeding cycle will be of the desired experimental genotype. As a result, obtaining sufficiently large and synchronous experimental cohorts for drug testing and tumorigenesis studies presents a significant challenge. Circumventing this difficulty in order to achieve appropriate statistical power requires maintenance of large breeding colonies at considerable expense. Studying the genetic interaction of two or more genes in tumorigenesis thus becomes exponentially more challenging, slow and expensive.
Nongermline GEMMs (nGEMMs), and specifically chimeric models, have the potential to circumvent many of the breeding-related obstacles of traditional GEMMs, because they do not rely on breeding cycles to generate experimental cohorts. Chimeric models are produced through injection of genetically engineered ES cells into wild-type blastocysts. The resulting chimeric mice consist of a mixture of ES-derived and host-derived cells, and can be directly used as the experimental cohort (Fig. 3). If the ES cells are engineered to have elevated tumor susceptibility (by expression of oncogenes or ablation of tumor-suppressor genes), they will give rise to tumors in the context of surrounding host blastocyst-derived normal tissues. By injecting ES cells into blastocysts of albino background, the degree of chimerism in the resulting animals can be estimated by coat color. Alternatively, fluorescent and/or luminescent reporters can be inserted into ES cells to allow for direct assessment and imaging of chimerism.
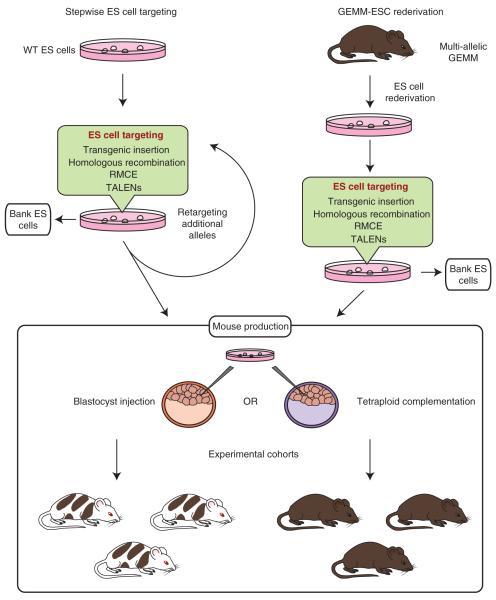
FIGURE 3.
Nongermline GEMMs for rapid production of multiallelic experimental cohorts. Multiallelic ES cell lines harboring knockout alleles, inducible transgenes, and/or shRNAs can be generated from validated wild-type ES cell lines by multiple rounds of targeting and selection (left panel). Alternatively, ES cells can be rederived from multiallelic GEMMs of the desired genotype produced by conventional breeding (right panel). GEMM-derived ESCs can then be targeted in vitro by homologous recombination, RMCE, TALEN nucleases, or transgene insertion to insert additional genetic elements without the need for several additional rounds of breeding. After engineering by either method, ES cells can be banked or used to generate nongermline GEMMs (lower panel). Injection of ES cells into blastocysts produces chimeric mice that consist of a mixture of ES- and host-derived cells. Injection of multiple blastocysts enables the production of synchronous experimental cohorts. Tetraploid complementation, in which ES cells are injected into tetraploid blastocysts that can only contribute to extraembryonic tissues, may be used instead of blastocyst injection to generate entirely donor ES cell–derived experimental mice.
Zhou and colleagues employed this strategy in conjunction with stepwise manipulation of ES cells followed by blastocyst injection to generate chimeric models of lung cancer (Zhou et al. 2009). In this case, the authors began with wild-type ES cells and performed sequential rounds of recombination to ablate the Cdkn2a locus on both alleles. This was followed by cotransfection of vectors allowing for tetracycline-inducible and lung-specific expression of the HER2V659E or KrasG12D oncogenes. At each step, the selected clones were assessed for maintenance of ES cell pluripotency and appropriate transgene activity. Treatment of the resulting chimeric animals with dox induced lung tumors arising from the genetically engineered ES cells in the context of normal lung tissue. A similar approach was also taken to generate a chimeric model of breast cancer that was subsequently employed to study the molecular basis of sensitivity to γ-secretase inhibitor treatment (Watters et al. 2009).
