Abstract
Objective
To characterize endocrine dysfunction in pediatric patients with brain tumors who received proton beam (PB) radiation therapy and to compare those treated with PB radiotherapy only versus combined conventional and PB irradiation.
Methods
A retrospective review of medical records of patients ≤18 years of age who received PB radiation therapy for a brain tumor between 2000 and 2008 was performed. Variables analyzed included patient demographics, tumor type, therapeutic modalities, radiation doses, and types and timing of endocrine dysfunction.
Results
Thirty-eight patients were identified, of whom 31 (19 boys and 12 girls; mean age, 11.9 ± 3.3 years) had undergone endocrine evaluation. Of these patients, 19 received PB radiotherapy only and 12 received conventional plus PB irradiation. Before irradiation, a cranial surgical procedure was performed in 28 study subjects, and 22 received chemotherapy. The mean duration of follow-up after radiation therapy was 1.8 ± 0.8 years. Nine patients (47%) in the PB only group and 4 (33%) in the conventional plus PB group developed endocrine dysfunction (no significant difference) after cranial irradiation. Children with endocrine sequelae treated with PB irradiation alone received fewer cobalt gray equivalents than those treated with conventional plus PB irradiation (5,384 ± 268 versus 5,775 ± 226, respectively; P<.02), and pituitary hormone deficiencies were detected later during follow-up in those who received PB radiotherapy only versus conventional plus PB irradiation (1.17 ± 0.4 years versus 0.33 ± 0.11 year, respectively; P<.01).
Conclusion
A high rate of endocrine sequelae was seen in our study. Children with brain tumors treated with conventional plus PB irradiation developed endocrine dysfunction faster and received a higher radiation dose than those receiving PB radiotherapy only. Prior surgical treatment and chemotherapy were additional risk factors. Large prospective studies are needed to evaluate further the incidence of endocrine sequelae after PB irradiation in children.
INTRODUCTION
Brain tumors constitute approximately 17% of all malignant tumors in patients younger than 20 years. The treatment modalities for these tumors include surgical resection, chemotherapy, and irradiation, all of which are associated with a high risk of endocrine sequelae. Specifically, radiation therapy is associated with high rates of hypothalamic-pituitary dysfunction (1). Up to 70% of children who receive radiation therapy develop anterior pituitary hormone dysfunction (2), and those who receive >4,000 cGy are at risk for multiple pituitary hormone deficiencies (1,3,4). Historically, nearly 100% of children treated with a conventional radiation dose exceeding 3,000 cGy have developed growth hormone (GH) deficiency (5). Twenty percent to 50% of children with brain tumors who receive irradiation eventually have gonadotropin deficiency (1), and approximately 19% have hypothalamic-pituitary-adrenal axis abnormalities (6). Rates of central hypothyroidism after conventional irradiation are reported to range from 6% to 36%, depending on the duration of follow-up (7). Overall, 5-year and 10-year survival rates for children with brain tumors have increased from approximately 60% to 75% during a 20-year period (8). As survival rates in children with brain tumors increase, the morbidity associated with treatment becomes more pronounced.
Proton beam (PB) radiation therapy is a newer treatment modality that provides targeted delivery to the tumor site while reducing radiation exposure to normal tissues, such as the hypothalamus and pituitary gland (9). This outcome eventuates because of a rapid decline in the radiation exit dose after maximal dose deposition at the target site (10). Theoretically, this type of radiation modality could prevent the development of neuroendocrine dysfunction. Studies regarding endocrine deficits associated with this type of irradiation, especially in children, however, are limited. At our institution, it has become standard practice to use PB irradiation for all children who require radiation therapy. The use of conventional irradiation is limited to those who are too clinically unstable to travel to a PB radiation site. Whether PB radiation therapy truly reduces morbidity in children with brain tumors, however, is unknown.
In this study, our objective was to characterize the rate of occurrence and type of pituitary hormone dysfunction in children with brain tumors who received PB irradiation. We then compared the rates and type of dysfunction in this patient population with those in children with brain tumors who received both conventional and PB radiation therapy.
