Summary
Myosin interacting-heads (MIH) motifs are visualized in 3D-reconstructions of thick filaments from striated muscle. These reconstructions are calculated by averaging methods using images from electron micrographs of grids prepared using numerous filament preparations. Here we propose an alternative method to calculate the 3D-reconstruction of a single thick filament using only a tilt series images recorded by electron tomography. Relaxed thick filaments, prepared from tarantula leg muscle homogenates, were negatively stained. Single-axis tilt series of single isolated thick filaments were obtained with the electron microscope at a low electron dose, and recorded on a CCD camera by electron tomography. An IHRSR 3D-recontruction was calculated from the tilt series images of a single thick filament. The reconstruction was enhanced by including in the search stage dual tilt image segments while only single tilt along the filament axis is usually used, as well as applying a band pass filter just before the back projection. The reconstruction from a single filament has a 40 Å resolution and clearly shows the presence of MIH motifs. In contrast, the electron tomogram 3D-reconstruction of the same thick filament –calculated without any image averaging and/or imposition of helical symmetry- only reveals MIH motifs infrequently. This is –to our knowledge- the first application of the IHRSR method to calculate a 3D reconstruction from tilt series images. This single filament IHRSR reconstruction method (SF-IHRSR) should provide a new tool to assess structural differences between well-ordered thick (or thin) filaments in a grid by recording separately their electron tomograms.
Keywords: 3D-reconstruction, electron microscopy, electron tomography, myosin interacting-heads (MIH) motif, negative staining, muscle thick filament
1. Introduction
Striated muscle consists of overlapping arrays of thick (myosin-containing) and thin (actin-containing) filaments. The contraction of muscle occurs when both sets of filaments slide relative to each other to produce sarcomere shortening (Huxley, 1969). This process is regulated by molecular switches located on either the thin filaments (troponin/tropomyosin) or the thick filaments, usually in the regulatory light chain of the myosin. To advance towards the understanding of the molecular mechanism of the myosin-linked regulation of muscle contraction, the determination of the structure of the myosin thick filaments is a requirement. The thick filaments form by tight packing of the long C-terminal, α-helical coiled-coils of the myosin heavy chains. The N-terminal domains of each heavy chain form a globular head, which contains the actin binding and ATPase activities. The tails pack together forming the backbone of the thick filament, and the myosin heads protrude from the backbone surface, often as an organized helical array (Craig and Padrón, 2004).
The structure of the thick filaments has been studied by X-ray diffraction (Huxley and Brown, 1967) which revealed the helical organization of the myosin heads on the surface of the thick filament. Initial structural studies by electron microscopy of negatively stained thick filaments (Huxley, 1963) revealed that the thick filaments were bipolar, with a central bare zone naked of myosin heads and disordered heads protruding on both sides. The improvement of the negative staining technique preserved helices of myosin heads in Limulus (Stewart et al., 1981), tarantula (Crowther et al., 1985) and scorpion (Kensler et al., 1985) thereby facilitating calculation of Fourier-Bessel 3D image reconstructions of the thick filaments (Stewart et al., 1981; Crowther et al., 1985; Kensler et al., 1985). However the resolution achieved (50 Å) was insufficient to resolve individual heads of each myosin molecule.
Rapidly freezing thick filaments and observing them frozen-hydrated in the cryoelectron microscope at low electron dose can achieve higher resolution. However, due to problems separating overlapping Bessel functions when using Fourier-Bessel 3D-reconstruction procedures on such low contrast specimens (Padrón and Alamo, 2004), the improved specimen preservation could not be fully used to resolve both myosin heads. It was a different approach, the Iterative Helical Real Space Reconstruction (IHRSR) method (Egelman, 2000) that finally allowed individual myosin heads to be resolved in 3-D from electron micrographs of frozen-hydrated thick filaments (Woodhead et al., 2005). The two heads of each myosin molecule were found to assume an asymmetric structure we call the myosin interacting-heads (MIH) motif, similar to the one found in myosin 2D-crystals (Wendt et al., 1999; Wendt et al., 2001; Liu et al., 2003). This motif has been detected by cryo-electron microscopy (EM) in Limulus and scallop (Zhao et al., 2009; Woodhead et al., 2013) and by negatively staining in mouse cardiac, scorpion and human cardiac (Zoghbi et al., 2008; Pinto et al., 2012; AL-Khayat et al., 2013) muscle thick filaments. A modification of the IHRSR method that takes into account the out-of-plane angle of the filaments (i.e. the angle that the filament made with the grid plane), increased the number of image segments that could be included in the reconstruction, and achieved a resolution of 20 Å (Alamo et al., 2008). The use of this modification together with band-pass filtering have facilitated detection of MIH motifs in scorpion thick filaments preserved using negative staining instead cryo-EM (Pinto et al., 2012).
This IHRSR 3D-reconstruction approach requires the recording of numerous electron micrographs from many different thick filament preparations imaged from several grids. Therefore the reconstructed MIH motifs are the average of potentially multiple different head conformations distributed on different thick filaments. Here we have recorded a tilt series of a single thick filament by electron tomography and enhanced the IHRSR method to calculate a 3D-reconstruction from it. This method successfully made possible to obtain a 3D-reconstruction from a single thick filament and validates the conclusion that the MIH motifs are the dominant structure on these relaxed thick filaments.
2. Materials and methods
Solutions
Relaxing solution contained 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 5 mM PIPES, 5 mM NaH2PO4, 1 mM NaN3 and 5 mM Mg.ATP, pH 7.0. The permeabilizing solution consisted of relaxing solution containing 0.1 % (w/v) saponin.
