Abstract
Background
Social learning models of substance use propose that drug-use behaviors are learned by observing and mimicking the behavior of others. The aim of this study was to examine the acquisition of cocaine self-administration in three groups of experimentally naïve rats: rats that were tested in isolation, rats that were tested in the presence of another rat that had access to cocaine and had previously been trained to self-administer cocaine, and rats that were tested in the presence of another rat that did not have access to cocaine.
Methods
Male rats were reared in isolated or pair-housed conditions and implanted with intravenous catheters. Pair-housed rats were then assigned to drug-experienced or drug-naïve conditions. In the drug-experienced condition, one rat of each pair was trained to self-administer cocaine in isolation before the reintroduction of its partner. In the drug-naïve condition, one rat of each pair did not have access to cocaine for the duration of the study. For each group, the acquisition of cocaine self-administration was measured over 15 days in rats with access to cocaine but no prior operant training.
Results
Rats tested with a drug-experienced partner were faster to acquire cocaine self-administration and emitted more active lever presses than rats tested with a cocaine-naïve partner. Data for the isolated control group fell between the other two groups on these measures.
Conclusion
These data indicate that the acquisition of cocaine self-administration can either be facilitated or inhibited by social contact. Collectively, these results support a social-learning model of substance use.
Keywords: cocaine, rat, self-administration, social, social learning
1. INTRODUCTION
Social learning models of substance use propose that drug use is learned, in part, by observing and mimicking the behavior of others (see reviews by Andrews and Hops, 2010; Kandel, 1986; Pandina et al., 2010). Despite the popularity of these models, very few experimental studies have examined the role of social learning in drug use, possibly due to a lack of animal models that allow subjects to observe and mimic the drug use behavior of another subject. We recently described the use of custom-built, operant conditioning chambers that permit two rats to be tested simultaneously during periods of intravenous, drug self-administration (Smith, 2012). Using these chambers, we reported that cocaine self-administration could either be increased or decreased based on the behavior of a partner. Specifically, we reported that cocaine self-administration was facilitated when a rat was paired with another rat with simultaneous access to cocaine, but cocaine self-administration was inhibited when a rat was paired with another rat without access to cocaine. Such data support a social learning model by showing that the behavior of a peer, as opposed to merely the presence of a peer, determines whether social contact increases or decreases drug self-administration.
In our previous study, all rats received lever-press training using food reinforcement before self-administration testing, and this prevented us from examining the role of social learning on the acquisition of drug self-administration. This is relevant because a rapid transition from initial drug exposure to regular patterns of use is an important prognosticator of whether an individual will later develop problems with substance use (U.S. Congress, Office of Technology Assessment, 1994). The acquisition of regular patterns of intake after initial drug exposure is often modeled in the laboratory by exposing a subject to noncontingent drug infusions and then permitting the subject to self-administer that drug during free-operant test sessions. Importantly, factors that increase the rate of acquisition in the laboratory are considered to be risk factors for developing problems with substance use in humans, whereas factors that decrease the rate of acquisition in the laboratory are considered to be protective against substance abuse in humans. For example, social isolation (Kosten et al., 2000) and social stress (Tidey and Miczek, 1997) reliably increase the rate of acquisition in laboratory animals and serve as risk factors in human populations (Chartier et al., 2010; Dube et al., 2006). In contrast, access to alternative, nondrug reinforcers decreases the rate of acquisition in laboratory animals (Carroll and Lac, 1993; Cosgrove et al., 2002), and access to nondrug social activities decreases the acquisition of drug and alcohol use in human adolescents (D’Amico et al., 2012; St. Pierre et al., 1992; for similar studies using the conditioned place preference procedure, see Bahi, 2013; Geuzaine and Tirelli, 2014; Ribeiro Do Couto et al., 2009). To date, the role of social learning in the acquisition of drug self-administration, at least in regard to intravenous drug self-administration, has not been examined.
The purpose of the present study was to examine the contribution of social learning to the establishment of stable patterns of drug intake after initial drug exposure. To this end, the acquisition of cocaine self-administration was examined in three groups of experimentally naïve rats: (1) rats that were tested in isolation, (2) rats that were tested in the presence of another rat that had access to cocaine and had previously been trained to self-administer cocaine (drug-experienced), and (3) rats that were tested in the presence of another rat that did not have access to cocaine (drug-naïve). Behavioral testing advanced through three stages designed to systematically increase the probability that self-administration would be acquired. In phase 1 (days 1–5), responding was reinforced with 0.25 mg/kg cocaine; in phase 2 (days 6–10), responding was reinforced with 0.75 mg/kg cocaine; and in phase 3 (days 11–15), responding was reinforced with 1.5 mg/kg cocaine. Our previous data (Smith, 2012) suggested that cocaine self-administration is enhanced in socially housed rats if both members of the pair have access to cocaine, but cocaine self-administration is inhibited if only one member of the pair has access to cocaine. Consequently, we hypothesized that the rate of acquisition would occur most rapidly in the drug-experienced group and most slowly in the drug-naïve group.
2. METHOD
2.1Animals and Apparatus
Male, Long-Evans rats were obtained at weaning (~21 days) from Charles River Laboratories and assigned to isolated or pair-housed conditions. Both isolated and pair-housed rats were housed in standard laboratory cages (interior dimensions: 50 × 28 × 20 cm) until the beginning of self-administration testing. At that time, all rats were transferred to custom-built, operant conditioning chambers that served as home cages for the remainder of the study. All rats were kept in a temperature- and humidity-controlled colony room on a 12 hr light/dark cycle (lights on: 0500) for the duration of the study. All animals were maintained in accordance with The Guide for Care of Laboratory Animals (Institute of Laboratory Animals Resources, 2011).