PATIENTS AND METHODS
Study Subjects
After obtaining institutional review board approval for the study, we conducted a retrospective review of children ≤18 years old who underwent PB irradiation between January 1, 2000, and October 1, 2008. Children were included if they had at least 1 subsequent follow-up visit for cancer treatment at our institution and had undergone assessment for endocrine abnormalities. From the hematology-oncology and endocrinology medical records, we extracted the following variables: age, sex, race, tumor type, type of therapeutic modalities including a surgical procedure and chemotherapy, dose of PB irradiation, dose of conventional radiation therapy, and presence and time to development of pituitary hormone deficiency.
Study Procedures and Definitions
Because some of the patients had received PB radiation therapy only and some had received both conventional and PB irradiation, they were classified into 2 groups based on these types of radiation exposure. Cobalt gray equivalents (CGE) were based on the uniform relative biologic equivalence of 1.1 for protons (11). In accordance with the radiation oncology literature, cGy and CGE doses were considered equivalent for the purposes of statistical analysis. The diagnosis of pituitary hormone dysfunction was based on standard criteria. Specifically, children were diagnosed with GH deficiency if a peak stimulated GH level was <10 ng/mL (before 2005) or <5 ng/mL (after 2005) after the sequential administration of 2 secretagogues. Central hypothyroidism was diagnosed if the patient had a low free thyroxine value in the setting of a normal level of thyroid-stimulating hormone (TSH). Central adrenal insufficiency was diagnosed if a patient had a peak stimulated cortisol level of <18 μg/dL after low-dose (1 μg) adrenocorticotropic hormone (ACTH) stimulation testing. Hypogonadotropic hypogonadism was diagnosed if patients had absence of puberty by age 13 years in girls and age 14 years in boys with low serum gonadotropin levels.
Statistical Analysis
Data are reported as means ± standard deviations. Independent-sample t tests (for continuous variables) and Fisher exact tests (for categorical variables) were used for comparisons between the 2 groups. P values <.05 were considered significant.
RESULTS
Of the 38 patients who had received PB radiation therapy, 31 (19 boys and 12 girls) had at least 1 formal endocrinology evaluation and were included in our analysis. The mean age of these patients at the time of review was 11.9 ± 3.3 years. The mean duration of follow-up from PB irradiation until the date of review was 1.8 ± 0.8 years. The most common types of brain tumor were craniopharyngioma (n = 7), medulloblastoma (n = 6), and glioma (n = 4). Twenty-eight patients had surgical treatment before irradiation. Twenty-two patients received chemotherapy before or with radiation therapy. The clinical characteristics of the children are summarized in Table 1.
Table 1.
Clinical Characteristics of Children Who Received Proton Beam Irradiation as Part of Brain Tumor Treatment
| Characteristic | Age (y)a and range | No. of cases |
|---|---|---|
| Sex | ||
| Boys | 10.1 ± 1.5 (3.6–17.4) | 19 |
| Girls | 14.8 ± 3.9 (3.8–16) | 12 |
| Race | ||
| White | 29 | |
| Black | 1 | |
| Hispanic | 1 | |
| Tumor type | ||
| Craniopharyngioma | 7 | |
| Medulloblastoma | 6 | |
| Glioma | 4 | |
| Rhabdomyosarcoma | 3 | |
| Ependymoma | 2 | |
| Astrocytoma | 2 | |
| Primitive neuroectodermal tumor | 2 | |
| Retinoblastoma | 2 | |
| Glioblastoma | 1 | |
| Germinoma | 1 | |
| Ewing sarcoma | 1 | |
Shown as mean ± standard deviation.
