Abstract
Objective
Obesity associates with increased numbers of inflammatory cells in adipose tissue (AT), including T cells, but the mechanism of T cell recruitment remains unknown. This study tested the hypothesis that the chemokine receptor CXCR3 participates in T-cell accumulation in AT of obese mice, and thus in the regulation of local inflammation and systemic metabolism.
Approach/Results
Obese wild-type mice exhibited higher mRNA expression of CXCR3 in peri-epididymal AT-derived stromal vascular cells, compared to lean mice. We evaluated the function of CXCR3 in AT inflammation in vivo using CXCR3-deficient and wild-type control mice that consumed a high-fat diet (HFD). Peri-epididymal AT from obese CXCR3-deficient mice contained fewer T cells than obese controls after 8 and 16 weeks on HFD, as assessed by flow cytometry. Obese CXCR3-deficient mice had greater glucose tolerance than obese controls after 8 weeks, but not after 16 weeks. CXCR3-deficient mice fed HFD had reduced mRNA expression of pro-inflammatory mediators such as MCP-1 and RANTES, and of anti-inflammatory genes such as Foxp3, IL-10, and arginase-1 in peri-epididymal AT, compared to obese controls.
Conclusions
These results demonstrate that CXCR3 contributes to T-cell accumulation in peri-epididymal AT of obese mice. Our results also suggest that CXCR3 regulates the accumulation of distinct subsets of T cells, and that the ratio between these functional subsets across time likely modulates local inflammation and systemic metabolism.
Keywords: CXCR3, T cell, inflammation, fat, obesity
Introduction
During obesity, adipose tissue (AT) accumulates inflammatory cells, including macrophages1, 2 and T cells3-6, which interact with endothelial cells and adipocytes in a local inflammatory network. This cellular crosstalk augments local production of multiple chemokines and cytokines, such as monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor alpha (TNFα), key elements in maintaining and propagating the inflammatory response in AT7, 8.
Various studies have examined the mechanisms of macrophage accumulation in AT of obese mice. Production of large amounts of MCP-1 by adipocytes in culture and by fat from obese mice suggested that this chemokine participates in the increased local macrophage traffic. Indeed, obese animals with deficiency of MCP-1 or its receptor, CCR2, have fewer macrophages in AT, less local inflammation, and improved insulin sensitivity, compared with obese controls9, 10. T lymphocytes also accumulate in AT of obese mice. We and others have shown increased T-cell numbers in AT from obese mice, relative to lean controls3, 4 Nishimura et al. demonstrated that immunological and genetic depletion of CD8+ T cells lowered macrophage accumulation and AT inflammation, and improved systemic insulin resistance6. Interferon-gamma (IFNγ), a prototypical cytokine of the Th1 subpopulation and an established orchestrator of the inflammatory response in atherosclerosis and other immune conditions, regulates fat inflammation4 — suggesting that adaptive immunity participates in the pathophysiology of obesity.
The exact mechanism of lymphocyte accumulation in AT remains unknown. Increased expression of regulated on activation, normal T cell expressed and secreted (RANTES) and its receptor (CCR5) in AT of obese mice and humans suggests that this duo participates in local T-cell migration3 — a conjecture not yet supported by definitive evidence. Moreover, RANTES recruits not only T cells, but also dendritic cells, natural killer cells, mast cells, eosinophils, and basophils, to sites of inflammation and infection11.
In contrast to the broad specificity of RANTES, the CXCR3 chemokine ligands — monokine induced by IFNγ (MIG), IFNγ-inducible protein 10 (IP-10) and IFN-inducible T-cell α chemoattractant (I-TAC) — selectively induce chemoattraction of T cells12. Overexpression of CXCR3 and its ligands occurs in a wide array of infectious and inflammatory diseases, including atherosclerosis13. Indeed, apolipoprotein E (ApoE)-deficient mice with deletion of either CXCR3 or IP-10 have significantly less atherosclerosis than do control ApoE-deficient mice14, 15. Treatment with a CXCR3 antagonist (NBI-74330) mitigates plaque development in association with reduced accumulation of effector T cells and macrophages in lesions of low-density lipoprotein receptor (LDLR)-deficient mice16. Recently, we showed that the adipocyte-derived mediator adiponectin, which diminishes in the plasma of obese subjects, inhibits the production of CXCR3 ligands by macrophages, and reduces T-cell accumulation in atheromata — suggesting a link between hypoadiponectinemia and T-cell accumulation via CXCR317.
These observations led us to test the hypothesis that CXCR3 participates in T-cell accumulation in AT of obese animals. Our results unveil CXCR3 as a crucial player in this process, demonstrating that it regulates local inflammation and affects systemic metabolic pathways that operate in obesity.
Materials and Methods
Briefly, male C57BL/6J mice and CXCR3-deficient mice (CXCR3-/-) in the C57BL/6J background were fed ad libitum a standard low-fat diet (LFD) (PicoLab Rodent Chow 5053; 13% kcal from fat) after weaning. At 8 weeks of age, mice were switched to a high-fat diet (HFD) (D12108 from Research Diets; 40% kcal from fat, 1.25% cholesterol, 0% cholate) and were kept on this diet for 8 or 16 additional weeks. After harvesting, the following experiments were performed: analysis of AT-derived stromal vascular cells (SVCs) by flow cytometry; analysis of inflammatory cells in AT by immunohistochemistry; quantification of gene expression by reverse transcription-quantitative PCR (RT-qPCR); peripheral cell blood count; measurements of blood cytokines and metabolic parameters, and assessment of indirect calorimetry, physical activity, and food intake. For extended review of materials and methods, please refer to the online supplement.