All drug self-administration sessions were conducted in custom-built, operant conditioning chambers described previously (Smith, 2012). Briefly, chambers for isolated rats were cubic in design with two response levers on the rear wall. Chambers for pair-housed rats were constructed from two isolated chambers separated by a 14-gauge wire-screen panel. The wire screen allowed pair-housed rats visual, auditory, olfactory, and limited tactile contact with each other, but prevented one rat from accessing the tethering system of its companion. Each rat had individual access to two response levers mounted on the rear wall. The response levers were positioned 13 cm apart and 6 cm from each sidewall. For pair-housed rats with access to cocaine, the inner lever (i.e., the lever in closest physical proximity to the partner) was designated the active lever, whereas the outer lever was designated the inactive lever. Drug infusions were delivered via Tygon tubing protected by a stainless steel spring and connected to a counter balanced swivel suspended above the chamber. An infusion pump (3.33 rpm) was mounted behind the cage and connected to interfacing equipment provided by Med Associates, Inc. (St Albans, VT, USA). Fresh food was placed inside the cages daily, and water dispensers were continuously available inside the cage.
Lever-press training for cocaine-experienced rats (see below) was conducted in standard, commercially available, operant conditioning chambers from Med Associates, Inc. These chambers were equipped with two response levers, two white stimulus lights above the response levers, a house light, and a food pellet receptacle located between the two response levers.
2.2 Group Assignments
Isolated rats were housed individually and tested in individual test chambers with no visual contact with other rats. Pair-housed rats were randomly assigned to cocaine-experienced and cocaine-naïve groups approximately five weeks after arrival. Rats in the isolated and cocaine-naïve groups remained undisturbed in their home cage until surgery and catheter implantation.
For rats in the cocaine-experienced group, one rat of each pair (the cocaine-experienced rat) was trained to press a lever using food reinforcement. Approximately five weeks after arrival, these rats were food restricted to no less than 90% of their free-feeding body weight, placed in operant conditioning chambers, and trained to press a response lever on a fixed ratio (FR1) schedule of reinforcement. Training sessions lasted 2 hr or until 40 reinforcers were delivered, whichever occurred first. Daily training sessions continued in this manner until a rat received the maximum number of 40 reinforcers during any four training sessions. Once this criterion was met, training was discontinued and the rat was placed back on unrestricted feed. The experimentally naïve partners of the cocaine-experienced rats were left undisturbed in the home cage throughout this time period.
Cocaine-experienced rats were surgically implanted with intravenous catheters six weeks after arrival and five days before their experimentally naïve partners (see below). Three days after surgery, cocaine-experienced rats were placed in custom-built, operant conditioning chambers (in isolation and without their experimentally naïve partner) during daily training sessions. Each session began with a priming infusion of cocaine, and the insertion of two retractable levers into the home cage. Each response on the inner (active) lever produced an infusion of cocaine and retraction of the lever for 20 s. All infusions, including the priming infusion, delivered 0.5 mg/kg cocaine over a duration of 2.5–3.0 seconds (based on body weight). Each session lasted 2 hr with no limit placed on the maximum number of infusions that could be earned. Training continued in this manner for 5 consecutive days, at which time the cocaine-experienced rats were joined by their experimentally naïve partners during daily test sessions (see below).
Rats that lost catheter patency before the end of testing were removed from the study and not included in the statistical analysis. For socially housed rats, if one member of the pair lost catheter patency before the end of testing, then both members of the pair were removed from the study and not included in the statistical analysis. This practice led to the removal of a greater number of socially housed rats than isolated rats from the study (rats removed: n = 1 experimentally naïve isolated rat; n = 5 cocaine-experienced rats and their 5 experimentally naïve partners; n = 3 cocaine-naïve rats and their 3 experimentally naïve partners). A total of 62 rats completed all phases of testing (n = 24 experimentally naïve isolated rats; n = 9 cocaine-experienced rats and their 9 experimentally naïve partners; n = 10 cocaine-naïve rats and their 10 experimentally naïve partners). All data (e.g., % of rats meeting the acquisition criterion) reflect only those animals that completed all phases of the study.
2.3 Surgery
All rats were surgically implanted with intravenous catheters between six and seven weeks after arrival. Rats were deeply anesthetized with a combination of ketamine HCL (100 mg/kg, ip) and xylazine HCl (8.0 mg/kg, ip). An intravenous catheter was implanted into the right jugular vein and exited the body via a port implanted on the dorsal surface of the scapulae. Ketoprofen (3.0 mg/kg, sc) was given immediately after surgery as an analgesic, and a solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily for 7 days to maintain patency and prevent infection. After 7 days, ticarcillin was discontinued and only heparinized saline was used to maintain catheter patency. All rats were allowed to recover for three days before beginning acquisition testing. We employ a three-day recovery period because general indices of health and behavioral activity (e.g., feeding, drinking, wheel running) return to pre-surgical levels within three days using our surgical protocol.
2.4 Acquisition of Cocaine Self-Administration
Immediately prior to the beginning of acquisition testing, all rats were transferred to the custom-built, operant conditioning chambers, which served as home cages for the remainder of the study. All cocaine self-administration sessions began promptly at the beginning of the dark phase of the light/dark cycle (lights off: 1700).
All rats in the isolated group, all experimentally naïve rats in the cocaine-experienced group, and one member of each pair of the cocaine-naïve group were tested for the acquisition of cocaine self-administration. Each session began with the insertion of two retractable levers into the chamber and a noncontingent infusion of cocaine. During all sessions, responding was reinforced on a FR1 schedule of reinforcement. On this schedule, each response on the active (inner) lever produced an infusion of cocaine and retraction of the response lever for 20 s to signal a timeout during which cocaine was not available. For all sessions, responses on the inactive (outer) lever were recorded but had no programmed consequences. Sessions lasted 2 hr, with no limit placed on the maximum number of infusions that could be earned. One session was conducted each day for 15 consecutive days.