Preparation of filament suspensions from tarantula striated muscle
Tarantulas (Gramostola rosea) were obtained from Carolina Biological Supply (Burlington, NC). Leg muscles were permeabilized for 3 hours in relaxing-saponin solution and then washed for 1 hour in relaxing solution. Saponin permeabilized muscle was homogenized for ~1 sec in 3 ml of relaxing solution at setting of 5 on a Polytron homogenizer (Kinematica AG, Luzern, Switzerland). The homogenate was centrifuged at 15,000g (11,500 RPM) on a JA-20 rotor in an Eppendorf model 5415 D centrifuge (Eppendorf, Hauppauge, NY) for 2 min in a 4° C cold room to remove large debris. The supernatant containing the thick filaments was stored and used the same day. The relaxed state of the thick filaments as judged by their helical order was not easily obtained. The best pH for getting relaxed state was 6.8. Most of the negative stained preparations were disordered.
Electron tomography
A thick filament suspension was applied to glow discharged holey carbon grids with a thin carbon film suspended over the holes and negatively stained with 2% uranyl acetate. Conventional single-axis tilt tomographic series (total accumulated electron dose ~ 473 e−/A2 for 66 tilt images) were obtained with a FEI CM-120 electron microscope (FEI Company, Eindhoven, The Netherlands) using a 3° Saxton scheme (Saxton et al., 1984) from +69.6° to −69.3° and recorded on a 2K TVIPS CCD camera (Tietz Video and Image Processing Systems GmbH, Gauting, Germany) at a 8.5 µm defocus. The step size starts at 0.3° at the high positive tilt angles and 1.1° for the negative ones. The pixel size with respect to the original specimen is 0.668 nm. The tilt series was aligned using marker-free alignment and tomograms calculated by weighted back projection using the Protomo software package (Winkler and Taylor, 2006; Winkler, 2007).
Single filament IHRSR (SF-IHRSR) 3D reconstruction method and validation
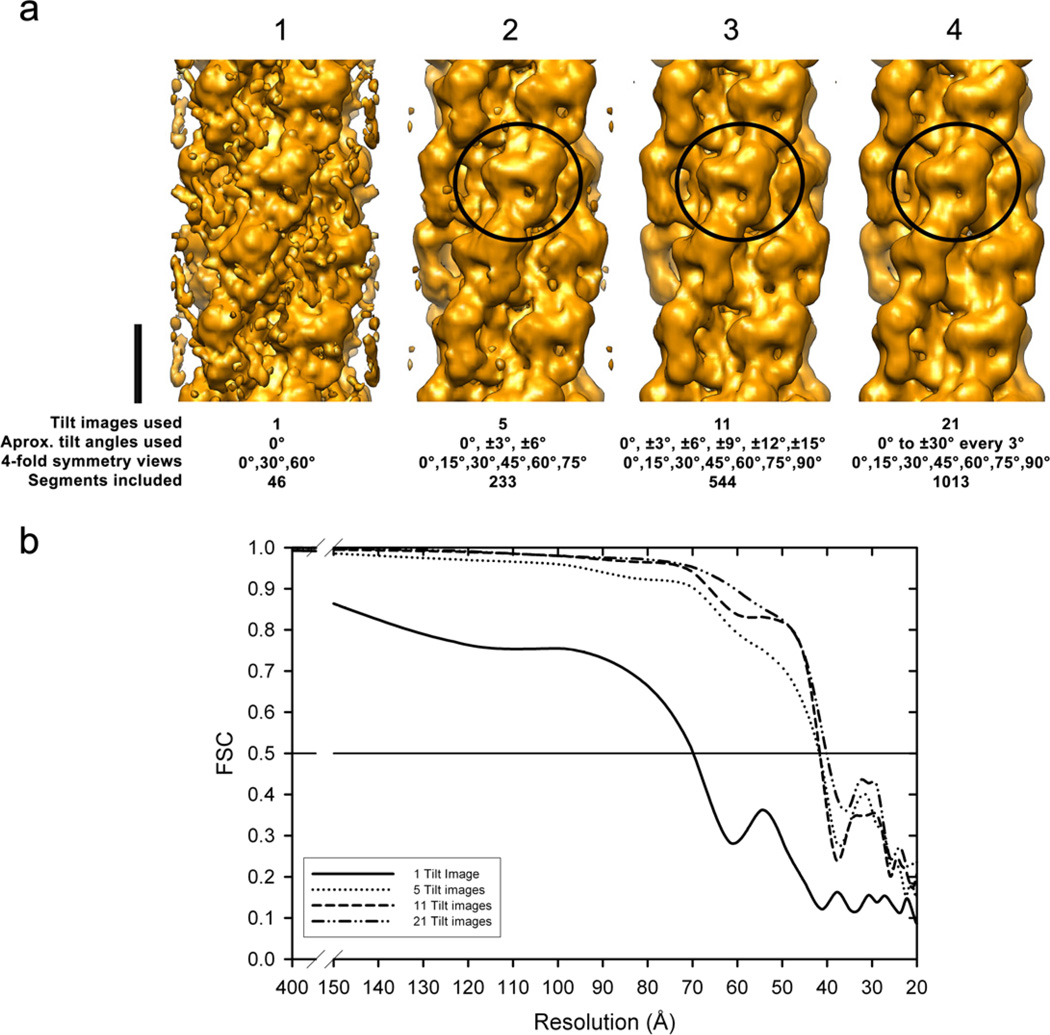
We have enhanced the IHRSR method (Egelman, 2000) to fully reconstruct a single myosin thick filament from images within a tilt series. This “single filament IHRSR” 3D reconstruction method (“SF-IHRSR” method, Fig. 2) consists of: (1) collecting a single tilt series of images from a half of one single thick filament using electron tomography instead of collecting many electron micrographs from different thick filaments from many grids; (2) calculating an IHRSR 3D-recontruction from this tilt series of images by including in the search stage additional image segments with out-of-plane angles which are not normally included (Alamo et al., 2008), as well as applying a band pass filter just before the back projection (Pinto et al., 2012). The SF-IHRSR method was implemented in a SPIDER environment (Frank et al., 1996) and the authors can provide the SPIDER script if required. The IHRSR 3D-reconstruction shown in Fig. 4a was computed using only a subset of 21 images from −28.9° to +28.9° (Supplementary movie 1) of a well preserved region of one half of the filament. The 0° image is shown in Fig. 1a. The minimum filament tilt images required to obtain a reasonable reconstruction is 11 (Fig. 3a, c) corresponding to a ~ 30° tilt, this covers 0° to 90° rotational angles required by 4-fold symmetry in 3 crowns. For the SF-IHRSR 3D-reconstruction an arbitrary initial reference was used (Yang et al., 2003; Egelman, 2007). Supplementary Fig. 1c shows that the number of segments required for a stable IHRSR reconstruction was achieved in less than 10 cycles. After 40 cycles, 1013 segments from a total of 1673 were enough to obtain a resolution of 40 Å. In Fig. 3 it is shown the effect of reducing the number of images from the tilt series included in the SF-IHRSR 3D-reconstruction. It is seen that to obtain an informative 3D-reconstruction requires at least 5 tilt images (Fig. 3a). In contrast, the IHRSR 3D-reconstruction from one image of a single thick filament tilt series was very noisy (Fig. 3a). The Fourier Shell Correlation (FSC) plots of these IHRSR 3D-maps (Fig. 3b) show the increasing resolution achieved with the number of tilt images included in the 3D-reconstruction. To calculate the FSCs we used the SPIDER operator "BP 32F" that randomly splits the data sets from 21 tilt series images in two halves and calculate the corresponding volume map, then used the operator "FSC" to calculate the plot. Even though these two data sets were not completely independent (van Heel, 1987; Chen et al. 2013; Penczek, 2010) the low res map (40 Å) agrees with previous reconstructions using independent data sets (Pinto el al., 2012)
Figure 2.