Results
Obese wild-type mice exhibit higher CXCR3 expression in peri-epididymal AT than lean wild-type mice
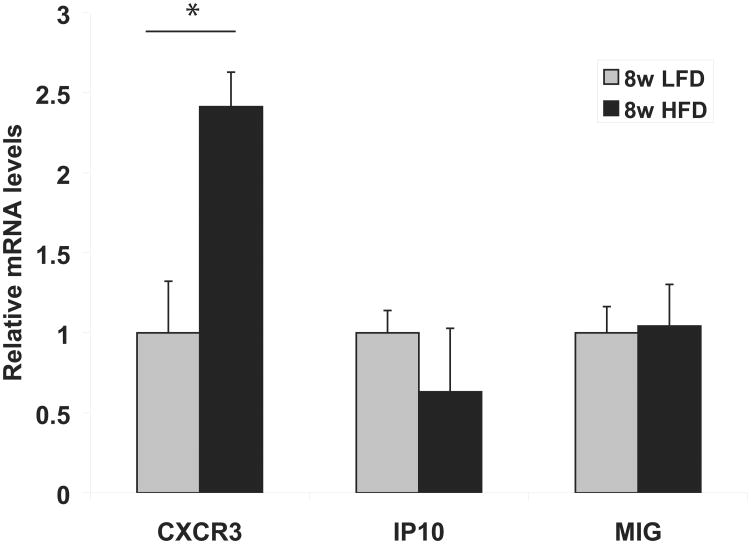
Peri-epididymal AT-derived SVCs from obese C57BL6 mice had significantly higher levels of CXCR3 mRNA than SVCs from lean controls after 8 weeks of HFD or LFD, respectively. Levels of mRNAs encoding the T-cell chemoattractants and the CXCR3 ligands IP-10 and MIG did not differ between the lean and obese animal groups at this time point (Figure 1).
Figure 1.
Peri-epididymal adipose tissue-derived stromal vascular cells (SVCs) from obese mice express more CXCR3 than SVCs from lean mice.
RNA was extracted from adipose tissue-derived SVCs isolated from lean (low-fat diet, LFD) or obese (high-fat diet, HFD) C57BL/6 mice. The graph represents relative mRNA levels of CXCR3, IP-10, and MIG in the LFD group (gray bars) and HFD group (black bars) after 8 weeks of diet, normalized to GAPDH mRNA expression. Data are expressed relative to LFD. *p <0.05 vs. LFD; n=5/group.
Obese CXCR3-deficient mice accumulate fewer T cells in peri-epididymal AT than obese wild-type mice
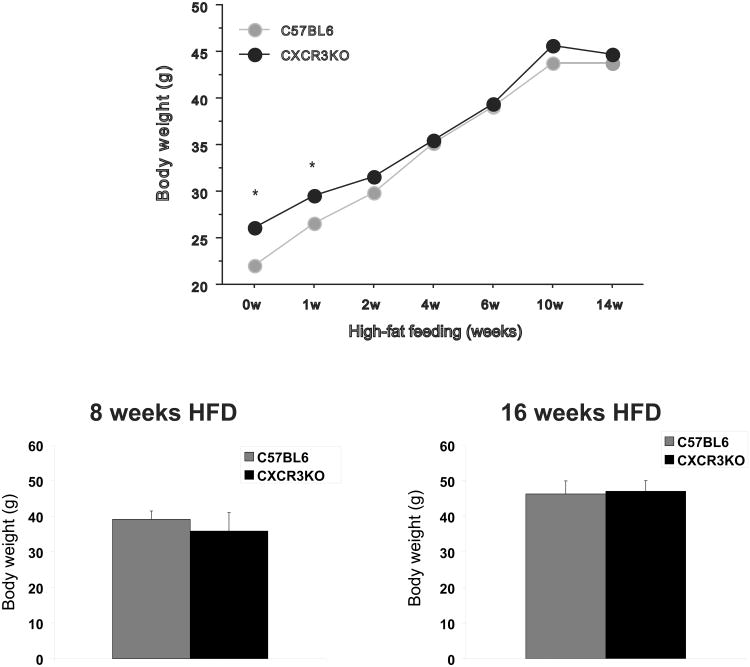
CXCR3-deficient mice and C57BL6 controls began receiving HFD at 8 weeks of age. With the exception of the first week of HFD feeding, body weights between the two groups were not different (Figure 2).
Figure 2.
Obese CXCR3-deficient mice and controls presented similar body weight after 8 weeks and after 16 weeks of high-fat diet. CXCR3-deficient mice and C57BL/6J controls were fed ad libitum standard low-fat diet (LFD) after weaning until 8 weeks of age. Mice were then switched to a high-fat diet (HFD) and were kept on it for 8 or 16 additional weeks. Body weight of each animal was measured at baseline and at 1, 2, 4, 6, 8, 10, 14, and 16 weeks after starting HFD. Graphs A and B represent the mean body weight of CXCR3-deficient mice (black bars) and their respective controls (gray bars) after 8 and 16 weeks of HFD, respectively.
