Abstract
One of the major challenges in preclinical studies of alcohol abuse and dependence remains the development of paradigms that will elicit high ethanol intake and mimic the progressive transition from low or moderate social drinking to excessive alcohol consumption. Exposure of outbred rats to repeated cycles of free-choice ethanol intake and withdrawal with the use of intermittent access to 20% ethanol in a 2-bottle choice procedure (IA2BC) has been shown to induce a gradual escalation of voluntary ethanol intake and preference, eventually reaching ethanol consumption levels of 5–6 g/kg/24 h, and inducing pharmacologically relevant blood ethanol concentrations (BECs). This procedure has recently been gaining popularity due to its simplicity, high validity, and reliable outcomes. Here we review experimental and methodological data related to IA2BC, and discuss the usefulness and advantages of this procedure as a valuable pre-training method for initiating operant ethanol self-administration of high ethanol intake, as well as conditioned place preference (CPP). Despite some limitations, we provide evidence that IA2BC and related operant procedures provide the possibility to operationalize multiple aspects of alcohol abuse and addiction in a rat model, including transition from social-like drinking to excessive alcohol consumption, binge drinking, alcohol seeking, relapse, and neuroadaptations related to excessive alcohol intake. Hence, IA2BC appears to be a useful and relevant procedure for preclinical evaluation of potential therapeutic approaches against alcohol abuse disorders.
Keywords: ethanol, animal models, binge drinking, excessive drinking, intermittent access, operant self-administration, two-bottle choice, blood ethanol concentrations, neuroadaptations, relapse
Introduction
Alcohol abuse and dependence are characterized by a progressive escalation from low or moderate to excessive alcohol consumption, and by repeated cycles of intoxication, withdrawal, craving, and relapse (Koob, 2003; Koob & Volkow, 2010; Vengeliene, Bilbao, Molander, & Spanagel, 2008). Therefore, animal models that can demonstrate escalation to excessive ethanol consumption via repeated cycles of free-choice access to ethanol and withdrawal are particularly useful as valid models of these aspects of alcohol abuse. Voluntary consumption of alcohol in laboratory rats has traditionally been obtained by preceding initiation procedures, such as sucrose fading, or water/food deprivation (Samson, 1987). However, ethanol intake usually declines upon the removal of the initiation factors. Procedures without an initiation stage involving intermittent access to ethanol in 2-bottle choice (IA2BC) were first presented in the early 1970s (Wayner et al., 1972; Wise, 1973). These early studies showed that the repeated cycles of free-choice ethanol intake and withdrawal over a period of several weeks lead to a gradual escalation of ethanol intake and preference, which reach a stable baseline after several weeks. Most importantly, when compared with protocols using a continuous access to ethanol in 2-bottle choice, the IA2BC procedure yields considerably higher levels of ethanol intake (Wayner et al., 1972; Wise, 1973). However, this paradigm was revived only in the last decade (Carnicella, Amamoto, & Ron, 2009; Carnicella, Kharazia, Jeanblanc, Janak, & Ron, 2008; Simms et al., 2008), and has been gaining popularity due to its simplicity, high validity, and reliable results.
1. Intermittent access to 20% alcohol in 2-bottle choice
a. Training procedure
The typical IA2BC procedure is described here, but several other variations have been used (e.g., intermittent access to ethanol in 3-bottle choice procedure, (Palm, Roman, & Nylander, 2011), or alcohol access and abstinence periods of 48 h (Wayner et al., 1972). Rats are housed individually and receive at least a week of acclimatization and handling. Importantly, individual caging (social isolation) was recently reported to lead to increased ethanol intake in IA2BC in rats, regardless of the stage at which rats are socially isolated (juveniles or adults) (Chappell, Carter, McCool, & Weiner, 2013). Rats then receive three 24-h sessions of free access to 2-bottle choice (water and 20% ethanol) per week (typically Monday, Wednesday, and Friday), with 24-h and 48-h withdrawal periods during weekdays and weekends, respectively. During the withdrawal periods, rats receive one or two bottles of water. The placement of the ethanol bottle is alternated each drinking session to control for side preferences. Drinking sessions can begin during the light cycle (Barak, Ahmadiantehrani, Kharazia, & Ron, 2011; Barak, Carnicella, Yowell, & Ron, 2011; Carnicella, Amamoto, et al., 2009; Carnicella et al., 2008) or the dark cycle (Li, Bian, Dave, & Ye, 2011; Simms et al., 2008). Fluid intake is recorded at various time points, usually 30–60 min and 24 h after the beginning of the session. Rats typically consume stable, high levels of ethanol (> 4.5 g/kg/24 h) after 3–4 weeks of training (Carnicella, Amamoto, et al., 2009; Simms et al., 2008).
Depending on the aims of the study, rats that fail to reach a predefined criterion of alcohol intake can be excluded. For example, in studies where the research question concerns the effects of manipulations on excessive drinkers, binge drinking, or withdrawal from excessive drinking, the study population consists of excessive alcohol drinkers, and therefore the sample should include only high ethanol-drinking rats. In such cases, rats consuming less than 3.5–4 g/kg/24 h should be excluded from the study to obtain a group of excessive ethanol-drinking rats (Carnicella, Amamoto, et al., 2009; Carnicella et al., 2008). In Long-Evans rats, typically about 20% of the animals fail to reach this criterion. However, if the research question refers to the general population, such as the effects of certain manipulations on escalation in ethanol drinking (see Ahmadiantehrani, Barak, & Ron, 2013) or individual differences, then no selection of high drinkers should be conducted to avoid the loss of valuable data and misrepresentation of the population. Unfortunately, information as to whether and according to what criteria rats have been excluded from the study is not readily available in most IA2BC studies, and non-standard exclusion criteria might account for the variability in ethanol intake and BEC levels (see Table 1 and below). Future studies should therefore include this critical information.
Table 1.