The single filament IHRSR 3D reconstruction method (SF-IHRSR). This figure, based on the IHRSR method (Egelman, 2000), highlights the three modifications we have implemented: (1) generating out-of-plane angle and Y shift projections (Alamo et al., 2008) instead of only azimuthally rotation projections (i.e. 4095 instead of 90 reference projections); (2) using a subset of the tilt series images from an electron tomogram of a single filament instead of using thousands of segments from many thick filaments; and (3) applying a Fermi band pass filter to each image before the back projection (Pinto et al., 2012). The Egelman programs and Spider operators used in each case are enclosed between parenthesis and square brackets respectively.
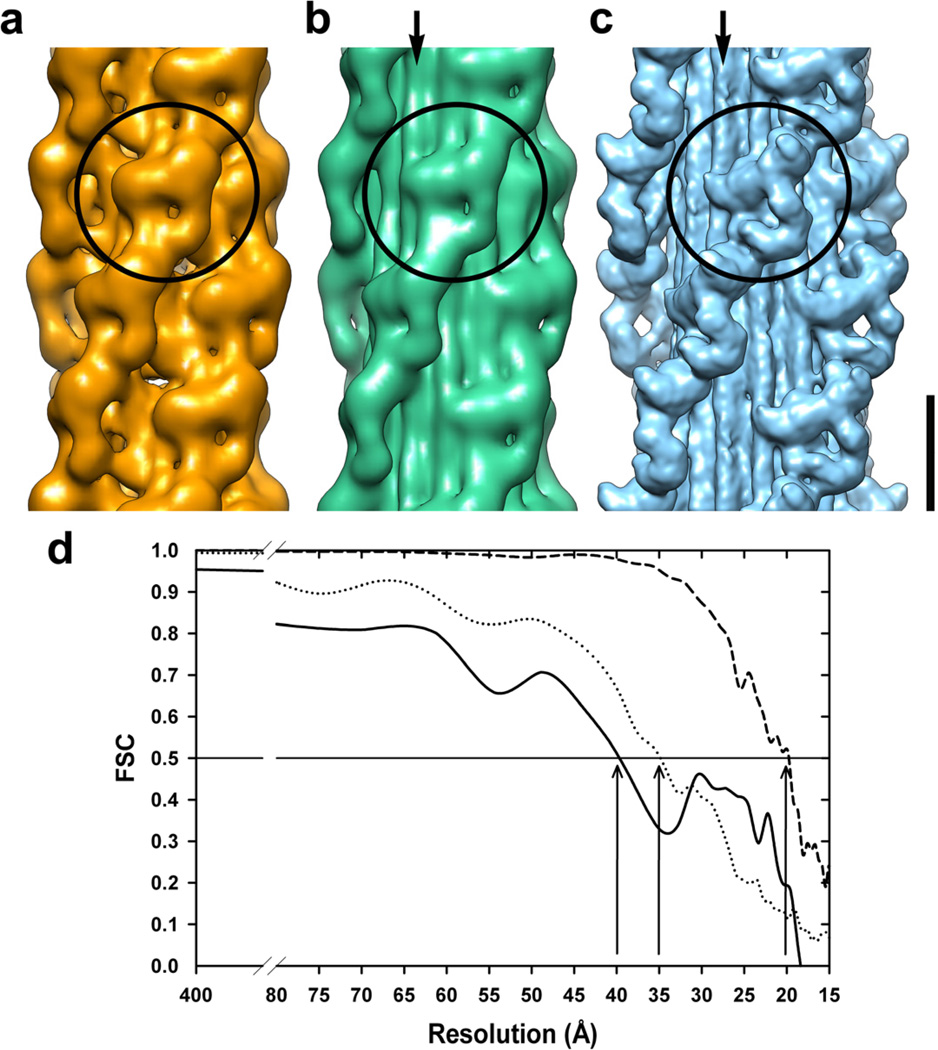
Figure 4.