One member of each pair of the cocaine-naïve group was randomly assigned to be cocaine naïve and did not have access to cocaine for the duration of the experiment. Cocaine naïve rats were implanted with catheters, flushed daily with heparinized saline, and connected to Tygon tubing in the operant conditioning chambers. For these rats, both levers were inactive, and responding had no programmed consequences. Cocaine-experienced rats (i.e., those rats that received prior lever-press training and cocaine self-administration training) were tested with cocaine in the exact same manner as their experimentally naïve partners.
Acquisition testing advanced through three consecutive, five-day phases. During the first phase, (days 1–5), each infusion, including the priming infusion, delivered 0.25 mg/kg cocaine. During the second phase (days 6–10), each infusion, including the priming infusion, delivered 0.75 mg/kg cocaine. During the third phase (days 11–15), each infusion, including the priming infusion, delivered 1.5 mg/kg cocaine. Progression through the three stages was the same for all rats with access to cocaine, regardless of when they acquired cocaine self-administration.
Acquisition was operationally defined as obtaining a minimum of 8 infusions on each of two consecutive days, with the first of those days marking the date of acquisition. Thus, it was possible for a rat to meet the acquisition criterion on the first day, provided that at least 8 infusions were obtained on both the first and second day of testing. Any rat that obtained a minimum of 8 infusions for the first time on the 15th day received one additional test day. If a rat obtained at least 8 infusions on the 16th day, then it was considered to have met the acquisition criterion on the 15th day.
2.5 Data Analysis
Total active lever presses and total inactive lever presses were compared across experimentally naïve rats from the three groups via one-factor ANOVA. Active and inactive lever presses were also compared across experimentally naïve rats from the three groups using mixed-factor ANOVA, with group serving as the between-subjects factor and dose and day serving as within-subjects factors. Time to acquisition was compared across groups using the non-parametric Kruskal-Wallis test, followed by the Mann-Whitney U-test for planned comparisons. All post-hoc tests were conducted using the Bonferroni adjustment for multiple comparisons.
Additional tests were conducted in pair-housed rats to compare experimentally naïve rats with their cocaine-experienced or cocaine-naïve partners (the study was not powered to perform simultaneous comparisons between all five possible groups). The total number of active lever presses were compared between cocaine-experienced rats and their experimentally naïve partners, and between cocaine-naïve rats and their experimentally naïve partners, using independent-samples t-tests. Active lever presses were also compared via mixed-factor ANOVA, with group serving as the between-subjects factor and dose and day serving as within-subjects factors. For cocaine-naïve rats, the inner lever was arbitrarily defined as the “active” lever.
3. RESULTS
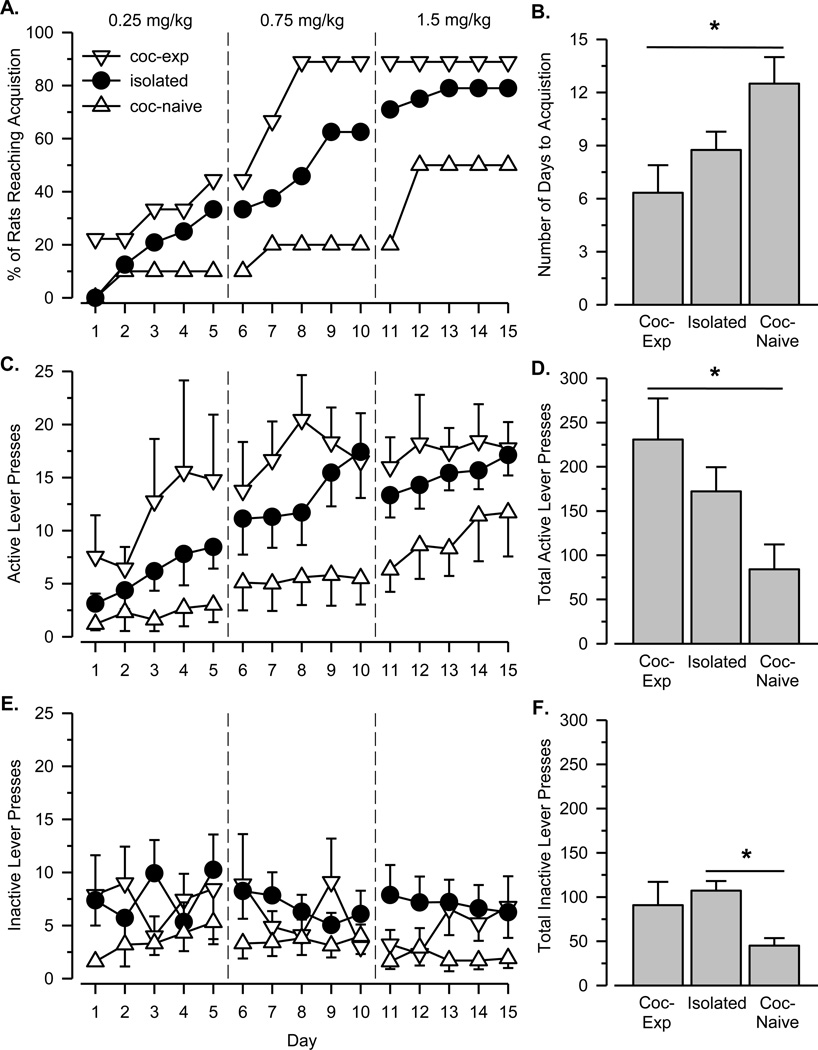
The percentage of rats acquiring cocaine self-administration differed across the three groups on all 15 days of testing, with the cocaine-experienced group having the greatest percentage and the cocaine-naïve group having the lowest percentage (Figure 1A). The three groups also differed in the rate at which they acquired cocaine self-administration [Figure 1B; χ2(2, N = 43) = 6.94, p = .031], with rats from the cocaine-experienced group acquiring significantly faster than rats from the cocaine-naïve group (p = .012). The number of active lever presses increased in all three groups across the 15 days of testing [Figure 1C; main effect of dose: F(2, 80) = 10.594, p < .001; main effect of day: F(4, 160) = 12.193, p < .001], and differed significantly across groups [main effect of group: F(2, 40) = 3.306, p = .047]. When only the total number of active lever presses was considered, the cocaine-experienced group emitted significantly more active lever presses than the cocaine-naïve group (Figure 1D; p = .047). In contrast, the number of inactive lever presses did not vary significantly across the 15 days of testing [no main effect of dose or day; Figure 1E], but significant group effects were observed [F(2, 40) = 4.459, p = .018]. When only the total number of inactive lever presses was considered, the isolated group emitted significantly more inactive lever presses than the cocaine-naïve group (Figure 1F; p = .014).