Intermittent access to 20% ethanol in 2-bottle choice – comparative table from representative reports
| Strain | Initial ethanol intake (1st week) g/kg/24 h |
Final ethanol intake; last week(s); g/kg/24 h |
Blood ethanol concentrations (mg%) and correlation with drinking (duration) |
Institution | Rat supplier |
Reference |
|---|---|---|---|---|---|---|
| Long Evans | ~2–3.5 | 5.1 ± 0.6 | Range 10 – 100 mg% R2 = 0.85; (30 min) | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Simms et al., 2008 |
| Long Evans | ~1.6 | 5.5 ± 1.5 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Carnicella et al., 2008 | |
| Long Evans | ~1.5 | 5.39 ± 0.37 | Range 7.1 - 158.6 mg% R2 = 0.63; (30 min) | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Carnicella, Amamoto, et al., 2009 |
| Long Evans | ~1.8 | ~5.5–6.5 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Carnicella et al., 2010 | |
| Long Evans | ~5.6–6 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Barak, Ahmadiantehrani, et al., 2011 | ||
| Long Evans | 4.95–6.18 ± 0.11–0.75 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Barak, Carnicella, et al., 2011 | ||
| Long Evans | ~2–3.5 | ~5–5.5 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Nielsen et al., 2012 | |
| Long Evans | 4.03 ± 0.61 (4th session) | 5.48 ± 0.88 | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Ahmadiantehrani et al., 2013 | |
| Long Evans | 5.90 ± 0.76 | 112.28 ± 32.27 mg%; (60 min) | Tufts University MA | Charles River; Wilmington, MA | Hwa et al., 2013 | |
| Long Evans | ~2–4 | ~4–5.5 | UCLA, CA | Harlan; Indianapolis, IN | Meyer et al., 2013 | |
| Long Evans | 5.7 ± 0.23 | Range 26–249 mg% R2 = 0.714 (30 min) | University of Medicine and Dentistry of New Jersey, NJ | Harlan; Indianapolis, IN | Li et al., 2012 | |
| Wistar | ~1.5–3 | 5.8 ± 0.8 | Range 4–93 mg% R2 = 0.63 (30 min) | Gallo Research Center, UCSF, CA | Harlan; Indianapolis, IN | Simms et al., 2008 |
| Wistar | ~3–4 | ~5.2 | Range ~7–61 mg% R2 = 0.85 (60 min) | NIAAA | Charles River; Wilmington, MA | Cippitelli et al., 2012 |
| Wistar | ~1 | ~4.2 | Sir George Williams University, Montreal, Quebec, Canada | Wise, 1973 | ||
| Wistar | ~1 | ~3.6 | ~58 mg% (120 min) | The Scripps Research Institute, La Jolla, CA | George et al., 2012 | |
| Wistar | ~3.7–4.2 | 3.4 ± 0.56 | University of Gothenburg, Sweden | Taconic; Ejby, Denmark | Adermark et al., 2011 | |
| Sprague Dawley | ~1.7 | 4.8 ± 0.4 | Range 6–122 mg% R2 = 0.7607 (30 min) | Gallo Research Center, UCSF, CA | Charles River; Wilmington, MA | Bito-Onon et al., 2011 |
| Sprague Dawley | 5.63 ± 0.3 | University of Medicine and Dentistry of New Jersey | Taconic Farm; Hudson NY | Li et al., 2010 | ||
| Sprague Dawley | 3.2 ± 0.1 | 4.3 ± 0.2 | Range ~15–87 mean = 30.2 ± 6.4 mg% R2 = 0.67 (60 min) | University of Medicine and Dentistry of New Jersey | Taconic Farm; Hudson NY | Li, Zou, et al., 2011 |
| P Rats | ~4.5 | ~8 | Range 11–63 mg% R2 = 0.93 (30 min) | Gallo Research Center, UCSF, CA | Indiana University, IN | Simms et al., 2008 |
| Sardinian P rats | ~4–5 | ~9 | Range ~49–125 mean = 81.1 ± 6.81 mg% R2 = 0.919 (60 min) | Boston University, MA | University of Cagliari, Italy | Sabino et al., 2013 |
| Sardinian P rats | ~5.5 | ~9–10 | Neuroscience Institute, Cagliari, Italy | University of Cagliari, Italy | Loi et al., 2010 |
b. Escalation in alcohol drinking and BECs
At the early stages of this procedure, rats consume relatively low levels of ethanol (< 2.5 g/kg/24 h). However, within 3–4 weeks of training they gradually escalate to consume considerably higher amounts, namely, 5–6 g/kg/24 h, with ~50% ethanol preference (Carnicella, Amamoto, et al., 2009; Carnicella et al., 2008; Simms et al., 2008). This gradual escalation from moderate to excessive ethanol drinking can potentially model the transition from moderate “social”-like drinking to excessive alcohol drinking in humans (e.g., Ahmadiantehrani et al., 2013; Barak et al., submitted). Interestingly, Carnicella, Amamoto, and colleagues (2009) showed that about one-third of the total ethanol amount consumed throughout the 24-h session is consumed within the first 30 min, generating a BEC of > 80 mg%, which meets the criteria of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) for binge drinking in humans (National Institute on Alcohol Abuse and Alcoholism, 2004). Thus, this procedure is also used to model binge-like alcohol drinking in rats (Ahmadiantehrani et al., 2013; Barak, Ahmadiantehrani, et al., 2011; Ben Hamida et al., 2012; Carnicella, Amamoto, et al., 2009; George et al., 2012; Neasta, Ben Hamida, Yowell, Carnicella, & Ron, 2010, 2011; Nielsen et al., 2012; Simms, Nielsen, Li, & Bartlett, 2013). Moreover, in procedures that start the session in the light cycle, rats seem to consume lower levels of ethanol for several hours after the first 30 min of binge-like drinking (possibly until the dark cycle begins), and then consume high levels during the dark cycle (Barak, Ahmadiantehrani, et al., 2011; Carnicella, Amamoto, et al., 2009). This drinking pattern should be carefully considered for studies assessing the effects of alcohol drinking on other variables, as well as the effects of various manipulations on alcohol consumption in this procedure. For example, some manipulations might affect the binge-like phase but not the later drinking phase, or vice versa (e.g., Barak, Ahmadiantehrani, et al., 2011, and see below).
c. Strain differences
Most of the studies that employed the IA2BC procedure used Long-Evans rats (Barak, Ahmadiantehrani, et al., 2011; Carnicella et al., 2008; Li, Bian, et al., 2011; Meyer, Long, Fanselow, & Spigelman, 2013; Simms et al., 2008) and Wistar rats (Cippitelli et al., 2012; George et al., 2012; Hopf, Chang, Sparta, Bowers, & Bonci, 2010; Shirazi, Dickson, & Skibicka, 2013; Simms et al., 2008; Wise, 1973). However, some studies used Sprague-Dawley (Bito-Onon, Simms, Chatterjee, Holgate, & Bartlett, 2011; Li, Zou, & Ye, 2011) or alcohol-preferring (P or Sardinian) rats (Sabino, Kwak, Rice, & Cottone, 2013; Simms et al., 2008). Simms and colleagues (2008) compared ethanol drinking in Wistar, Long-Evans, and alcohol-preferring (P) rats, and found similar intake levels in Long-Evans (5.1 ± 0.6 g/kg/24 h) and Wistar rats (5.8 ± 0.8 g/kg/24 h). P rats initiate drinking at higher levels compared to Long–Evans and Wistar rats (Simms et al., 2008), as well as to Sprague-Dawley rats (Bito-Onon et al., 2011) (see Table 1). However, P rats show only a trend toward an escalation in ethanol intake over time, and reach intake levels of ~8 g/kg/24 h (Simms et al., 2008). In contrast, other studies showed that the TSRI Sardinian alcohol-preferring rats show a very sharp escalation, starting at ~4 g/kg/24 h, and reaching ethanol intake levels of 9–10 g/kg/24 h within 3–6 sessions (1–2 weeks) (Loi et al., 2010; Sabino et al., 2013). Finally, escalation in ethanol intake was also observed in Sprague-Dawley rats (Bito-Onon et al., 2011; Li et al., 2011). However, the percentage of rats not showing drinking escalation seems to be higher in this strain compared to the Wistar and Long-Evans strains (Moorman & Aston-Jones, 2009).
Interestingly, the correlation between the levels of ethanol intake and BEC was stronger for Long-Evans, Sardinian alcohol-preferring, and P rats (R2 = 0.85, R2 = 0.84, and R2 = 0.93, respectively) compared to Wistar (R2 = 0.63) and Sprague-Dawley rats (R2 = 0.76) (Bito-Onon et al., 2011; Sabino et al., 2013; Simms et al., 2008). Furthermore, the levels of ethanol intake required to reach high BECs were lower for Long-Evans rats compared to Wistar, Sprague-Dawley, and P rats (Bito-Onon et al., 2011; Simms et al., 2008), as well as Sardinian alcohol-preferring rats (Sabino et al., 2013), possibly due to strain differences in ethanol metabolism. In fact, 40% of the Long-Evans rats reached BECs usually seen in rat strains selectively bred for alcohol preference (Bell, Rodd, Lumeng, Murphy, & McBride, 2006; Simms et al., 2008). This finding suggests that Long-Evans rats might be the ideal outbred strain for this model. It should be noted, however, that recent studies reported lower ethanol intake levels (3–4 g/kg/24 h) in both Long-Evans (Meyer et al., 2013) and Wistar (e.g., Adermark, Jonsson, Ericson, & Söderpalm, 2011; George et al., 2012) rats. Furthermore, Palm and colleagues (2011) reported 2-fold differences in ethanol intake in a 3-bottle choice procedure (water, 5% ethanol, and 20% ethanol) among Wistar rats from five different suppliers. In the same study, rats from all strains showed very little or no escalation in ethanol intake over time (Palm et al., 2011). Thus, there seems to be inter-strain, inter-supplier, and inter-laboratory variability in the amount of ethanol consumed and in the drinking escalation function (see Table 1).
d. Neuroadaptations
i. Neurophysiological and neurochemical adaptations
Stuber et al. (2008) showed that excessive ethanol consumption in the IA2BC procedure enhances postsynaptic AMPA receptor function in the ventral tegmental area (VTA) 12–24 h after the last self-administration bout. Subsequently, spontaneous, but not evoked, glutamate release was enhanced by ethanol consumption (Stuber et al., 2008).