A comparison of the SF-IHRSR 3D-reconstruction (a) calculated from an image subset of the electron tomographic series corresponding to Fig. 1b with the IHRSR 3D-reconstructions from several thick filaments either (b) negatively stained (Pinto et al., 2012) or (c) frozen hydrated (EMD-1535, (Alamo et al., 2008)). The circles indicate the volumes corresponding to a MIH motif, and the arrows show the subfilaments. Bar 14.5 nm. (d) The Fourier shell correlation (FSC) plots of the 3D-maps shown in a–c calculated from the electron tomographic series of one negatively stained thick filament (solid line), several negatively stained (dotted line) or frozen-hydrated (dashed line) thick filaments. According to the FSC = 0.5 criteria, the achieved resolutions (vertical arrows) are similar for both negatively stained IHRSR 3D-reconstructions (40 and 35 Å), but much lower than for the frozen-hydrated one (20 Å).
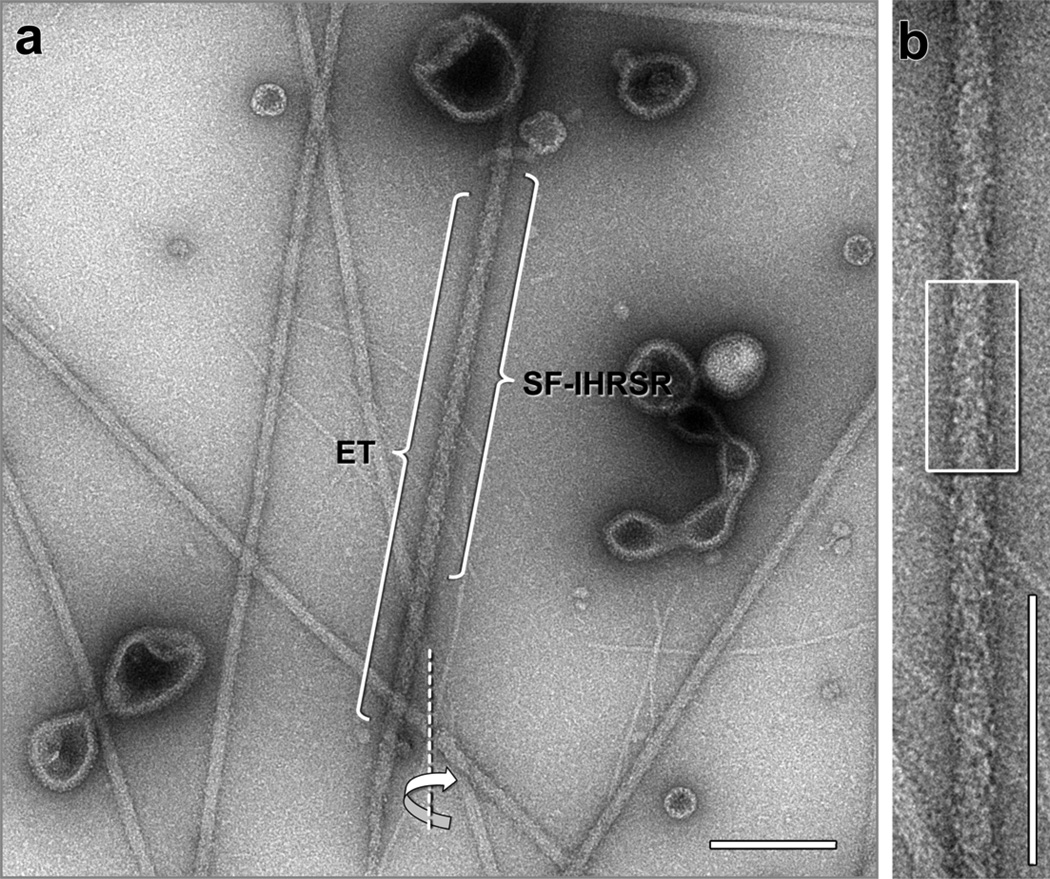
Figure 1.
(a) Electron micrograph showing several negatively stained thick filaments from tarantula muscle. An electron tomographic tilt series was recorded from the thick filament labeled “ET” along a tilt axis (dotted line). The tip of this filament is at the bottom and its bare zone is towards the top (not shown). The image shown corresponds to the 0° tilt image of the tomogram. A single filament IHRSR (SF-IHRSR) 3D-reconstruction was calculated from a subset of the tilt series of the same filament (labeled SF-IHRSR), enlarged in (b). Alternatively, a tomographic 3D-reconstruction was calculated for this thick filament using the tilt series images (Fig. 6a). The box in “b” also corresponds to the enlarged view shown in the Fig. 6a (bracket), Bare zone at the top. Bars 200 nm.
Figure 3.
Effect of the number of tilt images (1, 5, 11 and 21) from the tilt series images used for the SF-IHRSR 3D-reconstructions (a). Bar 14.5 nm. In (b) are the corresponding calculated Fourier shell correlation (FSC) plots for these four reconstructions. The myosin interacting-heads (MIH) motifs are clearly resolved (circles) when 5 or more tilt images are used (resolution 40 Å), while only a hint of the motif is seen when only one tilt image is used (resolution 70 Å).
In Fig. 4 we compare this SF-IHRSR 3D-reconstruction calculated for one filament (Fig. 4a) with a multiple filament IHRSR 3D-reconstruction obtained either from negative stained (Fig. 4b, (Pinto et al., 2012)) or frozen-hydrated (Fig 4c, (Alamo et al., 2008)). The multiple negatively stained filament IHRSR 3D-reconstruction was calculated with the same overlapping and IHRSR 3D-reconstruction parameters used in the single filament case (Fig. 4a) but using 2569 segments from a total of 3709 obtained from 31 filaments digitized at 0.662 nm/pixel from 11 electron micrographs of negatively stained thick filaments (Fig. 4b, (Pinto et al., 2012)). The multiple frozen-hydrated filament IHRSR 3D-reconstruction was calculated similarly but from a total of 10,700 segments obtained from 15,504 filaments segments digitized at 0.248 nm/pixel from 1008 frozen-hydrated thick filament halves (Fig. 4b, (Alamo et al., 2008)). The FSC plots of the IHRSR 3D-maps shown in Fig. 3b and 4d were calculated for several negatively stained or frozen-hydrated thick filaments from two independent data sets as usually done.