The advantage of the stepwise ES cell targeting strategy is the ability to model spontaneous autochthonous tumorigenesis without required breeding. Although sequential targeting of ES cells by homologous recombination is a slow process, once generated and tested, the multiallelic ES cells can be expanded and banked for future use. However, a drawback of this method is the extensive time the ES cells spend in culture over the multiple rounds of targeting and selection. Although extended culture does not preclude the identification of pluripotent clones, the overall efficiency of chimerism may decline because of accumulated mutations, epigenetic changes, or other mechanisms. Eventually, whole-genome sequencing can be used to validate the integrity of individual clones.
GENERATION OF MULTIALLELIC nGEMMs VIA ES CELL REDERIVATION
In an alternative approach for generating multiallelic nGEMMs, ES cells can be rederived from existing GEMMs harboring one or more engineered alleles of interest. The ES cells can then be subjected to a single round of genetic manipulation in culture (such as homologous recombination, RMCE, or mutagenesis), followed by blastocyst injection to generate chimeras (Huijbers et al. 2011; Premsrirut et al. 2011). ES cells are typically derived from the inner cell mass (ICM) of 3 d blastocysts (Martin 1981; Brook and Gardner 1997). ES cells are generally cultured in the presence of leukemia inhibitory factor (LIF) (Williams et al. 1988), on top of a mitotically inactive fibroblast feeder layer. It was recently shown that ES cell differentiation can further be suppressed via inhibition of Gsk3 and Mek (Ying et al. 2008), and deriving ES cells in media containing inhibitors of these two pathways (known as 2i media) can improve the ES cell derivation efficiency for difficult strains (see Meissner et al. 2009 for protocol for ES cell derivation). Additionally, the efficiency of this process may also be affected by the allelic composition of the GEMM used for derivation. For instance, constitutive deletion of genes regulating proliferation and viability may yield less robust ES cells, and cells harboring tissue-specific promoter expressing Cre recombinase may show unwanted gene excision due to promoter activation in ES cells (Huijbers et al. 2011). Thus, rigorous testing of the resulting clones for aberrant karyotypes or recombination events, as well as correct expression of pluripotency markers, is required. GEMM-derived ES cell lines that meet these criteria can be used for subsequent genetic targeting, selection (Meissner et al. 2009) and blastocyst injection according to standard protocols (Reid and Tessarollo 2009).
A caveat of producing experimental cohorts of chimeric animals via blastocyst injection is the variability in chimerism. In some cases, the degree of chimerism may be too low to induce sufficient tumor penetrance. In such situations, tetraploid complementation can be employed instead. Tetraploid complementation involves injection of ES cells into tetraploid blastocysts that cannot contribute to the embryonic tissues (Nagy et al. 1993; Eakin and Hadjantonakis 2006; Zhao et al. 2010). The resulting offspring are derived entirely from the injected ES cells. As with chimeras, the resulting mice could be used directly as part of an experimental cohort. If desired, they can also be bred to the parental GEMMs in order to establish colonies of mice harboring the newly engineered genetic elements.
Our laboratory has successfully rederived ES cells from a mouse model of lung adenocarcinoma that carried four alleles: LSL-KrasG12D, rCCSP-rtTA, Rosa26-LSL-Luciferase, and the ColA1-FRT homing cassette (Premsrirut et al. 2011). The ColA1 homing cassette was then targeted with an shRNA against sequences contained within exon 1β of the INK4a/ARF locus and thus capable of suppressing p19ARF. Mice generated from these ES cells via tetraploid complementation developed tumors upon treatment with adenoviral Cre recombinase to activate the latent Kras allele. Treatment with dox induced ARF knockdown and increased tumor burden, whereas subsequent ARF restoration by dox withdrawal induced partial tumor regression, indicating that ARF loss can cooperate with KrasG12D in lung tumorigenesis. The ColA1 homing cassette may be inserted into other ES cell lines, such as the complex multiallele lines rederived from existing GEMMs, or can also be introduced by breeding followed by ES cell rederivation. Each cell line modified in this manner creates a flexible platform for rapidly analyzing multiple genes in future experiments.