Ron and colleagues recently demonstrated that training rats in the IA2BC procedure for several weeks leads to neurochemical adaptations in the mesolimbic system (Barak, Carnicella, et al., 2011). More specifically, using in vivo microdialysis, they showed that following long-term excessive ethanol consumption in the IA2BC procedure (7 weeks; average consumption 5.5–6 g/kg/24 h), withdrawal from ethanol for 24 h led to a substantial decrease in dopamine (DA) overflow in the nucleus accumbens (NAc) (Barak, Carnicella, et al., 2011). Remarkably, although rats tested immediately after a 24-h ethanol-drinking session did not show DA deficiency, the DA levels in these rats declined within 2 h to levels similar to those of their counterparts measured after 24 h of withdrawal (Barak, Carnicella, et al., 2011). This report on withdrawal-associated DA deficiency agrees with previous studies that used other ethanol exposure protocols, showing that withdrawal from chronic exposure to high levels of ethanol leads to a substantial reduction in the activity of DA-ergic VTA neurons projecting to the NAc (Diana, Pistis, Carboni, Gessa, & Rossetti, 1993; Shen, Choong, & Thompson, 2007). This results in a reduction in DA levels in the NAc, which has been associated with ethanol craving during relapse (Diana et al., 1993; Rossetti, Melis, Carboni, Diana, & Gessa, 1992; Weiss et al., 1996).
Interestingly, Ahmed and Koob suggested that long-term excessive consumption of drugs leads to an allostatic decrease in the reward system, so that the levels of drug intake must be progressively increased to achieve a satisfying rewarding outcome (Ahmed & Koob, 1998, 2005). Moreover, the authors suggested that these allostatic changes lead to a transition from positive to negative reinforcement mechanisms in addiction (Koob, 2003; Koob & Le Moal, 2001). The results of Ron and colleagues suggest that the deficient VTA DA-ergic neuron firing and the consequent deficient DA release in the NAc are associated with the reduction in reward function after a long history of excessive ethanol consumption (Barak, Carnicella, et al., 2011), leading to ethanol-seeking behavior motivated by negative reinforcement mechanisms. Thus, the IA2BC procedure seems to generate allostatic changes in the reward system that are correlated with neurochemical allostatic deficiencies in the mesolimbic pathway.
ii. Molecular and biochemical neuroadaptations
Molecular and biochemical adaptations were reported following training in the IA2BC procedure for several weeks. Ron and colleagues showed that the mammalian target of rapamycin complex 1 (mTORC1), which controls translation of specific synaptic proteins and has been implicated in learning and memory processes (Hoeffer & Klann, 2010), is activated in the NAc of rats following 3 months’ training in the IA2BC procedure (Neasta et al., 2010). Moreover, the levels of the mTORC1-mediated synaptic proteins, Homer and GluR1, were increased in the NAc (Neasta et al., 2010). Interestingly, a similar increase in mTORC1 activity was observed after 24 h of abstinence and after 30 min of binge-like drinking (Neasta et al., 2010), suggesting that this neuroadaptation may be due to long-term ethanol exposure, rather than due to withdrawal or acute exposure to ethanol. Moreover, the same group found that the activity of H-Ras and AKT signaling, the main upstream activator of mTORC1, is increased in the NAc of rats trained in the IA2BC procedure, after 24 h of abstinence (Neasta et al., 2011).
Moreover, George and colleagues (2012) found in rats trained in the IA2BC procedure a robust increase in FOS protein expression, a marker of neuronal activity, in the medial prefrontal cortex (mPFC) and central nucleus of the amygdala (CeA), when measured after 24 h of abstinence. This neuroadaptation was completely abolished after 2 h of ethanol drinking, and the intake levels positively correlated with the increase in FOS expression (George et al., 2012). Thus, the IA2BC procedure seems to generate electrophysiological and neurochemical adaptations that underlie alcohol-seeking behavior seen in this procedure following 24 h of abstinence (Carnicella, Amamoto, et al., 2009; Simms et al., 2008). Finally, the mRNA expression of glial cell line-derived neurotrophic factor (GDNF) was shown to fluctuate as a function of stages in the IA2BC procedure (Ahmadiantehrani et al., 2013, and see below).
iii. Behavioral adaptations
Given the neuroadaptations detailed above, it is not surprising that several studies have demonstrated behavioral alterations after prolonged training, particularly following a short period of abstinence. Specifically, acute (24–72 h), but not protracted (16–68 days), abstinence in rats trained in the IA2BC was reported to cause working memory deficits in tasks known to depend on the integrity of the mPFC (Y maze-based spontaneous alteration task and operant-based delayed non-match to sample task) (George et al., 2012). In contrast, no changes in anxiety-like behavior (measured in an elevated plus maze test, a putative amygdala-related task) were found in the same study (George et al., 2012). These findings, taken together with a higher increase in FOS expression in the mPFC compared to the CeA, led the authors to suggest that the mPFC was more sensitive to the effect of acute ethanol abstinence than the CeA.
Several signs of physical withdrawal (tail stiffness and walking with broad gait) were reported after acute but not protracted abstinence (Steensland et al., 2012), raising the possibility that rats under this procedure develop ethanol dependency, at least to some extent. It will be beneficial for the validity of the model to further characterize the behavioral adaptations that occur after short withdrawal periods, e.g., in cognitive flexibility, motivated behaviors, and social behaviors.
Finally, we recently found that rats with a history of excessive ethanol consumption in the IA2BC procedure show ethanol-conditioned place preference (CPP) after the termination of the IA2BC training (Barak, Carnicella, et al., 2011). Typically, for ethanol-CPP experiments, rats need to be habituated to ethanol by a daily administration for several days or weeks before the experiment in order to prevent aversive responding of the rats to alcohol (e.g., Biala & Kotlińska, 1999; Reid, Hunter, Beaman, & Hubbell, 1985; Zarrindast, Meshkani, Rezayof, Beigzadeh, & Rostami, 2010). Conducting the CPP experiment in rats with a history of excessive ethanol (via IA2BC training) provides a more behaviorally relevant way to habituate the animals to ethanol. Furthermore, we showed that similar habituation to ethanol via IA2BC training leads to high ethanol consumption in an operant self-administration procedure (Barak et al., 2013; Carnicella et al., 2008; Carnicella, Yowell, & Ron, 2011) (see below).