3. Results and Discussion
We took advantage of the very well ordered thick filaments from tarantula striated muscle, to interpret the structural information available on the tilt series images of the electron tomograms from negatively stained thick filaments.
3.1 Electron tomogram of a single thick filament
The electron micrographs of negatively stained thick filaments from tarantula striated muscle reveal helical tracks of myosin heads (Fig. 1) (Crowther et al., 1985). A well-preserved and ordered thick filament half was chosen for recording an electron tomographic series (ET, Fig. 1a). The tilt series images reveal similar features along the thick filament (Supplementary movie 1).
3.2 SF-IHRSR reveals MIH motifs
The 3D-reconstruction calculated using the SF-IHRSR method from a subset of images of the tomographic series is shown in Fig. 4a. According to the FSC plot (0.5 criteria) this 3D-map has a resolution of 40 Å (Fig. 4d). The 3D-map shows 4 helices of detailed features and demonstrates that it is possible to obtain an informative 3D-reconstruction from only a single thick filament. This 3D-map clearly resolves four helices of MIHs (Fig. 4a, circle).
In Fig. 4 we compare the SF-IHRSR 3D reconstruction (Fig. 4a) with the IHRSR 3D-map calculated from several negatively stained (Fig. 4b) or frozen-hydrated (Fig. 4c) tarantula thick filaments. The Fourier Shell Correlation (FSC) curves of the negatively stained and frozen-hydrated specimens are shown in Fig. 4d. The 3D-map resolution (40 Å) from the electron tomogram of a single negatively stained thick filament (Fig. 4d) is lower than the one achieved under similar amount of data and acquisition settings from many negatively stained (35 Å) or frozen-hydrated (20 Å) thick filaments. However, the contour of the MIH motifs seen in the SF-IHRSR 3D-map from the electron tomographic series of a single thick filament (circle, Fig. 4a) is similar to the one seen in the IHRSR 3D-reconstructions calculated using several thick filaments (circles, Fig. 4b,c), showing clearly the MIH motif but at low resolution and lacks the 12 subfilaments feature in the backbone of the higher resolution IHRSR 3D-reconstructions (cf. Fig. 4a with b,c, arrows). Failure to resolve the subfilaments in this case could be due to the lower resolution and the smaller number of segments used in the SF-IHRSR 3D-map from the electron tomographic series (see Material and methods).
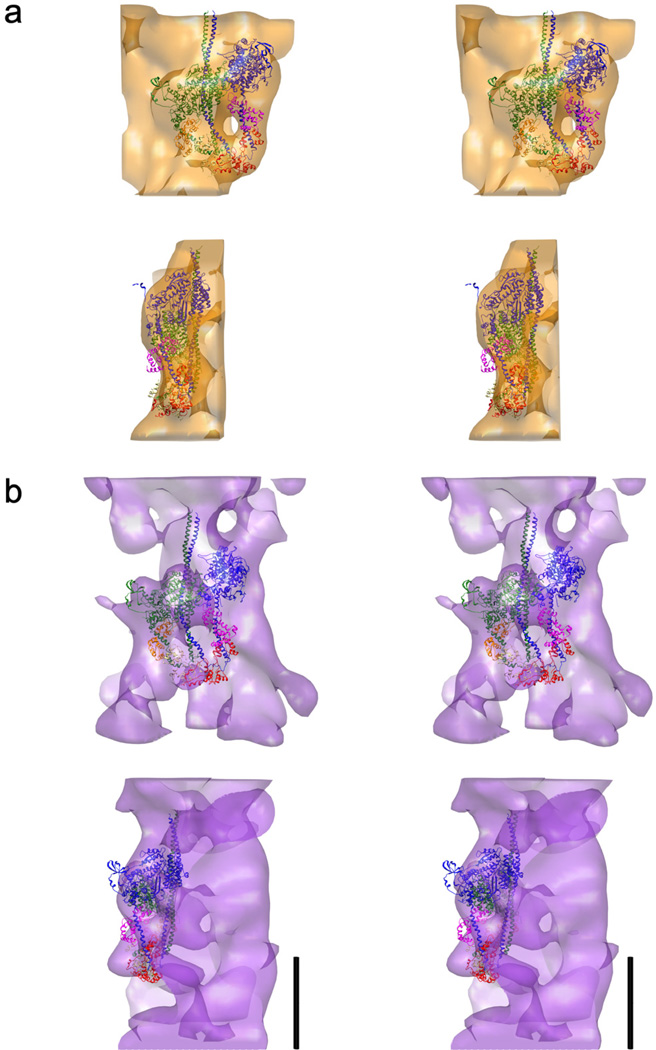
The atomic fitting of the PDB: 3DTP MIH motif atomic model (Alamo et al., 2008) to the SF-IHRSR 3D-map from a single negatively stained thick filament electron tomographic series is shown in Fig. 5a and Supplementary movie 2. The fitting reveals that the features in the surface of the SF-IHRSR 3D-map correspond to the MIH motifs, as was demonstrated before (Woodhead et al., 2005; Alamo et al., 2008; Pinto et al., 2012) (Supplementary movie 2).
Figure 5.
Wide-eye stereo pairs of (a) the SF-IHRSR 3D-reconstruction and (b) the tomographic 3D-reconstruction of a single thick filament. The MIH motif structure (PDB: 3DTP, (Alamo et al., 2008) fits very well to the SF-IHRSR 3D-reconstruction, as seen in the front (top) and lateral (bottom) views in (a). In contrast, the fitting to the tomographic 3D-reconstruction (b) is less good. Bars 14.5 nm for (a) and (b).