CXCR3-deficient mice and controls showed no consistent differences in VO2, VCO2 production, or RER before or 4 weeks after the initiation of HFD (Supplementary Figure 1). Physical activity was lower, and a small but statistically significant decrease occurred in food intake among the CXCR3-deficient mice compared to controls on HFD.
After 8 weeks of HFD, both groups of mice had similar mean body weights (Figure 2) but different peri-epididymal fat weights (not shown). The number of SVCs isolated from the peri-epidydimal adipose tissue of obese CXCR3-deficient mice compared to respective controls after 8 weeks of HFD did not differ: 2.41×106 (±1.4×106) SVCs and 2.76×106 (±1.2×106) SVCs, respectively (p=0.5; n=11-13 in each group). This lack of significant difference persisted even when the cell count was adjusted for body weight or the amount of fat used in the experiment (not shown).
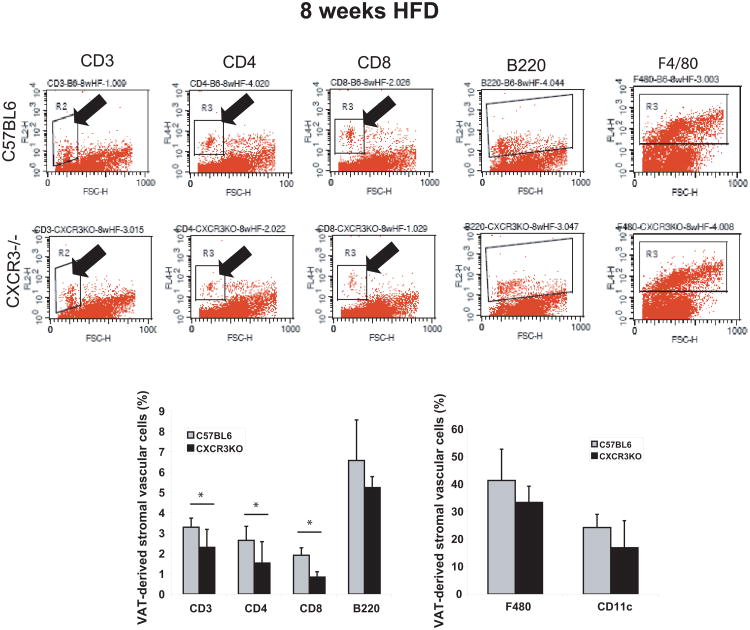
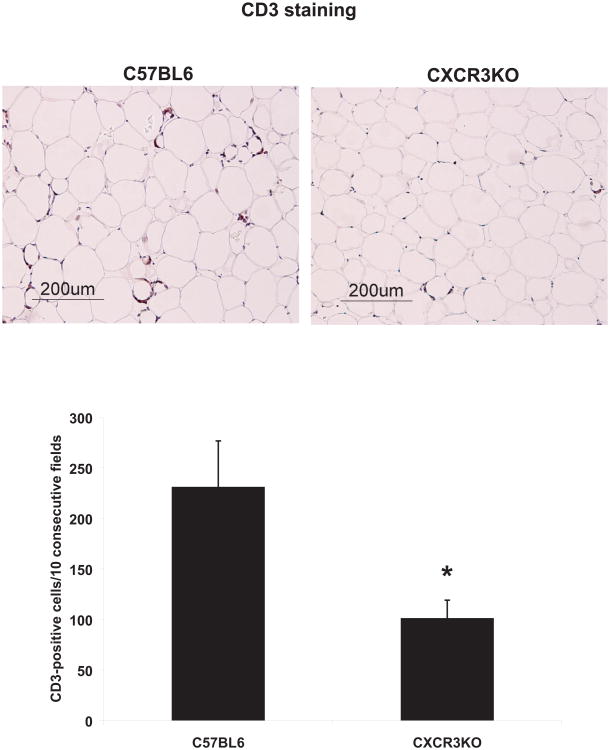
Obese CXCR3-deficient animals contained fewer CD3+ T lymphocytes in their peri-epididymal AT (represented as % of AT-derived SVCs) (Figure 3) compared to obese control mice (2.3+0.9 vs 3.3±0.5; p<0.01), as assessed by flow cytometry. Both CD4+ and CD8+ T-cell subsets were also decreased in the same AT depot of obese CXCR3-deficient mice, compared to obese wild-type counterparts (1.5±1 vs 2.6±0.7; p<0.02, and 0.8±0.3 vs 1.9±0.4; p<0.001, respectively) (Figure 3). Proportions of B220+ B cells, F4/80+ macrophages, and CD11c+ dendritic cells did not differ between the two groups (Figure 3). Consistent with the flow cytometry results, quantitative immunohistochemistry also showed fewer CD3+ T cells in peri-epididymal AT from obese CXCR3-deficient mice, compared to obese controls (Figure 4). Complete and differential blood counts did not reveal any difference in cell subsets between obese CXCR3-deficient mice and obese wild-type controls in peripheral blood (Supplementary Table 2). Likewise, both groups of mice had similar proportions of splenic cells expressing F4/80, CD3, CD4, CD8, and B220 (Supplementary Figure 2).
