Abstract
Light-driven oxidation of water into dioxygen, catalysed by the oxygen-evolving complex (OEC) in photosystem II, is essential for life on Earth and provides the blueprint for devices for producing fuel from sunlight. Although the structure of the OEC is known at atomic level for its dark-stable state, the mechanism by which water is oxidized remains unsettled. Important mechanistic information was gained in the past two decades by mass spectrometric studies of the H218O/H216O substrate–water exchange in the four (semi) stable redox states of the OEC. However, until now such data were not attainable in the transient states formed immediately before the O–O bond formation. Using modified photosystem II complexes displaying up to 40-fold slower O2 production rates, we show here that in the transient  state the substrate–water exchange is dramatically slowed as compared with the earlier S states. This further constrains the possible sites for substrate–water binding in photosystem II.
state the substrate–water exchange is dramatically slowed as compared with the earlier S states. This further constrains the possible sites for substrate–water binding in photosystem II.
 The oxygen-evolving complex of photosystem II converts water into oxygen during photosynthesis, but how this process occurs is not yet fully understood. Here, the authors use modified complexes with reduced reaction rates to study the process of oxygen evolution in more detail.
The oxygen-evolving complex of photosystem II converts water into oxygen during photosynthesis, but how this process occurs is not yet fully understood. Here, the authors use modified complexes with reduced reaction rates to study the process of oxygen evolution in more detail.
Photosynthesis provides the driving force for most life on Earth by converting sunlight into chemical energy. Cyanobacteria, algae and higher plants couple two photosystems in series to exploit water as the electron and proton source for the synthesis of carbohydrates from CO2. In the process they replenish the atmosphere with the dioxygen we live on. The complex four-electron four-proton chemistry of water oxidation is catalysed in photosystem II (PSII) by an inorganic cluster containing the earth-abundant metals Mn and Ca, which are bridged by five oxygen1,2,3,4,5,6,7,8,9. The structure of this Mn4CaO5 cluster, which together with its ligands forms the oxygen-evolving complex (OEC), is now known at the atomic scale in its dark-stable state4. Density functional theory-based refinements have provided OEC structures (Fig. 1a) that can rationalize the vast majority of the available spectroscopic data6,7,8,10,11,12,13,14.
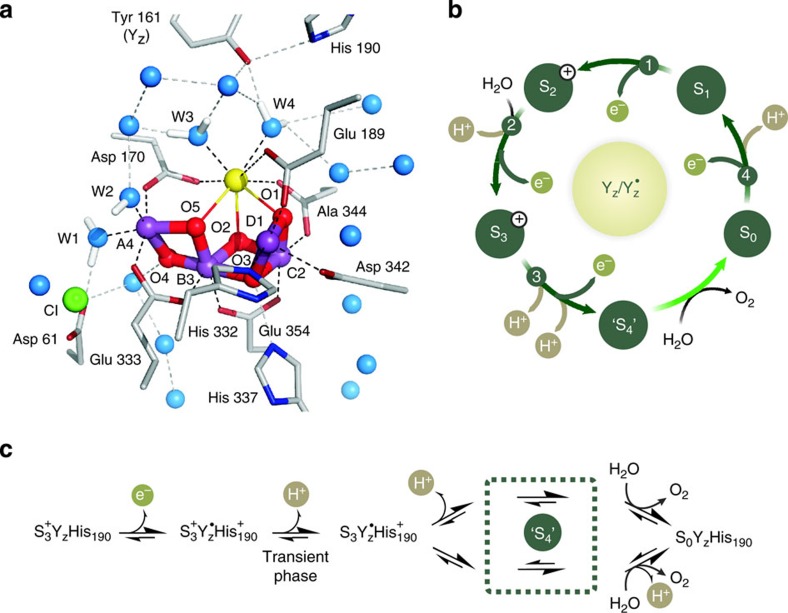
Figure 1. The water-oxidizing complex in PSII and its reaction sequence.
(a) Density functional theory-based model of the Mn4CaO5 cluster in the ‘open cube’ (S2 EPR multiline) configuration together with its water derived ligands W1–W412. The model is inserted into the 1.9-Å crystal structure of photosystem II4. MnIII/IV, purple; Ca2+, yellow; oxo-bridges, red; water–oxygens, blue; Cl−, green; amino acid backbones, grey; carboxy-oxygen, red; and His-nitrogen, dark blue. (b) Kok cycle for water oxidation in PSII including proton release to the bulk8,22 (see also Supplementary Note 1) and water-binding events27,28. The Si states (i=0, …, 4) denote the oxidation state of the Mn4CaO5 cluster relative to the S0 state, while the plus sign indicates a positive extra charge. (c) Detailed sequence of known and postulated states during molecular oxygen formation. Protons released in sequence c may in part be taken up by internal bases created in earlier Si state transitions29.
Water oxidation to dioxygen is energetically driven by light-induced charge separations within the reaction centre of PSII. These occur between the chlorophyll-containing photo-oxidant P680/ and the primary electron acceptor pheophytin. The OEC and P680 are connected via the redox-active tyrosine residue 161 of the D1 protein (YZ/
and the primary electron acceptor pheophytin. The OEC and P680 are connected via the redox-active tyrosine residue 161 of the D1 protein (YZ/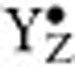 ) (Fig. 1a). In this way the OEC steps in response to short light flashes almost in synchrony through the four (semi)-stable oxidation states S0YZ, S1YZ, S2+YZ and S3+YZ (Fig. 1b), where the subscript signifies the number of stored oxidizing equivalents, and the plus sign indicates an extra charge caused by the lack of proton release during the transition from the dark-stable S1YZ state to the S2+YZ state. These (semi)-stable intermediates of water oxidation can be trapped with high yield and are thereby readily accessible for biophysical investigation. Although it is largely agreed that the S-state transitions between S0YZ and S3+YZ involve Mn-centred oxidation of the Mn4CaO5 cluster6,9,15, some experimental results suggest ligand (oxo-bridge) participation and/or a structural change during the S2+YZ ⇒ S3+YZ transition16,17,18. The dark-stable S1YZ state is generally considered to have the formal oxidation states Mn4(III,III,IV,IV) (high oxidation state scenario)6,15,19, but also the lower valent Mn4(II,III,III,IV)/Mn4(III,III,III,III) options are discussed20,21.
) (Fig. 1a). In this way the OEC steps in response to short light flashes almost in synchrony through the four (semi)-stable oxidation states S0YZ, S1YZ, S2+YZ and S3+YZ (Fig. 1b), where the subscript signifies the number of stored oxidizing equivalents, and the plus sign indicates an extra charge caused by the lack of proton release during the transition from the dark-stable S1YZ state to the S2+YZ state. These (semi)-stable intermediates of water oxidation can be trapped with high yield and are thereby readily accessible for biophysical investigation. Although it is largely agreed that the S-state transitions between S0YZ and S3+YZ involve Mn-centred oxidation of the Mn4CaO5 cluster6,9,15, some experimental results suggest ligand (oxo-bridge) participation and/or a structural change during the S2+YZ ⇒ S3+YZ transition16,17,18. The dark-stable S1YZ state is generally considered to have the formal oxidation states Mn4(III,III,IV,IV) (high oxidation state scenario)6,15,19, but also the lower valent Mn4(II,III,III,IV)/Mn4(III,III,III,III) options are discussed20,21.