The enhanced method described here to calculate the SF-IHRSR 3D-reconstruction from a subset of images from a single electron tomographic series (see Material and methods) allows an independent assessment of the myosin head arrangement present in a subset of the images of the tomographic tilt series. We conclude that the 3D-reconstruction obtained by the SF-IHRSR method from tilt series images of one stained myosin thick filament clearly reveals the presence of the MIH motifs as reported for frozen-hydrated tarantula thick filaments (Woodhead et al., 2005; Alamo et al., 2008).
3.3 Can the MIH motifs be visualized directly in the electron tomographic 3D-reconstruction?
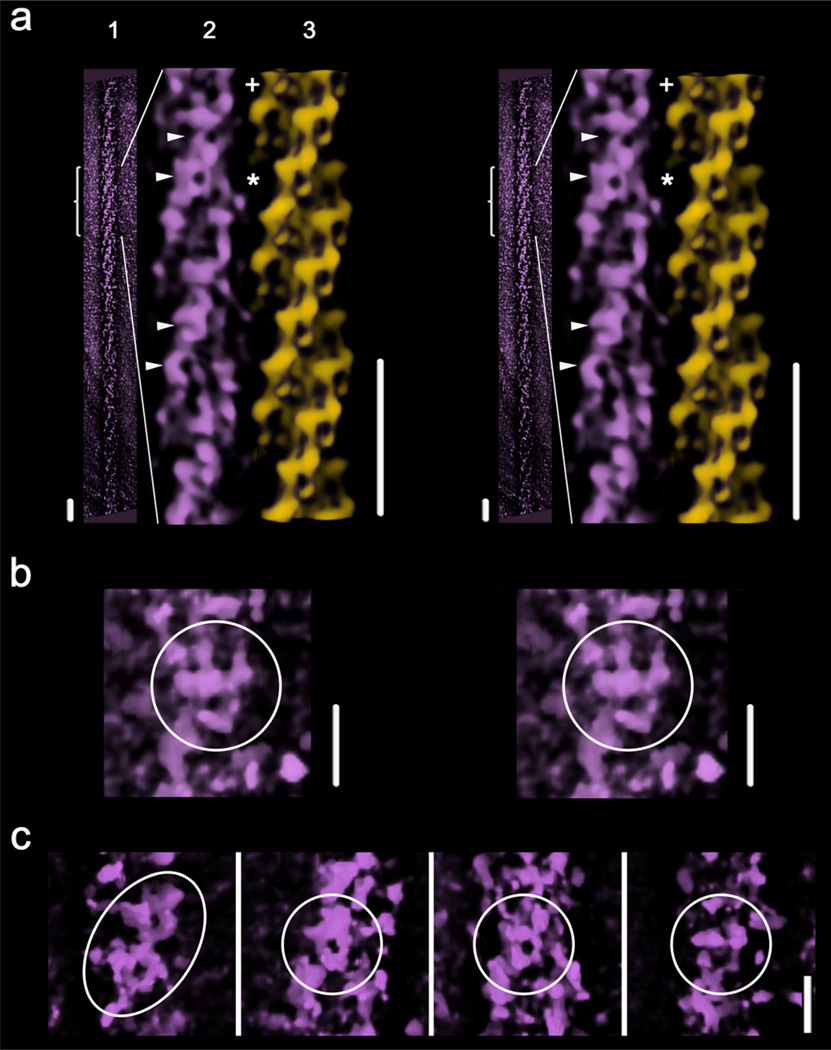
The outer shell of the electron tomographic 3D-reconstruction of a thick filament shows oblique helical tracks (Fig. 6a-1). We used the IHRSR 3D-reconstruction (Fig. 4a) and fitting (Fig. 5a, Supplementary movie 2, top) of the MIH motif to interpret the tomographic reconstruction (Fig. 6a3 vrs 6a2). After inspecting different segments along this tomographic reconstruction (Fig. 6a-1) we found that shapes which approximately resemble the MIH motif were preserved in regions of the top half of the tomogram (bracket, Fig. 6a-1, 6a-2, Supplementary movie 3). For instance, a shape is seen in the tomographic reconstruction (Fig. 6a-2 asterisk) as compared with the SF-IHRSR 3D-reconstruction (Fig. 6a-3, asterisk). These MIH motifs are only resolved in some well preserved regions of the tomogram, like in the region labeled “+” in Fig. 6a-2 (enlarged in Fig. 6b, Supplementary movie 3) and the one pointed by the four arrowheads (enlarged in Fig. 6c). This is probably due to the fact that the filament is not strictly straight along the plane of the grid and there are probably also some differences in the thickness of the sample along the grid (see Supplementary movie 1). It was possible to approximately fit the 3DTP model into the motif shape featured on the electron tomographic 3D-reconstruction as show in Fig. 5b (Supplementary movie 2, bottom).
Figure 6.
Wide-eye stereo views of (a): (1) the near outer shell of the tomographic 3D-reconstruction of one thick filament; (2) an enlarged view of (1), filtered and cylindrically masked (box, Fig. 1b); and (3) an equivalent segment of the SF-IHRSR 3D-reconstruction calculated from the tomogram images subset (Fig. 4a). The asterisk points the MIH motif used to fit the 3DTP model into the tomographic 3D-reconstruction shown in Fig. 5b and Supplementary movie 2. Bars 43.5 nm. The near outer shell of the tomogram reveals in some places shapes (labeled with 4 arrowheads and a “+”) which approximately resemble the MIH motif. The one labeled with a “+” is best seen after left hand rotation of the region by ~ 21° as shown in the enlarger wide-eye stereo view (b) (see Suppl. movie 3). A panel of several shapes (labeled with arrowheads Fig. 6a) is shown in (c). Bars 14.5 nm for (b) and (c)
The presence of shapes resembling the MIH motif in Fig. 6b,c in a electron tomographic 3D-reconstruction obtained without any image averaging or imposition of helical symmetry demonstrates that these motifs are sometimes directly visible in well-preserved regions of the filament tomogram itself. It seems clear that the MIH motif is such a prominent feature of the thick filament that its shape is also seen in the best-preserved regions of the single filament tomographic reconstruction. We conclude that the MIH motifs that form the oblique track features can be sparingly observed directly in the outer shell of the electron tomographic reconstruction (Fig. 6a-2, Supplementary movie 3).
