Abstract
The Northern black-tailed rattlesnake (Crotalus molossus molossus) venom is mainly hemotoxic, hemorrhagic, and neurotoxic. Its effects in the central nervous system are unknown and only poorly described for all Viperidae species in general. This is why we are interested in describe the damage induced by C. m. molossus venom in rat brain, particularly in the area postrema capillaries. Four C. m. molossus venom doses were tested (0.02, 0.05, 0.10 and 0.20mg/kg) injected intramuscularly at the lower limb, incubated by 24 hours and the brains were harvested. Area postrema coronal sections were stained with Haematoxylin and Eosin, and examined to observe the venom effect in quantity of capillaries and porphology. Starting from the 0.10mg/kg treatment we observed lysed extravasated erythrocytes and also capillary breakdown, as a consequence of hemorrhages appearance. The number of capillaries decreased significantly in response to the venom dose increment. Hemorrhages could be caused by the metalloproteinase activity on the basal membrane and the apoptosis generated by L-amino acid oxidases. Hemolysis could be caused by phospholipase A2 hemotoxic effect. We conclude that C. m. molossus crude venom produces hemolysis, capillary breakdown, hemorrhages, and the reduction in number of capillaries in the area postrema.
Keywords: Crotalus molossus molossus, area postrema, metalloproteinase, L-amino acid oxidase, phospholipase A2
INTRODUCTION
In Mexico, 27,480 cases of ophidic accidents were reported in 2007, in which 0.5% of the cases ends in death (Luna-Bauza, 2007). Although mortality due to an ophidic accident is not a major problem, in many cases it can result in functional disability, loss of extremities, and costly recovery (Sánchez et al., 2003). C. m. molossus is distributed in the southwestern United States in Arizona, New Mexico, and Texas, and northern Mexico in Sonora, Chihuahua, and Coahuila (Lemos-Espinal and Smith, 2007). Crotalus venom contains a complex mixture of proteins with and without enzymatic activity, the most representative are the P-I and P-III metalloproteinases, serine proteinases, L-amino acid oxidases, phospholipase A2, disintegrin, cysteine-rich secretory protein, and others (Mackessy, 2010). C. m. molossus venom has a phosphodiesterase (Ferlan et al., 1983a) two metalloproteinases (Rael et al., 1992; Chen and Rael, 1997; Sánchez et al., 2001), a disintegrin called molossin (Scarborough et al., 1993), and three phospholipase A2 (Ferlan et al., 1983b; Tsai et al., 2001), that had been isolated and characterized. Together all these proteins cause platelet aggregation (Hardy et al., 1982; Corrigan et al., 1983), hemorrhages, proteolysis (Soto et al., 1989), and fibrinolysis (Perez et al., 2001).
The neurological effect of the crotalids venom on the central nervous system has not been well described, but there are some medical cases were ischemic stroke (Thomas et al., 2006), intracranial hemorrhages, and cerebral infarctions (Del Brutto and Del Brutto, 2012; Rebahi et al., 2014) are reported. Experimentally, it has been shown that Bothrops colombiensis venom can cause erythrocyte extravasation at leptomeninges (Rodríguez-Acosta et al., 2003), and Hypnale zara venom can cause ischemic neuronal degeneration in cerebral cortex (Silva et al., 2012). Moreover, experimentation with specific venom toxins reveals damage in the brain, for example, phospholipase A2 from Echis carinatus generates vacuole formation in the cytoplasm of prefrontral cortex cells (Perumal Samy et al., 2010), and gyroxin, a Crotalus durissus terrificus serine protease, can cause histological changes in cerebellum and prefrontal cortex (Ruiz de Torrent et al., 2007) and, temporally, is able to disturb blood brain barrier permeability (Alves da Silva et al., 2011). These evidences suggest that the venom components could pass through blood-brain barrier and cause this effect.
Area postrema is a brain structure that lacks of blood-brain barrier, is highly vascularized and contains fenestrated capillaries without tight junctions between endothelial cells, through which molecules may pass freely from the circulation into the central nervous system (Cottrell and Ferguson, 2004; Maolood and Meister, 2009). It is chemosensitive to toxins in blood, also controls respiratory and renal functions, among others (Willis et al., 2007). Thus, we described the damage in the area postrema capillaries of rat induced by the black-tailed rattlesnake C. m. molossus crude venom.