Water oxidation starts only after the fourth oxidizing equivalent has been accumulated in the OEC, that is, once the transient 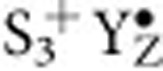 state is reached (Fig. 1c). It is widely agreed that this dioxygen-forming reaction sequence starts with a proton release during the
state is reached (Fig. 1c). It is widely agreed that this dioxygen-forming reaction sequence starts with a proton release during the 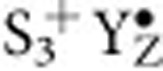 →
→ transition22,23,24,25, followed by the formation of a Ca- or Mn-bound oxyl radical or of a MnV-oxo group due to oxidation of the cluster by
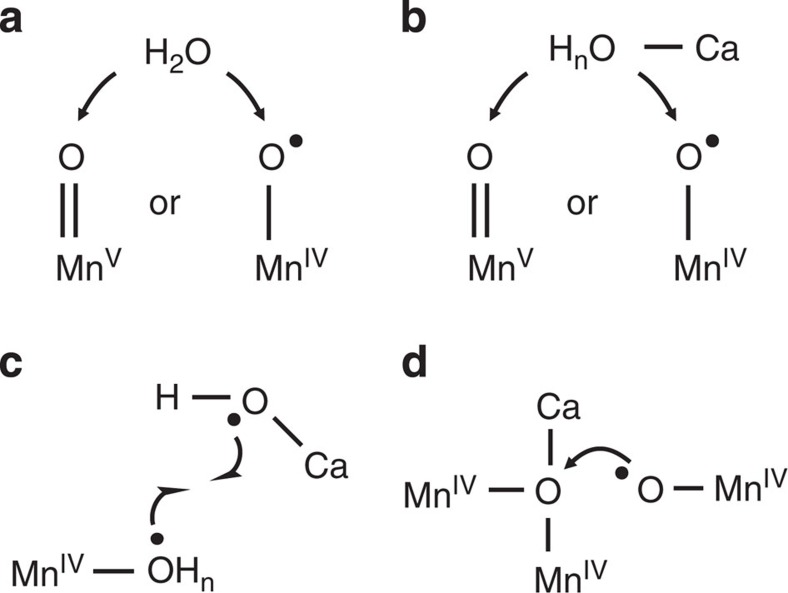
transition22,23,24,25, followed by the formation of a Ca- or Mn-bound oxyl radical or of a MnV-oxo group due to oxidation of the cluster by 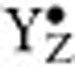 6,14,26. Although the following reaction steps towards O2 must include the formation of a bound peroxidic intermediate, dioxygen formation and release, and the rebinding of substrate water27,28 (Fig. 1c), the exact nature of the chemistry involved remains unsettled, as these transient states have so far largely eluded biophysical investigations6,14,28,29,30,31,32. O–O formation by nucleophilic attack may occur, for example, either between a free (Fig. 2a)29 or a Ca-bound (Fig. 2b)32,33,34,35 substrate water (the term ‘substrate water’ is used throughout the manuscript irrespective of the protonation state of the substrate oxygen) and a MnV=O or MnIV-oxyl radical. Alternatively, the O–O bond may be formed via radical coupling between either a Ca-oxyl radical and an Mn-bound oxyl radical (Fig. 2c)14, or between an Mn-oxyl radical and an Mn-oxo (bridge) (Fig. 2d)6,30,31,36.
6,14,26. Although the following reaction steps towards O2 must include the formation of a bound peroxidic intermediate, dioxygen formation and release, and the rebinding of substrate water27,28 (Fig. 1c), the exact nature of the chemistry involved remains unsettled, as these transient states have so far largely eluded biophysical investigations6,14,28,29,30,31,32. O–O formation by nucleophilic attack may occur, for example, either between a free (Fig. 2a)29 or a Ca-bound (Fig. 2b)32,33,34,35 substrate water (the term ‘substrate water’ is used throughout the manuscript irrespective of the protonation state of the substrate oxygen) and a MnV=O or MnIV-oxyl radical. Alternatively, the O–O bond may be formed via radical coupling between either a Ca-oxyl radical and an Mn-bound oxyl radical (Fig. 2c)14, or between an Mn-oxyl radical and an Mn-oxo (bridge) (Fig. 2d)6,30,31,36.
Figure 2. Conceivable O–O bond formation mechanisms in the ‘S4’ state of PSII.
(a) Nucleophilic attack by a bulk water onto a MnV=O or MnIV-oxyl radical29, (b) nucleophilic attack by a Ca bound water onto a MnV=O or MnIV-oxyl radical26,32,33,34,35, (c) coupling of a Ca-hydroxyl radical with a Mn-bound radical substrate14, (d) direct coupling between a terminal Mn-oxyl radical with an oxo bridge between Ca and Mn6,28,30,31,36.
Substrate–water exchange experiments, which monitor the rate of incorporation of isotopically labelled bulk water into the substrate sites of the OEC, have allowed determining the relative binding characteristics of the two substrate–water molecules in all (meta)-stable SiYZ states under various conditions, and have thereby provided unique insight into the mechanism of photosynthetic water oxidation28,29,30,31,37,38,39,40. In these experiments, PSII samples are preset to the desired SiYZ state by light flashes, and are then rapidly mixed with H218O. After desired incubation times they are further advanced by flashes to produce dioxygen. The isotopic composition of the product O2 (16,16O2, 16,18O2, 18,18O2) is monitored by membrane-inlet mass spectrometry41, and the substrate–water exchange rates are calculated from the H218O incubation time dependence of the m/z 34 and m/z 36 signals26,42. Assessing whether the two substrate waters can still exchange with the bulk water just before O–O bond formation, that is, in the transient 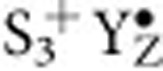 and
and  states, is expected to provide additional information on the nature and the binding sites of the two substrate waters, and therefore on the chemistry of water oxidation.
states, is expected to provide additional information on the nature and the binding sites of the two substrate waters, and therefore on the chemistry of water oxidation.
Thus far, only very few time-resolved studies were able to probe these two transient states22,24,25,43,44 and no information about substrate–water binding in the 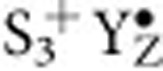 and
and  states is available as yet. This is mainly because the halftimes of these states in native PSII samples are too short (≤1–2 ms) with respect to the mixing dead time in the water-exchange experiments (t1/2=3 ms; see Supplementary Fig. 1). Recent studies set conditions though whereby the lifetimes of these transient states can be extended while preserving the overall function of the enzyme45,46. It was found that Thermosynechococcus elongatus cells growing on Sr2+-containing media devoid of Ca2+ incorporate Sr2+ in place of Ca2+ into the OEC and that Cl− can be exchanged biochemically against Br− or I− (Fig. 1a); importantly, these substitutions have only minor structural effects9,47,48,49,50, but extend the half-lifetime of the
states is available as yet. This is mainly because the halftimes of these states in native PSII samples are too short (≤1–2 ms) with respect to the mixing dead time in the water-exchange experiments (t1/2=3 ms; see Supplementary Fig. 1). Recent studies set conditions though whereby the lifetimes of these transient states can be extended while preserving the overall function of the enzyme45,46. It was found that Thermosynechococcus elongatus cells growing on Sr2+-containing media devoid of Ca2+ incorporate Sr2+ in place of Ca2+ into the OEC and that Cl− can be exchanged biochemically against Br− or I− (Fig. 1a); importantly, these substitutions have only minor structural effects9,47,48,49,50, but extend the half-lifetime of the  state to 7 ms (Sr/Br-PSII) or even 45 ms (Sr/I-PSII)45,46. These samples thereby provide the opportunity to probe the rates of substrate–water exchange in this last transient before O2 formation (Fig. 1c).
state to 7 ms (Sr/Br-PSII) or even 45 ms (Sr/I-PSII)45,46. These samples thereby provide the opportunity to probe the rates of substrate–water exchange in this last transient before O2 formation (Fig. 1c).
Here we show that the exchange of both substrate waters is strongly retarded in the transient  state as compared with the semi-stable S3+YZ state. Four possible mechanisms for this simultaneous retardation of the exchange of both substrate waters induced by YZ oxidation and the subsequent deprotonation of the catalytic site are presented and evaluated. On the basis of this evaluation and present literature data we conclude that W2 is most likely to be the fast exchanging substrate water (Wf), while O5 (or W3) can be assigned to be the slowly exchanging substrate water (Ws).
state as compared with the semi-stable S3+YZ state. Four possible mechanisms for this simultaneous retardation of the exchange of both substrate waters induced by YZ oxidation and the subsequent deprotonation of the catalytic site are presented and evaluated. On the basis of this evaluation and present literature data we conclude that W2 is most likely to be the fast exchanging substrate water (Wf), while O5 (or W3) can be assigned to be the slowly exchanging substrate water (Ws).