Figure 3.
Peri-epididymal adipose tissue from obese CXCR3-deficient mice contains fewer T cells than obese controls after 8 weeks of high-fat diet, as detected by flow cytometry.
Stromal vascular cells from 1 g of adipose tissue of CXCR3-deficient mice and wild-type control mice fed a high-fat diet (HFD) for 8 weeks were labeled with conjugated antibodies to CD3, CD4, CD8, B220, F480, and CD11c, and analyzed by flow cytometry. The graphs represent percentages of gated cells in the CXCR3-deficient group (black bars) and the control group (gray bars). Data are shown as mean ± SD. *p <0.02; n=9/group.
Figure 4.
Peri-epididymal adipose tissue from obese CXCR3-deficient mice contains fewer T cells than obese controls, as revealed by immunohistochemistry.
Sections of peri-epididymal fat tissue from obese CXCR3-deficient mice and obese controls were stained for CD3, as described in the text (representative images are shown). Positive cells were counted under a microscope in 10 consecutive high-power fields. Data are shown as mean ± SD. *p <0.01; n=3-4/group.
Both groups had similar mean body weight (Figure 2) and peri-epididymal fat weight (not shown) after 16 weeks of HFD. CXCR3-deficient mice yielded significantly fewer adipose tissue-derived SVCs compared to controls: 3.43×106 (±3.4×105) SVCs and 5.30×106 (±1.7×106) SVCs, respectively (p=0.045; n=5-6 in each group). This difference persisted after adjustment of cell count for body weight, but was attenuated after correction of the cell count for the weight of fat used in the experiment (not shown).
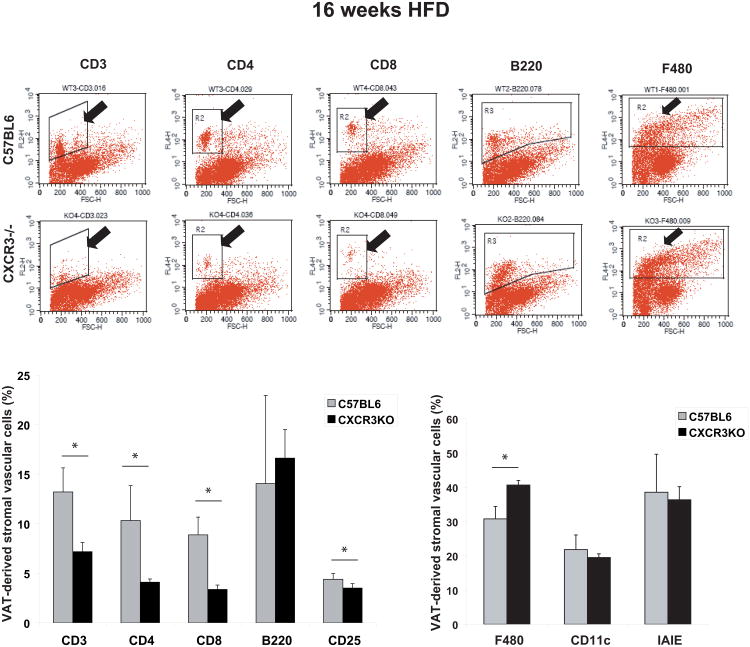
Similar to the results at the 8-week time point, AT from 16-week HFD-fed CXCR3-deficient mice had significantly fewer CD3+ T cells (7±0.9 vs 13±2.4; p<0.01), including both subsets — CD4+ (4±0.3 vs 10±3.5; p<0.02) and CD8+ (3.4±0.4 vs 8.9±1.8; p<0.001) — compared to control mice on the same diet (Figure 5). Fat of CXCR3-deficient mice had fewer CD25+ cells (panel not shown), representing both stimulated T cells and T regulatory cells (Tregs), than control mice (3.5±0.44 vs 4.4±0.58; p=0.02). AT from CXCR3-deficient mice had more F4/80+ macrophages than did control mice at the same time point (Figure 5). The proportion of cells positive for CD11c or MHCII did not differ between the two groups.
Figure 5.
Peri-epididymal adipose tissue from obese CXCR3-deficient mice contains fewer T cells than obese controls after 16 weeks of high-fat diet, as detected by flow cytometry. Stromal vascular cells from 1 g of peri-epididymal adipose tissue of CXCR3-deficient and wild-type controls consuming a high-fat diet (HFD) for 16 weeks were labeled with conjugated antibodies to CD3, CD4, CD8, B220, CD25, F480, CD11c, and IAIE, and analyzed by flow cytometry. The graphs represent percentages of gated cells in the CXCR3-deficient group (black bars) and the control group (gray bars). Data are shown as mean ± SD. *p ≤0.02; n=5-6/group.
When consuming LFD, the body weight of CXCR3-deficient and wild-type mice showed a small yet statistically significant difference up to 9 weeks (not shown). At the time of harvesting, after approximately 20 weeks of LFD, the body weights of the CXCR3-deficient and control groups did not differ, although the peri-epididymal fat weighed less in the CXCR3-deficient mice than in controls (not shown).
