Abstract
Human tumors frequently express membrane-bound or soluble NK group 2, member D (NKG2D) ligands. This results in chronic engagement of NKG2D on the surfaces of NK and CD8+ T cells and rapid internalization of the receptor. Although it is well appreciated that this phenomenon impairs NKG2D-dependent function, careful analysis of NKG2D-independent functions in cells chronically stimulated through NKG2D is lacking. Using a mouse model of chronic NKG2D ligand expression, we show that constant exposure to NKG2D ligands does not functionally impair NK cells and CD8+ T cells in the context of viral infection.
Natural killer cells are fundamental players in the recognition of transformed or virally infected cells. As members of the innate arm of the immune system, NK cells’ hallmark is their ability to directly recognize target cells through a plethora of inhibitory and activating receptors, the balance of which determines the outcome of the encounter (1). Inhibitory NK cell receptors typically bind to self-MHC molecules and mediate the “missing-self recognition of foreign cells or self-cells that have downregulated their levels of MHC class I expression, as occurs in transformed or virally infected cells that seek to avoid CD8+ T cell recognition (2). Activating NK cell receptors can recognize a variety of distinct ligands. Although a minority of activating receptors can bind to “non-self ligands, such as Ly49H binding to the mouse CMV (MCMV) m157 protein (3,4), a majority of NK activating receptors bind to self-ligands that have been upregulated under certain conditions and mediate “induced-self recognition (reviewed in Ref. 1).
A well-characterized activating receptor is NK group 2, member D (NKG2D), a homodimeric, type II transmembrane glycoprotein belonging to the C-type lectin-like receptor family (5, 6). NKG2D is expressed on all mouse NK cells, most NKT cells, and activated CD8+ T cells. To signal, the NKG2D receptor associates noncovalently with specialized signaling adapters DAP10 and DAP12, which signal via and the YINM motif-linked PI3K and the ITAM-induced Syk–Zap70 pathways, respectively (7–9). NKG2D recognizes numerous cellular ligands that belong to distinct families with homology to MHC class I molecules (reviewed in Refs. 6, 10). These ligands are often induced on tumor cells or virally infected cells and result in NK cell lysis of the target cell. Mouse NKG2D ligands include the GPI-anchored Rae-1α-ε molecules as well as the transmembrane proteins mouse UL16-binding protein-like transcript 1 and H60 (11–13). In contrast to healthy adult tissues, many primary human and mouse tumors express NKG2D ligands constitutively, leading to NK cell recognition and elimination of tumor cells. Indeed, ectopic expression of NKG2D ligands on tumors renders them susceptible to NK cell killing in vitro and in vivo (14, 15). In addition, mice treated with NKG2D blocking Ab or genetically deficient in NKG2D have an increased susceptibility to chemically induced or oncogene-driven tumorigenesis, respectively (16, 17). Using transgenic models of constitutive NKG2D ligand expression (18, 19) and coincubation of NK cells with NKG2D ligand-bearing targets (20–22), we and others have shown that sustained NKG2D engagement results in downregulation of the receptor and impairment of its function. These findings are supported by growing evidence from human cancer patients that constitutive NKG2D ligand expression on tumors and shedding of NKG2D ligand from the tumor cells result in decreased NKG2D receptor expression and is associated with poor prognosis (23–29).
In addition to impaired NKG2D function, recent studies have suggested that sustained NKG2D engagement also impairs NKG2D–independent functions. In one study, Oppenheim et al. (19) noted a defect in NK cell missing-self recognition in mice of the FVB strain constitutively expressing the nonsyngeneic Rae-1ε ligand. In another study, Coudert et al. (30) designed an in vitro system to examine the effect of chronic NKG2D stimulation on NK cells. NK cells were incubated with H60-expressing RMA cells in the presence of IL-2. After 3 d, NK cells were used as effector cells in cytotoxicity assays against a variety of NKG2D-dependent and independent targets. In addition to impaired NKG2D function, RMA-H60–exposed NK cells showed reduced cytotoxicity toward DAP10- and DAP12-independent pathways, such as Ab-dependent cellular cytotoxicity and missing-self recognition, and slightly reduced or normal cytotoxicity toward DAP10- and DAP12-dependent pathways, such as Ly49H and Ly49D recognition. The finding that sustained NKG2D engagement might affect NK cells globally warrants further studies, because this would have important implications for the treatment of human cancer patients.
In this study, we addressed whether constitutive NKG2D engagement globally impairs NK cells. We used a mouse in which Rae-1ε is driven by the β-actin promoter (referred to as Rae-1 Tg) to investigate this question (31, 32). Rae-1 Tg mice were analyzed for their capacity to mediate NKG2D-independent functions, such as missing-self recognition, as well as to mount an immune response to MCMV infection, a pathogen normally controlled by NK cells.
Materials and Methods
Mice and infections
Inbred C57BL/6 (B6) mice were purchased from the National Cancer Institute (Frederick, MD) or Charles River Laboratories (Wilmington, MA). Rae-1ε Tg mice were generated in our laboratory as previously described (32) and are maintained by continuous backcross of heterozygous transgenics to B6 wild-type (WT) mates. Rae-1 Tg mice were screened by staining peripheral blood lymphocytes with an anti-pan–Rae-1 mAb (clone 186107). B2m−/− C57BL/6 mice were bred at University of California at San Francisco. All of the mice were maintained and treated according to University of California at San Francisco Committee on Animal Research guidelines. Mice were infected by i.p. injections of MCMV (Smith strain, 5 × 104 PFU, salivary gland-derived) or MCMV Δm151-m158 (MC96.73, 6 × 106 PFU, tissue culture-derived) (33). NK cells were depleted by injections with 200 µg anti-NK1.1 mAb PK136.
Ex vivo NK cell stimulation assay
NK cells were enriched from spleen using rat anti-mouse IgG Abs against CD8, CD4, and Ter119 and magnetically depleting labeled cells with anti-rat IgG along with anti-mouse IgG magnetic beads to deplete B cells (Qiagen, Valencia, CA). A total of 1 × 106 enriched splenic NK cells were incubated in a 96-well plate coated with 10 µg/ml Abs or with equal numbers of Ba/F3 or m157-transfected Ba/F3 (m157-Ba/F3) cells (3). After 4 h at 37°C in the presence of monensin (BD GolgiStop), cells were washed and stained for intracellular IFN-γ by using an Intracellular Staining kit (34).
51Cr release assay
To measure NK cell-mediated cytotoxicity ex vivo, freshly isolated enriched splenic NK cells were incubated in triplicate with 51Cr-labeled Ba/F3 cells or m157-Ba/F3 cells. Four hours later, supernatants were harvested and assayed for the release of 51Cr. Spontaneous and total lysis were measured by incubating target cells in the absence of NK cells and in the presence of 1% SDS in water, respectively. The percentage of specific lysis = (experimental lysis – spontaneous lysis)/(maximum lysis – spontaneous lysis) × 100. To measure NK cell-mediated cytotoxicity of in vitro activated NK cells, enriched splenic NK cells were incubated with DX5 (anti-CD49b) mAb-coated beads (Miltenyi Biotec, Auburn, CA) and positively selected by using magnetic cell sorting with a MACS column (Miltenyi Biotec). Purified NK cells were cultured in RPMI 1640 supplemented with 10% FCS in the presence of 4000 U/ml human rIL-2 (generously provided by the National Cancer Institute Biological Resources Branch Preclinical Repository) for 5–6 d.