3.4 Possible applications for the proposed approach
Detection of differences in the myosin heads arrangement
The SF-IHRSR method could be used for determining differences in the arrangement of the myosin heads in different thick filaments present in a grid by recording separately their tomograms, or alternatively it could be used to choose different regions along a single thick filament to record their tomograms. For instance, it could be associated with the rapid negative staining technique (Zhao and Craig, 2003) or rapid freezing (Hidalgo et al., 2001; Woodhead et al., 2005) to quickly trap structural changes on the MIH motifs due to the activation of thick filaments, as for instance, capturing the release of the swaying free heads in the relaxed state (Brito et al., 2011; Sulbarán et al., 2013). The method could be also used to study the structure of thick filaments in which the MIH motifs have differences in their regulatory domains, i.e. the scorpion and Limulus striated muscle. In scorpion, a 33 Å resolution IHRSR 3D-reconstruction has shown that its thick filaments have four helices of MIH motifs (Pinto et al., 2012). The region of the myosin regulatory light chains (RLCs) in scorpion is different from the corresponding one in tarantula. This is probably due to the fact that tarantula myosin has only one highly expressed 26 KDa RLC ((Craig et al., 1987), cf. (Zhu et al., 2009)) whereas scorpion myosin has two (16 and 27 KDa) RLCs (Linarez, 2002). Therefore in tarantula all MIH motifs are identical (i.e. containing two 26 KDa RLCs) while in scorpion the MIH motifs could be either identical (with only 16 KDa or 27 KDa RLCs present on each MIH motif), hybrid (with 16 KDa RLC on the free head and 27 KDa on the blocked head, or vice versa) or randomly distributed. IHRSR 3D-reconstructions from different electron tomographic series recorded from different single scorpion thick filaments could help clarify this point.
The visualization of frozen-hydrated tarantula thick filaments by using a 300KV FEG cryo-electron microscope and the method presented here would provide a higher resolution IHRSR 3D-reconstruction. This approach would enable –for instance-visualization of the myosin tails in each of the 12 subfilaments vs detecting only the subfilament envelope (Woodhead et al., 2005). It could also help resolve any asymmetric features along the thick filament.
The SPIDER script to implement the SF-IHRSR method from a tilt series images described in this paper may be obtained from the authors.
5. Conclusions
The SF-IHRSR method allows calculating the 3D-reconstruction for the tilt image series of a single filament obtained by electron tomography.
The MIH motif is present in the single filament IHRSR (SF-IHRSR) 3D-reconstruction calculated from the electron tomographic series of a single thick filament of tarantula striated muscle.
The electron tomographic 3D-reconstruction of a single tarantula striated muscle thick filament reveals directly the presence of shapes resembling the MIH motif only very scarcely in the best preserved regions.
The proposed method – applicable to both negative stained and frozen-hydrated samples - may be used for visualizing endogenous or bound proteins in the thick filament, as well as for the detection of differences in the myosin heads arrangement.
Supplementary Material
Parameters of the SF-IHRSR 3D-reconstruction calculation for a single (filled circles), or several negatively stained (open circles): (a) Rotation angle vs. cycle number, (b) Axial repeat vs. cycle number, (c) Number of segments included in each cycle.
Electron tomograms tilt series of a negatively stained tarantula thick filament (Fig. 1a shows the 0° tilt image). This movie was produced from a subset of 21 images from the tilt series aligned using the IMOD package version 4.5 (The Boulder Laboratory for 3-D Electron Microscopy of Cells) (Kremer et al., 1996) and the NIH ImageJ package.
The SF-IHRSR (top) and tomographic (bottom) 3D-reconstructions calculated from a single thick filament showing the best fitting of the MIH structure PDB: 3DTP (Alamo et al., 2008) for each case.
Front outer shell of the tomographic 3D-reconstruction of a negatively stained tarantula thick filament (“1”, see Fig. 6a-1) and the equivalent part (“2”, see Fig. 6a-2) of the filtered tomographic 3D-reconstruction and the SF-IHRSR 3D-reconstruction of a single thick filament (“3”, see Fig. 6a-3). The good fitting of one MIH structure PDB: 3DTP (Alamo et al., 2008) is shown in “1” and “3”. The movie corresponds to the region labeled “+” in Fig. 6a-2. The rigid docking of 3DTP to both 3D-reconstructions was done automatically with the Chimera "Fit in Map" module.
Acknowledgements
Electron microscopy was carried out at the Biological Science Imaging Resource of the Department of Biological Science, Florida State University. Molecular graphics images (Figs. 3a, 4 and 5, and supplementary movies 2 and 3) were produced using the UCSF Chimera package (Pettersen et al., 2004) from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081). This work was supported in part by NIH grants U54 GM64346 to the Cell Migration Consortium, R01 AR47421 (to K. T.) and Howard Hughes Medical Institute (HHMI), U.S.A. (to R.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data.