Obese CXCR3-deficient mice exhibit decreased expression of inflammation-related genes in peri-epididymal AT compared to obese wild-type mice
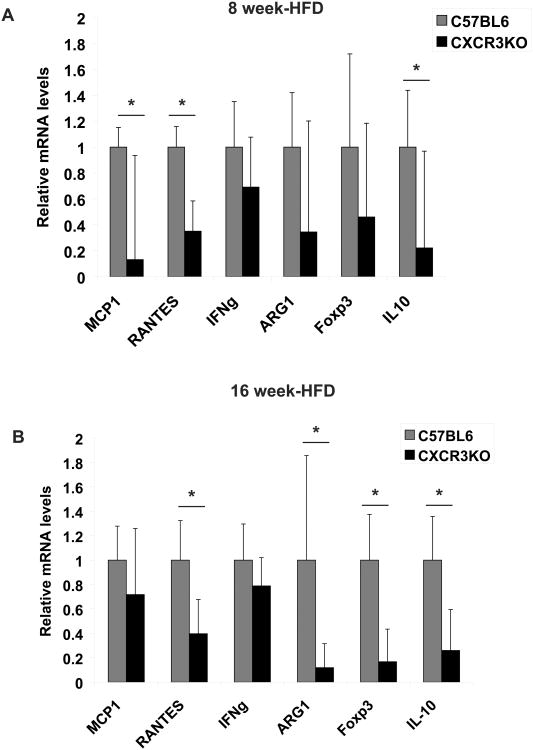
CXCR3 deficiency led to a decrease in expression of several genes related to inflammation. After 8 weeks of HFD, obese CXCR3-deficient mice exhibited lower mRNA expression of chemokines such as MCP-1 and RANTES in AT, compared to their wild-type counterparts (Figure 6A). Compared to controls, CXCR3-deficient mice also had lower levels of interleukin-10 (IL-10) (Figure 6A), a cytokine with anti-inflammatory functions. Tregs and M2 macrophages can elaborate IL-10 in several inflammatory conditions, and seem to exert a metabolic protective effect in obesity. This finding prompted us to measure mRNA levels of the T regulatory cell marker Foxp3 (a forkhead family transcription factor) and arginase 1, one of the signature products of M2 macrophages in mice. The expression of these markers did not differ significantly between CXCR3-deficient mice and wild-type controls after 8 weeks of HFD (Figure 6A). After 16 weeks of HFD, CXCR3-deficient mice had significantly reduced expression of IL-10, Foxp3, and arginase 1 mRNAs in peri-epididymal AT compared to controls (Figure 6B). IFNγ, a signature of T-helper 1 cells, showed a trend towards decreased mRNA expression at both 8 weeks and 16 weeks of HFD, but the differences did not reach statistical significance (Figure 6). The mRNA expression levels of TNF-α, IL-6, IP-10, MIG, and MHC II did not differ between the CXCR3-deficient and control groups at both time points (not shown).
Figure 6.
Obese CXCR3-deficient mice exhibit decreased expression of inflammation-related genes in their peri-epididymal adipose tissue, compared to obese wild-type mice. mRNA levels of MCP-1, RANTES, IFNγ, ARG1, Foxp3, and IL-10 in adipose tissue from CXCR3-deficient mice (black bars) and control mice (gray bars) at 8 weeks (A) and at 16 weeks of high-fat diet (HFD) (B) were quantified by RT-qPCR and normalized to GAPDH mRNA expression. Fold change was calculated relative to wild-type controls. *p<0.05 vs. wild-type controls; n=6/group.
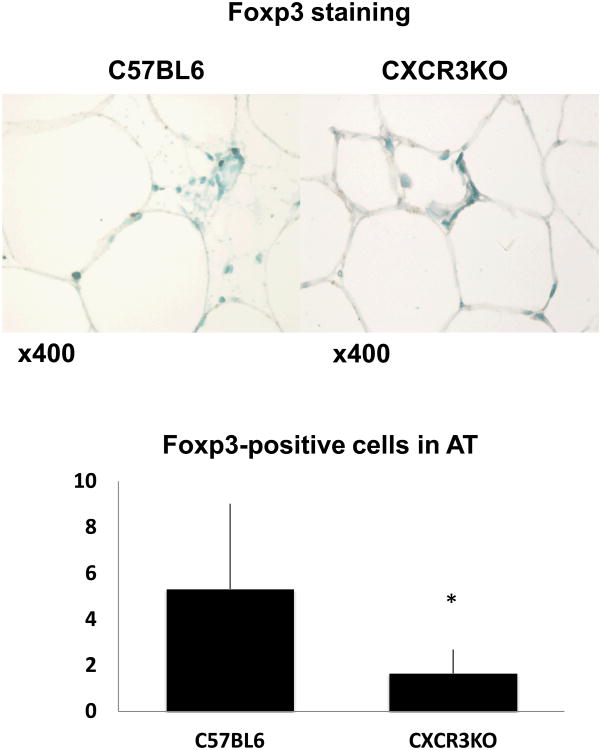
In agreement with the reduced mRNA expression of Foxp3 in the peri-epididymal fat of CXCR3-deficient mice compared to controls after 16 weeks of diet, immunohistochemistry also showed significantly fewer Foxp3-positive cells in the adipose tissue of CXCR3-deficient animals (Figure 7).