Results
Substrate–water exchange in the S3 +YZ state
Substrate–water exchange measurements were performed for the S3+YZ state to determine the influence of co-factor substitution on the substrate–water binding affinity in PSII core samples of T. elongatus. For this, the dark-adapted PSII samples were excited with two saturating flashes to advance the PSII complexes from the dark-stable S1YZ state into the semi-stable S3+YZ state. H218O was then injected at defined times before inducing dioxygen formation by giving one additional flash to the enzyme26,28,37.
Figures 3a,b compare the substrate–water exchange kinetics in the S3+YZ state of native Ca/Cl samples of T. elongatus with samples in which these cofactors were replaced by either Sr/Br or Sr/I. The native Ca/Cl-PSII shows the typical biphasic kinetics for the rise of the mixed labelled 16,18O2 (Fig. 3a), and the corresponding monophasic rise for the doubly labelled 18,18O2 species (Fig. 3b). As described previously, the fast 16,18O2 rise reflects the exchange of the fast exchanging substrate water (Wf), while the subsequent slow increase of the 16,18O2 signal and the rise of the double-labelled 18,18O2 species (Fig. 3b) reflect the exchange of the slowly exchanging substrate water (Ws)26.
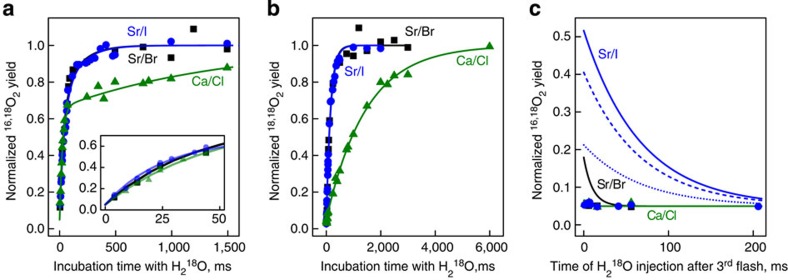
Figure 3. Substrate–water exchange in T. elongatus PSII core particles containing different ionic cofactors.
(a,b) The substrate water exchange in the S3+YZ state at m/z 34 (16,18O2; a) and m/z 36 (18,18O2; b), while c displays the exchange in the  state at m/z=34. Symbols mark the data points (green triangles, Ca/Cl-PSII; black squares, Sr/Br-PSII; blue circles, Sr/I-PSII), while full lines are fits representing the fast and slow substrate–water exchange (a,b; for rate constants see Table 1) or simulations of the expected experimental outcome assuming the exchange rates are identical in the S3+YZ and
state at m/z=34. Symbols mark the data points (green triangles, Ca/Cl-PSII; black squares, Sr/Br-PSII; blue circles, Sr/I-PSII), while full lines are fits representing the fast and slow substrate–water exchange (a,b; for rate constants see Table 1) or simulations of the expected experimental outcome assuming the exchange rates are identical in the S3+YZ and  states (c). The blue dashed and dotted lines in c represent simulations where either the slow (dashed) or fast (dotted) rate of exchange was set to be 1,000 times slower than that measured in the S3+YZ state (Table 1). All data points (n=1) are normalized to values reached after complete isotopic equilibration. Each time course was measured once, but consists of many separately measured data points that were in part obtained on different days.
states (c). The blue dashed and dotted lines in c represent simulations where either the slow (dashed) or fast (dotted) rate of exchange was set to be 1,000 times slower than that measured in the S3+YZ state (Table 1). All data points (n=1) are normalized to values reached after complete isotopic equilibration. Each time course was measured once, but consists of many separately measured data points that were in part obtained on different days.
The biological substitution of Ca2+ by Sr2+ leads to a tenfold acceleration of the slow exchange without significantly affecting Wf (Fig. 3a,b and Table 1). This is in good agreement with an earlier higher plant study, in which biochemical replacement of Ca2+ by Sr2+ was found to cause a fourfold acceleration of Ws exchange51. This specific effect on the slow substrate–water exchange is useful, as it allows the detection of the exchange of both substrate waters in the  state (see below). Finally, the substitution of Br− for I− has only a modest effect on the water exchange in the S3+YZ state as evidenced by the similar exchange characteristics obtained with the Sr/Br- and Sr/I-PSII samples (Fig. 3a,b).
state (see below). Finally, the substitution of Br− for I− has only a modest effect on the water exchange in the S3+YZ state as evidenced by the similar exchange characteristics obtained with the Sr/Br- and Sr/I-PSII samples (Fig. 3a,b).
Table 1. Rate constants of proton and electron transfer and water exchange during O2 formation in PSII.
Substrate–water exchange in the  state
state
On the basis of the exchange rates determined for the semi-stable S3+YZ state, substrate water-exchange experiments in the transient  state (Fig. 3c) were performed for all three sample types (Ca/Cl, Sr/Br, Sr/I). In these experiments, the dark-adapted PSII samples were advanced from the S1YZ state by three saturating flashes into the transient
state (Fig. 3c) were performed for all three sample types (Ca/Cl, Sr/Br, Sr/I). In these experiments, the dark-adapted PSII samples were advanced from the S1YZ state by three saturating flashes into the transient 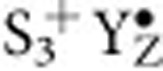 state to initiate the O2-forming reaction sequence (Fig. 1c). After this third flash, H218O was injected at various delay times, and the 18O incorporation into the product O2 was monitored. The symbols in Fig. 3c show that no incorporation of the 18O-label occurred for any of the three sample types, not even in the relatively long-lived
state to initiate the O2-forming reaction sequence (Fig. 1c). After this third flash, H218O was injected at various delay times, and the 18O incorporation into the product O2 was monitored. The symbols in Fig. 3c show that no incorporation of the 18O-label occurred for any of the three sample types, not even in the relatively long-lived  state of the Sr/I-PSII samples. This shows that the bulk H218O exchanges too slowly with the already bound 16O-substrate to allow incorporation of the 18O-label into the O2 product.
state of the Sr/I-PSII samples. This shows that the bulk H218O exchanges too slowly with the already bound 16O-substrate to allow incorporation of the 18O-label into the O2 product.
The extent to which the substrate-water exchange is slowed in the  state versus S3+YZ is illustrated by the computed exchange curves in Fig. 3c. The longer lifetimes of both the
state versus S3+YZ is illustrated by the computed exchange curves in Fig. 3c. The longer lifetimes of both the 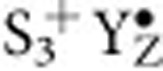 and
and  states should have resulted in relative 16,18O2 signals of 51(±6)% and 18(±2)% in the Sr/I-PSII and Sr/Br-PSII samples, respectively, if the exchange rates were the same as in the S3+YZ state. Such yields are well above the detection limit, which allows the detection of 16,18O2 formation even at natural abundance (Fig. 3c and Supplementary Fig. 2). Interestingly, the dashed and dotted blue curves show that a 1,000-fold slowing of only one of the exchange rates, while keeping the other one unchanged, cannot explain the data. Thus, our results imply that the exchange rates of both substrate waters are significantly retarded during the
states should have resulted in relative 16,18O2 signals of 51(±6)% and 18(±2)% in the Sr/I-PSII and Sr/Br-PSII samples, respectively, if the exchange rates were the same as in the S3+YZ state. Such yields are well above the detection limit, which allows the detection of 16,18O2 formation even at natural abundance (Fig. 3c and Supplementary Fig. 2). Interestingly, the dashed and dotted blue curves show that a 1,000-fold slowing of only one of the exchange rates, while keeping the other one unchanged, cannot explain the data. Thus, our results imply that the exchange rates of both substrate waters are significantly retarded during the 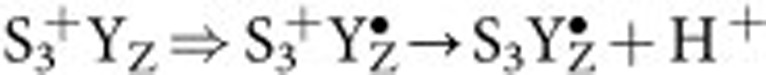 transitions. These simulations demonstrate that the corresponding other substrate water exchange rate needs to be additionally slowed by a factor >30 for Wf (if Ws is slowed by a factor of 1,000) and >10 for Ws (if Wf is slowed by a factor of 1,000), to make them consistent with the data.
transitions. These simulations demonstrate that the corresponding other substrate water exchange rate needs to be additionally slowed by a factor >30 for Wf (if Ws is slowed by a factor of 1,000) and >10 for Ws (if Wf is slowed by a factor of 1,000), to make them consistent with the data.