Figure 1.
A. Percent of experimentally naïve rats reaching the acquisition criterion over 15 days of testing in each of the three groups. B. Number of days to reach the acquisition criterion. C. Number of active lever presses per session over 15 days of testing. D. Total number of active lever presses over 15 days of testing. E. Number of inactive lever presses per session over 15 days of testing. F. Total number of inactive lever presses over 15 days of testing. Asterisks (*) indicate significant difference between groups. For panels A, C, and E, vertical reference lines represent transitions between different experimental events: self-administration testing with 0.25 mg/kg/infusion cocaine (days 1–5); self-administration testing with 0.75 mg/kg/infusion cocaine (days 6–10); self-administration testing with 1.5 mg/kg/infusion cocaine (days 11–15). For all panels, vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
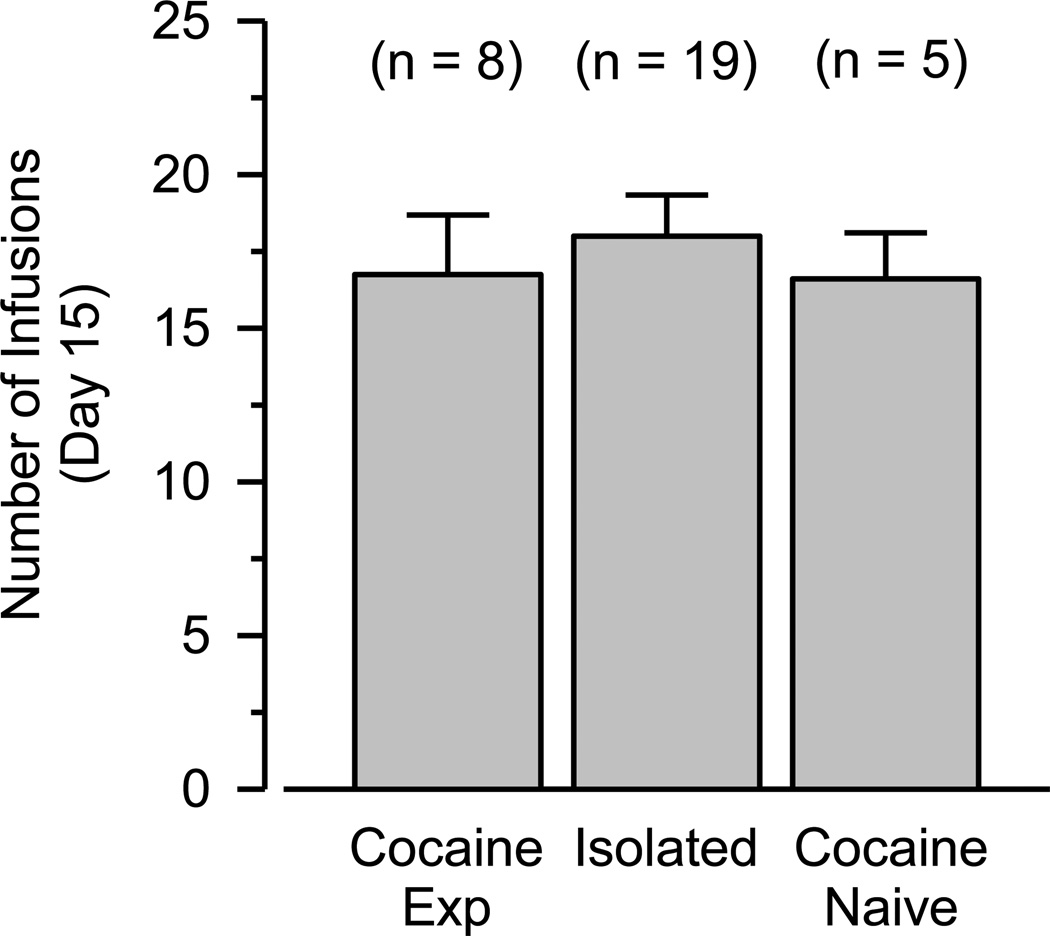
The maintenance of cocaine self-administration was examined by comparing the number of infusions obtained on day 15 across the three groups using only data from rats that met the acquisition criterion. In the 5 cocaine-naïve rats, 19 isolated rats, and 8 cocaine-experienced rats meeting the acquisition criterion, cocaine self-administration did not differ across the three groups (Figure 2). Analysis of individual event records revealed stable response patterns with regular post-reinforcement pauses in the majority of animals (data not shown).
Figure 2.
Cocaine self-administration during the 15th session in only those rats meeting the acquisition criterion. Vertical axis indicates number of infusions during 2-hr test session. Data are shown from 8 experimentally naïve rats from the cocaine-experienced group, 19 experimentally naïve rats from the isolated group, and 5 experimentally naïve rats from the cocaine-naïve group.