Figure 7.
Peri-epididymal adipose tissue from CXCR3-deficient mice contains fewer Foxp3-positive cells than controls, as revealed by immunohistochemistry. Sections of peri-epididymal fat tissue from CXCR3-deficient mice and controls were stained for Foxp3, as described in the text (representative pictures are shown). Positive cells were counted under a microscope. Data are shown as mean ± SD. *p <0.05; n=6-9/group.
Obese CXCR3-deficient mice exhibit improved glucose tolerance and decreased plasma levels of leptin and total cholesterol compared to obese wild-type mice
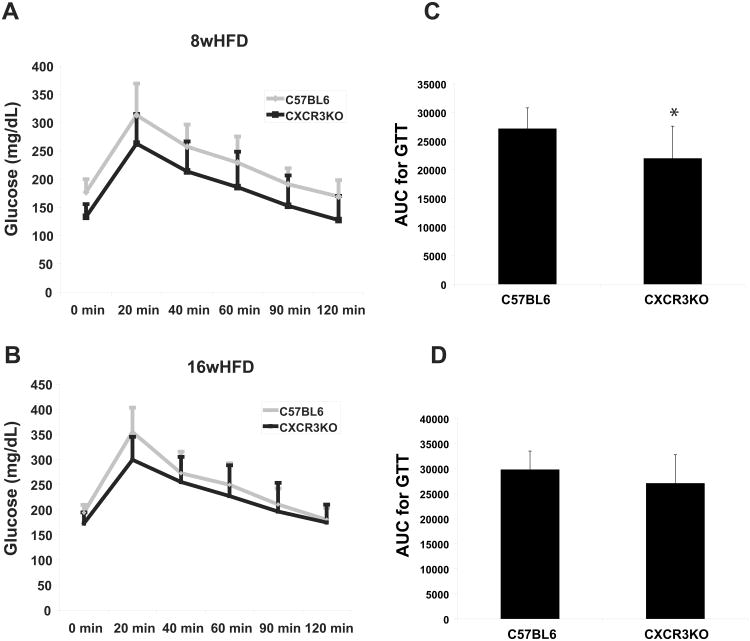
Despite having comparable body weights, CXCR3-deficient mice had greater glucose tolerance in response to intraperitoneal glucose load than did control mice after 8 weeks of HFD (according to the area under the curve and all the individual time points on the curve) (Figures 8A and 8C). Mice that consumed HFD for 16 weeks showed a similar trend, but the difference was not statistically significant; although there was a difference at baseline and at 20 minutes after glucose loading, all other time points on the glucose tolerance curve did not differ significantly between the groups, resulting in a non-significant difference between the areas under the curve (Figures 8B and 8D).
Figure 8.
Obese CXCR3-deficient mice exhibit improved glucose tolerance compared to obese wild-type mice.
Average glucose tolerance curves of CXCR3-deficient mice (black curves) and wild-type control mice (gray curves) after 8 weeks (A) and after 16 weeks (B) of high-fat diet (HFD). Areas under the glucose tolerance curves (AUC) were calculated for each mouse, and the average of each group is represented in C and D. Data are shown as mean ± SD.*p≤0.01; n=19-22/group.
CXCR3-deficient mice had lower concentrations of plasma leptin than their obese wild-type counterparts after both 8 and 16 weeks of HFD (Supplementary Figure 3). Similarly, total plasma cholesterol levels, which increased over time in both groups of mice, were lower in CXCR3-deficient mice compared to controls (Supplementary Figure 3). Plasma concentrations of adiponectin and insulin did not differ significantly between the two groups at either time point (Supplementary Figure 3).
Discussion
Despite the increasing recognition of the participation of T lymphocytes in the pathophysiology of obesity, the mechanism by which they accumulate in fat under obese conditions remains unclear. MCP-1 and RANTES, chemokines abundantly expressed in AT of obese mice, can induce migration of several cell types (including T lymphocytes) during inflammatory processes. Indeed, both of these chemokines appear to participate pivotally in macrophage accumulation in AT of obese mice and humans3, 9, 18. The finding that T-cell accumulation in AT of obese mice precedes the appearance of macrophages,5, 6 however, suggests the operation of a chemokine/receptor system with higher selectivity for T cells than that of RANTES/CCR5 or MCP1/CCR2. Our results demonstrate that CXCR3 participates in T-cell accumulation in AT in the context of obesity. The exclusive ligation of CXCR3 with the T-cell chemoattractants IP-10, MIG, and ITAC, and its abundant expression in activated T cells, make this chemokine system an ideal candidate to initiate T-cell recruitment in AT of obese animals, and therefore to orchestrate fat inflammation.
Our data show a significant increase of CXCR3 mRNA levels in peri-epididymal AT-derived SVCs from obese C57BL6 mice, compared to lean controls. Because CXCR3 expression associates with T-cell activation, this finding suggests that obesity enhances accumulation of activated T cells in AT.