Supplementary data associated with this article can be found, in the online version, at http:://
Contributor Information
G. Márquez, Email: eltavogus@gmail.com.
A. Pinto, Email: pinto.misle@gmail.com.
L. Alamo, Email: alamo@ivic.gob.ve.
B. Baumann, Email: baumann@sb.fsu.edu.
F. Ye, Email: feye@ucsd.edu.
H. Winkler, Email: winkler@sb.fsu.edu.
K. Taylor, Email: taylor@bio.fsu.edu.
R. Padrón, Email: raul.padron@gmail.com.
References
- AL-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc. Natl. Acad. Sci. U. S. A. 2013;110:318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamo L, Wriggers W, Pinto A, Bartoli F, Salazar L, Zhao FQ, Craig R, Padrón R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J. Mol. Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito R, Alamo L, Lundberg U, Guerrero JR, Pinto A, Sulbaran G, Gawinowicz MA, Craig R, Padron R. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J. Mol. Biol. 2011;414:44–61. doi: 10.1016/j.jmb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Padrón R. Molecular structure of the sarcomere. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd ed. McGraw-Hill, Inc.; 2004. pp. 129–166. 3rd ed., McGraw-Hill, Inc., pp. 129 166. [Google Scholar]
- Craig R, Padrón R, Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J. Cell Biol. 1987;105:1319–1327. doi: 10.1083/jcb.105.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA, Padrón R, Craig R. Arrangement of the heads of myosin in relaxed thick filaments from tarantula muscle. J. Mol. Biol. 1985;184:429–439. doi: 10.1016/0022-2836(85)90292-x. [DOI] [PubMed] [Google Scholar]
- Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- Egelman EH. The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. J. Struct. Biol. 2007;157:83–94. doi: 10.1016/j.jsb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Padron R, Horowitz R, Zhao FQ, Craig R. Purification of native myosin filaments from muscle. Biophys. J. 2001;81:2817–2826. doi: 10.1016/S0006-3495(01)75923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley HE. Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. J. Mol. Biol. 1963;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley HE, Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J. Mol. Biol. 1967;30:383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Kensler RW, Levine RJ, Stewart M. Electron microscopic and optical diffraction analysis of the structure of scorpion muscle thick filaments. J. Cell Biol. 1985;101:395–401. doi: 10.1083/jcb.101.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Linarez N. M. Sc. Thesis. Caracas, Venezuela: Centro de Estudios Avanzados (CEA), IVIC; 2002. Estudio de la estructura del aparato contractil y de la fosforilación de las cadenas ligeras reguladoras de miosina de músculo estriado de escorpión. [Google Scholar]
- Liu J, Wendt T, Taylor D, Taylor K. Refined model of the 10S conformation of smooth muscle myosin by cryo-electron microscopy 3D image reconstruction. J. Mol. Biol. 2003;329:963–972. doi: 10.1016/s0022-2836(03)00516-3. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pinto A, Sanchez F, Alamo L, Padron R. The myosin interacting-heads motif is present in the relaxed thick filament of the striated muscle of scorpion. J. Struct. Biol. 2012;180:469–478. doi: 10.1016/j.jsb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Saxton WO, Baumeister W, Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13:57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kensler RW, Levine RJ. Structure of Limulus telson muscle thick filaments. J. Mol. Biol. 1981;153:781–790. doi: 10.1016/0022-2836(81)90418-6. [DOI] [PubMed] [Google Scholar]
- Sulbarán G, Biasutto A, Alamo L, Riggs C, Pinto A, Mendéz F, Craig R, Padrón R. Different head environments in tarantula thick filaments support a cooperative activation process. Biophys. J. 2013;105:2114–2122. doi: 10.1016/j.bpj.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Taylor D, Messier T, Trybus KM, Taylor KA. Visualization of head-head interactions in the inhibited state of smooth muscle myosin. J. Cell Biol. 1999;147:1385–1390. doi: 10.1083/jcb.147.7.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Taylor D, Trybus KM, Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. 3D reconstruction and processing of volumetric data in cryo-electron tomography. J. Struct. Biol. 2007;157:126–137. doi: 10.1016/j.jsb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Winkler H, Taylor KA. Accurate marker-free alignment with simultaneous geometry determination and reconstruction of tilt series in electron tomography. Ultramicroscopy. 2006;106:240–254. doi: 10.1016/j.ultramic.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Woodhead JL, Zhao FQ, Craig R. Structural basis of the relaxed state of a Ca2+-regulated myosin filament and its evolutionary implications. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8561–8566. doi: 10.1073/pnas.1218462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- Yang S, Yu X, Galkin VE, Egelman EH. Issues of resolution and polymorphism in single-particle reconstruction. J. Struct. Biol. 2003;144:162–171. doi: 10.1016/j.jsb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Craig R. Capturing time-resolved changes in molecular structure by negative staining. J. Struct. Biol. 2003;141:43–52. doi: 10.1016/s1047-8477(02)00546-4. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Craig R, Woodhead JL. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J. Mol. Biol. 2009;385:423–431. doi: 10.1016/j.jmb.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Sun Y, Zhao FQ, Yu J, Craig R, Hu S. Analysis of tarantula skeletal muscle protein sequences and identification of transcriptional isoforms. BMC. Genomics. 2009;10:117. doi: 10.1186/1471-2164-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameters of the SF-IHRSR 3D-reconstruction calculation for a single (filled circles), or several negatively stained (open circles): (a) Rotation angle vs. cycle number, (b) Axial repeat vs. cycle number, (c) Number of segments included in each cycle.
Electron tomograms tilt series of a negatively stained tarantula thick filament (Fig. 1a shows the 0° tilt image). This movie was produced from a subset of 21 images from the tilt series aligned using the IMOD package version 4.5 (The Boulder Laboratory for 3-D Electron Microscopy of Cells) (Kremer et al., 1996) and the NIH ImageJ package.
The SF-IHRSR (top) and tomographic (bottom) 3D-reconstructions calculated from a single thick filament showing the best fitting of the MIH structure PDB: 3DTP (Alamo et al., 2008) for each case.
Front outer shell of the tomographic 3D-reconstruction of a negatively stained tarantula thick filament (“1”, see Fig. 6a-1) and the equivalent part (“2”, see Fig. 6a-2) of the filtered tomographic 3D-reconstruction and the SF-IHRSR 3D-reconstruction of a single thick filament (“3”, see Fig. 6a-3). The good fitting of one MIH structure PDB: 3DTP (Alamo et al., 2008) is shown in “1” and “3”. The movie corresponds to the region labeled “+” in Fig. 6a-2. The rigid docking of 3DTP to both 3D-reconstructions was done automatically with the Chimera "Fit in Map" module.