MATERIAL AND METHODS
Materials
Folin-Ciocalteu´s phenol reagent, bovine serum albumin, acrylamide, bis-acrylamide, TEMED, Coomassie brilliant blue R-250, paraformaldehyde, Sodium hydroxide, sodium borate decahydate, haematoxylin and eosin Y were from Sigma (St. Louis, MO). Sodium carbonate, sodium tartrate, cupric sulphate decahydrate were from J. T. Baker (Center Valey, PA). Sodium duodecyl sulfate, Tris-HCl, ammonium persurfate were from Gibco BRL (Grand Island, NY). Tissue-Tek® OCT was from Sakura Finetek (Torrance, CA)
Venom
Venom was extracted from two female adult C. m. molossus specimens maintained at the Laboratorio de Ecología y Biodiversidad Animal of Universidad Autónoma de Ciudad Juárez. The snakes were allowed to bite into a paraffin membrane over a beaker; the venom was pooled, transferred to 1.5ml microtube, and stored at -20oC. For experiments, the venom was used within four months of the collection. Protein concentration of venom was determined by the method of Lowry et al (1951). Venom proteins were visualized by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) performed on 12% (w/v) polyacrylamide slab gel following the method of Laemmli (1970). Protein bands were observed by Coomassie Brilliant Blue R-250 staining procedure.
Animals
The animals used in this study were 15 female Sprague-Dawley rats (150–180gm body weight) of three month old obtained from animal housing facility of Universidad Autónoma de Ciudad Juárez and maintained at room temperature with food and water ad libitum. Ethical clearance for the study was obtained from the Ethics review committee of the Instituto de Ciencias Biomédicas of Universidad Autónoma de Ciudad Juárez.
Treatments and histopathological examination
Five treatments of three rats were tested, all rats were intramuscularly injected in the lower limb with 100μl of 0.00 (as a control), 0.02, 0.05, 0.10, and 0.20mg/kg of venom dissolved in physiological saline solution. After 24hrs, the animals were sacrificed with an intraperitoneal pentobarbital sodium injection (380mg/kg). The brains were quickly harvested, fixed in paraformaldehyde (4%, v/v, paraformaldehyde, 0.1M sodium borate decahydrate, 0.4%, w/v, sodium hydroxide, pH 9.5) for 24hrs, dehydrated with 30% (w/v) sucrose for 24hrs. The brains were frozen in Tissue-Tek® OCT and cut (10μm) in a cryostat Leica CM1510 S. Three coronal sections of area postrema from each brain were obtained at -13.80 to -14.04mm bregma coordinates (Paxinos and Watson, 2005). Sections were stained with Harris’ Haematoxylin and Eosin (Allen, 1995), and examined with a light microscope Leica DM 2000.
Capillary quantification
Capillaries where quantified from each area postrema section, using three sections per rat and three rats per treatment, considering approximately a 0.18mm2 surface of the structure. Means of capillaries per treatment were calculated and ANOVA test (P < 0.05) was applied.
RESULTS
Venom
In the SDS-PAGE 8 bands were visualized of 113.2, 94.5, 55.1, 40.4, 26.2, 20.2, 13.69 and 11.0kDa (Figure 1).
Figure 1.

SDS-PAGE of C. m. molossus venom. All toxins are indicated on the right side of the gel (arrow heads) and molecular mass marker (MMM) on the left. Samples were separated using 12% (w/v) polyacrylamide gels and stained with Coomassie Brilliant Blue R-250.