CXCR3 deficiency in HFD-fed obese mice associated with significantly fewer CD3+ cells in peri-epididymal fat pads, represented by reduced numbers of both T lymphocyte subtypes — CD4+ and CD8+ cells. This reduction occurred at both early (8 weeks) and late (16 weeks) time points, with a more marked fall at 16 weeks. Therefore, despite the diversity and potential importance of other chemotactic pathways in fat inflammation, these results indicate a high degree of selectivity among them, and support a crucial role of CXCR3 in the accumulation of T cells in AT of obese animals across time. Unlike T-cell numbers, macrophage and dendritic cell numbers were not lower in AT of obese CXCR3-deficient mice, compared to controls. After 16 weeks of HFD, macrophages were more numerous in the fat of CXCR3-deficient mice, relative to controls. This finding suggests that changes in specific T-cell subsets, such as the reduction of the Treg cellular pool in peri-epididymal fat of CXCR3-deficient mice compared to controls, impacts the local number of macrophages later. Indeed, Treg cells can have suppressive effects on macrophages and T-effector cells. The similar numbers of T cells and other leukocyte populations in the spleen and blood of obese CXCR3-deficient mice and control mice further support a local effect of CXCR3 on T-cell accumulation in AT of obese animals, independent of the circulating cell number.
T lymphocytes display functional heterogeneity, and different T-cell subsets can exert pro-inflammatory or anti-inflammatory actions on other lymphocyte subpopulations and other cells of the innate immune system19. CXCR3 deficiency in the context of obesity associated with reduction of the mRNA expression levels of RANTES (a product of cytotoxic T lymphocytes) and of MCP-1, predominantly secreted by activated macrophages. Conversely, obese CXCR3-deficient mice also had decreased expression of IL-10 (a prototypical anti-inflammatory cytokine) and Foxp3 (a Treg marker). These results indicate that CXCR3 participates in the accumulation of various T-cell subsets, and thus helps define the expression profiles of pro-inflammatory and anti-inflammatory molecules present in AT of obese mice. CXCR3 deficiency also reduces the expression of arginase 1, a marker of alternatively activated macrophages (M2) induced by Th2 cytokines such as IL-1020, typically involved in tissue repair and inflammation blockade21, 22. This finding suggests that the CXCR3/IP10-Mig-ITAC chemokine system indirectly regulates macrophage function in AT of obese mice.
CXCR3 deficiency correlated with significant changes in several systemic metabolic variables. Obese CXCR3-deficient mice had reduced levels of plasma leptin, and cholesterol. In most atherosclerosis susceptible mouse strains (e.g. C57BL/6) plasma HDL-C levels decrease and total cholesterol levels increase substantially after initiating a high-fat diet. The majority of the increase in plasma cholesterol derives from increase of VLDL and LDL fractions23, 24. But, considering that in normal mice the HDL fraction carries most of cholesterol and that HDL-C levels fall under high-fat diet, we cannot exclude a contribution of HDL-C decrease among the CXCR3-deficient mice to our findings.
Obese CXCR3-deficient mice also had greater glucose tolerance than their obese wild-type counterparts after 8 weeks of HFD. But differences in glucose tolerance curves between the two groups became non-significant after 16 weeks of HFD, coinciding with a substantial fall in the expression of anti-inflammatory markers — including IL-10 (also significantly decreased after 8 weeks of HFD), arginase 1, and Foxp3 — in the fat tissue of CXCR3-deficient mice, compared with controls. In agreement with this finding, the adipose tissue of CXCR3-deficient mice had significantly fewer Foxp3-positive cells than did that of controls, as determined by immunohistochemical analysis, suggesting that CXCR3 plays a role in Treg accumulation in AT. Several studies have shown that reduced numbers of anti-inflammatory cells (such as M2 macrophages and Tregs) and their products in AT associate with deterioration of metabolic homeostasis25-26. Feuerer et al. demonstrated that AT of obese mice contains fewer Tregs than AT of lean mice, and this diminished pool of cells may cause excessive inflammation and its metabolic consequences in obesity25. Moreover, treatment with a CD3-specific antibody reduced the predominance of Th1 cells over Foxp3 cells in obese mice, reversing insulin resistance27.
Our findings suggest that CXCR3 deficiency interferes with the accumulation of distinct T-cell subsets with antagonistic functions, and temporal changes in the size of distinct T-cell pools may alter the balance of expression of pro-inflammatory and anti-inflammatory molecules. The net effect of this duality between pro-inflammatory and anti-inflammatory forces within the AT likely impacts local and systemic metabolism. In our study, reduced numbers of effector T cells in the AT of CXCR3-deficient mice may have prevailed at 8 weeks of HFD, eliciting improved glucose tolerance, whereas a reduced number of anti-inflammatory T cells may have compensated the effect at 16 weeks of HFD, abrogating the improvement in glucose tolerance.
Our study has limitations. We have not analyzed the impact of CXCR3 deficiency on fat depots beyond the peri-epididymal adipose tissue. Despite the clear importance of this fat pad in mice as an inflammatory source in the context of obesity, the present results do not discount potential contributions of other adipose tissue depots. Accumulating evidence supports a differential role of distinct fat depots in a range of systemic or local effects28. Whereas fat storage in the visceral area associates with increased local and systemic inflammation and higher cardiometabolic risk in humans, accumulation of fat in lower-body subcutaneous adipose tissue likely has less adverse metabolic consequences. Moreover, accumulation of fat in adipose tissue depots around or near organs, such as perivascular or pericardial fat, likely affects primarily the adjacent tissue through lipotoxicity and secretion of inflammatory mediators.