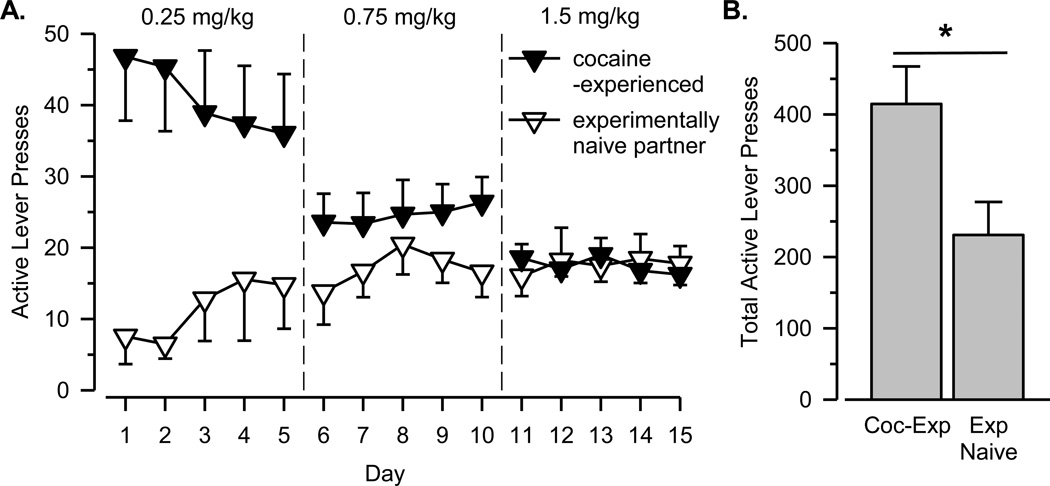
Cocaine-experienced rats in the cocaine-experienced group exhibited high levels of responding on the first day of testing (Figure 3A). As expected, the number of infusions decreased in these rats as the dose of cocaine increased. In contrast, only increases in responding were observed in their experimentally naïve partners over the 15 days of testing, resulting in significant differences between the two groups during early but not later stages of testing [dose × group interaction: F(2, 32) = 10.832, p < .001]. When data from all 15 days were considered, cocaine-experienced rats emitted significantly more total active lever presses than their experimentally naïve partners [Figure 3B; t(16) = 2.626, p = .018].
Figure 3.
A. Number of active lever presses per session over 15 days of testing for the cocaine-experienced group. B. Total number of active lever presses over 15 days of testing. Asterisks (*) indicate significant difference between groups. See Figure 1 for additional details.
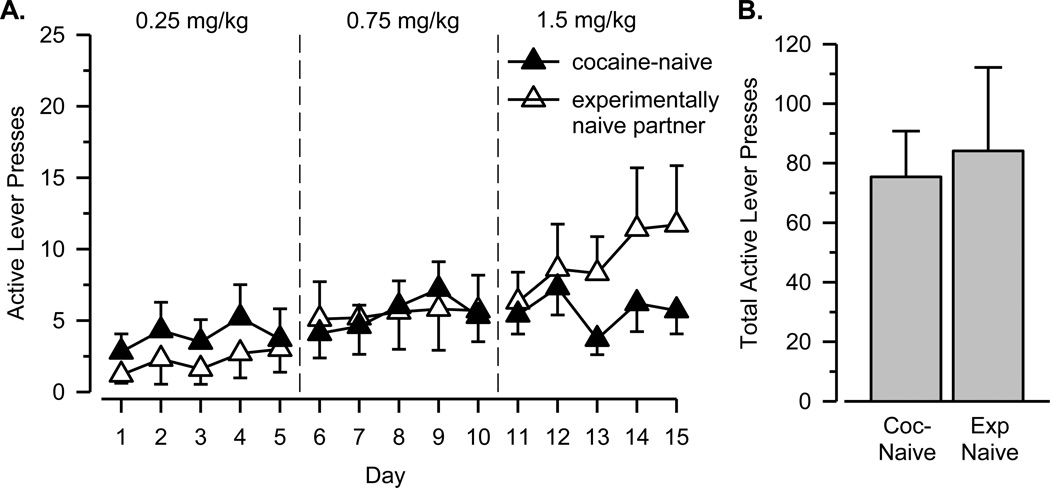
Cocaine-naïve rats and their experimentally naïve partners exhibited low levels of responding that increased significantly, albeit slowly, over the 15 days of testing (Figure 4A; main effect of dose: F(2, 36) = 4.343, p = .020; main effect of day: F(4, 72) = 3.922, p = .006). Although numerically greater levels of responding were observed in rats with access to cocaine by the end of the testing period, no significant main effect of group or significant interaction was observed. Similarly, no differences were observed between cocaine naïve rats and their experimentally naïve partner in the total number of “active” lever presses (Figure 4B).
Figure 4.
A. Number of active lever presses per session over 15 days of testing for the cocaine-naïve group. B. Total number of active lever presses over 15 days of testing. See Figure 1 for additional details.
4. DISCUSSION
The principal finding of this study is that the behavior of a peer, as opposed to merely the presence of a peer, determines whether the acquisition of cocaine self-administration will be increased or decreased by social contact. In the present study, experimentally naïve rats paired with a cocaine-experienced partner met the acquisition criterion twice as quickly (6 vs. 12 days) than experimentally naïve rats paired with a cocaine-naïve partner. Furthermore, rats paired with a cocaine-experienced partner emitted approximately 150 more active lever presses (231 vs. 84 responses) than rats paired with a cocaine-naïve partner. These differences were substantially greater than the differences observed in the number of inactive lever presses (90 vs. 45 responses), which failed to reach statistical significance between these groups. Importantly, these differences could not be attributed to differences in sensitivity to cocaine. When only rats meeting the acquisition criterion were compared on the final day of testing, cocaine self-administration did not differ between the three groups.