Area postrema
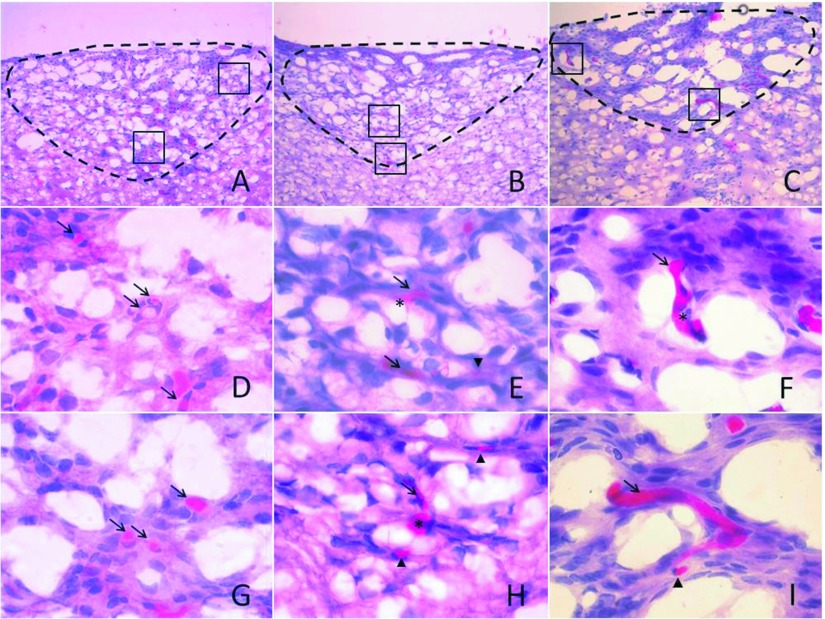
The area postrema showed no histological damage in control, 0.02, and 0.05mg/kg treatments. In the 0.10 and 0.20mg/kg treatments, extravasated erythrocytes and capillary breakdown was induced, and as consequence hemorrhages appeared in both treatments (Figure 2). Hemolysis was observed in the 0.10 and 0.20mg/kg treatments (Figure 3) at peripheral zones of the area postrema such as central canal and the space between the spinal cord and the cerebellum.
Figure 2.
Venom effect in capillaries of the area postrema. Haematoxylin and Eosin staining of 10µm area postrema coronal sections. Control (A, D and G), 0.10mg/kg treatment (B, E, H) and 0.20mg/kg treatment (C, F and I). Area postrema in A, B and C are delimited by a discontinuous line, boxes in A, B and C indicate higher magnifications as shown in D, G; E, H and F, I, respectively. Control show intact capillaries (arrow) only, 0.10 and 0.20mg/kg treatments show capillaries with hemorrhages (asterisk) and extravasated erythrocytes (arrow head). A, B and C were observed in 20x magnification, D, E, F, G, H and I were observed in 100x magnification.
Figure 3.
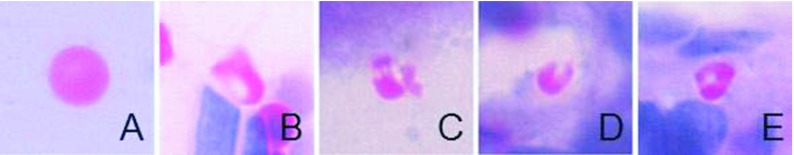
Hemolysis caused by C. m. molossus venom. Undamaged erythrocyte (A) from control, damaged erythrocytes are shown on 0.10 (B) and 0.20 (C, D and E) mg/kg treatments.
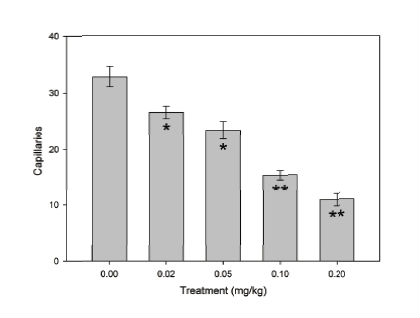
Figure 4 shows the effect of the venom in all capillaries counted per area postrema per treatment. The control treatment showed the maximum quantity of capillaries of all treatments and it significantly decreased (P < 0.05) in response to the venom dose increase, being the 0.20mg/kg dose the treatment with the lower quantity of capillaries. Between 0.02 and 0.05mg/kg treatments no significant difference was observed, as occurred among 0.10 and 0.20mg/kg treatments.
Figure 4.
Effect of the C. m. molossus venom on the quantity of the area postrema capillaries. Bars are mean ±S.E.M. of area postrema capillaries from three coronal sections (n=3 per treatment). 0.02 and 0.05mg/kg treatments showed no significant difference (*) between them, neither between 0.10 and 0.20mg/kg treatments (**) (P < 0.05).
DISCUSSION
Following the results of Mackessy (2010) all the bands of our C. m. molossus venom sample were identified: the 11.0kDa band corresponds to a disintegrin (Scaborough et al., 1993) or a phospholipase A2 (Tsai et al., 2001); the 11.6 and 13.7 are different isomers of phospholipase A2; the 26.2kDa to a PI metalloproteinase (Chen and Rael, 1997; Sánchez et al., 2001); the 40.4kDa to a serine proteinase; the 55.1kDa band to a PIII metalloproteinase; the 94.5kDa band to a L-amino acid oxidase and the 113.13kDa to a nuclease or a phosphodiesterase (Ferlan et al., 1983a).