Another unanswered question regards the origin of adipose tissue inflammation in fat-fed mice – obesity or the high-fat diet itself. Data from this manuscript and from previous feeding investigations do not isolate the excess adiposity from the high-fat diet itself as the driver of adipose tissue inflammation in diet-induced obese mice, another limitation inherent in the design of such studies.
Conclusion
This study demonstrates an important role of CXCR3 in T-cell accumulation in peri-epididymal AT of obese mice, with a significant metabolic impact. Our results suggest that CXCR3 may regulate the accumulation of distinct subsets of T cells — the relative proportions of which, across time, likely influence local and systemic metabolism. Understanding the roles of both the innate and adaptive immune arms in the pathophysiology of obesity may pave the road toward novel therapeutic alternatives against this condition.
Supplementary Material
Significance.
Obesity associates with macrophages and T-cells in adipose tissue (AT), and these inflammatory cells likely contribute to the metabolic consequences of obesity. Although the mechanisms of macrophage traffic in AT have undergone extensive exploration, the mechanism of local T cell accumulation remains unknown.
This study demonstrates that the chemokine receptor CXCR3 contributes importantly to T-cell accumulation in peri-epididymal AT of obese mice. Our results also suggest that CXCR3 mediates the accumulation of distinct subsets of T cells, including T-regulatory cells, and therefore may influence local expression of pro- and anti-inflammatory mediators. The ratio between these functional T-cell subsets across time modulates local inflammation and systemic metabolism.
Acknowledgments
We thank Elissa Simon-Morrissey, Eugenia Shvartz, and Yevgenia Tesmenitsky for skillful technical assistance, and Sara Karwacki for excellent editorial assistance.
Sources of Funding: This work was supported by the Donald W. Reynolds Foundation; by grants from the National Institutes of Health (R01 HL080472 to P.L., CA-069212 to A.D.L., and DK-48873 and DK-56626 to D.E.C.); by a grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (BEX 1594 04/4; to V.Z.R.); and by the Harvard Digestive Diseases Center (P30 DK034854).
Nonstandard Abbreviations and Acronyms
- AT
Adipose tissue
- LFD
Low-fat diet
- HFD
High-fat diet
- MCP-1
Monocyte chemoattractant protein-1
- TNFα
Tumor necrosis factor alpha
- IFNγ
Interferon-gamma
- RANTES
Regulated on activation, normal T cell expressed and secreted
- MIG
Monokine induced by IFNγ
- IP-10
IFNγ-inducible protein 10
- I-TAC
IFN-inducible T-cell α chemoattractant
- ApoE
Apolipoprotein E
- LDLR
Low-density lipoprotein receptor
- SVC
Stromal vascular cells
- IL-10
Interleukin-10
- Foxp3
Forkhead family transcription factor
- Treg
T regulatory cell
Footnotes
Disclosures: None.
References
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal t cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 4.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a th1 cytokine, regulates fat inflammation: A role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. Cd8+ effector t cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 7.Rocha VZ, Folco EJ. Inflammatory concepts of obesity. International journal of inflammation. 2011;2011:529061. doi: 10.4061/2011/529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 9.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. Mcp-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr Ccr2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appay V, Rowland-Jones SL. Rantes: A versatile and controversial chemokine. Trends in immunology. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor cxcr3: An unexpected enigma. Current topics in developmental biology. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 13.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three t lymphocyte-activating cxc chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors ccr2 and cxcr3 in development of atherosclerosis in vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 15.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine cxcl10 promotes atherogenesis by modulating the local balance of effector and regulatory t cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 16.van Wanrooij EJ, de Jager SC, van Es T, de Vos P, Birch HL, Owen DA, Watson RJ, Biessen EA, Chapman GA, van Berkel TJ, Kuiper J. Cxcr3 antagonist nbi-74330 attenuates atherosclerotic plaque formation in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:251–257. doi: 10.1161/ATVBAHA.107.147827. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, Libby P. Adiponectin inhibits the production of cxc receptor 3 chemokine ligands in macrophages and reduces t-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 18.Keophiphath M, Rouault C, Divoux A, Clement K, Lacasa D. Ccl5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:39–45. doi: 10.1161/ATVBAHA.109.197442. [DOI] [PubMed] [Google Scholar]
- 19.Long SA, Buckner JH. Cd4+foxp3+ t regulatory cells in human autoimmunity: More than a numbers game. J Immunol. 2011;187:2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls t-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 22.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class a macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paigen B, Holmes PA, Mitchell D, Albee D. Comparison of atherosclerotic lesions and hdl-lipid levels in male, female, and testosterone-treated female mice from strains c57bl/6, balb/c, and c3h. Atherosclerosis. 1987;64:215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishina PM, Wang J, Toyofuku W, Kuypers FA, Ishida BY, Paigen B. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids. 1993;28:599–605. doi: 10.1007/BF02536053. [DOI] [PubMed] [Google Scholar]
- 25.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory t cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. International journal of cardiology. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.