As a consequence, the exchange rate of Wf in the  state is<0.83 s−1, and is thus at least tenfold slower than the exchange rate of Ws in the S3+YZ state of the Sr/I samples, and at least as slow as the exchange of Ws in the Ca/Cl-PSII. It is emphasized that neither the overall oxygen production yield nor the period four oscillations were markedly affected by the cofactor substitutions45,46 (see also Supplementary Fig. 3), showing that this observation is made with enzyme that despite the slowed final O2-producing transition functions normally.
state is<0.83 s−1, and is thus at least tenfold slower than the exchange rate of Ws in the S3+YZ state of the Sr/I samples, and at least as slow as the exchange of Ws in the Ca/Cl-PSII. It is emphasized that neither the overall oxygen production yield nor the period four oscillations were markedly affected by the cofactor substitutions45,46 (see also Supplementary Fig. 3), showing that this observation is made with enzyme that despite the slowed final O2-producing transition functions normally.
Discussion
In catalysis, either in biology or in chemistry, isotope labelling studies are instrumental for elucidating reaction mechanisms52. In such experiments the substrate is labelled with a (stable) isotope and the propagation of this label into intermediates and/or the product(s) is followed. When studying the mechanism of water oxidation, H218O is typically added to a reactive species that has been pre-formed in unlabelled water. As an example, this method provided the demonstration that in a synthetic Mn-oxo complex, O2 is produced by nucleophilic attack of hydroxide on MnV≡O (ref. 53). The characteristic signature for this mechanism is the evolution of 16,18O2 at a ratio equal to the 18O-enrichment of bulk water. In contrast, we did not observe here any incorporation of 18O into the dioxygen product above the natural abundance level, when injecting H218O into PSII suspensions poised in the  state, even when the lifetime of the
state, even when the lifetime of the  state was significantly lengthened by the exchange of Ca2+ by Sr2+ and Cl− by I− (Fig. 3c). It is noted that the
state was significantly lengthened by the exchange of Ca2+ by Sr2+ and Cl− by I− (Fig. 3c). It is noted that the  state of PSII is comparable to MnV in the above model complex in the sense that the O-O bond is formed without the acquisition of any additional oxidizing equivalents. In absence of an unprecedented diffusion barrier54,55, which would need to arise during the
state of PSII is comparable to MnV in the above model complex in the sense that the O-O bond is formed without the acquisition of any additional oxidizing equivalents. In absence of an unprecedented diffusion barrier54,55, which would need to arise during the 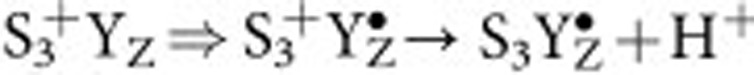 transitions, the observed lack of 16,18O2 formation directly excludes that in PSII the O–O bond is formed in the same way as in the MnV≡O model system53. In other terms, the nucleophilic attack of free water onto an electrophilic oxygen species (Fig. 2a) does not occur in PSII. This conclusion is consistent with previous data that showed that both substrate waters are bound to the OEC already in the S2 and S3 states56,57. However, nucleophilic attack of a Ca-bound water/hydroxo onto a
transitions, the observed lack of 16,18O2 formation directly excludes that in PSII the O–O bond is formed in the same way as in the MnV≡O model system53. In other terms, the nucleophilic attack of free water onto an electrophilic oxygen species (Fig. 2a) does not occur in PSII. This conclusion is consistent with previous data that showed that both substrate waters are bound to the OEC already in the S2 and S3 states56,57. However, nucleophilic attack of a Ca-bound water/hydroxo onto a 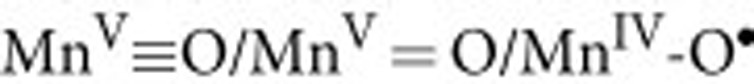 group (Fig. 2b)26,32,33,34,35 or coupling of a Ca-bound oxyl radical with a high valent Mn-oxo group (Fig. 2c)14 may take place, if conditions are present in PSII that slow down the exchange of the Ca-bound substrate water seven to eight orders of magnitude over the exchange rates reported for water ligated to Ca2+ in aqueous solutions37,39,40,58. Alternatively, both substrate –waters may be Mn-ligated and form the O–O bond via radical coupling (Fig. 2d)6,30,31,36.
group (Fig. 2b)26,32,33,34,35 or coupling of a Ca-bound oxyl radical with a high valent Mn-oxo group (Fig. 2c)14 may take place, if conditions are present in PSII that slow down the exchange of the Ca-bound substrate water seven to eight orders of magnitude over the exchange rates reported for water ligated to Ca2+ in aqueous solutions37,39,40,58. Alternatively, both substrate –waters may be Mn-ligated and form the O–O bond via radical coupling (Fig. 2d)6,30,31,36.
The lack of 16,18O2 and 18,18O2 production (Fig. 3c) demonstrates that the exchange of both substrate waters is significantly slower than the decay of the  state into S0YZ+O2, even when the O2 production is severely slowed by cofactor exchange. The fact that the exchange of both substrate waters is slowed down so significantly despite a constant redox state of the Mn4CaO5 cluster is important, and we discuss below the four possible mechanisms for ‘arresting’ both substrate waters under these conditions.
state into S0YZ+O2, even when the O2 production is severely slowed by cofactor exchange. The fact that the exchange of both substrate waters is slowed down so significantly despite a constant redox state of the Mn4CaO5 cluster is important, and we discuss below the four possible mechanisms for ‘arresting’ both substrate waters under these conditions.
The simplest possibility (mechanism 1) for slowing down significantly the exchange of both substrate waters would be the existence of the O–O bond (peroxide) already in the  state59,60. At this point, the 18O-label from the injected water could not be incorporated into the product and thus no 16,18O2 would be observed. Although internally consistent, this hypothesis is at odds with a recent time resolved X-ray spectroscopy experiment, which excluded Mn reduction (formation of the formal S1(O2)2−
state59,60. At this point, the 18O-label from the injected water could not be incorporated into the product and thus no 16,18O2 would be observed. Although internally consistent, this hypothesis is at odds with a recent time resolved X-ray spectroscopy experiment, which excluded Mn reduction (formation of the formal S1(O2)2−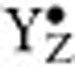 intermediate) during the transient phase (Fig. 1c). This implies that the O–O bond is formed at a later stage of the reaction cycle8,43, and rules out peroxide formation in the
intermediate) during the transient phase (Fig. 1c). This implies that the O–O bond is formed at a later stage of the reaction cycle8,43, and rules out peroxide formation in the  state as an explanation for the arrested water change.
state as an explanation for the arrested water change.