The capillary damage in the area postrema was progressive in response to the increase of venom dose. The capillary breakdown and hemorrhages showned in 0.10 and 0.20mg/kg treatments could be caused, firstly, by the degradation of basal membrane structural proteins as fibrin, nidogen, laminin and IV collagen by the P-I and P-III metalloproteinases (Sánchez et al., 2001; Escalante et al., 2006; Baldo et al., 2010). These enzymes can generate endothelial cells apoptosis (Díaz et al., 2005; Tanjoni et al., 2005). Secondly, hydrogen peroxide production by the activity of the L-amino acid oxidase could cause a cytotoxic environment for the endothelial cells ending in apoptosis (Guo et al., 2012).
Hemolysis found in the 0.10 and 0.20mg/kg treatments could be caused by phospholipase A2 activity over erythrocyte cell membrane phospholipids (Du et al., 1998). Furthermore, L-amino acid oxidase contributes to hemolysis (Ali et al., 2000); particularly, C. m. molossus venom has been reported as a cause of this effect in vitro (Macias-Rodriguez et al., 2014). The quantitative analysis of area postrema capillaries demonstrates a significant decrease of the number of capillaries as a consequence of the increment of the C. m. molossus venom dose. This event could be originated by the serine proteinase activity over the blood coagulation pathway (Pérez et al., 2007) and P-III metalloproteinase can promote prothrombin activation (Fox and Serrano, 2010). Suggesting that the ischemic stroke described in some medical cases (Thomas et al., 2006; Del Brutto and Del Brutto, 2012) could be caused by this phenomena, not only in the area postrema but in the whole central nervous system. Even though neurotoxic affections are not observed in envenomation by C. m. molossus (Hardy et al., 1982; Yarema and Curry, 2005) probably because only three clinical cases have been reported, but several cerebrovascular complications have been reported by crotalid envenomation (Thomas et al., 2006; Cardoso-Vale et al., 2013; Del Brutto and Del Brutto, 2012).
Morphological damage in the area postrema could generate a physiological alteration probably does not result in a renal failure or a respiratory paralysis, but maybe to predispose these symptoms of the envenomation by Crotalus sp venom (Sarmiento-Acuña, 2012).
CONCLUSIONS
C. m. molossus crude venom causes hemolysis, capillary breakdown, hemorrhages, and reduction in number of capillaries in the area postrema of rats starting from a low venom dose after 24hrs. Even though, affection in the area postrema could not be the main cause of the renal and respiratory dysfunction in ophidic accidents, also it is important to know all the possible venom effects because many of the brain injuries are not reported or underestimate to affecting the patient health.
ACKNOWLEDGEMENTS
This work was supported by Departamento de Ciencias Químico Biológicas through SF-CGIP Animal Origin Toxins project UACJ 2009, CONABIO GT032, and to the SEMARNAT (SGPA/DGVS/04134/11) for the rattlesnakes collection permit.
STATEMENT OF COMPETING INTERESTS
None declared.
REFERENCES
- Ali SA, Stoeva S, Abbasi A, et al. Isolation, structural, and functional characterization of an apoptosis-inducing L-amino acid oxidase from leaf-nosed viper (Eristocophis macmahoni) snake venom. Arch Biochem Biophys. 2000;384:216–226. doi: 10.1006/abbi.2000.2130. [DOI] [PubMed] [Google Scholar]
- Allen T. Hematoxilina y eosina. In: Prophet ED, Mills B, Arrington JB, Sobin LH, editors. Métodos Histotecnológicos. Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América; 1995. pp. 55–60. (Eds) [Google Scholar]
- Alves-da-Silva JA, Oliveira KC, Camillo MA. Gyroxin increases blood-brain barrier permeability to Evans blue dye in mice. Toxicon. 2011;57:162–167. doi: 10.1016/j.toxicon.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Baldo C, Jamora C, Yamanouye N, Zorn TM, Moura-da-Silva AM. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and In Situ hydrolysis. PLoS Negl Trop Dis. 2010;4:e727. doi: 10.1371/journal.pntd.0000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Vale T, Ferreira-Leite A, Ribeiro-da-Hora P, et al. Bilateral posterior circulation stroke secondary to a crotalid envenomation: Case report. Rev Soc Bras Med Trop. 2013;46:255–256. doi: 10.1590/0037-8682-1667-2013. [DOI] [PubMed] [Google Scholar]