This method of chimeric mouse model production maintains the advantages of the stepwise model: The ability to model autochthonous tumorigenesis, immediate production of experimental cohorts without breeding, and the ability to bank generated ES cell lines. Both approaches thus facilitate the modeling of antagonistic and cooperative genetic interactions in tumorigenesis. For instance, researchers can compare the effects of different oncogenic mutations on a genetically equivalent background by introducing them in parallel onto the same parental genetically engineered ES cell line. Using such an experimental strategy, Zhou et al. (2009) characterized differences in signaling pathways between Kras and Erbb2 family-driven lung adenocarcinomas. The ES cell rederivation–based method also adds the advantage of minimizing culture time and sequential selection and targeting steps that may reduce ES cell quality.
FUTURE PERSPECTIVES.
The technological developments described here have the potential to dramatically accelerate the pace of mouse cancer model production. The ability to rederive and genetically manipulate ES cells from multiallelic mouse models is a major step toward this goal. The continued improvement of ES culture conditions and mouse chimera production will further increase the efficiency of this process. Eventually, large repositories of such multiallelic ES cells can be established for distribution, as is already the case for conditional knockout ES cell lines (Skarnes et al. 2011). Such ES cell lines can be further engineered using more efficient TALEN nucleases. Additionally, the modular and reversible nature of the in vivo RNAi systems allow for an unprecedented level of spatiotemporal control over gene expression in tumorigenesis. However, it is important to remember that shRNA-mediated silencing should not be equated with a null allele, because it does not completely eliminate its target gene expression. RNAi-GEMMs therefore serve a complementary role to traditional germline models, enabling rapid analysis of gene function not currently possible with traditional GEMMs.
ACKNOWLEDGMENTS
We thank Luke Dow for his critical reading of the manuscript. G.L. is supported by a postdoctoral fellowship, PF-13-037-01-DMC, from the American Cancer Society. S.W.L. is supported by a UO1 consortium grant from the National Cancer Institute–Mouse Model of Human Cancer Consortium. He is Geoffrey Beene Professor in Cancer Biology and an investigator in the Howard Hughes Medical Institute.
REFERENCES
- Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. doi: 10.1158/0008-5472.CAN-04-0807. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim Sungjoon, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat Med. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-White JL, Webb CP, Patacsil VS, Miranti CK, Williams BO, Holmen SL. Delivery of short hairpin RNA sequences by using a replication-competent avian retroviral vector. J Virol. 2004;78:4914–4916. doi: 10.1128/JVI.78.9.4914-4916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002a;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002b;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Zhang L, Conklin DS, Hannon GJ, Rosenquist TA. Germline transmission of RNAi in mice. Nat Struct Biol. 2003;10:91–92. doi: 10.1038/nsb896. [DOI] [PubMed] [Google Scholar]
- Chang K, Elledge SJ, Hannon GJ. Lessons from Nature: MicroRNA-based shRNA libraries. Nat Meth. 2006;3:707–714. doi: 10.1038/nmeth923. [DOI] [PubMed] [Google Scholar]
- Chang L-Y, Lin Y-C, Mahalingam J, Huang C-T, Chen T-W, Kang C-W, Peng H-M, Chu Y-Y, Chiang J-M, Dutta A, et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8+ T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Teng CF, Tsai WH, Yeh CW, Wu MP, Hsu HC, Hung CF, Chang WT. Multitarget therapy of malignant cancers by the head-to-tail tandem array multiple shRNAs expression system. Cancer Gene Ther. 2009;16:516–531. doi: 10.1038/cgt.2008.102. [DOI] [PubMed] [Google Scholar]
- Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, Dickins RA, Narita M, Zhang M, Lowe SW. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumoul X, Shukla V, Li C, Wang R-H, Deng C-X. Conditional knockdown of Fgfr2 in mice using Cre-LoxP induced RNA interference. Nucleic Acids Res. 2005;33:e102. doi: 10.1093/nar/gni100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czauderna F, Santel A, Hinz M, Fechtner M, Durieux B, Fisch G, Leenders F, Arnold W, Giese K, Klippel A, et al. Inducible shRNA expression for application in a prostate cancer mouse model. Nucleic Acids Res. 2003;31:e127. doi: 10.1093/nar/gng127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, Lowe SW. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37:1289–1295. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- Dickins RA, McJunkin K, Hernando E, Premsrirut PK, Krizhanovsky V, Burgess DJ, Kim Sang Yong, Cordon-Cardo C, Zender L, Hannon GJ, et al. Tissue-specific and reversible RNA interference in transgenic mice. Nat Genet. 2007;39:914–921. doi: 10.1038/ng2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Premsrirut PK, Zuber J, Fellmann C, McJunkin K, Miething C, Park Y, Dickins RA, Hannon GJ, Lowe SW. A pipeline for the generation of shRNA transgenic mice. Nat Protoc. 2012;7:374–393. doi: 10.1038/nprot.2011.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Podsypanina K, Huang S, McGrath A, Toneff MJ, Bogoslovskaia E, Zhang X, Moraes RC, Fluck M, Allred DC, et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci. 2006;103:17396–17401. doi: 10.1073/pnas.0608607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin GS, Hadjantonakis A-K. Production of chimeras by aggregation of embryonic stem cells with diploid or tetraploid mouse embryos. Nat Protoc. 2006;1:1145–1153. doi: 10.1038/nprot.2006.173. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2883166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Evers B, Speksnijder EN, Schut E, Ciampricotti M, Smalley MJ, Derksen PWB, Jonkers J, de Visser KE. A tissue reconstitution model to study cancer cell-intrinsic and -extrinsic factors in mammary tumouri-genesis. J Pathol. 2010;220:34–44. doi: 10.1002/path.2655. [DOI] [PubMed] [Google Scholar]
- Fellmann C, Zuber J, McJunkin K, Chang K, Malone CD, Dickins RA, Xu Q, Hengartner MO, Elledge SJ, Hannon GJ, et al. Functional identification of optimized RNAi triggers using a massively parallel sensor assay. Mol Cell. 2011;41:733–746. doi: 10.1016/j.molcel.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fletcher BS. Delivery of small interfering RNA (siRNA) using the sleeping beauty transposon. Cold Spring Harb Protoc. 2010 doi: 10.1101/pdb.prot5521. 2010. pdb.prot5521. [DOI] [PubMed] [Google Scholar]
- Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–141. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): Mechanism of action. Apoptosis. 2000;5:107–114. doi: 10.1023/a:1009664109241. [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan K-P, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Hasuwa H, Kaseda K, Einarsdottir T, Okabe M. Small interfering RNA and gene silencing in transgenic mice and rats. FEBS Lett. 2002;532:227–230. doi: 10.1016/s0014-5793(02)03680-3. [DOI] [PubMed] [Google Scholar]
- Heggestad AD, Notterpek L, Fletcher BS. Transposon-based RNAi delivery system for generating knockdown cell lines. Biochem Biophys Res Commun. 2004;316:643–650. doi: 10.1016/j.bbrc.2004.02.090. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Fridman JS, Zilfou JT, Hernando E, Paddison PJ, Cordon-Cardo C, Hannon GJ, Lowe SW. An epiallelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- Hitz C, Wurst W, Kühn R. Conditional brain-specific knockdown of MAPK using Cre/loxP regulated RNA interference. Nucleic Acids Res. 2007;35:e90. doi: 10.1093/nar/gkm475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL. Essential role for ras signaling in glioblastoma maintenance. Cancer Res. 2005;65:8250–8255. doi: 10.1158/0008-5472.CAN-05-1173. [DOI] [PubMed] [Google Scholar]