An alternative interpretation (mechanism 2) is that the oxidation state of YZ strongly influences the substrate–water exchange via the H-bonding network around the Mn4CaO5 cluster, which includes the two water ligands of Ca (W3 and W4; Fig. 1a). The oxidation of YZ (that is, 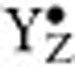 formation) is coupled to the transfer of its phenolic proton to the nearby D1-His190 (Fig. 1a)61,62. This proton movement undoubtedly changes the H-bonding network4,63,64 around the YZ/Ca site and, as a consequence, may affect the exchange of the two substrate waters. This line of thought is partially supported by earlier data obtained with the alkaline-induced
formation) is coupled to the transfer of its phenolic proton to the nearby D1-His190 (Fig. 1a)61,62. This proton movement undoubtedly changes the H-bonding network4,63,64 around the YZ/Ca site and, as a consequence, may affect the exchange of the two substrate waters. This line of thought is partially supported by earlier data obtained with the alkaline-induced 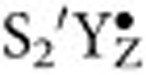 state (the dash denotes a likely difference in protonation state and/or water ligation with respect to the S2+YZ state), which was generated by addition of base to the preformed S3+YZ state65. These studies demonstrated that the substrate–water exchange is 5- to 20-fold slower in the
state (the dash denotes a likely difference in protonation state and/or water ligation with respect to the S2+YZ state), which was generated by addition of base to the preformed S3+YZ state65. These studies demonstrated that the substrate–water exchange is 5- to 20-fold slower in the 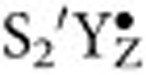 state than in the S2+YZ state42,65. The magnitude of this change is, however, too small to account for the 1000-fold decrease needed for at least one of the two substrates to explain the lack of 18O-labelling of the dioxygen observed here (Fig. 3c). Nevertheless, this option cannot be completely ruled out, and, if true, would be a remarkable demonstration of the interconnectivity of all components of the OEC.
state than in the S2+YZ state42,65. The magnitude of this change is, however, too small to account for the 1000-fold decrease needed for at least one of the two substrates to explain the lack of 18O-labelling of the dioxygen observed here (Fig. 3c). Nevertheless, this option cannot be completely ruled out, and, if true, would be a remarkable demonstration of the interconnectivity of all components of the OEC.
A third option (mechanism 3) correlates the arrest of the substrate–water exchange with the deprotonation event on the Mn4CaO5(HnO)4 cluster that was previously reported to occur during the 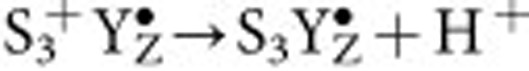 transition (Fig. 1c)22 and is demonstrated here to also occur in the Sr/Br-PSII samples (Supplementary Figs 4–10, Supplementary Note 1 and Supplementary Methods). This proton release could explain a 1,000-fold decrease of one of the substrate–water exchange rates, if Wf or Ws is deprotonated. Notably, this could also account for a simultaneous slowing of the other substrate water (that is, at least 10- to 30-fold; see above), if the exchanges of both substrate waters are coupled (see ref. 28), or if the exchange of the other substrate is simultaneously slowed by mechanism 2.
transition (Fig. 1c)22 and is demonstrated here to also occur in the Sr/Br-PSII samples (Supplementary Figs 4–10, Supplementary Note 1 and Supplementary Methods). This proton release could explain a 1,000-fold decrease of one of the substrate–water exchange rates, if Wf or Ws is deprotonated. Notably, this could also account for a simultaneous slowing of the other substrate water (that is, at least 10- to 30-fold; see above), if the exchanges of both substrate waters are coupled (see ref. 28), or if the exchange of the other substrate is simultaneously slowed by mechanism 2.
Finally (mechanism 4), a recent theoretical study concluded that substrate–water exchange can only occur in PSII, if at least one Mn ion within the Mn4CaO5 cluster is in the MnIII redox state40. Thus, to exchange a water ligand in the S3+YZ state, the Mn4CaO5 cluster must first be transiently reduced by YZ to generate the exchange-competent 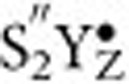 state (which may differ from the S2+YZ and
state (which may differ from the S2+YZ and 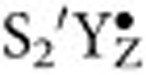 states discussed above in its protonation state and/or water binding). This requires the
states discussed above in its protonation state and/or water binding). This requires the 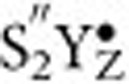 state and S3+YZ state to be almost isoenergetic with low transition barrier, allowing for a fast redox equilibrium with an appreciable probability to form the
state and S3+YZ state to be almost isoenergetic with low transition barrier, allowing for a fast redox equilibrium with an appreciable probability to form the 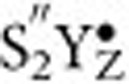 state. If this is the mechanism for substrate exchange in the S3+YZ state, then the substrate–water exchange in the
state. If this is the mechanism for substrate exchange in the S3+YZ state, then the substrate–water exchange in the  state would indeed be expected to be impeded, simply because in this state the Mn4CaO5 cluster cannot be transiently re-reduced by the oxidized
state would indeed be expected to be impeded, simply because in this state the Mn4CaO5 cluster cannot be transiently re-reduced by the oxidized 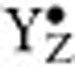 . As such, our data provide the first experimental support to this theoretical prediction. We note, however, that mechanism 4 critically depends on the condition that both substrate waters are ligated to Mn and that all Mn ions in the S3+YZ and
. As such, our data provide the first experimental support to this theoretical prediction. We note, however, that mechanism 4 critically depends on the condition that both substrate waters are ligated to Mn and that all Mn ions in the S3+YZ and  states are in the oxidation state MnIV, while the alternative explanations (mechanisms 2 and 3) do not.
states are in the oxidation state MnIV, while the alternative explanations (mechanisms 2 and 3) do not.
Experiments and theoretical calculations conducted by several groups have suggested that W2, W3, O5 (Fig. 1a) or WX (a water that is proposed to bind to the Mn4CaO5 cluster during the S2+YZ⇒S3+YZ transition) are likely candidates for the two substrate waters4,6,28,39,40,66,67. In a very recent work, WX was ruled out as the immediate substrate by clearly demonstrating that both substrates are already bound in the S2+YZ state57. Our present substrate–water exchange experiments in the S3+YZ state (Fig. 3a,b and Table 1) show that the exchange of Wf is only marginally affected by biological Ca/Sr substitution, while the exchange of Ws occurs ten times faster in the Sr-PSII sample. The fact that the difference between Wf and Ws is even stronger after biological substitution than after chemical exchange51 considerably strengthens the previous suggestion30,51 that Wf is not a ligand of Ca2+. This point is further supported by the data presented here on water exchange in the  state, which show that the exchange rate of Wf is at least commensurate with the exchange rate of Ws in the S3+YZ state. Thus, from the short list above, W2 appears to be the most probable candidate for Wf39,57. In contrast, the tenfold dependence of the binding affinity of Ws on biosynthetic Ca/Sr substitution reported here provides additional strong support for a direct bond between Ca/Sr and Ws51. This makes W3 (ref. 39) and O5 the most likely candidates for Ws with O5 being favoured, owing to the SiYZ state dependence of the Ws exchange rate28,30,58,67 even though a definitive assignment will require additional experimental support.
state, which show that the exchange rate of Wf is at least commensurate with the exchange rate of Ws in the S3+YZ state. Thus, from the short list above, W2 appears to be the most probable candidate for Wf39,57. In contrast, the tenfold dependence of the binding affinity of Ws on biosynthetic Ca/Sr substitution reported here provides additional strong support for a direct bond between Ca/Sr and Ws51. This makes W3 (ref. 39) and O5 the most likely candidates for Ws with O5 being favoured, owing to the SiYZ state dependence of the Ws exchange rate28,30,58,67 even though a definitive assignment will require additional experimental support.
For the first time, the exchangeability of the substrate–water molecules has been probed in the last transient state before the O–O bond formation. This provides important additional constraints for the ongoing identification of the substrate–water binding sites at the Mn4CaO5 cluster and for the elucidation of the mechanism of water oxidation in PSII. The discovery that both substrate waters are non-exchangable in the last transient state before O2 formation suggests that arresting the exchange of both substrate water molecules, rather than just one, is a mechanistic requirement. We propose, in line with the finding that the slowing down of oxygen evolution on Ca/Sr substitution stems from a change in entropy68, that this lack of exchange with the bulk water reflects a highly ordered arrangement of the OEC that is essential for low-energy O–O bond formation.