- Chen T, Rael ED. Purification of M5, a fibrinolytic proteinase from Crotalus molossus molossus venom that attacks complement. Int J Biochem Cell Biol. 1997;29:789–799. doi: 10.1016/s1357-2725(96)00139-2. [DOI] [PubMed] [Google Scholar]
- Corrigan JJ, Jetter M, Ferlan I. In vitro effect of Crotalus molossus molossus (Blacktail rattlesnake) venom on human platelets, fibrinolysis and fibrinogen. Comparison with C. atrox and C. adamanteus. Toxicon. 1983;1:77–80. [Google Scholar]
- Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Del Brutto OH, Del Brutto VJ. Neurological complications of venomous snake bites: a review. Acta Neurol Scand. 2012;125:363–372. doi: 10.1111/j.1600-0404.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- Díaz C, Valverde L, Brenes O, Rucavado A, Gutiérrez JM. Characterization of events associated with apoptosis/anoikis induced by snake venom metralloproteinase BaP1 on human endothelial cells. Cell Biochem. 2005;94:520–528. doi: 10.1002/jcb.20322. [DOI] [PubMed] [Google Scholar]
- Du XY, Zhong XY, Ruan KC, Wu XF, Zhou YC. Advances in the study of phospholipase A2 from the venom of Agkistrodon halys Pallas. Toxin Rev. 2002;17:15–22. [Google Scholar]
- Escalante T, Shannon JD, Moura-da-Silva AM, Gutiérrez JM, Fox JW. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: a biochemical and immunocytochemical study. Arch Biochem Biophys. 2006;455:144–153. doi: 10.1016/j.abb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ferlan I, Ferlan A, Rusell FE. Purification and characterization of phosphodiesterase from Crotalus molossus molossus venom. Toxicon. 1983a;1:137–140. [Google Scholar]
- Ferlan I, Ferlan A, Capel MS, Russell FE. Isolation and characterization of two phospholipases from Crotalus molossus molossus venom. Toxicon. 1983b;1:129–132. [Google Scholar]
- Fox JW, Serrano SM. Snake venom metalloproteinases. In: Mackessy SP, editor. Handbook of venoms and toxins of reptiles. CRC Press, New York, United States of America, first edition; 2010. pp. 95–113. (Ed) [Google Scholar]
- Guo C, Liu S, Yao Y, Zhang Q, Sun MZ. Past decade study of venom L-amino acid. Toxicon. 2012;60:302–311. doi: 10.1016/j.toxicon.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Hardy DL, Jeter M, Corrigan JJ. Envenomation by the northern blacktail rattlesnake (Crotalus molossus molossus): Report of two cases and the in vitro effects of the venom on fibrinolysis and platelet aggregation. Toxicon. 1982;20:487–493. doi: 10.1016/0041-0101(82)90012-5. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The brain-blood barrier/Neurovascular unit in health and disease. Pharmacol Rev. 2005;57:179–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemos-Espinal JA, Smith HM. Universidad Nacional Autónoma de México, Mexico city, Mexico, first edition; 2007. Anfibios y reptiles del estado de Chihuahua, México. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luna-Bauza MM. Bases para el tratamiento por intoxicación por veneno de serpiente. Revista de la facultad de medicina de la UNAM. 2007;50:1–4. [Google Scholar]
- Macias-Rodríguez EF, Martínez-Martínez A, Gatica-Colima A, Bojórquez-Rangel G, Plenge-Tellechea LF. Análisis comparativo de la actividad hemolítica entre las subespecies Crotalus molossus molossus y Crotalus molossus negriscens . Revista Bio Ciencias. 2014;2:302–312. [Google Scholar]
- Mackessy SP. The field of reptile toxinology. Snakes, lizards, and their venoms. In: Mackessy SP, editor. Handbook of Venoms and Toxins of Reptiles. CRC Press, New York, United States of America, first edition; 2010. pp. 3–23. (Ed) [Google Scholar]
- Maolood N, Meister B. Protein components of the blood-brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitaries region. J Chem Neuroanat. 2009;37:182–195. doi: 10.1016/j.jchemneu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Paxinos W, Watson C. The rat brain in stereotaxic coordinates. Elsevier academic press, Boston, USA, fifth edition; [Google Scholar]
- Perez JC, McKeller MR, Pérez JC, Sánchez EE. An internet database of crotaline venom found in the United States. Toxicon. 2001;39:621–632. doi: 10.1016/s0041-0101(00)00186-0. [DOI] [PubMed] [Google Scholar]