e. Advantages, limitations, and perspectives
The IA2BC procedure provides one of the most efficient behavioral protocols to train animals to voluntarily consume clinically relevant excessive ethanol levels. More specifically, this procedure is advantageous for several reasons. First, it makes it possible to train rats to voluntarily consume excessive, stable levels of ethanol without an initiation procedure that might have confounding issues (see below). Second, rats of different strains trained in this procedure will initially consume moderate levels of ethanol and will progress to excessive alcohol drinking, providing a useful model for transition from social-like to excessive alcohol intake. Third, long-term training in the IA2BC leads to binge-like drinking episodes generating high levels of BEC, which show high positive correlations with alcohol intake levels. Fourth, training in this procedure produces neuroadaptations in the molecular, cellular, and behavioral levels, which are relevant to alcohol abuse disorders. Taken together, these characteristics suggest that IA2BC training is useful to model escalation to excessive drinking, as well as for binge drinking, in the rat. Importantly, the model shows three aspects of validity: face validity, given the similarity to the drinking pattern of human alcoholics (Koob, 2003; Koob & Volkow, 2010; Vengeliene, Bilbao, Molander, & Spanagel, 2008); construct validity, given the high correlation of BEC and alcohol intake levels, and the neuroadaptations found following IA2BC training; and predictive validity, given the accumulating findings in the literature reporting that drugs approved by the US Food and Drug Administration for the treatment of alcoholism (i.e., naltrexone and acamprosate) suppress alcohol intake in this model (e.g., Li et al., 2010; Sabino et al., 2013; Simms et al., 2008).
Several limitations should, however, be taken into account when using the IA2BC model. First, only 50–80% (depending on strains, breeds, and laboratory) of the rats typically escalate to excessive alcohol drinking, and from the latter cohort, only about half show BECs of > 80 mg%. On the one hand, this variability may contribute to the validity of the model, which can detect individual differences. Specifically, the fact that some rats do not escalate their drinking allows dissociation between high- and low-drinking rats – an advantage of the procedure when the research question refers to individual differences. However, a major problem of this approach is that by the time this differentiation can be concluded, high and low drinkers will not have the history of alcohol exposure. This confounding issue must be considered in any subsequent behavioral and/or neurobiological assessment of the two phenotypes.
On the other hand, this variability limits the usefulness of the model, as a high percentage of the animals cannot be used for many studies. Second, although rats reach very high levels of ethanol consumption, and although some signs of physical withdrawal have been reported, it is likely that this procedure cannot model alcohol dependence as other models have done, e.g., models using vapor chambers (Gilpin, Richardson, Cole, & Koob, 2008). In addition, the lack of behavioral effects after long-term withdrawal (George et al., 2012; Steensland et al., 2012) and the fact that alcohol deprivation effects are typically not observed in this model (Li et al., 2011; Meyer et al., 2013; Simms et al., 2008, but see Barak et al., 2013), further suggest that this procedure models alcohol abuse, rather than alcohol dependence.
Furthermore, it should be noted that most of the studies using the IA2BC procedure to investigate molecular, neuronal, neurochemical, or behavioral adaptations related to repeated excessive ethanol intake, or to test the potential inhibitory impact of molecules on such behavior, did not include a formal low ethanoldrinking control group (Barak, Carnicella, et al., 2011; Neasta et al., 2010; Seif et al., 2013; Stuber et al., 2008, but see George et al., 2012; Hopf et al., 2010). The lack of a non-escalated ethanol-drinkers group, usually used as a control in models of escalation of drug use (e.g., Ahmed & Koob, 1998), does not allow the investigator to conclusively infer that the mechanisms evidenced in these studies are specifically associated with the development and/or maintenance of high alcohol intake. It appears, therefore, important for future studies to use, when possible, a continuous-ethanol access group in which rats do not escalate their ethanol intake (Simms et al., 2008; Wise, 1973) in order to accurately dissect potential mechanisms implicated in normal or excessive alcohol drinking behaviors.
Finally, given the fact that the IA2BC is a non-operant self-administration procedure, it lacks the more advanced analyses that operant self-administration procedures provide. Thus, the next section will present the advantages of a combination of IA2BC training with an operant ethanol self-administration procedure.
2. Operant self-administration in rats pre-trained in IA2BC
The IA2BC paradigm in rats appears to be a useful and relevant approach for studying the psychobiological mechanisms and the neuroadaptations underlying alcohol use disorders, as well as the effects of systemic or intra-cerebral manipulations on excessive alcohol intake and binge-like drinking behaviors. This procedure does not, however, afford a strong insight into the motivational and reinforcing processes that govern alcohol seeking and drinking behaviors. Because these aspects, which are critical for the study of addiction, are classically evaluated under instrumental conditions, we discuss below how to shift 20% ethanol intermittent-access drinking rats from free-choice drinking to operant procedures, and we discuss the validity of this approach for preclinical studies of alcohol abuse and addiction.
a. Training procedure
Rats are first subjected to IA2BC with a 20% (v/v) ethanol solution as described above for 7 weeks. Animals consuming less than 4 g/kg/24 h at the baseline are excluded from the study (Carnicella et al., 2008; Carnicella et al., 2011), as they are considered resilient to excessive alcohol intake. Exclusion of rats according to this predefined criterion is critical as rats drinking low levels of ethanol in the IA2BC do not acquire operant 20% ethanol self-administration (S. Carnicella, unpublished observation). Thus, as emphasized in section 1a above, the selection of animals that drink higher levels of ethanol is derived from the reference population of studies. Hence, studies using this operant procedure refer to high or excessive ethanol drinkers rather than the general population, and conclusions drawn from studies using this procedure should be considered accordingly. Next, rats are trained to orally self-administer the 20% ethanol solution in operant self-administration chambers, with an active, reinforced lever (for which presses result in the delivery of 0.1 mL of the ethanol solution), and an inactive, non-reinforced lever, to control for non-specific behavioral activity. No discrete cues are required to indicate the delivery of ethanol and to trigger responding on the active lever, unless a cue-induced reinstatement of ethanol-seeking test (see below) is conducted. Two or three overnight sessions under a fixed ratio 1 (FR1) allow rapid acquisition of the instrumental contingency between the manipulandum (lever presses, nosepokes) and the delivery of the ethanol solution into a dipper receptacle. Then, operant sessions are conducted 5 days per week, with the schedule requirement increased to FR3 and the length of session shortened from 60 to 30 min over the first 2 weeks, as we found that the majority of rats terminate their operant activity after 30 min (Carnicella et al., 2008). One month of training under these parameters (FR3, 30 min), usually results in a stable baseline of operant ethanol self-administration (Barak, Carnicella, et al., 2011; Barak et al., 2013; Carnicella et al., 2011). Animals pressing for less than 0.4 g/kg/30 min at the baseline are excluded from the study (Carnicella et al., 2011). The operant responding criterion is standard for most operant procedures, including ethanol operant self-administration using sucrose-fading pre-training (e.g., Bertholomey, Verplaetse, & Czachowski, 2013; McCool & Chappell, 2009; Radwanska et al., 2008; Simms, Bito-Onon, Chatterjee, & Bartlett, 2010).