There is not a standardized procedure for measuring the acquisition of drug self-administration in laboratory animals, but many studies include noncontingent infusions of the drug at the onset of testing, either through priming infusions or autoshaping procedures, and then permit the animal to respond for contingent infusions of the drug under free-operant conditions (e.g., Campbell and Carroll, 2000; Carroll and Lac, 1993; Perry et al., 2005; Roth and Carroll, 2004; Schenk et al., 2001; Smith and Pitts, 2011; Soria et al., 2006). This method is considered to model human patterns of acquisition in which a drug is initially provided freely (such as by a friend or a dealer), and then the monetary or behavioral costs of obtaining the drug increase as the individual shifts to regular patterns of use (see review and discussion by Carroll and Meisch, 2011). It must be noted that noncontingent infusions are not necessary for the acquisition of drug self-administration, and many studies describe the acquisition of responding in the absence of priming infusions or autoshaping procedures (e.g., Belluzi et al., 2005; Bird and Schenk, 2013; Crombag et al., 2008; Kabbaj et al., 2001; Schippers et al., 2012). Moreover, such procedures model the acquisition of drug self-administration under conditions in which drugs are available only after a monetary or behavioral price has been paid (e.g., paying for the drug, exchanging services for the drug, or traveling to a location to procure the drug).
Studies with animals have consistently shown that increasing the dose of the drug increases the likelihood that self-administration will be acquired (Campbell et al., 1998; Carroll and Lac, 1997). In the present study, behavioral testing advanced through three distinct phases in which the dose of cocaine was systematically increased. Importantly, these phases were not designed to measure the impact of increasing doses of cocaine on acquisition; rather, they were designed to progressively increase the likelihood that self-administration would be acquired. Consistent with this aim, the percentage of rats acquiring self-administration progressively increased across all three phases of testing. As noted above, differences between groups were apparent during each of these phases.
Our experimental chambers are constructed so that the active levers for socially housed rats are in close physical proximity to their partners. This is done to maximize the effects of social learning in acquiring the response, given that previous studies have indicated that social learning is enhanced if the model and observer are in close physical proximity ( Aisner and Terkel, 1992; Coussi-Korbel and Fragaszy, 1995; Hausberger et al., 1995). It is important to note that we did not see evidence that rats were inadvertently pressing the active lever by trying to interact with their partner, because there was no preference for the active versus inactive lever in the early days of testing prior to the acquisition of drug self-administration, nor was there a preference for one lever or the other in cocaine naïve rats that never received cocaine.
Experimentally naïve rats paired with cocaine-experienced rats were the fastest to acquire cocaine self-administration and emitted the most active lever presses over the 15-day testing period. Decades of research in social learning have uncovered a number of behavioral mechanisms that may account for the rapid acquisition of cocaine self-administration in this group of animals. A popular explanation that is often found in social learning models of substance use proposes that drug-use behaviors are acquired by observing and imitating the drug use behaviors of others (Kandel, 1985). Imitation could account for the elevated levels of responding and subsequent faster rates of acquisition in these rats because their cocaine-experienced partners exhibited high rates of active lever pressing throughout the duration of testing. As an alternative explanation, social facilitation is a process by which responding is increased by the presence of another individual. For instance, alcohol self-administration is facilitated in prairie voles housed with a same-sex sibling compared to those housed in isolation (Hostetler et al., 2012). Importantly, social facilitation is greatest if both individuals are performing the same behavior (Hake and Laws, 1967). In the present study, only experimentally naïve rats in the cocaine-experienced group were paired with other rats that were engaged in cocaine self-administration, and social facilitation would be expected to be greatest in this group. Finally, stimulus enhancement is a process by which one individual directs the attention of another to a specific stimulus in the environment that is associated with reinforcement. For example, rats acquire nicotine self-administration at a faster and greater rate in the presence of a demonstrator that is drinking a scented solution associated with nicotine reinforcement; however, this effect is abolished if the demonstrator is drinking a scented solution that is not associated with nicotine reinforcement (Chen et al., 2011). In the present study, cocaine-experienced rats emitted high levels of responding on the active response lever, which likely increased the salience of the active lever for their experimentally naïve partners. It should be noted that these explanations are not mutually exclusive of one another, and all likely contributed to the elevated levels of responding and faster rates of acquisition in these rats.
Experimentally naïve rats paired with cocaine-naïve rats were the slowest to acquire cocaine self-administration and emitted the fewest lever presses over the 15-day testing period. These data are consistent with studies reporting social housing in the home cages decreases rates of drug self-administration (Gipson et al., 2011), and slows the acquisition of cocaine self-administration (Howes et al., 2000). Environmental enrichment provided by social housing could account for the lower rates of active lever pressing and acquisition of cocaine self-administration in this group. Numerous studies have reported that environmental enrichment produces functional changes in dopamine binding proteins (Del Arco et al., 2007; Gomez et al., 2012; Zhu et al., 2004), alters sensitivity to direct and indirect dopamine agonists (Del Arco et al., 2007; Hoffmann et al., 2009), and decreases amphetamine (Bardo et al., 2001; Green et al., 2002) and cocaine (Puhl et al., 2012, but see Smith et al., 2009) self-administration. Alternatively, the presence of another rat may have served as an alternative, nondrug reinforcer in this group. Many studies have reported that access to an alternative nondrug reward decreases drug-maintained responding (e.g., Comer et al., 1996; Cosgrove et al., 2002; Cosgrove and Carroll, 2003) and slows the acquisition of drug self-administration (e.g., Campbell et al., 1998; Carroll et al., 1989). It must be noted that this effect was only apparent in rats paired with a cocaine-naïve partner that never had access to cocaine, suggesting that a cocaine-experienced (or a cocaine-intoxicated) partner does not serve as an alternative, nondrug reinforcer to decrease cocaine self-administration.