- Pérez AV, Saravia P, Rucavado A, et al. Local and systemic pathophysiological alterations induced by a serine proteinase from the venom of the snake Bothrops jararacussu. Toxicon. 2007;49:1063–1069. doi: 10.1016/j.toxicon.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Perumal Samy R, Gopalakrishnakone P, Bow H, Puspharaj PN, Chow VT. Identification and characterization of a phospholipase A2 from the venom of the Saw-scaled viper: Novel bactericidal and membrane damaging activities. Biochemie. 2010;92:1854–1866. doi: 10.1016/j.biochi.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Rael ED, Martinez M, Molina O. Isolation of a fibrinolytic protease, M4, from venom of Crotalus molossus molossus (northern black tail rattlesnake) Haemostasis. 1992;22:41–49. doi: 10.1159/000216290. [DOI] [PubMed] [Google Scholar]
- Rebahi H, Nejmi H, Abouelhassan T, Hasni K, Samkaoui MA. Severe envenomation by Cerastes cerastes viper: An unusual mechanism of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:169–72. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Acosta A, Monterrey F, Céspedes G, Finol HJ. Alteraciones estructurales y ultraestructurales del encéfalo ocasionados por veneno de la serpiente mapanare (Bothrops colombiensis) Revista de Toxicología. 2003;20:199–203. [Google Scholar]
- Ruiz de Torrent RM, Bongiovanni B, Leiva LC, et al. Neurotoxicological effects of a thrombin-like enzyme isolated from Crotalus durissus terrificus venom (preliminary study) Toxicon. 2007;50:144–152. doi: 10.1016/j.toxicon.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Soliz LA, Ramírez MS, Pérez JC. Partial characterization of a basic protein from Crotalus molossus molossus (northern blacktail rattlesnake) venom and production of a monoclonal antibody. Toxicon. 2001;39:523–537. doi: 10.1016/s0041-0101(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Galá JÁ, Pérez JC, et al. The efficacy of two antivenoms against the venom of North American snakes. Toxicon. 2003;41:357–365. doi: 10.1016/s0041-0101(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Sarmiento-Acuña K. Aspectos biomédicos del accidente ofidico. Univ. Med. Bogota. 2012;53:68–85. [Google Scholar]
- Scaborough RM, Rose JW, Naughton MA, et al. Characterization of the integrin specificities of disintegrins isolated from American pit vipers venoms. J Biol Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- Silva A, Gunawardena P, Weilgama D, Maduwage K, Gawarammana I. Comparative in-vitro toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Crotalinae: Hypnale) BMC Res Notes. 2012;29:471. doi: 10.1186/1756-0500-5-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto JG, Perez JC, Lopez MM, et al. Comparative enzymatic study of HPLC-fractionated Crotalus venoms. Comp Biochem Physiol. 1989;93:847–855. doi: 10.1016/0305-0491(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Tanjoni I, Weinlich R, Della-Casa MS, et al. Jararhagin, a snake venom metalloproteinase, induces a specialized form of apoptosis (anoikis) selective to endothelial cells. Apoptosis. 2005;10:851–861. doi: 10.1007/s10495-005-2945-1. [DOI] [PubMed] [Google Scholar]
- Thomas L, Chausson N, Uzan J, et al. Thrombotic stroke following snake bites by the “Fer-de-lance” Bothrops lanceolatus in Martinique despite antivenom treatment: A report of three recent cases. Toxicon. 2006;48:23–28. doi: 10.1016/j.toxicon.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Tsai IH, Chen YH, Wang YW, Tu MC, Tu AT. Purification, sequencing, and phylogenetic analyses of novel Lys-49 phospholipases A2 from the venoms of rattlesnakes and other pit vipers. Arch Biochem Biophys. 2001;394:236–244. doi: 10.1006/abbi.2001.2524. [DOI] [PubMed] [Google Scholar]
- Willis CL, Garwood CJ, Ray DE. A size selective vascular barrier in the rat area postrema formed by perivascular macrophages and the extracellular matrix. Neuroscience. 2007;150:498–509. doi: 10.1016/j.neuroscience.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Yarema MC, Curry SC. Envenomation by the northern blacktail rattlesnake (Crotalus molossus molossus): Case repot. Pediatr Emerg Care. 2003;21:40–42. doi: 10.1097/01.pec.0000150989.03981.06. [DOI] [PubMed] [Google Scholar]