As mentioned above, approximately 20% of Long-Evans rats typically fail to escalate their ethanol intake in the intermittent-access 2-bottle choice procedure, while 10% do not successfully acquire operant self-administration, leading to a success rate of ~70% (Carnicella et al., 2011). Critically, acquisition of operant 20% ethanol self-administration under these conditions without pre-exposure to a 20% ethanol solution in an intermittent-access procedure leads to a lower success rate of only 40% (Carnicella et al., 2011). Interestingly, the level of ethanol intake in non-pre-exposed rats that successfully acquire operant self-administration is comparable to the level obtained after the intermittent-access procedure (Carnicella et al., 2011). It therefore indicates that rats can readily self-administer ethanol without sucrose fading (see also Simms et al., 2010) and that pre-exposure to a 20% ethanol solution in the IA2BC procedure significantly reduces the rate of attrition.
b. Self-administration pattern and BECs
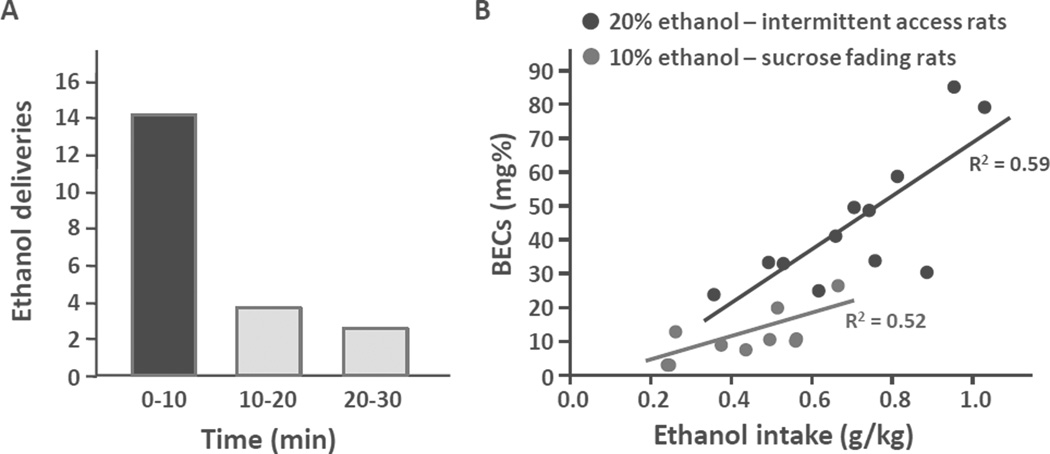
At the baseline, ethanol consumption ranges from 0.8 to more than 1 g/kg in 30 min (Barak, Carnicella, et al., 2011; Carnicella, He, Yowell, Glick, & Ron, 2010; Carnicella et al., 2011; Neasta et al., 2011; Wang et al., 2012). Interestingly, the majority of this consumption occurs at the beginning of the operant session, as approximately 70% of the ethanol deliveries are made within the first 10 min (Figure 1A), suggesting a voluntary fast ethanol loading, as observed in ethanol-dependent rats (e.g., Weiss et al., 1996). Indeed, operant self-administration of a 20% ethanol solution with this pattern leads to pharmacologically relevant BECs, ranging from 25 up to 85 mg%, with a mean of 60 mg% and with a strong correlation between BECs and the level of ethanol intake (Figure 1B).
Figure 1. Pattern of ethanol intake and BECs during a 30-min operant 20% ethanol self-administration session.
A. Number of 20% ethanol deliveries during 10-min intervals. The graph summarizes data collected and published in Carnicella et al., 2008 and Neasta et al., 2011; n = 15.
B. Correlations between BECs and the ethanol consumed by 20% ethanol IA2BC-trained rats during a 30-min operant 20% ethanol self-administration session (black, n = 12), or by rats pre-trained with a sucrose-fading 10% ethanol procedure, during a 1-h operant 10% ethanol self-administration session (gray, n = 10).
These BEC and intake levels are higher than those commonly obtained with a standard 10% ethanol self-administration preceded by sucrose-fading initiation procedures (Carnicella et al., 2008; Simms et al., 2010; Slawecki, Samson, & Hodge, 1997; Weiss et al., 1996, and Figure 1B; but see Czachowski, Santini, Legg, & Samson, 2002), but not as high as the values obtained with the IA2BC procedure (see Table 1). This decrease in intake following the shift of paradigm may be associated with some instrumental constraints (e.g., greater effort to obtain ethanol, repeated alternations between seeking and consummatory behaviors, short refractory period between two ethanol deliveries). It is also likely due, at least in part, to an increase in the frequency of ethanol access (every weekday ethanol-access schedule). Indeed, increasing the interval between two ethanol self-administration sessions by keeping an every-other-day schedule, allows for the maintenance of ethanol intakes comparable to the ones obtained in the intermittent-access 2-bottle choice procedure (Carnicella & Ron, unpublished observations).
c. Other intermittent procedures
Other procedures for operant ethanol self-administration using intermittency have recently been developed and have produced similar outcomes. For example, slight variations of the procedure described above were used to induce high levels of ethanol intake during operant self-administration (0.8–1.0 g/kg/30 min) by pre-exposing Sprague-Dawley or Wistar rats to a 20% ethanol IA2BC (Bito-Onon et al., 2011; Steensland et al., 2012). The main difference from the procedure described above is the use of a 3-sec stimulus light and a 3-sec tone as ethanol-associated cues that are paired with the delivery of ethanol. Moreover, after 2 months of daily 30-min operant sessions, Steensland and colleagues reduced ethanol access to only 3 times per week (Monday, Wednesday, and Friday), but increased the duration of operant sessions to 60 min (Bito-Onon et al., 2011; Steensland et al., 2012). While the reason for this modification was not indicated, we speculate that it was done to maintain high levels of ethanol intake. In another study, Hopf and colleagues also shifted Wistar rats from a longer-term (1.5 or 3–4 months) IA2BC schedule to operant self-administration, but tested the rats directly under a progressive ratio paradigm (see below), after only two overnight sessions for acquisition of the lever-ethanol contingency, without several weeks of short training sessions under FR reinforcement schedules (Hopf et al., 2010). Another variant of the procedure was introduced by Simms and colleagues who showed in Long-Evans rats that underwent intermittent access to 20% ethanol in 12 overnight operant self-administration sessions, produced ethanol intake as high as 1.5 g/kg in subsequent daily 30-min operant sessions, with a mean BEC of 60 mg%, as observed in our procedure, but ranging up to 150 mg% (Simms et al., 2010). Intriguingly, the intermittent schedule in this condition did not appear crucial as 12 consecutive overnight operant self-administration sessions led to similar results (Simms et al., 2010), suggesting that extensive overnight operant training with a 20% ethanol solution may be sufficient to induce high levels of ethanol intake.
d. Instrumental manipulations
After acquisition of self-administration, several instrumental manipulations can be performed to accurately investigate the motivational and reinforcing processes associated with alcohol seeking and drinking behaviors. Below are those that have been validated in the present procedure.
i. Progressive ratio
Steensland and colleagues (2012) and Hopf and colleagues (2010) have recently used a progressive ratio schedule of reinforcement as an index of motivation (Hodos, 1961). During the progressive ratio test, the response requirement for obtaining an ethanol reward increases after each reward earned, according to an exponential function that was specifically adapted for ethanol self-administration (5 × e(0.1 × number of rewards previously earned) − 5; Bowers et al., 2008). This paradigm, in which the workload to obtain ethanol increases until animals eventually cease operant responding, can provide interesting insights into the motivation of rats to seek and consume ethanol. Using this procedure, Hopf and colleagues (2010) showed that motivation to seek and consume ethanol was reduced by quinine adulteration in rats exposed to 1.5, but not 3–4, months of IA2BC, suggesting the potential development of abnormal ethanol seeking behaviors following a long-term exposure to the intermittent-access procedure.