Methods
Preparation of the PSII samples
The T. elongatus strain used was the ΔpsbA1ΔpsbA2 deletion mutant69 constructed from the T. elongatus 43-H strain that had a His6-tag on the carboxy terminus of CP43 (ref. 70). The biological Ca/Sr and the biochemical Cl/Br exchanges were achieved as previously described45,46,68. Ca/Cl-PSIIs, Sr/Cl-PSIIs and Sr/Br-PSIIs were purified with the protocol already described68. For the Cl−/I− exchange46, Sr/Cl-PSII’s bound to the Ni column were washed overnight with ~\n8–10 column volumes of a buffer containing 10% glycerol, 1 M betaine, 100 mM NaCl, 15 mM CaCl2, 15 mM, MgCl2, 40 mM MES, 1 mM L-histidine, 0.03% β-dodecyl maltoside, pH 6.5 (pH adjusted with NaOH). Next, the PSII core complexes bound to the resin were washed with one volume equivalent of a buffer containing 10% glycerol, 1 M betaine, 1 mM NaI, 15 mM Ca(OH)2, 15 mM Mg(OH)2, 1 mM L-histidine, 0.03% β-dodecyl maltoside, MES 40 mM, pH 6.5 (adjusted by addition of NaOH). The PSII’s were then eluted with a buffer containing, 1 M betaine, 1 mM NaI, 15 mM Ca(OH)2, 15 mM Mg(OH)2, 200 mM L-histidine, 0.03% β-dodecyl maltoside, pH 6.5 (adjusted by addition of MES powder). The eluted PSII samples were then washed by using Amincon-ultra-15 100 K concentrators in a buffer containing 1 M betaine, 1 mM NaI, 15 mM Ca(OH)2, 15 mM Mg(OH)2, 40 mM MES 40 mM, pH 6.5 (pH adjusted with NaOH). PSII samples were frozen at 77 K in liquid nitrogen until use.
Substrate–water exchange measurements
An isotope ratio mass spectrometer (ThermoFinnigan Delta plus XP) connected to a membrane-inlet cuvette (165 μl) via a cooling trap (liquid N2) was used to measure substrate–water exchange at 20 °C26,28,41. The substrate–water exchange in the S3+YZ state was studied by illuminating PSII, highly enriched in the 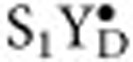 state by a preflash and subsequent dark-adaptation, with two saturating Xe-flashes (2 Hz), followed by H218O injection at various times before the third flash, which was given at a fixed time after the second flash (6 s for Ca/Cl-PSII, 3 s for Sr/Br-PSII, 2 s for Sr/I-PSII). Four flashes (2 Hz) were given 5 min after the third turnover flash for normalization purpose. For the
state by a preflash and subsequent dark-adaptation, with two saturating Xe-flashes (2 Hz), followed by H218O injection at various times before the third flash, which was given at a fixed time after the second flash (6 s for Ca/Cl-PSII, 3 s for Sr/Br-PSII, 2 s for Sr/I-PSII). Four flashes (2 Hz) were given 5 min after the third turnover flash for normalization purpose. For the  state measurements the
state measurements the 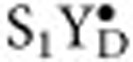 -enriched PSII samples were illuminated with three flashes (2 Hz) followed by H218O injection at various times after the third flash, and the normalizing flash sequence. The S3+YZ data were treated and fit within an Excel spread sheet employing equations 34Y=0.66 (1−exp(−34kf•t))+0.34 (1−exp(−34ks•t)) and 36Y=1−exp(−36k•t), where 34Y and 36Y signify the incubation time dependent 16,18O2 and 18,18O2 yields, respectively26,42. The expected 16,18O2 yields for the
-enriched PSII samples were illuminated with three flashes (2 Hz) followed by H218O injection at various times after the third flash, and the normalizing flash sequence. The S3+YZ data were treated and fit within an Excel spread sheet employing equations 34Y=0.66 (1−exp(−34kf•t))+0.34 (1−exp(−34ks•t)) and 36Y=1−exp(−36k•t), where 34Y and 36Y signify the incubation time dependent 16,18O2 and 18,18O2 yields, respectively26,42. The expected 16,18O2 yields for the  state experiments were calculated in 1 ms intervals and then summed up over the whole decay (3,000 ms) within Excel by folding the monoexponetial
state experiments were calculated in 1 ms intervals and then summed up over the whole decay (3,000 ms) within Excel by folding the monoexponetial  decay with the increasing H218O enrichment in the two binding sites. By varying the delay time between
decay with the increasing H218O enrichment in the two binding sites. By varying the delay time between  formation (third flash) and start of H218O enrichment (injection), the expected 16,18O2 yield was calculated for delays up to 210 ms. Injection artefacts, Chl dilution and H218O mixing were accounted for as in the S3+YZ experiments.
formation (third flash) and start of H218O enrichment (injection), the expected 16,18O2 yield was calculated for delays up to 210 ms. Injection artefacts, Chl dilution and H218O mixing were accounted for as in the S3+YZ experiments.
Author contributions
The experiment was conceived by F.R., A.B. and J.M. The samples were prepared by A.B. and the water-exchange experiments were performed and analysed by H.N. under the supervision of J.M. Proton release measurements were performed by F.R. and A.B. The manuscript was written by F.R., A.B. and J.M. with contributions of H.N.
Additional information
How to cite this article: Nilsson, H. et al. Substrate–water exchange in photosystem II is arrested before dioxygen formation. Nat. Commun. 5:4305 doi: 10.1038/ncomms5305 (2014).
Supplementary Material
Supplementary Figures 1-10, Supplementary Note 1, Supplementary Methods and Supplementary References
Acknowledgments
Miwa Sugiura is acknowledged for the gift of the His-tagged strain. We thank Nick Cox and Dimitrios Pantazis for discussions and suggestions regarding the manuscript. Dimitrios Pantazis has provided data for Fig. 1a and Dmitriy Shevela prepared the final versions of all figures. We acknowledge Per Siegbahn, Ron Pace and Jerome Lavergne for ongoing discussions concerning the mechanism of water oxidation, and the Max-Planck Institute for Chemical Energy Conversion for the loan of the isotope ratio mass spectrometer (to J.M).
HN and JM were supported by the Artificial Leaf Project Umeå (K&A Wallenberg foundation), the Solar Fuels Strong Research Environment Umeå (Umeå University), Vetenskapsrådet and Swedish Energy Agency (Energimyndigheten). AB was supported in part by the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INSB-05-01 and by the ‘Bioénergie’ program from CEA/DSV. FR acknowledges financial support from the CNRS and the ‘Initiative d’Excellence’ program from the French state (Grant ‘ DYNAMO’, ANR-11-LABX-0011-01).