In vivo cytotoxicity
Target cells were labeled with CFSE (Molecular Probes) (hi, 5 µM; lo, 0.5 µM) for 8 min at 37°C in PBS with 0.1% BSA. Cells were then incubated with ice-cold RPMI 1640 with 10% FCS for 5 min prior to washing three times with PBS. A total of 4 × 106 of each target cell (1:1 ratio) were injected i.v. in 100 µl PBS. Cells were harvested from spleen or peripheral blood at the indicated time points and analyzed by flow cytometry.
MCMV titers
Mice were infected with 5 × 104 PFU MCMV-Smith or 6 × 106 PFU Δm151-m158 (MC96.73) MCMV mutant (35). After 3 d, whole spleens and one lobe of liver were harvested and snap-frozen on dry ice in 1:1 DMEM and skim milk media. Samples were stored at −80°C, thawed, weighed, homogenized, plated on 3T3 cells in 10-fold serial dilutions in DMEM without FCS, and incubated for 2 h at 37°C. DMEM with 10% FCS and 0.75% carboxymethyl cellulose was added, and samples were incubated for 5–7 d. Plaques were visualized by staining with crystal violet dye. Titers were adjusted for weight of the tissue.
Ex vivo CD8+ T cell stimulation
Spleen, lymph nodes, and liver cells were harvested from MCMV-infected mice. A total of 2 × 106 spleen cells and 1 × 106 lymph node and liver cells were cultured in a round-bottom 96-well plate in 200 µl RPMI 1640 with 10% FCS containing 1 × 10−8 M MCMV peptide (H-2Db–restricted, M45 peptide 985HGIRNASFI993) (36) and BD GolgiPlug (34). After 4 h at 37°C, cells were stained for surface markers followed by intracellular IFN-γ.
Abs and flow cytometry
Single-cell suspensions were used for flow cytometry. FcRs were blocked with anti-CD16 + CD32 mAb (clone 2.4G2) at a concentration of 10 µg/ml prior to surface staining with the indicated Abs (all purchased from BD Biosciences, San Jose, CA; eBioscience, San Diego, CA; or BioLegend, San Diego, CA). Samples were acquired on an LSR II flow spectrometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
All of the statistical differences were determined by the unpaired, two-tailed Mann-Whitney U test. Results with p < 0.05 were considered significant. Statistics were calculated with Prism software (GraphPad, San Diego, CA).
Results
NK cells from Rae-1 Tg mice express reduced NKG2D but normal levels of other NK cell receptors
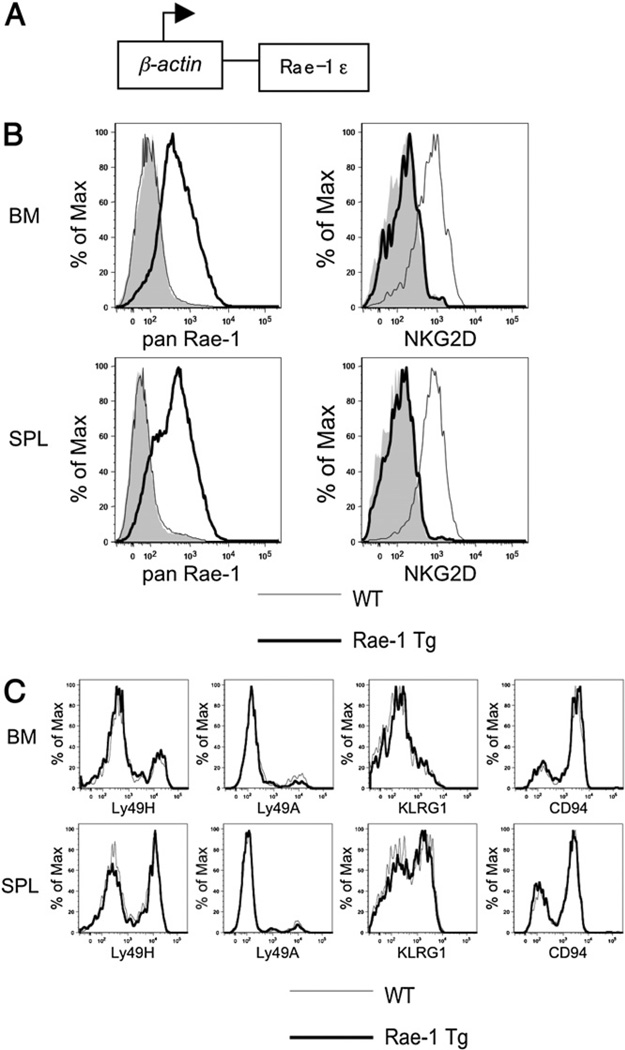
In this study, we used B6 Rae-1 Tg mice, in which the mouse NKG2D ligand Rae-1ε is driven by the human β-actin promoter and CMV enhancer (Fig. 1A), resulting in Rae-1 expression in all of the tissues tested. In particular, Rae-1 is expressed on lymphocytes from the bone marrow and spleen and results in a marked decrease of NKG2D receptor levels on NK1.1+TCRβ− NK cells (Fig. 1B). To determine whether other receptors were affected by constitutive Rae-1 expression, we stained bone marrow and spleen NK cells for the activating receptor Ly49H, the inhibitory receptors Ly49A and CD94, and the activation marker KLRG1 (Fig. 1C). We found no change in surface levels of these receptors along with various other receptors tested (data not shown). Together, these data indicate that constitutive Rae-1 expression results in specific down-modulation of the NKG2D receptor.
FIGURE 1.
Ubiquitous Rae-1ε expression results in NKG2D downmodulation on NK cells but normal expression of other NK receptors. A, Schematic of the Rae-1ε Tg mouse driven by the β-actin promoter. B, Bone marrow and spleen lymphocytes from Rae-1 Tg (bold line) or WT littermate control (thin line) were stained with anti–Rae-1 or an isotype-matched control Ig (shaded histogram) (left panel). Bone marrow and spleen NK cells (NK1.1+TCRβ−) from Rae-1 Tg (bold line) or WT littermate control mice (thin line) were stained for NKG2D or an isotype-matched control Ig (shaded histogram) (right panel). C, Bone marrow and spleen NK cells (NK1.1+TCRβ−) from Rae-1 Tg (bold line) or WT littermate control mice (thin line) were stained for a panel of NK cell receptors. Data are representative of at least three experiments.