ii. Dose-response curve
When levels of self-administration are stable, changing the concentration of the ethanol solution strongly affects operant behavior. We found that varying the concentration of ethanol from 2.5 up to 60% (v/v) after acquisition of 20% ethanol self-administration leads to a typical inverted U-shaped dose-response curve (Carnicella et al., 2011). As observed for other drugs of abuse, beyond a certain concentration (20% ethanol here), rats adapt their level and pattern of responding to ethanol concentration changes in order to obtain a constant level of intake and BEC (Barak, Carnicella, et al., 2011; Carnicella et al., 2011), suggesting that their operant behavior is mainly driven by the motivation to obtain a specific pharmacological effect of ethanol. Using this method, Simms and colleagues (2010) showed that animals trained to self-administer 20% ethanol consume significantly more ethanol than rats trained to self-administer 10% ethanol with a sucrose-fading initiation procedure, regardless of the ethanol concentration, indicating an upward shift in the dose-response curve. This upward shift, as observed for example in cocaine-treated rats after a long history of access (Ahmed & Koob, 1998), could reflect profound changes within the reward systems indicative of an allostatic mechanism (Kenny, 2007), and is usually considered a hallmark of drug abuse (Carnicella et al., 2011; Piazza, Deroche-Gamonent, Rouge-Pont, Le Moal, 2000).
iii. Extinction test
Rats can be tested in extinction (by not reinforcing the conditioned response during a short self-administration session) after a period of withdrawal of several days (Carnicella, Ahmadiantehrani, et al., 2009), or just the day after an ethanol self-administration session (Wang, Lanfranco, et al., 2010). By avoiding consummatory behaviors, it provides, in a simple manner, a specific insight into ethanol-seeking behaviors. For example, Carnicella, Ahmadiantehrani, and colleagues (2009) found that cabergoline, a dopaminergic agonist that increases GDNF levels in the ventral tegmental area (Carnicella, Ahmadiantehrani, et al., 2009), reduced the instrumental response in extinction following a 10-day period of withdrawal from ethanol, which is indicative of a potent action on the motivation to seek ethanol after a period of abstinence.
iv. Ethanol priming and cue-induced reinstatement of ethanol seeking
Reinstatement is considered to be a particularly relevant model of relapse (Marchant, Li, & Shaham, 2013), one of the core features of addiction, and a major challenge for the treatment of alcohol-use disorders (McLellan, Lewis, O'Brien, & Kleber, 2000; O'Brien, 2008). Interestingly, exposing rats that first underwent a 20% ethanol intermittent-access period and were then subjected to extinction of their conditioned response, to a small (0.2 mL) non-contingent delivery of ethanol in the reward port, induces a rapid return of operant responding on the lever previously associated with ethanol, indicating a robust reinstatement of ethanol seeking by the polysensory properties of a small ethanol volume (Wang, Lanfranco, et al., 2010). Using this method, we were able to show that pharmacological blockade of the NR2B subunit of the NMDA receptor in the dorsomedial striatum, a region in which this subunit shows long-lasting hyperactivation following IA2BC, reduces the reinstatement of ethanol-seeking behavior (Wang, Lanfranco, et al., 2010). With a similar operant ethanol self-administration paradigm, Steensland and colleagues (2012) efficiently induced reinstatement of ethanol-seeking behavior with an ethanol-associated cue.
v. Reconsolidation
Very recently, Barak and colleagues (2013) introduced a procedure based on prior training in IA2BC followed by operant 20% ethanol self-administration, designed to investigate the mechanisms of reconsolidation of ethanol-associated memories. Specifically, after 7 weeks of IA2BC training followed by 4 weeks of self-administration training, rats were subjected to 10 days of abstinence in the home cage. On the 11th day, rats had a 5-min “reactivation” session, conducted in the operant chambers under extinction conditions (lever presses were not reinforced), with a small amount of ethanol that served as an odor-taste cue, given at the beginning of the session. They found that the retrieval of alcohol-associated memories increased the activation of the mammalian target of rapamycin complex 1 (mTORC1), a complex that controls synaptic translational machinery and is known to play a crucial role in learning and memory processes (Hoeffer & Klann, 2010), and the effect was restricted to the prefrontal cortex and central nucleus of the amygdala (CeA). In a subsequent series of experiments, the mTORC1 inhibitor rapamycin was administered immediately after the reactivation session either systemically or into the CeA in order to disrupt the reconsolidation of ethanol-associated memories. Twenty-four and 48 h later, rats underwent a 30-min retention test under extinction as described above, and a 30-min reacquisition test (in which lever presses were reinforced), which measured relapse to ethanol seeking and drinking, respectively. They found that mTORC1 inhibition disrupted the reconsolidation of alcohol-associated memories and reduced lever pressing in both relapse tests (Barak et al., 2013).
e. Advantages, limitations and perspectives
The major advantage of the IA2BC-initiated operant self-administration procedure is the absence of sucrose-fading pre-training. Indeed, sucrose is a powerful reinforcer (e.g., Lenoir, Serre, Cantin, & Ahmed, 2007) and consequently, may be a potent confounding factor in preclinical studies of alcohol addiction (Simms et al., 2010). For instance, different psychobiological mechanisms can underlie the acquisition and maintenance of operant ethanol self-administration after a sucrose-fading procedure. The classical view is that rats self-administer ethanol for its primary reinforcing or motivational properties. However, it is not unlikely that ethanol becomes a discriminative stimulus (Macenski & Shelton, 2001), due to its strong association with sucrose (i.e., sucrose available in the presence of ethanol), thereby acting as a strong conditioned reinforcer (e.g., McCusker & Bell, 1988). This potential confound remains largely under-estimated when a sucrose-fading procedure is used, while it may lead to alternative interpretations concerning the effect of pharmacological agents on operant ethanol self-administration, or the investigation of the neurobiological mechanisms associated with ethanol drinking and seeking behaviors. It should be noted that Logrip and Zorrilla (2012) recently introduced an operant procedure that produces rapid stable ethanol (10%) self-administration without sucrose-fade pre-training. However, the level of ethanol intake during the FR3 baseline was 0.52 ± 0.07 g/kg/60 min in the control group (Logrip & Zorrilla, 2012), and is therefore considerably lower than the 0.8–1.0 g/kg/30 min usually obtained under a similar, FR3 schedule following IA2BC.
Animals trained in operant self-administration that were pre-trained in the IA2BC procedure consume relatively high amounts of ethanol during 30 min, which generate pharmacologically relevant BECs, well within the range reported with ethanol vapor- or liquid diet-dependent rats (Gilpin, Richardson, Cole, et al., 2008; Roberts, Heyser, Cole, Griffin, & Koob, 2000; Weiss et al., 1996) or with alcohol-preferring rats (Gilpin, Richardson, Lumeng, & Koob, 2008). Thus, IA2BC-trained rats outperform animals trained to self-administer 10% ethanol with a sucrose-fading initiation procedure. However, except for the study of Simms and colleagues with a related but different method (Simms et al., 2010), animals of these two procedures were never directly compared by self-administering the same ethanol concentration, but rather with their respective 20% and 10% ethanol solution. In other words, it remains to be determined whether rats trained to self-administer 10% ethanol with a sucrose-fading procedure maintain a lower ethanol intake than IA2BC-trained rats, when the concentration of the ethanol solution is increased to 20%. This point is particularly relevant as it has already been shown that shifting sucrose-fading pre-trained rats from 10% to 20% ethanol substantially increases their ethanol intake during operant self-administration (Samson, Pfeffer, & Tolliver, 1988; Samson, Sharpe, & Denning, 1999; Simms et al., 2010). Thus, it appears crucial to compare levels of operant self-administration of rats pre-trained with 20% ethanol IA2BC with those of rats pre-trained with sucrose fading to self-administer 10% ethanol, in a full dose-response curve study to ensure that 20% ethanol IA2BC leads to higher levels of ethanol consumption. This point is particularly critical, as sucrose-fading pre-trained rats may be used as the operant control counterparts of the non-escalating ethanol-drinking rats of the IA2BC procedure in future studies, particularly if sucrose-fading pre-trained rats self-administer less ethanol (20%) than IA2BC-pre-treated rats. Indeed, rats under a 20% ethanol continuous-access drinking protocol are unlikely to acquire operant ethanol self-administration due to their low level of ethanol intake (S. Carnicella, unpublished observation with Long-Evans rats), and the 10% ethanol sucrose-fading procedure can potentially be used as a standard control for other models of escalating ethanol intake, such as in procedures using ethanol vapor chambers (Roberts et al., 2000) or liquid diet (Weiss et al., 1996). Such controls will therefore promote a comparison across different models of a high level of ethanol self-administration.