The neurobiological mechanisms responsible for the effects of social learning on drug self-administration are not known, but candidate systems are those that process information regarding both drug and social stimuli (see reviews by Bardo et al., 2013; Burkett and Young, 2012; McGregor and Bowen, 2012). For instance, the amygdaloid complex plays a critical role in the assimilation of interoceptive (i.e., drug effects) and exteroceptive (i.e., social environment) stimuli and may serve as a locus of integration for drug and social reinforcement (Badiani, 2013). Alternatively, the nucleus accumbens mediates the positive reinforcing effects of both self-administered drugs and social contact and may serve to mediate the effects of social learning on drug self-administration. Supporting this latter possibility, both oxytocin and dopamine D2 receptors in the nucleus accumbens are required for the development of social attachment in rodents, and stimulant-induced increases in dopamine concentrations in the nucleus accumbens are attenuated by central administration of oxytocin (Liu and Wang, 2003; Qi et al., 2008).
The present findings are consistent with our previous study reporting that cocaine self-administration is enhanced in socially housed rats if both members of the pair have access to cocaine, but cocaine self-administration is inhibited if only one member of the pair has access to cocaine (Smith, 2012). In our previous study, all rats were trained to press a response lever prior to self-administration training, so there was no opportunity to examine the role of social learning on the acquisition of a novel drug-reinforced response (i.e., all rats responded on the first day in which cocaine was available and received the maximum number of infusions available). Collectively, these studies suggest that social learning plays a role in both the acquisition of drug use and the maintenance of drug use once established.
From a translational perspective, the present findings suggest that social contact with individuals who engage in substance use may accelerate a person’s substance use and increase the likelihood that he or she will become a habitual user of drugs. On the other hand, these data also suggest that social contact with individuals who practice abstinence, regardless of reason, may delay an individual’s substance use and decrease the odds that he or she will become a regular user of drugs. These results are consistent with epidemiological studies reporting that having friends who use drugs is a risk factor for adolescent drug use (Bahr et al., 2005; Simons-Morton and Chen, 2006; Walden et al., 2004), but that participation in community, religious, and after-school activities is associated with lower rates of adolescent drug use (Brown et al., 2001; D'Amico et al., 2012; Mellor and Freeborn, 2011; St. Pierre et al., 1992; Tebes et al., 2007). Coupled with the present data, such studies argue for the expanded use of socially based interventions in drug abuse prevention programs.
Acknowledgement
The authors thank Maryam Witte and Mary Kathryn Brophy for expert technical assistance, Amy Sullivan for animal care and maintenance, Chris Van Rooyen for instrumentation support, and the National Institute on Drug Abuse for supplying the study drug.
Role of Funding Source
Funding for this study was provided by NIH Grants DA031725 and DA027485; the NIH had no further role in designing the study, collecting the data, interpreting the results, writing the manuscript, or submitting the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors
Mark Smith conceived of the study and wrote the manuscript. Ryan Lacy and Justin Strickland contributed to the experimental design, collected data, and edited the final draft of the manuscript. All authors read and approved the final draft of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest in regard to this research.
REFERENCES
- Aisner R, Terkel J. Ontogeny of pine cone opening behavior in the black rat, Rattus rattus. Anim. Behav. 1992;44:327–336. [Google Scholar]
- Andrews JA, Hops H. The influence of peers on substance abuse. In: Scheier LM, editor. Handbook of Drug Use Etiology: Theory, Methods, and Empirical Findings. Washington DC: American Psychological Association; 2010. pp. 403–420. [Google Scholar]
- Badiani A. Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr. Opin. Neurobiol. 2013;23:588–596. doi: 10.1016/j.conb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Bahi A. Increased anxiety, voluntary alcohol consumption and ethanol-induced place preference in mice following chronic psychosocial stress. Stress. 2013;16:441–451. doi: 10.3109/10253890.2012.754419. [DOI] [PubMed] [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X. Parental and peer influences on the risk of adolescent drug use. J. Prim. Prev. 2005;26:529–551. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl.) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisenwander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Bird J, Schenk S. Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict. Biol. 2013;18:654–664. doi: 10.1111/j.1369-1600.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- Brown TL, Parks GS, Zimmerman RS, Phillips CM. The role of religion in predicting adolescent alcohol use and problem drinking. J. Stud. Alcohol. 2001;62:696–705. doi: 10.15288/jsa.2001.62.696. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical, and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl.) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp. Clin. Psychopharmacol. 2000;8:312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Thompson SS, Carroll ME. Acquisition of oral phencyclidine (PCP) self-administration in rhesus monkeys: effects of dose and an alternative non-drug reinforcer. Psychopharmacology (Berl.) 1998;137:132–138. doi: 10.1007/s002130050602. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl.) 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping of i.v. cocaineself-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology (Berl.) 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl.) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Acquisition of drug self-administration. In: Olmstead MCC, editor. Animal Models of Drug Addiction. Neuromethods. Humana Press; New York: 2011. pp. 237–265. [Google Scholar]
- Chartier KG, Hesselbrock MN, Hesselbrock VM. Development and vulnerability factors in adolescent alcohol use. Child Adolesc. Psychiatr. Clin.N. Am. 2010;19:493–504. doi: 10.1016/j.chc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on iv cocaine self-administration in rats maintained under FR schedules. Psychopharmacology (Berl.) 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology (Berl.) 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences. Pharmacol. Biochem. Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Coussi-Korbel S, Fragaszy DM. On the relations between social dynamics and social learning. Anim. Behav. 1995;50:1441–1453. [Google Scholar]