NK cells from Rae-1 Tg mice develop normally
NK cell development occurs primarily in the bone marrow and proceeds through the loss and acquisition of various surface markers (reviewed in Ref. 37). To determine whether NK cell development is affected in Rae-1 Tg mice, we stained Rae-1 Tg and WT NK cells from bone marrow, spleen, and liver for developmental markers. NK1.1 is expressed early on during NK cell development, and its levels were unchanged in Rae-1 Tg mice (Fig. 2A). Similarly, the levels of CD122 and CD117 (c-Kit), which appear before and after NKG2D expression, respectively, were also unaltered in Rae-1 Tg mice (Fig. 2A). Peripheral NK cells have recently been classified based on their expression of Mac-1 (CD11b) and CD27, with CD27loMac-1hi NK cells being the most mature population (38, 39). Absolute numbers and percentages of these NK cell subsets in Rae-1 Tg mice were similar to those of WT mice (Fig. 2B). Moreover, we did not observe elevated proportions of NK cells in Rae-1 Tg mice expressing CD69 or KLRG1 (Fig. 1C and data not shown). In accordance to previous findings (19), our results indicate that NK cells that develop in the presence of Rae-1 are as mature as their WT counterparts and do not exhibit properties of activated or exhausted NK cells.
FIGURE 2.
NK cells from the bone marrow, liver, and spleen of Rae-1 Tg mice are phenotypically as mature as their WT counterparts. A, Histograms show the expression of the developmental markers CD122 (top panels), NK1.1 (middle panels), and c-Kit (bottom panels) on NK cells (NK1.1+TCRβ−) in the bone marrow, liver, and spleen of Rae-1 Tg (bold line) or WT littermate control (thin line) mice. B, Plots show the expression of the maturation markers CD27 and Mac-1 (CD11b) on NK cells (NK1.1+TCRβ−) in the bone marrow, liver, and spleen of Rae-1 Tg and WT mice. Quadrants were set to determine the percentage of the most immature (CD27hiMac-1−) to the most mature (CD27−Mac-1hi) NK cells. Data are representative of two different experiments.
Partial rescue of impaired NKG2D function in Rae-1 Tg mice by IL-2 or polyinosinic-polycytidylic acid treatment
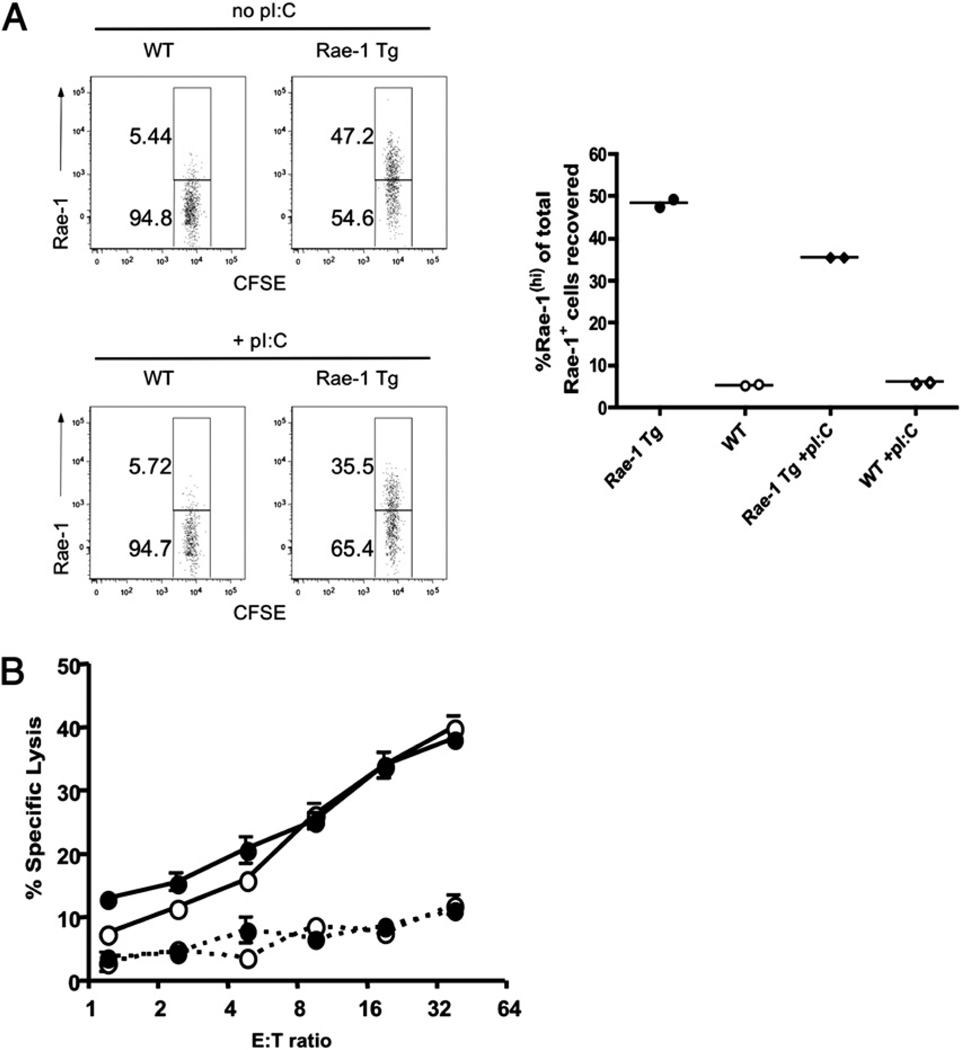
It has previously been shown that constitutive NKG2D ligand expression downregulates NKG2D expression on the cell surface of NK and T cells and impairs NKG2D function (18, 19, 22, 24, 30). To confirm this finding in our B6 Rae-1 Tg mouse model, we performed in vivo killing assays examining the ability of WT or Rae-1 Tg mice to reject adoptively transferred Rae-1+ Tg splenocytes. Splenocytes from Rae-1 Tg or WT mice were labeled with CFSE (lo or hi, respectively) and injected into either Rae-1 Tg or control recipients. At 48 h, spleens were collected and stained with anti–Rae-1 mAb. The percentage of CFSEIo splenocytes (Rae-1 Tg targets) expressing Rae-1 was analyzed. Although WT litter-mate control mice rejected Rae-1+ splenocytes efficiently, Rae-1 Tg mice failed to do so, and this defect was only slightly rescued when mice were activated 1 d prior to splenocyte transfer with polyinosinic-polycytidylic acid (poly I:C) (Fig. 3A). In addition, unlike WT mice, Rae-1 Tg mice were unable to reject NKG2D-bearing tumors (data not shown). Similar to previous reports (21, 22), we also found that growth of NK cells from Rae-1 Tg mice in IL-2 for 5 d restores cytotoxicity toward tumor cell targets expressing NKG2D ligands (Fig. 3B).
FIGURE 3.
Rae-1 Tg mice have impaired NKG2D-mediated functions restored by IL-2 in vitro and slightly rescued by poly I:C in vivo. A, Splenocytes from WT or Rae-1 Tg mice were harvested and differentially labeled as CFSE hi or CFSE Io, respectively. Cells were injected i.v. at equal ratios into either Rae-1 Tg or WT mice, some of which were pretreated on day −1 with poly I:C (left panel). Plots show recovery of CFSE Io-labeled Rae-1 Tg cells reclaimed from the spleen of Rae-1 Tg or WT mice after 48 h (right panel). B, IL-2–grown NK cells (day 5) from Rae-1 Tg (black circles) or WT littermate controls (open circles) were incubated for 4 h with 51Cr-labeled Ba/F3 (dashed line) or Ba/F3 cells expressing Rae-1ε (solid line). Data are representative of two experiments.