The procedures described here enable several instrumental manipulations with useful heuristic values concerning drug abuse and addiction. However, the compulsive aspect of addiction remains to be investigated. As shown in Figure 1 (see also Simms et al., 2010), there is great inter-individual variability in the level of ethanol intake, with only a small subset of rats reaching intoxication consistently. As for cocaine (Belin, Mar, Dalley, Robbins, & Everitt, 2008; Deroche-Gamonet, Belin, & Piazza, 2004), it therefore may be of interest to determine whether specific, vulnerable subjects developed compulsive ethanol seeking and taking by using a procedure of punishment (i.e., mild footshock) associated with the reinforced response. By using a quinine adulteration procedure, Hopf and colleagues (2010) suggested that a prolonged intermittent access to ethanol (3–4 months) may lead to the development of compulsive seeking and taking behaviors.
3. Studies on GDNF as a proof concept
The glial-derived neurotrophic factor (GDNF) is a growth factor that plays an essential role in the development, survival, and maintenance of midbrain DA neurons (Airaksinen & Saarma, 2002; Lin, Doherty, Lile, Bektesh, & Collins, 1993). GDNF signals via the Ret receptor tyrosine kinase and GFRα1 co-receptor, and the growth factor was reported to regulate DA transmission in the nigrostriatal DA pathway in the adult brain (Airaksinen & Saarma, 2002) and mesolimbic system (Barak, Carnicella, et al., 2011; Wang, Carnicella, et al., 2010). Ron and colleagues have conducted comprehensive work on the role of GDNF in alcohol abuse disorders, mostly using the two procedures described above. This series of studies demonstrates the strengths and advantages of these models in the characterization of the role of a specific signaling pathway in alcohol addiction-related behaviors. Specifically, the group found that infusion of GDNF into the ventral tegmental area (VTA) in the midbrain, where the receptors of the growth factor are highly abundant, suppresses ethanol intake in animals trained in the IA2BC procedure (Carnicella, Amamoto, et al., 2009). Furthermore, the group reported that the effect of GDNF is rapid (within 10 min), and sustained for at least 24 h (Barak, Ahmadiantehrani, et al., 2011; Carnicella, Amamoto, et al., 2009). More specifically, the group showed that infusion of GDNF into the VTA 10 min before the initiation of an IA2BC session reduces the binge-like drinking behaviors measured in the first 30 min after the presentation of ethanol (Carnicella, Amamoto, et al., 2009), and that GDNF also suppressed ethanol intake in the remainder of the 24-h drinking session (Barak, Ahmadiantehrani, et al., 2011; Carnicella, Amamoto, et al., 2009). Interestingly, they found that the exogenous, recombinant GDNF infused into the VTA accounts for the immediate suppressive effects of the growth factor on ethanol intake. By contrast, the sustained decrease in ethanol consumption was mediated by a molecular positive autoregulatory feedback loop that led to the recurring synthesis of endogenous GDNF (Barak, Ahmadiantehrani, et al., 2011). Taken more generally, these findings suggest that mechanisms that control the "binge phase" in the IA2BC model may be different from the mechanisms controlling ethanol consumption in the later phase of the drinking session.
Moreover, the same group recently showed that training rats in the IA2BC procedure causes fluctuations in the mRNA levels of GDNF in the VTA during the course of training (Ahmadiantehrani et al., 2013). Specifically, they found that GDNF expression levels in the VTA were increased following an ethanol-drinking session in rats trained in the IA2BC procedure for 1 week. After 7 weeks of training in this procedure, GDNF expression levels were reduced when tested following a 24-h abstinence, but not after a 24-h drinking session, whereas the levels were elevated after a 30-min binge-like drinking session (Ahmadiantehrani et al., 2013). Moreover, knockdown of GDNF within the VTA facilitated the escalation of ethanol drinking by ethanol-naïve rats (Ahmadiantehrani et al., 2013). These results suggest that GDNF is an ethanol-responsive gene in the VTA, which protects against development of excessive drinking at the early stages of the IA2BC procedure; however, this protection breaks with the progress of training and escalation in ethanol intake.
Ron and colleagues also looked into the mechanisms by which GDNF acts to suppress ethanol seeking and drinking behaviors, and particularly on the effects of the growth factor on ethanol-induced neuroadaptations occurring in the mesolimbic system. Thus, Barak and colleagues recently showed that infusion of GDNF into the VTA rapidly reverses allostatic DA-ergic deficits, both in the spontaneous firing of VTA DA-ergic neurons, and in the extracellular levels of DA in the NAc (Barak, Carnicella, et al., 2011; Barak et al., submitted), effects that likely account for the capacity of GDNF to suppress ethanol seeking and drinking. Moreover, the authors showed that GDNF suppresses ethanol-CPP in rats with a history of long-term excessive ethanol intake in IA2BC training (Barak, Carnicella, et al., 2011).
Finally, Ron and colleagues also demonstrated that intra-VTA infusion of GDNF decreases operant self-administration for a 20% ethanol solution in rats that were pre-trained in the IA2BC procedure (Carnicella et al., 2008). Interestingly, GDNF does not abolish ethanol self-administration but rather reduces ethanol intake to moderate, non-intoxicating levels. Consistently, intra-VTA infusion of GDNF induces a downward shift of the inverted U-shaped dose-response curve for ethanol self-administration, reducing the amount of ethanol consumed from 1.2 to 0.6 g/kg (Barak, Carnicella, et al., 2011). The normalization of accumbal DA levels and of spontaneous firing of VTA DA-ergic neurons in IA2BC-trained rats by intra-VTA GDNF infusion (Barak, Carnicella, et al., 2011; Barak et al., submitted) may account for this downward shift, indicative of a decreased motivation to seek and consume ethanol related to the reversal of an allostatic mechanism.
Conclusions
In summary, the IA2BC procedure is a low-cost, simple, accessible behavioral protocol that efficiently induces voluntary consumption of high amounts of ethanol in several strains of outbred rats, with clear advantages, such as the absence of initiation or forced exposure procedures. The progressive transition from social-like to excessive alcohol intake and binge-like drinking induced by intermittent access to ethanol provides an important heuristic value to this model, as it is reminiscent of the repeated cycles of intoxication, abstinence, craving, and relapse that characterize alcohol abuse and dependence (Koob, 2003; Koob & Volkow, 2010; Vengeliene et al., 2008). IA2BC appears also to be a powerful pre-training method for promoting the acquisition of robust and reliable operant ethanol self-administration and ethanol-CPP, in order to gain more insights into the rewarding, reinforcing, and motivational mechanisms that govern alcohol seeking and drinking behaviors. The point concerning CPP is of particular interest, as CPP to ethanol is generally difficult to induce in rats (Tzschentke, 2007). Indeed, the study of GDNF and its regulatory effects on ethanol seeking and intake provide a wealth of data pointing to the usefulness of the two models presented in this review, in modeling multiple aspects of alcohol abuse and addiction, including transition from social-like drinking to excessive alcohol consumption, binge drinking, alcohol seeking, relapse, and neuroadaptations related to alcohol intake. However, some criteria of standardization remain to be determined, such as the systematic use of non-escalating alcohol-drinking control groups, and the exclusion of low-drinking rats.