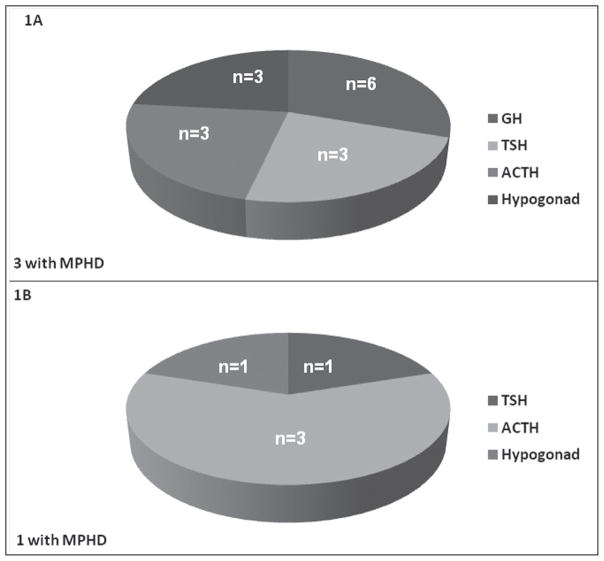
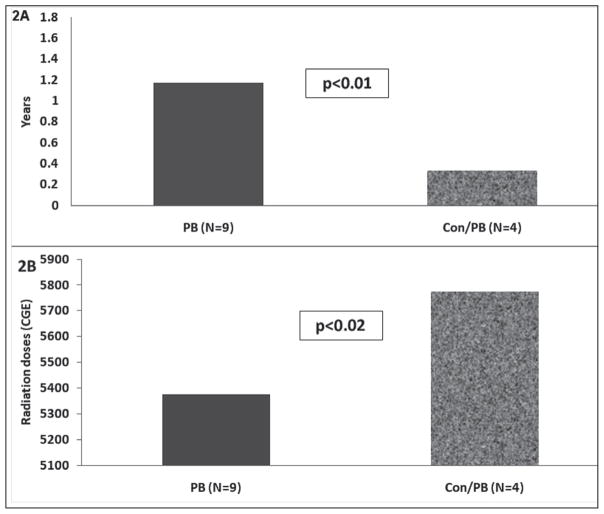
Of the 31 patients analyzed, 19 received only PB irradiation and 12 received conventional plus PB radiation therapy. Overall, brain tumor locations did not differ between the 2 groups. Before irradiation, 8 patients in the PB group already had evidence of pituitary hormone deficiencies, 2 of whom subsequently developed additional endocrinopathies after radiation exposure. No preradiation endocrinopathies were noted in the group of patients who received conventional plus PB irradiation. Of the remaining study subjects, 7 in the PB group and 4 in the conventional plus PB group developed pituitary dysfunction after radiation therapy (47% versus 33%; no significant difference), 4 of whom had multiple pituitary hormone deficiencies. Of those patients who developed pituitary hormone dysfunction in the PB group, 6 had craniopharyngiomas, 1 child had a medulloblastoma, 1 had a glioma, and 1 had a rhabdomyosarcoma. Specific abnormalities in the PB-treated group consisted of GH deficiency (n = 6), TSH deficiency (n = 3), ACTH deficiency (n = 3), and hypogonadotropic hypogonadism (n = 3). Of those with pituitary hormone deficiencies in the conventional plus PB irradiation group, 3 children had medulloblastomas and 1 had a glioblastoma. Pituitary hormone abnormalities in this group consisted of ACTH deficiency (n = 3), TSH deficiency (n = 1), and hypogonadotropic hypogonadism (n = 1). The distribution of these deficiencies among the groups is illustrated in Figure 1. Among those patients who developed pituitary hormone deficiencies, these abnormalities were detected sooner during follow-up in the conventional plus PB irradiation group than in the PB only group (0.33 ± 0.11 year versus 1.17 ± 0.4 years, respectively; P<.01) (Fig. 2 A). The conventional plus PB irradiation group also received a higher radiation dose than did the PB only group (5,775 ± 226 CGE versus 5,384 ± 268 CGE, respectively; P<.02) (Fig. 2 B).
Fig. 1.
A, Distribution of pituitary hormone deficiencies in proton beam irradiation-treated group of young patients with brain tumors (N = 9). B, Distribution of pituitary hormone deficiencies in young patients with brain tumors receiving conventional plus proton beam irradiation (N = 4). ACTH = adrenocorticotropic hormone; GH = growth hormone; Hypogonad = hypogonadotropic hypogonadism; MPHD = multiple pituitary hormone deficiencies; TSH = thyroid-stimulating hormone.