Normal rejection of B2m−/− splenocytes in Rae-1 Tg mice
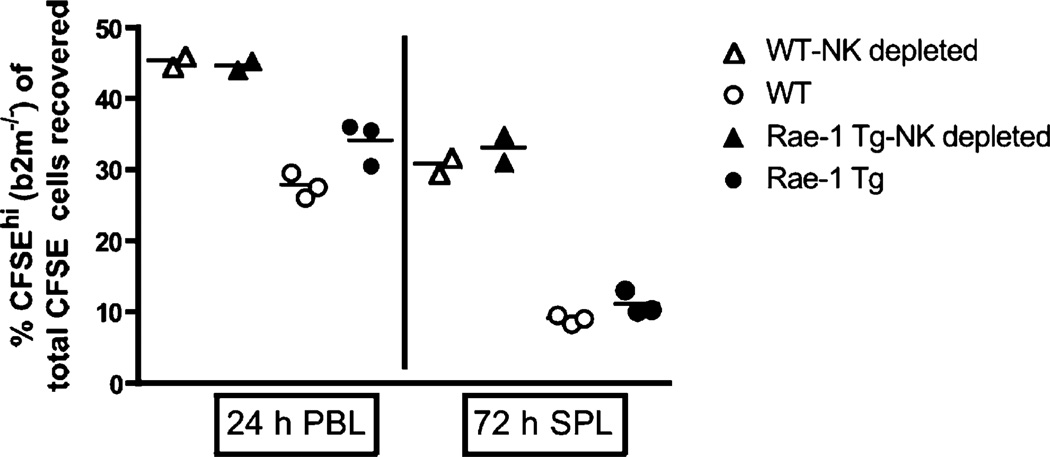
Having established that Rae-1 Tg mice develop normally and exhibit the expected impaired NKG2D function, we asked whether these mice had impaired missing-self recognition. Using an in vitro model, Coudert et al. (30) reported that NK cells chronically stimulated via NKG2D by coculture with NKG2D ligand-bearing stimulator cells in the presence of IL-2 have an impaired ability to kill RMA-S cells, indicating a defect in missing-self recognition. To determine whether Rae-1 Tg NK cells exhibited an impaired recognition of missing self, we labeled B2m−/− or WT splenocytes with CFSE and injected them into Rae-1 Tg or WT recipients with or without depleting NK cells in the recipients (Fig. 4). We then measured the percentage recovery of B2m−/− splenocytes. At 10 and 24 h, Rae-1 Tg had a slightly higher recovery of B2m−/− splenocytes in spleen and blood, respectively, indicating a reduced ability to kill these targets at early time points (Fig. 4, Supplemental Fig. 2); however, by 72 h, NK cells in Rae-1 Tg mice had cleared B2m−/− splenocytes to similar levels as WT NK cells (Fig. 4). These results indicate that constitutive NKG2D engagement slows recognition of missing self by NK cells but does not impair NK cell ability to reject MHC class I-deficient targets in vivo.
FIGURE 4.
Normal rejection of B2m−/− splenocytes in Rae-1 Tg mice. Splenocytes from B2m−/− or WT mice were harvested and differentially labeled as CFSE hi or CFSE lo, respectively. Cells were injected i.v. at equal ratios into either Rae-1 Tg or WT control mice, some of which were pretreated at day −1 with anti-NK1.1 mAb to deplete NK cells. Plots show percentages of CFSEhi-labeled B2m−/− cells (of total CFSE+ cells injected) reclaimed from the blood (24 h) and spleen (72 h) of untreated Rae-1 Tg (black circles) and WT control mice (open circles) or anti-NK1.1 treated Rae-1 Tg (black triangles) and WT control mice (open triangles). Data are representative of two experiments.
NK cells from Rae-1 Tg mice produce comparable amounts of IFN-γ and CD107a when stimulated with Ly49H
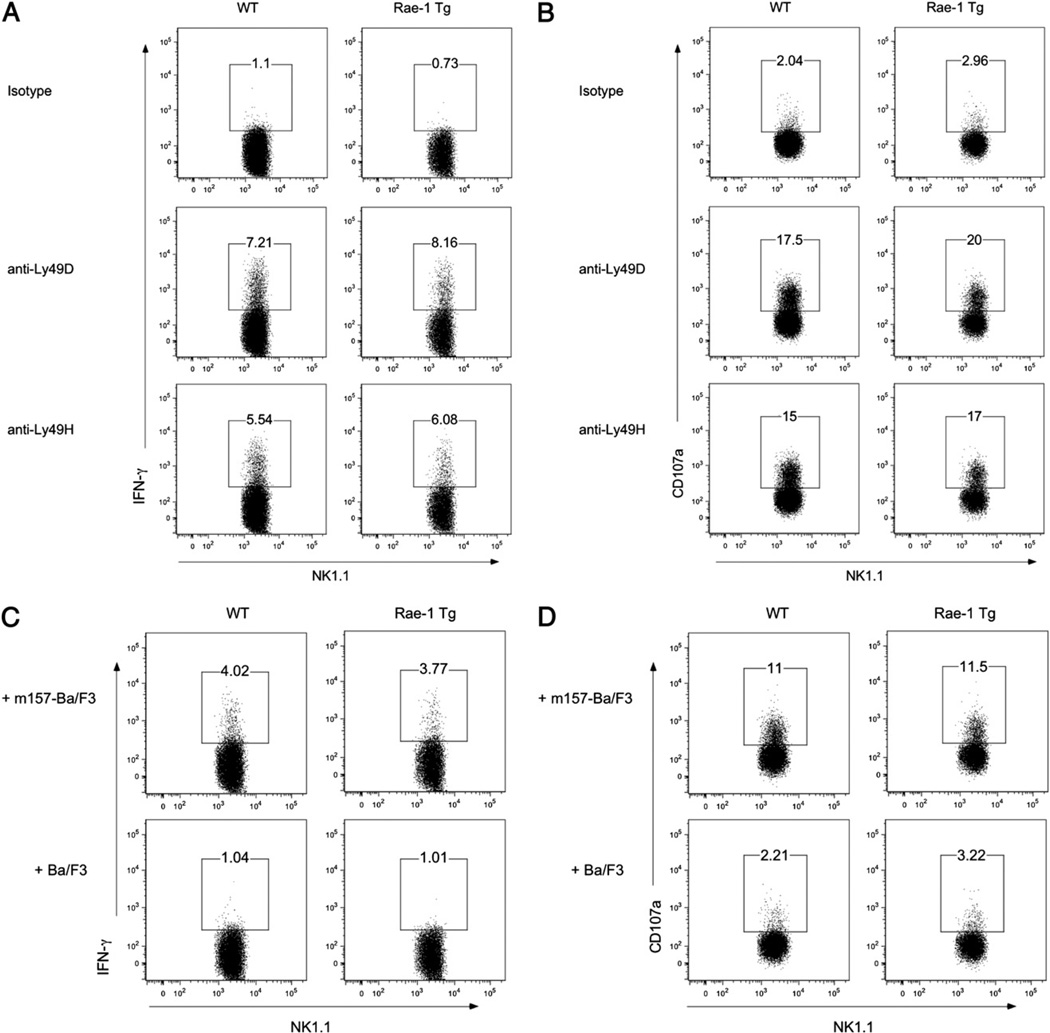
Ly49H, an activating receptor present on ∼50% of resting B6 NK cells, binds with high affinity to the MCMV protein m157 and pairs with the adapter molecules DAP12 and DAP10 for optimal function (40, 41). Coudert et al. (22, 30) reported that constitutive NKG2D ligation in vitro results in decreased presence of DAP10 and DAP12, but RMA-H60–exposed NK cells have a normal ability to kill RMA-m157 targets. We asked whether Rae-1 Tg mice showed an impaired Ly49H function. To test the function of Ly49H cells from Rae-1 Tg mice ex vivo, spleens were harvested and stimulated with Abs for 4 h in the presence of monensin, after which intracellular IFN-γ and surface CD107a staining were measured. Rae-1 Tg mice produced comparable amounts of IFN-γ and CD107a following activation by Abs of both Ly49H and another DAP12-associated receptor, Ly49D (Fig. 5A, 5B). We also incubated NK cells with m157-transduced Ba/F3 cells or parental Ba/F3 cells for 4 h in the presence of monensin. Rae-1 Tg and control mice produced equivalent amounts of IFN-γ and CD107a in response to m157-bearing cells (Fig. 5C, 5D). Finally, we performed ex vivo cytotoxicity assays using m157-expressing and nonexpressing Ba/F3 cells as targets and NK cells isolated from the spleens of either naive (Supplemental Fig. 1A) or day 4 MCMV-infected (Supplemental Fig. 1B) WT or Rae-1 Tg mice as effector cells. Both naive and MCMV-activated NK cells from WT or Rae-1 Tg mice killed m157-bearing targets equivalently. Altogether, these results indicate that NK cells chronically exposed to NKG2D ligands have normal Ly49H function ex vivo.