Several aspects of addiction-like features remain to be investigated, including signs of behavioral dependence during acute and protracted abstinence and the development of compulsive alcohol seeking and taking, especially after a prolonged and extensive IA2BC training (Hopf et al., 2010). Such insights will advance the validation of these approaches, and will help to better define the specific aspects of drug abuse and addiction that are recapitulated by the models.
Acknowledgments
Generation of data presented in Figure 1 was supported by NIH–NIAAA R01 grant AA014366 (D.R.) and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California San Francisco (D.R.). The authors thank Mr. Oren Even-Chen for his assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interests.
References
- Adermark L, Jonsson S, Ericson M, Söderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Ahmadiantehrani S, Barak S, Ron D. GDNF is a novel ethanol-responsive gene in the VTA: implications for the development and persistence of excessive drinking. Addiction Biology. 2013 doi: 10.1111/adb.12028. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nature Reviews. Neuroscience. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Barak S, Ahmadiantehrani S, Kharazia V, Ron D. Positive autoregulation of GDNF levels in the ventral tegmental area mediates long-lasting inhibition of excessive alcohol consumption. Translational Psychiatry. 2011;1:1–9. doi: 10.1038/tp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Carnicella S, Yowell QV, Ron D. Glial cell line-derived neurotrophic factor reverses alcohol-induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. The Journal of Neuroscience. 2011;31:9885–9894. doi: 10.1523/JNEUROSCI.1750-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, et al. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nature Neuroscience. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Wang J, Ahmadiantehrani S, Yowell Q, Ben Hamida S, Ron D. GDNF Is An Endogenous Protector Against Excessive Alcohol Consumption And Relapse. doi: 10.1111/adb.12152. (submitted) [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Ben Hamida S, Neasta J, Lasek AW, Kharazia V, Zou M, Carnicella S, et al. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. The Journal of Neuroscience. 2012;32:15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholomey ML, Verplaetse TL, Czachowski CL. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behavioural Brain Research. 2013;238:252–258. doi: 10.1016/j.bbr.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Kotlińska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol and Alcoholism. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addiction Biology. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through G betagamma. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He DY, Nielsen CK, Bartlett SE, Janak PH, et al. Cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biological Psychiatry. 2009;66:146–153. doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, He DY, Yowell QV, Glick SD, Ron D. Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration. Addiction Biology. 2010;15:424–433. doi: 10.1111/j.1369-1600.2010.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Yowell QV, Ron D. Regulation of operant oral ethanol self-administration: a dose-response curve study in rats. Alcoholism: Clinical and Experimental Research. 2011;35:116–125. doi: 10.1111/j.1530-0277.2010.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcoholism: Clinical and Experimental Research. 2013;(Suppl 1):E394–E403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, et al. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacology, Biochemistry, and Behavior. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current Protocols in Neuroscience. 2008;Chapter 9(Unit 9.29) doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends in Neurosciences. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcoholism: Clinical and Experimental Research. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Debold JF, Miczek KA. Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology. 2013;225:313–327. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends in Pharmacological Sciences. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addiction Biology. 2011;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH. Region-specific induction of FosB/ΔFosB by voluntary alcohol intake: effects of naltrexone. Alcoholism: Clinical and Experimental Research. 2010;34:1742–1750. doi: 10.1111/j.1530-0277.2010.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. The Journal of Pharmacology and Experimental Therapeutics. 2012;341:196–204. doi: 10.1124/jpet.111.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye JH. Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Research Bulletin. 2011;86:428– 434. doi: 10.1016/j.brainresbull.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addiction Biology. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, et al. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcoholism: Clinical and Experimental Research. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Shelton KL. Self-administered ethanol as a discriminative stimulus in rats. Drug and Alcohol Dependence. 2001;64:243–247. doi: 10.1016/s0376-8716(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Current Opinion in Neurobiology. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcoholism: Clinical and Experimental Research. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CG, Bell R. Conditioned ethanol preference in rats. Alcohol and Alcoholism. 1988;23:359–364. doi: 10.1093/oxfordjournals.alcalc.a044829. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. The Journal of the American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. National Institute on Alcohol Abuse and Alcoholism Newsletter. 2004;3:3. [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, Carnicella S, Ron D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biological Psychiatry. 2011;70:575–582. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, et al. δ-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats. The Journal of Neuroscience. 2012;32:4540–4552. doi: 10.1523/JNEUROSCI.5345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Review. Evidence-based treatments of addiction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3277–3286. doi: 10.1098/rstb.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Roman E, Nylander I. Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol. 2011;45:607–614. doi: 10.1016/j.alcohol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. The Journal of Neuroscience. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, et al. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33:1835–1846. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hunter GA, Beaman CM, Hubbell CL. Toward understanding ethanol's capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacology, Biochemistry, and Behavior. 1985;22:483–487. doi: 10.1016/0091-3057(85)90051-6. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL. Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcoholism: Clinical and Experimental Research. 1992;16:529–532. doi: 10.1111/j.1530-0277.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Sabino V, Kwak J, Rice KC, Cottone P. Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcoholism: Clinical and Experimental Research. 2013;37:635–643. doi: 10.1111/acer.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson H. Initiation of ethanol-maintained behavior: a comparison of animal models and their implication to human drinking. In: Thompson T, Dews B, Barrett J, editors. Advances in Behavioral Pharmacology: Volume 6: Neurobehavioral Pharmacology. Hillsdale, New Jersey: Erlbaum Associates; 1987. pp. 221–248. [Google Scholar]
- Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcoholism: Clinical and Experimental Research. 1988;12:591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology. 1999;147:274–279. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature Neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biological Psychiatry. 2007;61:93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PloS One. 2013;8:e61965. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Nielsen CK, Li R, Bartlett SE. Intermittent access ethanol consumption dysregulates CRF function in the hypothalamus and is attenuated by the CRF-R1 antagonist, CP-376395. Addiction Biology. 2013 doi: 10.1111/adb.12024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Samson HH, Hodge CW. Differential changes in sucrose/ethanol and sucrose maintained responding by independently altering ethanol or sucrose concentration. Alcoholism: Clinical and Experimental Research. 1997;21:250–260. [PubMed] [Google Scholar]
- Steensland P, Fredriksson I, Holst S, Feltmann K, Franck J, Schilström B, et al. The monoamine stabilizer (−)-OSU6162 attenuates voluntary ethanol intake and ethanol-induced dopamine output in nucleus accumbens. Biological Psychiatry. 2012;72:823–831. doi: 10.1016/j.biopsych.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcoholism: Clinical and Experimental Research. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. British Journal of Pharmacology. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, et al. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. The Journal of Neuroscience. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Ahmadiantehrani S, He DY, Barak S, Kharazia V, et al. Nucleus accumbens-derived glial cell line-derived neurotrophic factor is a retrograde enhancer of dopaminergic tone in the mesocorticolimbic system. The Journal of Neuroscience. 2010;30:14502–14512. doi: 10.1523/JNEUROSCI.3909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. The Journal of Neuroscience. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiology & Behavior. 1972;8:345–362. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. The Journal of Neuroscience. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Meshkani J, Rezayof A, Beigzadeh R, Rostami P. Nicotinic acetylcholine receptors of the dorsal hippocampus and the basolateral amygdala are involved in ethanol-induced conditioned place preference. Neuroscience. 2010;168:505–513. doi: 10.1016/j.neuroscience.2010.03.019. [DOI] [PubMed] [Google Scholar]