Fig. 2.
A, Time to detection of endocrinopathies after proton beam (PB) and conventional (Con) plus PB radiation therapy in children with brain tumors. B, Radiation doses administered in these 2 treatment groups (PB and Con plus PB). CGE = cobalt gray equivalents.
DISCUSSION
The goal of cranial irradiation in pediatric patients with brain tumor is to optimize treatment to the tumor site while sparing normal tissue. The advent of PB radiotherapy has been accompanied by considerable optimism regarding its potential to influence rates of endocrine late effects favorably in childhood brain tumor survivors. Whether this projection will be realized is unknown. Thus far, minimal information regarding clinical outcomes in pediatric recipients of PB radiation therapy is available. In one small study of children treated with PB irradiation for germ cell tumors, 4 of 4 who did not have endocrine abnormalities at baseline developed them after radiotherapy (12). In another group of 17 children with ependymomas, the use of PB radiotherapy was associated with lower radiation doses and greater sparing of normal tissue in comparison with traditional radiation therapy. Although no late toxicity was reported, the details in terms of neuroendocrine evaluation in this group of patients were not provided (9). To our knowledge, our current study is the first to compare endocrine outcomes between a group of patients treated with PB irradiation only and a group receiving combined conventional plus PB radiation therapy.
Our study had several limitations. In addition to radiation therapy, most of our study subjects underwent a cranial surgical procedure and received chemotherapy, both of which are notorious for increasing the risk of neuroendocrine problems (13–15). In addition, as evidenced by the presence of pretreatment endocrinopathies in some of the patients, brain tumors themselves can cause hypopituitarism (16). Thus, it is impossible to quantify the contribution of any one of these variables to the development of endocrine sequelae in these patients, and the risks attributed to PB radiation therapy may be overestimated. Another limitation is that, because of its retrospective nature, the timing and type of endocrine evaluation in our cohort of children with brain tumors were not standardized. This situation could lead to an underestimation of the rates of neuroendocrine dysfunction, and the precise temporal relationship between a central nervous system insult such as cranial irradiation and the development of an endocrinopathy in our study subjects could not be determined. Lastly, our mean duration of follow-up of approximately 2 years was short. Some pituitary hormone deficiencies associated with cranial irradiation, such as ACTH deficiency, can manifest up to 30 years after the initial treatment (17). As a result, children who did not have pituitary hormone deficiencies at the time of our study may develop them in the future. Thus, our findings should be considered preliminary.
In 2009, there were 5 PB treatment centers in the United States that offered therapy for children with brain tumors. Four new centers were near completion in 2010 (18). The cost of these centers is 1.7 to 2.1 times greater than that incurred for a traditional radiation therapy center (19), resulting in an increase in total health care costs for children with brain tumors. These health care costs could be reduced if there were further reductions in morbidity-related outcomes or if fewer doses were needed to treat the primary tumor (16). Further evaluation of treatment outcomes is needed to assess the total cost-to-benefit ratio of PB radiation therapy in childhood cancer patients.
CONCLUSION
Overall, we observed a high rate of presumed radiation-induced pituitary hormone dysfunction in children with brain tumors receiving either PB radiotherapy alone or combined conventional and PB irradiation (47% and 33%, respectively). These rates are similar to historical data of pituitary hormone deficiencies associated with conventional radiation therapy at comparable doses (1,5–7). Interestingly, we detected endocrinopathies in children in the conventional plus PB irradiation group sooner during follow-up than in the PB only group. Because these study subjects also received a higher dose of irradiation than those in the PB only group, however, it is difficult to attribute this difference to radiotherapy technique versus exposure. Regardless, our results suggest that children who undergo treatment with PB radiation therapy for brain tumors constitute a high-risk population for developing pituitary hormone deficiencies. As PB radiotherapy is increasingly used, larger prospective studies will be needed for further evaluation of its role in the treatment of brain tumors in young patients and its exact relationship to the subsequent development of neuroendocrine dysfunction in these patients.