FIGURE 5.
NK cells from Rae-1 Tg and WT mice produce comparable amounts of IFN-γ and CD107a when stimulated with Ly49H agonists. A and B, Freshly isolated splenocytes from Rae-1 Tg or WT littermate controls were enriched for NK cells and stimulated with plate-bound Abs against the NKRs NKG2D, Ly49D, and Ly49H or an isotype-matched control Ig in the presence of monensin (BD GolgiStop). After 4 h, cells were stained for intracellular IFN-γ and surface CD107a expression. Plots show the percentage of NK1.1+TCRβ− cells that produce (A) IFN-γ and (B) CD107a. C and D, NK cells enriched as in A were incubated with Ba/F3 or Ba/F3-m157 targets in the presence of monensin. After 4 h, cells were stained for intracellular IFN-γ and surface CD107a expression. Plots show the percentage of NK1.1+TCRβ− cells that produce (C) IFN-γ and (D) CD107a. Data are representative of three experiments.
Normal NK cell response to MCMV in Rae-1 Tg mice
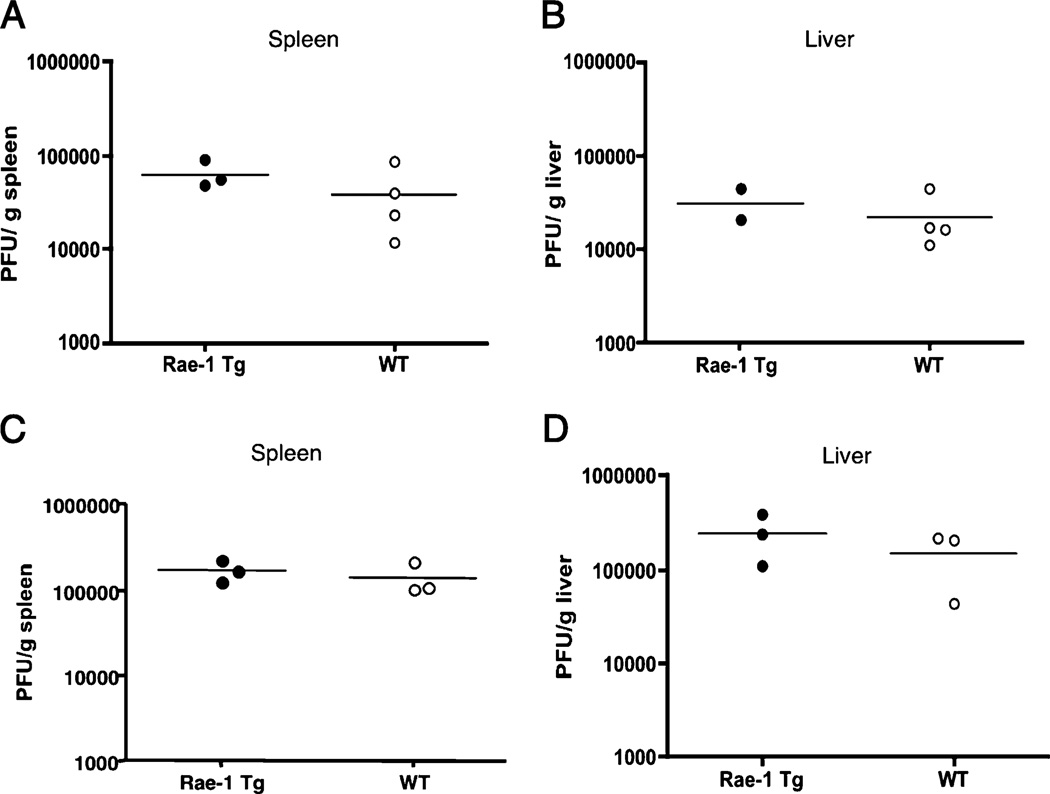
To investigate whether chronic NKG2D stimulation globally impairs NK cell function, as has been proposed previously based on in vitro studies (30), we addressed whether Rae-1 Tg mice are more susceptible to pathogens controlled by NK cells. NK cell deficiency renders human and mice susceptible to certain viruses, in particular herpesviruses, such as human CMV and MCMV, respectively (42, 43). The immune response induced by Ly49H binding to the viral protein m157 mediates resistance to MCMV in B6 mice (3). To determine whether chronic NKG2D stimulation impaired immunity to MCMV, we infected Rae-1 Tg mice with WT MCMV. At d 3 postinfection, viral titers in the spleen and liver of Rae-1 Tg mice were not statistically different from those of WT mice (Fig. 6A, 6B).
FIGURE 6.
Normal control of MCMV in spleen and liver of Rae-1 Tg mice. A and B, Rae-1 Tg and WT mice were infected with WT MCMV. Three days postinfection, MCMV titers in the (A) spleen and (B) liver were determined by plaque assay. C and D, Rae-1 Tg and WT mice were infected with MCMV Δm151-m158 deletion virus. Three days postinfection, MCMV titers in the (A) spleen and (B) liver were determined by plaque assay. Horizontal lines depict the means of the groups. Data are representative of two experiments.