References
- Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J. & Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004). [DOI] [PubMed] [Google Scholar]
- Yano J. et al. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314, 821–825 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskov A. et al. Cyanobacterial photosystem II at 2.9 Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Biol. Mol. Biol. 16, 334–342 (2009). [DOI] [PubMed] [Google Scholar]
- Umena Y., Kawakami K., Shen J. R. & Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–61 (2011). [DOI] [PubMed] [Google Scholar]
- Tsui E. Y. & Agapie T. Reduction potentials of heterometallic manganese-oxido cubane complexes modulated by redox-inactive metals. Proc. Natl Acad. Sci. USA 110, 10084–10088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegbahn P. E. M. Water oxidation mechanism in photosystem II, including oxidations, proton release pathways, O-O bond formation and O2 release. Biochim. Biophys. Acta 1827, 1003–1019 (2013). [DOI] [PubMed] [Google Scholar]
- Ames W. et al. Theoretical evaluation of structural models of the S2 state in the oxygen evolving complex of photosystem II: protonation states and magnetic interactions. J. Am. Chem. Soc. 133, 19743–19757 (2011). [DOI] [PubMed] [Google Scholar]
- Dau H., Zaharieva I. & Haumann M. Recent developments in research on water oxidation by photosystem II. Curr. Opin. Chem. Biol. 16, 3–10 (2012). [DOI] [PubMed] [Google Scholar]
- Cox N., Pantazis D. A., Neese F. & Lubitz W. Biological water oxidation. Acc. Chem. Res. 46, 1588–1596 (2013). [DOI] [PubMed] [Google Scholar]
- Luber S. et al. S1-state model of the O2-evolving complex of photosystem II. Biochemistry 50, 6308–6311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel S., Schraut J., Arbuznikov A. V., Siegbahn P. E. M. & Kaupp M. Density functional calculations of 55Mn, 14N and 13C electron paramagnetic resonance parameters support an energetically feasible model system for the S2 state of the oxygen-evolving complex of photosystem II. Chem. Eur. J. 16, 10424–10438 (2010). [DOI] [PubMed] [Google Scholar]
- Pantazis D. A., Ames W., Cox N., Lubitz W. & Neese F. Two interconvertible structures that explain the spectroscopic properties of the oxygen-evolving complex of photosystem II in the S2 state. Angew. Chem. Int. Ed. 51, 9935–9940 (2012). [DOI] [PubMed] [Google Scholar]
- Gatt P., Petrie S., Stranger R. & Pace R. J. Rationalizing the 1.9 Å crystal structure of photosystem II—a remarkable Jahn-Teller balancing act induced by a single proton transfer. Angew. Chem. Int. Ed. 51, 12025–12028 (2012). [DOI] [PubMed] [Google Scholar]
- Isobe H. et al. Theoretical illumination of water-inserted structures of the CaMn4O5 cluster in the S2 and S3 states of oxygen-evolving complex of photosystem II: full geometry optimizations by B3LYP hybrid density functional. Dalt. Trans. 41, 13727–13740 (2012). [DOI] [PubMed] [Google Scholar]
- Dau H. & Haumann M. The manganese complex of photosystem II in its reaction cycle—Basic framework and possible realization at the atomic level. Coord. Chem. Rev. 252, 273–295 (2008). [Google Scholar]
- Glatzel P. et al. Electronic structural changes of Mn in the oxygen-evolving complex of photosystem II during the catalytic cycle. Inorg. Chem. 52, 5642–5644 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glöckner C. et al. Structural changes of the oxygen-evolving complex in photosystem II during the catalytic cycle. J. Biol. Chem. 288, 22607–22620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger J. et al. Absence of Mn centered oxidation in the S2 to S3 transition: implications for the mechanism of photosynthetic water oxidation. J. Am. Chem. Soc. 123, 7804–7820 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L. V., Epel B., Lubitz W. & Messinger J. Electronic structure of the Mn4OxCa cluster in the S0 and S2 states of the oxygen-evolving complex of photosystem II based on pulse 55Mn-ENDOR and EPR spectroscopy. J. Am. Chem. Soc. 129, 13421–13435 (2007). [DOI] [PubMed] [Google Scholar]
- Kolling D. R. J., Cox N., Ananyev G. M., Pace R. J. & Dismukes G. C. What are the oxidation states of manganese required to catalyze photosynthetic water oxidation? Biophys. J. 103, 313–322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace R. J., Jin L. & Stranger R. What spectroscopy reveals concerning the Mn oxidation levels in the oxygen evolving complex of photosystem II: X-ray to near infra-red. Dalt. Trans. 41, 11145–11160 (2012). [DOI] [PubMed] [Google Scholar]
- Rappaport F., Blanchard-Desce M. & Lavergne J. Kinetics of electron transfer and electrochromic change during the redox transitions of the photosynthetic oxygen evolving complex. Biochim. Biophys. Acta 1184, 178–192 (1994). [Google Scholar]
- Koike H., Hanssum B., Inoue Y. & Renger G. Temperature dependence of the S-state transitions in a thermophilic cyanobacterium, Synechococcus vulcanus Copeland measured by absorption changes in the ultraviolet region. Biochim. Biophys. Acta 893, 524–533 (1987). [Google Scholar]
- Razeghifard M. R. & Pace R. J. EPR kinetic studies of oxygen release in thylakoids in PSII membranes: a kinetic intermediate in the S3 to S0 transition. Biochemistry 38, 1252–1257 (1999). [DOI] [PubMed] [Google Scholar]
- Dilbeck P. L. et al. The D1-D61N mutation in synechocystis sp PCC 6803 allows the observation of pH-sensitive intermediates in the formation and release of O2 from photosystem II. Biochemistry 51, 1079–1091 (2012). [DOI] [PubMed] [Google Scholar]
- Messinger J., Badger M. & Wydrzynski T. Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc. Natl Acad. Sci. USA 92, 3209–3213 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sugiura M. & Noguchi T. Monitoring water reactions during the S-state cycle of the photosynthetic water-oxidizing center: Detection of the DOD bending vibrations by means of Fourier transform infrared spectroscopy. Biochemistry 47, 11024–11030 (2008). [DOI] [PubMed] [Google Scholar]
- Cox N. & Messinger J. Reflections on substrate water and dioxygen formation. Biochim. Biophys. Acta 1827, 1020–1030 (2013). [DOI] [PubMed] [Google Scholar]
- Dau H. et al. The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724–761 (2010). [Google Scholar]
- Messinger J. Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data. Phys. Chem. Chem. Phys. 6, 4764–4771 (2004). [Google Scholar]
- Vinyard D. J., Ananyev G. M. & Dismukes G. C. Photosystem II: the reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 82, 577–606 (2013). [DOI] [PubMed] [Google Scholar]
- Sproviero E. M., Gascon J. A., McEvoy J. P., Brudvig G. W. & Batista V. S. Quantum mechanics/molecular mechanics study of the catalytic cycle of water splitting in photosystem II. J. Am. Chem. Soc. 130, 3428–3442 (2008). [DOI] [PubMed] [Google Scholar]
- McEvoy J. P. & Brudvig G. W. Water-splitting chemistry of photosystem II. Chem. Rev. 106, 4455–4483 (2006). [DOI] [PubMed] [Google Scholar]
- Taguchi T. et al. Preparation and properties of a monomeric high-spin MnV-oxo complex. J. Am. Chem. Soc. 134, 1996–1999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro V. L., Baldwin M. J., Caudle M. T., Hsieh W.-Y. & Law N. A. A proposal for water oxidation in photosystem II. Pure Appl. Chem. 70, 925–929 (1998). [Google Scholar]
- Siegbahn P. E. M. O-O bond formation in the S4 state of the oxygen-evolving complex in photosystem II. Chem. Eur. J. 12, 9217–9227 (2006). [DOI] [PubMed] [Google Scholar]
- Hillier W. & Wydrzynski T. 18O-Water exchange in photosystem II: Substrate binding and intermediates of the water splitting cycle. Coord. Chem. Rev. 252, 306–317 (2008). [Google Scholar]