- Huijbers IJ, Krimpenfort P, Berns A, Jonkers J. Rapid validation of cancer genes in chimeras derived from established genetically engineered mouse models. Bioessays. 2011;33:701–710. doi: 10.1002/bies.201100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Kunath T, Gish G, Lickert H, Jones N, Pawson T, Rossant J. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat Biotechnol. 2003;21:559–561. doi: 10.1038/nbt813. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim Sunyoung, et al. The nuclear RNase III Drosha initiates micro-RNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom K-H, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BC, Chinnasamy N, Morgan RA, Varmus HE. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector. J Virol. 2001;75:9339–9344. doi: 10.1128/JVI.75.19.9339-9344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003;17:3127–3138. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BC, Klimstra DS, Socci ND, Xu S, Koutcher JA, Varmus HE. The absence of p53 promotes metastasis in a novel somatic mouse model for hepatocellular carcinoma. Mol Cell Biol. 2005;25:1228–1237. doi: 10.1128/MCB.25.4.1228-1237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AH, Duch M, Pedersen FS. Transcriptional silencing of retroviral vectors. J Biomed Sci. 1996;3:365–378. doi: 10.1007/BF02258042. [DOI] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Lanza DG, Guest I, Uk-Lim C, Glinskii A, Glinsky G, Sell S. Characterization of mammary cancer stem cells in the MMTV-PyMT mouse model. Tumour Biol. 2012;33:1983–1996. doi: 10.1007/s13277-012-0458-4. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura S, Jones PA, Takai D. Establishment of conditional vectors for hairpin siRNA knockdowns. Nucleic Acids Res. 2003;31:e77. doi: 10.1093/nar/gng077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva OV, Kang Y, Spiridonov AN, Saetrom P, Nemtsov VA, Ogurtsov AY, Nechipurenko YD, Shabalina SA. Optimization of duplex stability and terminal asymmetry for shRNA design. PLoS One. 2010;5:e10180. doi: 10.1371/journal.pone.0010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Eminli S, Jaenisch R. Derivation and manipulation of murine embryonic stem cells. Methods Mol Biol. 2009;482:3–19. doi: 10.1007/978-1-59745-060-7_1. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim So Young, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman S, Fox DT, Wahl GM. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991;251:1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- Oumard A, Qiao J, Jostock T, Li J, Bode J. Recommended method for chromosome exploitation: RMCE-based cassette-exchange systems in animal cell biotechnology. Cytotechnology. 2006;50:93–108. doi: 10.1007/s10616-006-6550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison PJ, Silva JM, Conklin DS, Schlabach M, Li M, Aruleba S, Balija V, O’Shaughnessy A, Gnoj L, Scobie K, et al. A resource for large-scale RNA-interference-based screens in mammals. Nature. 2004;428:427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo H-K, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premsrirut PK, Dow LE, Kim Sang Yong, Camiolo M, Malone CD, Miething C, Scuoppo C, Zuber J, Dickins RA, Kogan SC, et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell. 2011;145:145–158. doi: 10.1016/j.cell.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Hoff Von DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MK, Pham J, Imam JS, MacLean JA, Murali D, Furuta Y, Sinha-Hikim AP, Wilkinson MF. Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis. Genes Dev. 2006;20:147–152. doi: 10.1101/gad1367806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun J-C, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Reid SW, Tessarollo L. Isolation, microinjection and transfer of mouse blastocysts. Methods Mol Biol. 2009;530:269–285. doi: 10.1007/978-1-59745-471-1_14. [DOI] [PubMed] [Google Scholar]
- Schlake T, Bode J. Use of mutated FLP recognition target (FRT) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry. 1994;33:12746–12751. doi: 10.1021/bi00209a003. [DOI] [PubMed] [Google Scholar]
- Scuoppo C, Miething C, Lindqvist L, Reyes J, Ruse C, Appelmann I, Yoon S, Krasnitz A, Teruya-Feldstein J, Pappin D, et al. A tumour suppressor network relying on the polyamine-hypusine axis. Nature. 2012;487:244–248. doi: 10.1038/nature11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler J, Schübeler D, Fiering S, Groudine M, Bode J. DNA cassette exchange in ES cells mediated by Flp recombinase: An efficient strategy for repeated modification of tagged loci by marker-free constructs. Biochemistry. 1998;37:6229–6234. doi: 10.1021/bi980288t. [DOI] [PubMed] [Google Scholar]