To address the NKG2D-dependent, Ly49H-independent immunity in the face of infection, we infected Rae-1 Tg and WT mice with Δm151-m158 deletion mutant MCMV (33). This mutant virus lacks the viral genes m152 and m157, which are responsible for NKG2D ligand downmodulation and Ly49H recognition, respectively (3, 44); therefore, cells infected with this virus would express NKG2D ligands on their cell surface for engagement by NKG2D on NK cells but would lack m157 so would not activate via Ly49H. Again, viral titers in the spleen and liver of Rae-1 Tg mice were not statistically different from those of WT mice at 3 d postinfection (Fig. 6C, 6D).
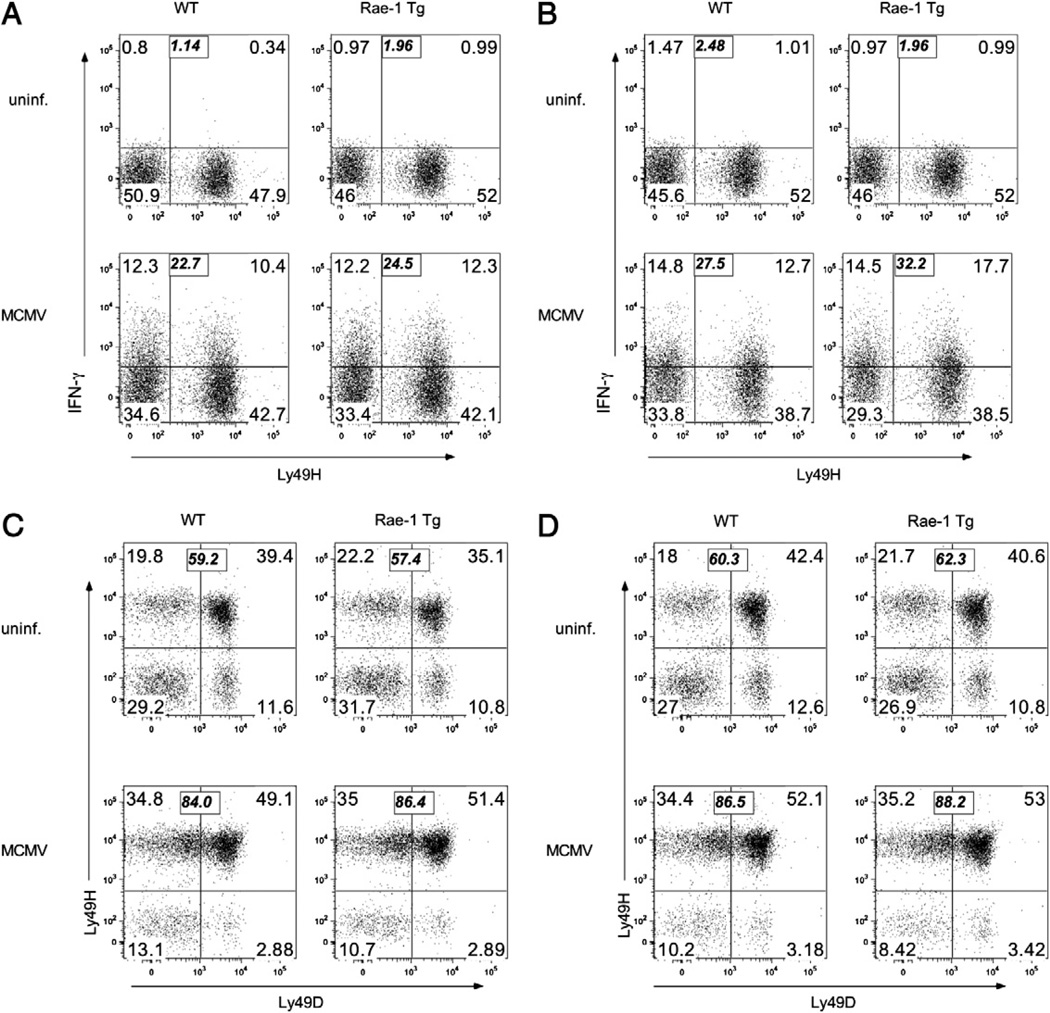
To determine whether Rae-1 Tg NK cells exhibit a normal cytokine response to WT MCMV infection, we infected Rae-1 Tg or control mice with WT MCMV and measured NK cell IFN-γ production at 36 h postinfection. Rae-1 Tg and WT NK cells produced comparable amounts of IFN-γ in both liver and spleen (Fig. 7A, 7B). At this time point, Ly49H–negative and positive NK cells contributed equivalently to IFN-γ production. We also asked whether Rae-1 Tg NK cells could expand similarly to WT NK cells. We harvested spleen and liver NK cells at 7 d postinfection with WT MCMV and stained NK cells for Ly49H and Ly49D expression. Similarly to WT NK cells, Ly49H+ Rae-1 Tg NK cells expanded to an average of 86% of the total NK cell population in the liver and spleen of infected mice, whereas the proportion of NK cells expressing an irrelevant Ly49D receptor remained unchanged compared with that in uninfected mice (Fig. 7C, 7D). These results indicate that chronic NKG2D stimulation does not impair Ly49H function during MCMV infection.
FIGURE 7.
Normal NK cell response to MCMV in Rae-1 Tg mice. Rae-1 Tg or WT littermate control mice were infected with 104 PFU per mouse of MCMV. A and B, Thirty-six hours postinfection, cells were harvested from (A) liver and (B) spleen and incubated with brefeldin A. After 4 h, NK cells were stained for intracellular IFN-γ and surface Ly49H expression. The bold italic numbers represent the total percentage of IFN-γ+ cells. C and D, Seven days postinfection, NK cells were harvested from (C) liver and (D) spleen and stained for surface Ly49H and Ly49D expression. The bold italic numbers represent the percentage of Ly49H+ cells. Data are representative of three experiments.