Acknowledgments
The initial results of this work were presented in poster form at the Annual Meeting of the Pediatric Academic Society; May 1–4, 2010; Vancouver, British Columbia, Canada.
Abbreviations
- ACTH
adrenocorticotropic hormone
- CGE
cobalt gray equivalents
- GH
growth hormone
- PB
proton beam
- TSH
thyroid-stimulating hormone
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LE. Endocrine late effects of cancer treatment. Endocrinol Metab Clin North Am. 2005;34:769–789. xi. doi: 10.1016/j.ecl.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer. 2004;11:589–602. doi: 10.1677/erc.1.00779. [DOI] [PubMed] [Google Scholar]
- 4.Madanat LM, Lähteenmäki PM, Hurme S, Dyba T, Salmi TT, Sankila R. Hypothyroidism among pediatric cancer patients: a nationwide, registry-based study. Int J Cancer. 2008;122:1868–1872. doi: 10.1002/ijc.23277. [DOI] [PubMed] [Google Scholar]
- 5.Clayton PE, Shalet SM. Dose dependency of time of onset of radiation-induced growth hormone deficiency. J Pediatr. 1991;118:226–228. doi: 10.1016/s0022-3476(05)80487-1. [DOI] [PubMed] [Google Scholar]
- 6.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Lange M, Poulsen HS, Müller J. Assessment of the hypothalamo-pituitary-adrenal axis in patients treated with radiotherapy and chemotherapy for childhood brain tumor. J Clin Endocrinol Metab. 2003;88:3149–3154. doi: 10.1210/jc.2002-021994. [DOI] [PubMed] [Google Scholar]
- 7.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Poulsen HS, Müller J. A population-based study of thyroid function after radiotherapy and chemotherapy for a childhood brain tumor. J Clin Endocrinol Metab. 2003;88:136–140. doi: 10.1210/jc.2002-020380. [DOI] [PubMed] [Google Scholar]
- 8.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71:979–986. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 10.Yock TI, Tarbell NJ. Technology insight: proton beam radiotherapy for treatment in pediatric brain tumors [published correction appears in Nat Clin Pract Oncol. 2005;2: 222] Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. [DOI] [PubMed] [Google Scholar]
- 11.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald SM, Trofimov A, Safai S, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121–129. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 13.Darzy KH, Shalet SM. Hypopituitarism as a consequence of brain tumours and radiotherapy. Pituitary. 2005;8:203–211. doi: 10.1007/s11102-006-6042-4. [DOI] [PubMed] [Google Scholar]
- 14.Haddy TB, Mosher RB, Nunez SB, Reaman GH. Growth hormone deficiency after chemotherapy for acute lymphoblastic leukemia in children who have not received cranial radiation. Pediatr Blood Cancer. 2006;46:258–261. doi: 10.1002/pbc.20485. [DOI] [PubMed] [Google Scholar]
- 15.Rose SR, Schreiber RE, Kearney NS, et al. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab. 2004;17:55–66. doi: 10.1515/jpem.2004.17.1.55. [DOI] [PubMed] [Google Scholar]
- 16.Merchant TE, Williams T, Smith JM, et al. Preirradiation endocrinopathies in pediatric brain tumor patients determined by dynamic tests of endocrine function. Int J Radiat Oncol Biol Phys. 2002;54:45–50. doi: 10.1016/s0360-3016(02)02888-2. [DOI] [PubMed] [Google Scholar]
- 17.Darzy KH. Radiation-induced hypopituitarism after cancer therapy: who, how and when to test. Nat Clin Pract Endocrinol Metab. 2009;5:88–99. doi: 10.1038/ncpendmet1051. [DOI] [PubMed] [Google Scholar]
- 18.Merchant TE. Proton beam therapy in pediatric oncology. Cancer J. 2009;15:298–305. doi: 10.1097/PPO.0b013e3181b6d4b7. [DOI] [PubMed] [Google Scholar]
- 19.Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol) 2003;15:S37–S50. doi: 10.1053/clon.2002.0174. [DOI] [PubMed] [Google Scholar]