- Seibler J, Kleinridders A, Küter-Luks B, Niehaves S, Brüning JC, Schwenk F. Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 2007;35:e54. doi: 10.1093/nar/gkm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler B, Schmidt A, Mayr U, Nakhai H, Schmid RM, Schneider G, Saur D. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc Natl Acad Sci. 2008;105:10137–10142. doi: 10.1073/pnas.0800487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. The mighty mouse: Genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang E, Zuber J, Rappaport A, Taylor M, Johns C, Lowe SW, Vakoc CR. The Polycomb complex PRC2 supports aberrant self-renewal in a mouse model of MLL-AF9;NrasG12D acute myeloid leukemia. Oncogene. 2012;32:930–938. doi: 10.1038/onc.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Li MZ, Chang Ken, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–1288. doi: 10.1038/ng1650. [DOI] [PubMed] [Google Scholar]
- Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, Thompson JD, Cheng JH, Bou Reslan H, Ho CCK, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: Closing the gap between preclinical data and trial outcomes. Cancer Res. 2012;72:2695–2700. doi: 10.1158/0008-5472.CAN-11-2786. [DOI] [PubMed] [Google Scholar]
- Siwko SK, Bu W, Gutierrez C, Lewis B, Jechlinger M, Schaffhausen B, Li Y. Lentivirus-mediated oncogene introduction into mammary cells in vivo induces tumors. Neoplasia. 2008;10:653. doi: 10.1593/neo.08266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Liu D. Hydrodynamic gene delivery: Its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan S, Kuehle J, Schambach A, Baum C, Bode J. Multiplexing RMCE: Versatile extensions of the Flp-recombinase-mediated cassette-exchange technology. J Mol Biol. 2010;402:52–69. doi: 10.1016/j.jmb.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Turan S, Galla M, Ernst E, Qiao J, Voelkel C, Schiedlmeier B, Zehe C, Bode J. Recombinase-mediated cassette exchange (RMCE): Traditional concepts and current challenges. J Mol Biol. 2011;407:193–221. doi: 10.1016/j.jmb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Van den Haute C, Eggermont K, Nuttin B, Debyser Z, Baekelandt V. Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Hum Gene Ther. 2003;14:1799–1807. doi: 10.1089/104303403322611809. [DOI] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lin L, Zheng C. Downregulation of Orai1 expression in the airway alleviates murine allergic rhinitis. Exp Mol Med. 2012;44:177–190. doi: 10.3858/emm.2012.44.3.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JW, Cheng C, Majumder PK, Wang R, Yalavarthi S, Meeske C, Kong L, Sun W, Lin J, Heyer J, et al. De novo discovery of a γ-secretase inhibitor response signature using a novel in vivo breast tumor model. Cancer Res. 2009;69:8949–8957. doi: 10.1158/0008-5472.CAN-09-1544. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S, Shih I-H, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wagner EJ, Cullen BR. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol Cell. 2002;9:1327–1333. doi: 10.1016/s1097-2765(02)00541-5. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Cai X, Cullen BR. Use of RNA polymerase II to transcribe artificial microRNAs. Meth Enzymol. 2005;392:371–380. doi: 10.1016/S0076-6879(04)92022-8. [DOI] [PubMed] [Google Scholar]
- Zhao X-Y, Lv Z, Li W, Zeng F, Zhou Q. Production of mice using iPS cells and tetraploid complementation. Nat Protoc. 2010;5:963–971. doi: 10.1038/nprot.2010.61. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Rideout WM, Zi T, Bressel A, Reddypalli S, Rancourt R, Woo J-K, Horner JW, Chin L, Chiu MI, et al. Chimeric mouse tumor models reveal differences in pathway activation between ERBB family and KRAS-dependent lung adenocarcinomas. Nat Biotechnol. 2009;28:71–78. doi: 10.1038/nbt.1595. http://www.nature.com/nbt/journal/vaop/ncurrent/full/nbt.1595.html. [DOI] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol. 2010;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, Shi J, Weiss-mueller S, Fellman C, Taylor MJ, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011a;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011b;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]