- Crombag HS, Ferrario CR, Robinson TE. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol. Biochem. Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico EJ, Tucker JS, Miles JNV, Zhou AJ, Shih RA, Harold DG., Jr Preventing alcohol use with a voluntary after-school program for middle school students: results from a cluster randomized controlled trial of CHOICE. Prev. Sci. 2012;13:415–425. doi: 10.1007/s11121-011-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, et al. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J. Neural Transm. 2007;114:43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- 27.Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and intitaing alcohol use during adolescence. J. Adolesc. Health. 2006;38:e1–e10. doi: 10.1016/j.jadohealth.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Geuzaine A, Tirelli E. Wheel-running mitigates psychomotor sensitization initiation but not post-sensitization conditioned activity and conditioned place preference induced by cocaine in mice. Behav. Brain Res. 2014;262:57–67. doi: 10.1016/j.bbr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl.) 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez AM, Midde NM, Mactutus CF, Booze RM, Zhu J. Environmental enrichment alters nicotine-mediated locomotor sensitization and phosphorylation of DARPP-32 and CREB in rat prefrontal cortex. PLOS One. 2012;7:e44149. doi: 10.1371/journal.pone.0044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green T, Gehrke B, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl.) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 32.Hake DF, Laws DR. Social facilitation of response during a stimulus paired with electric shock. J. Exp. Anal. Behav. 1967;10:387–392. doi: 10.1901/jeab.1967.10-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausberger M, Richard Yris MA, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings(Sturnus vulgaris) J. Comp. Psychol. 1995;109:222–241. [Google Scholar]
- 34.Hoffmann LC, Schutte SRM, Koch M, Schwabe K. Effect of "enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience. 2009;158:1589–1598. doi: 10.1016/j.neuroscience.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 35.Hostetler CM, Anacker AMJ, Loftis JM, Ryabinin AE. Social housing and alcohol drinking in male-female pairs of prairie voles(Microtus ochrogaster) Psychopharmacology (Berl.) 2012;224:121–132. doi: 10.1007/s00213-012-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl.) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Laboratory Animal Resources. Washington DC: National Academies; 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 38.Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl.) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- 39.Kandel DB. On processes of peer influences in adolescent drug use: a developmental perspective. Adv. Alcohol Subst. Abuse. 1985;4:139–163. doi: 10.1300/J251v04n03_07. [DOI] [PubMed] [Google Scholar]
- 40.Kandel DB. Processes of peer influences in adolescence. In: Silbereisen RK, Eyferth K, Rudinger G, editors. Development as Action in Context: Problem Behavior and Normal Youth Development. New York: Springer; 1986. pp. 203–228. [Google Scholar]
- 41.Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 43.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm. Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Mellor JM, Freeborn BA. Religious participation and risky health behaviors among adolescents. Health Econ. 2011;20:1226–1240. doi: 10.1002/hec.1666. [DOI] [PubMed] [Google Scholar]
- 45.Pandina RJ, Johnson VL, White HR. Peer influences on substance use during adolescence and emerging adulthood. In: Scheier LM, editor. Handbook Of Drug Use Etiology: Theory, Methods, And Empirical Findings. Washington DC: American Psychological Association; 2010. pp. 383–401. [Google Scholar]
- 46.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl.) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 47.Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav. Pharmacol. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2008;376:441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodrigues-Arias M, Minarro J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl.) 2009;207:485–498. doi: 10.1007/s00213-009-1678-1. [DOI] [PubMed] [Google Scholar]
- 50.Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl.) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- 51.Schenk S, Partridge B, Shippenburg TS. Effects of the kappa-opioid receptor agonist, U69593, on the development of sensitization and on the maintenance of cocaine self-administration. Neuropsychopharmacology. 2001;24:441–450. doi: 10.1016/S0893-133X(00)00190-1. [DOI] [PubMed] [Google Scholar]
- 52.Schippers MC, Binnekade R, Schoffelmeer AN, Pattij T, De Vries TJ. Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology (Berl.) 2012;219:443–452. doi: 10.1007/s00213-011-2444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simons-Morton B, Chen RS. Over time relationships between early adolescents and peer substance use. Addict. Behav. 2006;31:1211–1223. doi: 10.1016/j.addbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Smith MA. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl.) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT. Effects of environmental enrichment on sensitivity to cocaine in female rats: Importance of control rates of behavior. Behav. Pharmacol. 2009;20:312–321. doi: 10.1097/FBP.0b013e32832ec568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol. Biochem. Behav. 2011;100:237–243. doi: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:979–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- 58.St. Pierre TL, Kaltreider DL, Mark MM, Aikin KJ. Drug prevention in a community setting: a longitudinal study of the relative effectiveness of a three-year primary prevention program in Boys and Girls Clubs across the nation. Am. J. Community Psychol. 1992;20:673–706. [PubMed] [Google Scholar]
- 59.Tebes JK, Feinn R, Vanderploeg JJ, Chinman MJ, Shepard J, Brabham T, Genovese M, Connell C. Impact of a positive youth development program in urban after-school settings on the prevention of adolescent substance use. J. Adolesc. Health. 2007;41:239–247. doi: 10.1016/j.jadohealth.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of nucleus accumbens dopamine. Psychopharmacology (Berl.) 1997;142:31–40. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- 61.US Congress, Office of Technology Assessment. Washington DC: U.S. Government Printing Office; 1994. Technologies for Understanding and Preventing Substance Abuse and Addiction, OTA-EHR-597. [Google Scholar]
- 62.Walden B, McGue M, Iacono WG, Burt A, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J. Abnorm. Psychol. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- 63.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav. Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]