Normal CD8+ T cells response to MCMV in Rae-1 Tg mice
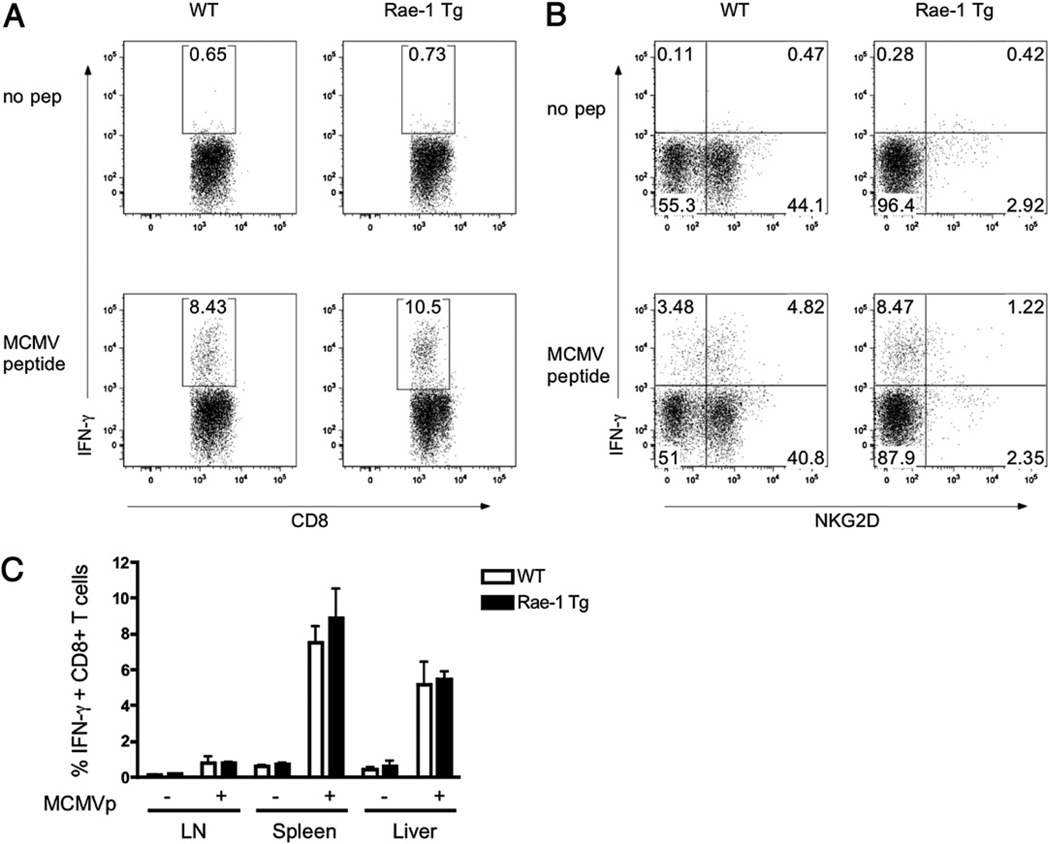
Upon activation, CD8+ T cells upregulate NKG2D, which has been suggested to mediate T cell costimulation (45). We asked whether Rae-1 Tg mice could efficiently generate MCMV-specific CTLs in the presence of chronic NKG2D stimulation. WT or Rae-1 Tg mice were infected with MCMV, and spleen, liver, and lymph nodes were harvested at 7 d postinfection. As shown in Fig. 8A, Rae-1 Tg and WT CD8+ T cells produced comparable amounts of IFN-γ following peptide stimulation in vitro, despite the lack of NKG2D upregulation on Rae-1 Tg CD8+ T cells (Fig. 8B). Our results show that constitutive NKG2D stimulation does not impair MCMV-specific CTL generation.
FIGURE 8.
Normal CD8+ T cells response to MCMV in Rae-1 Tg mice. Rae-1 Tg or WT littermate control mice were infected with MCMV. Splenocytes were harvested at day 7 postinfection and incubated in the presence of MCMV peptide and brefeldin A. A and B, After 4 h, CD8+ T cells were stained for intracellular (A) IFN-γ and surface (B) NKG2D expression. C, The graph indicates the percentage of IFN-γ–producing CD8+ T cells in response to MCMV peptide in the lymph node, spleen, and liver of mice infected as in A and B. Data are representative of two experiments.
Discussion
Human tumors often express cell surface or soluble NKG2D ligands. As a result of constant engagement of NKG2D with its ligands, NKG2D is frequently downregulated on NK and CD8+ T cells from human cancer patients. Although it is well established that tumor-induced modulation of NKG2D inhibits NKG2D-dependent functions, the global consequences of long-term exposure to NKG2D ligands in vivo remains unknown.
Prior studies have used in vitro models (22, 30) or mouse models using strains in which NK cells are hyporesponsive (19) to ask what effects chronic NKG2D engagement impairs NK cells globally. In this study, we use an in vivo model of constitutive ligand expression in C57BL/6 mice. We provide evidence that despite impaired NKG2D-mediated function, Rae-1 Tg mice have intact NKG2D-independent functions. Our group and others have previously generated mice in which a tissue-specific or a ubiquitous promoter drives the expression of an NKG2D ligand (18, 19). Whether human NKG2D ligand MICA was expressed under the H-2Kb promoter or mouse NKG2D ligand Rae-1ε was expressed under the β-actin promoter, these transgenic models provided strong evidence that sustained NKG2D ligand expression impairs NKG2D function. H-2Kb–MICA and β-actin–Rae-1ε mice had decreased NKG2D levels on NK cells and were unable to reject Rae-1–expressing RMA tumors or Rae-1 Tg splenocytes. Similar findings were observed when WT NK cells were exposed to NKG2D ligand-bearing cells in vitro and in vivo (21). NK cells rapidly downregulated surface NKG2D and were inefficient at lysing or producing IFN-γ in response to NKG2D ligand-expressing targets. In accordance with these studies, we have found that Rae-1 Tg mice expressed lower amounts of NKG2D on the surface of NK cells. This correlates with a defect in rejecting Rae-1+ splenocytes that is partially rescued by previous activation with poly I:C in vivo. In addition, we found that activation with IL-2 in vitro rescues NKG2D function, as had previously been shown (21).
Our study of Rae-1 Tg mice addresses whether sustained NKG2D engagement might affect NK cell development. Using mice expressing m157 in the bone marrow, Sun et al. (46) showed that Ly49H+ NK cells were phenotypically less mature than their WT counterparts. We found that NK cell developing in the presence of Rae-1 expressed similar levels of the developmental markers CD122, NK1.1, and c-Kit as WT mice, hence proceeding through similar stages of development. These results are in accordance with the studies using β-actin–driven Rae-1 (FVB strain) and m157 transgenic mice that also showed normal expression of NK cell developmental markers (19, 47). Furthermore, we found that subsets of CD27- and Mac-1–expressing NK cells were unchanged in Rae-1 Tg mice. This is contrasts with a prior study in which there was a slight decrease in the percentage of CD27Io NK cells in the spleen of Rae-1 Tg mice (48).
The effect of sustained NKG2D engagement on missing-self recognition has remained controversial. Using the H-2Kb–MICA transgenic mice, Wiemann et al. (18) reported normal cytotoxicity of preactivated transgenic NK cells toward RMA-S cells in vitro. Two reports subsequently showed impairment of missing-self recognition. In their β-actin–Rae-1ε Tg mice, Oppenheim et al. (19) observed a defect in the in vivo lysis of B2m−/− mice that was restored upon poly I:C treatment of the mice. Second, Coudert et al. (30) reported that after in vitro coculture with H60-transduced RMA cells NK cells were unable to efficiently kill MHC class I-deficient RMA-S targets. Our results indicate that on the C57BL/6 background, in the absence of prior activation, Rae-1 TgNK cells efficiently kill B2m−/− splenocytes in vivo, albeit less rapidly than WT NK cells. The differences between these studies might be explained in various ways. First, in Oppenheim et al. (19), the nonsyngeneic Rae-1ε transgene is on the FVB background, a mouse strain in which we have found WT NK cells to be generally hyporesponsive compared with C57BL/6 (data not shown) and thus may be easier to impair functionally. Also, Oppenheim et al. (19) measured killing of B2m−/− splenocytes at 10 h postinjection, at which time we also observe reduced, albeit much less pronounced, cytotoxicity by Rae-1 Tg NK cells. Finally, the study by Coudert et al. (30) used NK cells exposed to H60-transduced RMA cells in the presence of a high dose of IL-2 for 3 d. We have found that growth of NK cells in the presence of IL-2 affects NK cell receptor expression, including NKG2D (D. Hesslein, unpublished observation). The activating receptor that mediates killing of B2m−/− splenocytes is unknown; therefore, it is possible than the levels of responsible receptor(s) were decreased in the presence of IL-2 and NKG2D ligands, which would explain the observed defect in RMA-S killing. Along this line, Coudert et al. (30) measured decreased expression of CD16, the receptor that mediates Ab-dependent cellular cytotoxicity, following incubation with H60-transduced RMA cells and IL-2. This contrasts with our observation that freshly isolated NK cells from Rae-1 Tg and WT mice have identical levels of CD16 expression (data not shown). In accordance with this finding, IL-2–grown Rae-1 Tg NK cells mediate normal Ab-dependent cell-mediated cytotoxicity (data not shown).