- Petrie S., Stranger R. & Pace R. J. Hydration preferences for Mn4Ca cluster models of photosystem II: Location of potential substrate-water binding sites. Chem. Eur. J. 16, 14026–14042 (2010). [DOI] [PubMed] [Google Scholar]
- Sproviero E. M. et al. QM/MM computational studies of substrate water binding to the oxygen-evolving centre of photosystem II. Philos. Trans. R. Soc. Lond. B 363, 1149–1156 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegbahn P. E. M. Substrate water exchange for the oxygen evolving complex in PSII in the S1, S2, and S3 states. J. Am. Chem. Soc. 135, 9442–9449 (2013). [DOI] [PubMed] [Google Scholar]
- Beckmann K., Messinger J., Badger M. R., Wydrzynski T. & Hillier W. On-line mass spectrometry: membrane inlet sampling. Photosynth. Res. 102, 511–522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier W. & Wydrzynski T. Substrate water interactions within the photosystem II oxygen evolving complex. Phys. Chem. Chem. Phys. 6, 4882–4889 (2004). [Google Scholar]
- Haumann M. et al. Photosynthetic O2 formation tracked by time-resolved X-ray experiments. Science 310, 1019–1021 (2005). [DOI] [PubMed] [Google Scholar]
- Kern J. et al. Simultaneous femtosecond X-ray spectroscopy and diffraction of photosystem II at room temperature. Science 340, 491–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N. et al. Biosynthetic exchange of bromide for chloride and strontium for calcium in the photosystem II oxygen-evolving enzymes. J. Biol. Chem. 283, 13330–13340 (2008). [DOI] [PubMed] [Google Scholar]
- Boussac A., Ishida N., Sugiura M. & Rappaport F. Probing the role of chloride in Photosystem II from Thermosynechococcus elongatus by exchanging chloride for iodide. Biochim. Biophys. Acta 1817, 802–810 (2012). [DOI] [PubMed] [Google Scholar]
- Koua F. H. M., Umena Y., Kawakami K. & Shen J. R. Structure of Sr-substituted photosystem II at 2.1 Å resolution and its implications in the mechanism of water oxidation. Proc. Natl Acad. Sci. USA 110, 3889–3894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. W. et al. X-ray crystallography identifies two chloride binding sites in the oxygen evolving centre of Photosystem II. Energy Environ. Sci. 1, 161–166 (2008). [Google Scholar]
- Kawakami K., Umena Y., Kamiya N. & Shen J.-R. Location of chloride and its possible functions in oxygen-evolving photosystem II revealed by X-ray crystallography. Proc. Natl Acad. Sci. USA 106, 8567–8572 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkar Y. L., Yano J., Sauer K., Boussac A. & Yachandra V. K. Structural changes in the Mn4Ca cluster and the mechanism of photosynthetic water splitting. Proc. Natl Acad. Sci. USA 105, 1879–1884 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. & Wydrzynski T. 18O isotope exchange measurements reveal that calcium is involved in the binding of one substrate-water molecule to the oxygen-evolving complex in photosystem II. Biochemistry 42, 6209–6217 (2003). [DOI] [PubMed] [Google Scholar]
- Romain S., Vigara L. & Llobet A. Oxygen-oxygen bond formation pathways promoted by ruthenium complexes. Acc. Chem. Res. 42, 1944–1953 (2009). [DOI] [PubMed] [Google Scholar]
- Gao Y., Åkermark T., Liu J. H., Sun L. C. & Åkermark B. Nucleophilic attack of hydroxide on a MnV oxo complex: a model of the O-O bond formation in the oxygen evolving complex of photosystem II. J. Am. Chem. Soc. 131, 8726–8727 (2009). [DOI] [PubMed] [Google Scholar]
- Vassiliev S., Zaraiskaya T. & Bruce D. Molecular dynamics simulations reveal highly permeable oxygen exit channels shared with water uptake channels in photosystem II. Biochim. Biophys. Acta 1827, 1148–1155 (2013). [DOI] [PubMed] [Google Scholar]
- Persson E. & Halle B. Nanosecond to microsecond protein dynamics probed by magnetic relaxation dispersion of buried water molecules. J. Am. Chem. Soc. 130, 1774–1787 (2008). [DOI] [PubMed] [Google Scholar]
- Hendry G. & Wydrzynski T. The two substrate water molecules are already bound to the oxygen evolving complex in the S2 state of photosystem II. Biochemistry 41, 13328–13334 (2002). [DOI] [PubMed] [Google Scholar]
- Nilsson H., Krupnik T., Kargul J. & Messinger J. Substrate water exchange in photosystem II core complexes of the extremophilic red alga Cyanidioschyzon merolae. Biochim. Biophys. Acta 1837, 1257–1262 (2014). [DOI] [PubMed] [Google Scholar]
- Richens D. T. Ligand substitution reactions at inorganic centers. Chem. Rev. 105, 1961–2002 (2005). [DOI] [PubMed] [Google Scholar]
- Messinger J. & Renger G. inProcesses of Photosynthesis—Part 2: Basic Principles and Apparatus Comprehensive Series in Photochemical and Photobiological Sciences ed Renger G. 291–349The Royal Society of Chemistry (2008). [Google Scholar]
- Meyer T. J., Huynh M. H. V. & Thorp H. H. The possible role of proton-coupled electron transfer (PCET) in water oxidation by photosystem II. Angew. Chem. Int. Ed. 46, 5284–5304 (2007). [DOI] [PubMed] [Google Scholar]
- Rappaport F. et al. Probing the coupling between proton and electron transfer in photosystem II core complexes containing a 3-fluorotyrosine. J. Am. Chem. Soc. 131, 4425–4433 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays A.-M. A., Vassiliev I. R., Golbeck J. H. & Debus R. J. Role of D1-His190 in the proton coupled oxidation of tyrosine YZ in manganese depleted photosystem II. Biochemistry 38, 11851–11865 (1999). [DOI] [PubMed] [Google Scholar]
- Schilstra M. J., Rappaport F., Nugent J. H. A., Barnett C. J. & Klug D. R. Proton/hydrogen transfer affects the S-state dependent microsecond phases of P680+ reduction during water splitting. Biochemistry 37, 3974–3981 (1998). [DOI] [PubMed] [Google Scholar]
-
Christen G., Seeliger A. & Renger G.
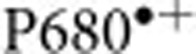 reduction kinetics and redox transition probability of the water oxidizing complex as a function of pH and H/D isotope exchange in spinach thylakoids. Biochemistry
38, 6082–6092 (1999). [DOI] [PubMed] [Google Scholar]
reduction kinetics and redox transition probability of the water oxidizing complex as a function of pH and H/D isotope exchange in spinach thylakoids. Biochemistry
38, 6082–6092 (1999). [DOI] [PubMed] [Google Scholar] - Sjöholm J., Havelius K. G. V., Mamedov F. & Styring S. Effects of pH on the S3 state of the oxygen evolving complex in photosystem II probed by EPR split signal induction. Biochemistry 49, 9800–9808 (2010). [DOI] [PubMed] [Google Scholar]
- Navarro M. P. et al. Ammonia binding to the oxygen-evolving complex of photosystem II identifies the solvent-exchangeable oxygen bridge (μ-oxo) of the manganese tetramer. Proc. Natl Acad. Sci. USA 110, 15561–15566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapatskiy L. et al. Detection of the water-binding sites of the oxygen-evolving complex of photosystem II using W-band 17O electron-electron double resonance-detected NMR spectroscopy. J. Am. Chem. Soc. 134, 16619–16634 (2012). [DOI] [PubMed] [Google Scholar]
- Rappaport F., Ishida N., Sugiura M. & Boussac A. Ca2+ determines the entropy changes associated with the formation of transition states during water oxidation by photosystem II. Energy Environ. Sci. 4, 2520–2524 (2011). [Google Scholar]
- Sugiura M., Boussac A., Noguchi T. & Rappaport F. Influence of Histidine-198 of the D1 subunit on the properties of the primary electron donor, P680, of photosystem II in Thermosynechococcus elongatus. Biochim. Biophys. Acta 1777, 331–342 (2008). [DOI] [PubMed] [Google Scholar]
- Sugiura M. & Inoue Y. Highly purified thermo-stable oxygen-evolving photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having his-tagged CP43. Plant Cell Physiol. 40, 1219–1231 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-10, Supplementary Note 1, Supplementary Methods and Supplementary References