Coudert et al. reported that NK cells cultured with H60-transfected RMA cells expressed lower amounts of DAP10 and DAP12 protein (30). We examined whether in Rae-1 Tg NK cells NKG2D modulation affected the Ly49H and Ly49D receptors that rely on these adapter molecules for expression and to signal. We found that Rae-1 Tg NK cells expressed identical amounts of Ly49D and Ly49H on the cell surface compared with those on WT NK cells and could produce IFN-γ and upregulate CD107a at similar levels to WT NK cells in response to plate-bound anti-Ly49D and anti-Ly49H stimulation. Likewise, Rae-1 Tg NK cells responded as well as WT NK cells to stimulation by m157-bearing Ba/F3 cells, as measured by degranulation of the NK cells and by standard 51Cr release assays. Therefore, chronic exposure of NK cells to NKG2D ligands in vivo does not impair non-NKG2D–dependent NK cell activation pathways.
It is well appreciated that NKG2D ligand expression by human tumors can lead to decreased NKG2D levels on NK cells and CD8+ T cells and result in impaired NK cell-mediated cytotoxic activity in cancer-bearing humans. Several studies have shown that high levels of soluble NKG2D ligand in the sera of cancer patients is an indicator of poor prognosis (49), but the effect of downregulation of NKG2D on NK cells and T cells in mounting immune responses to viral infections has not been investigated. We believe that our transgenic model mimics NKG2D ligand exposure by cancer patients, because we have found that NKG2D ligands can be shed both in the sera of Rae-1 Tg mice and in the supernatant of Rae-1–transduced B16 melanoma cells (50). In addition to controlling tumors, NK cells are potent players in response to viral infections, in particular herpesviruses, such as human CMVand MCMV (51). In this study, we found that Rae-1 Tg mice are fully capable of mounting a robust immune response to MCMV. Rae-1 Tg NK cells produced normal amounts of IFN-γ early after MCMV infection. Also, the expansion of Ly49H+ NK cells and generation of MCMV peptide-specific CD8+ T cells were unaltered in Rae-1 Tg mice.
In summary, we demonstrate that despite an impairment of NKG2D function sustained NKG2D engagement does not impact NKG2D-independent NK cell functions. In particular, missing-self recognition and response to MCMV were unaltered in Rae-1 Tg mice. These findings indicate that cancer patients expressing tumor-bound or soluble NKG2D ligands may not be at elevated risk to pathogens normally controlled by NK cells and T cells.
Supplementary Material
Acknowledgments
We thank the Lanier laboratory for insightful comments and Dr. Joseph Sun for critical review of the manuscript.
This work was supported by National Institutes of Health Grant CA095137. L.L.L. is an American Cancer Society Professor. J.N.B. is a Juvenile Diabetes Research Foundation Postdoctoral Fellow and was supported by National Institutes of Health Training Grant T32.
Abbreviations used in this paper
- B6
C57BL/6
- MCMV
mouse cytomegalovirus
- NKG2D
NK group 2, member D
- poly I:C
polyinosinic-polycytidylic acid
- WT
wild-type
Footnotes
Disclosures
L.L.L., K.O., and the University of California (San Francisco, CA) have licensed intellectual property rights relative to NKG2D for commercial applications.
References
- 1.Lanier LL. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo ME, Ploegh HL, Tirabassi RS. Viral immune evasion strategies and the underlying cell biology. Semin. Immunol. 2001;13:1–9. doi: 10.1006/smim.2000.0290. [DOI] [PubMed] [Google Scholar]
- 3.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 4.Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 6.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Song Y, Bakker ABH, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 8.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat. Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat. Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61:335–343. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 11.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J. Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 12.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 14.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J. Exp. Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiemann K, Mittru¨cker H-W, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee H-G, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J. Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL. NKG2D blockade prevents autoimmune diabetes in NOD mice. Cell. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 22.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, Held W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 23.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J. Exp. Med. 1991;173:1017–1020. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 25.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J. Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 26.Jinushi M, Takehara T, Kanto T, Tatsumi T, Groh V, Spies T, Miyagi T, Suzuki T, Sasaki Y, Hayashi N. Critical role of MHC class I-related chain A and B expression on IFN-α-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J. Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 27.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, Hayashi N. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int. J. Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 28.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 29.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 30.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 31.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat. Immunol. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrlich LI, Ogasawara K, Hamerman JA, Takaki R, Zingoni A, Allison JP, Lanier LL. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J. Immunol. 2005;174:1922–1931. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 33.Kielczewska A, Pyzik M, Sun T, Krmpotic A, Lodoen MB, Munks MW, Babic M, Hill AB, Koszinowski UH, Jonjic S, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cebo C, Da Rocha S, Wittnebel S, Turhan AG, Abdelali J, Caillat-Zucman S, Bourhis JH, Chouaib S, Caignard A. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J. Immunol. 2006;176:864–872. doi: 10.4049/jimmunol.176.2.864. [DOI] [PubMed] [Google Scholar]
- 35.Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski UH. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold MC, Munks MW, Wagner M, Koszinowski UH, Hill AB, Fling SP. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 2002;169:359–365. doi: 10.4049/jimmunol.169.1.359. [DOI] [PubMed] [Google Scholar]
- 37.Di Santo JP, Vosshenrich CA. Bone marrow versus thymic pathways of natural killer cell development. Immunol. Rev. 2006;214:35–46. doi: 10.1111/j.1600-065X.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 39.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol. Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 40.Orr MT, Sun JC, Hesslein DGT, Arase H, Phillips JH, Takai T, Lanier LL. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J. Exp. Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith KM, Wu J, Bakker ABH, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J. Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 42.Orange JS. Human natural killer cell deficiencies. Curr. Opin. Allergy Clin. Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 43.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 46.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J. Exp. Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayakawa Y, Watt SV, Takeda K, Smyth MJ. Distinct receptor repertoire formation in mouse NK cell subsets regulated by MHC class I expression. J. Leukoc. Biol. 2008;83:106–111. doi: 10.1189/jlb.0707496. [DOI] [PubMed] [Google Scholar]
- 49.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, et al. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin. Cancer Res. 2009;15:5208–5215. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 50.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat. Rev. Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.