Abstract
Functional imaging studies have implicated the orbitofrontal cortex (OFC) in the pathophysiology of borderline personality disorder (BPD). To date, however, volume-based magnetic resonance imaging (MRI) studies have yielded mixed results. We used a surface-based processing approach that allowed us to measure five morphometric cortical features of the OFC, including volumetric (cortical thickness and surface area) and geometric (mean curvature, depth of sulcus, and metric distortion – three indicators of cortical folding) parameters. Participants comprised 25 female BPD patients with no other current psychiatric comorbidity and 25 age- and gender-matched healthy controls who received structural MRI scans. Images were processed using the Freesurfer package. All BPD patients had a history of comorbid psychiatric disorder(s) and were currently on medications. Compared with controls, the BPD group showed reduced cortical thickness, surface area, mean curvature, depth of sulcus, and metric distortion in the right medial OFC. In the left medial OFC, the BPD group had reduced cortical thickness and mean curvature, but increased metric distortion. This study confirmed the utility of surface-based analysis in the study of BPD cortical structures. In addition, we observed extensive structural abnormalities in the medial OFC of female subjects with BPD, findings that were most pronounced in the right OFC, with preliminary data suggesting hemispheric asymmetry.
Keywords: Magnetic resonance imaging, Cortical thickness, Multimodal analysis
1. Introduction
Borderline personality disorder (BPD) is a devastating condition that affects 1% to 2% of the population and causes an intense disruption of patients’ lives and relationships (Minzenberg et al., 2007; Minzenberg et al., 2008). BPD patients often exhibit emotional instability, impulsive behavior, rapid mood changes, and a propensity toward intense negative emotional states like anger, anxiety and dysphoria (Lis et al., 2007; Silbersweig et al., 2007).
The orbitofrontal cortex (OFC) is involved in high-level aspects of cognition and emotional behavior (Elliott et al., 2000; Ongur and Price, 2000). OFC lesions result in emotional dysregulation, along with impulsivity and socially inappropriate behavior (Malloy et al., 1993). In patients with BPD, functional neuroimaging studies have consistently identified abnormal findings in the OFC (Soloff et al., 2000; Soloff et al., 2003; Soloff et al., 2005; New et al., 2007; New et al., 2009). To date, however, structural magnetic resonance imaging (MRI) investigations have yielded mixed results, with few studies showing reduced OFC volume (Tebartz van Elst et al., 2003; Chanen et al., 2008; Vollm et al., 2009; Brunner et al., 2010), while others failed to replicate similar findings (Rüsch et al., 2003; Goodman et al., 2011). Several factors could have contributed to the discrepant results, including the following: (1) Differences in the image-processing methods used in the various MRI studies, a factor that is especially important in the context of data indicating a differential effect of BPD on white vs. gray matter or right vs. left hemisphere. (2) Given the putative functional distinction between subregions of the OFC (e.g. medial for emotional processing vs. lateral for cognitive processing) (Elliott et al., 2000; Ongur and Price, 2000), subregions differences might have confounded region-of-interest analyses. (3) All previously studied MRI cohorts were carried out in BPD patients with a high level of comorbidity with several other psychiatric disorders.
To contribute to the understanding of such discrepancies between the above-mentioned volume-based preliminary findings, we used a well-validated surface-based processing method to measure five geometric parameters of the OFC in a homogeneous group of female BPD patients without psychiatric comorbidities. We hypothesized that the BPD group would present significant OFC alterations, mainly OFC reductions, of all morphometric parameters in comparison with controls. In addition, an exploratory analysis of the morphometrics of the OFC in relationship to the severity of the disorder was also conducted.
2. Methods
2.1. Participants and study design
BPD patients and healthy controls, aged 18–45, were enrolled in this cross-sectional, case-control neuroimaging study. Participants enrolled were recruited from 137 BPD patients at an outpatient clinic at a tertiary center (Department of Psychiatry of the Universidade Federal de São Paulo, Brazil). Out of 137 screened subjects, 25 BPD (18.2%) and 25 healthy subjects met the study criteria. The Ethics Committee of the Universidade Federal de São Paulo, Brazil, approved all study procedures. Before enrollment, informed consent was obtained from all subjects. A comprehensive medical and psychiatric evaluation was performed to determine eligibility. The Structured Clinical Interview for DSM-IV Axis I & II Disorders (SCID-I and SCID-II) was used to confirm diagnoses (Spitzer et al., 1989; First et al., 1998; American Psychiatric Association, 2004). In addition, the Clinical Global Impression scale (CGI) was used to document severity – on a scale that ranges from 1 (less severe) to 7 (more severe) (Guy, 1976). For the patients group, inclusion criteria were (1) female gender; (2) BPD diagnosis, as determined by SCID-II; (3) absence of other current psychiatric comorbidities, as determined by SCID-I and SCID-II; (4) no MRI contraindications; (5) medically stable; and (6) treatment on our unit for at least 6 months. A total of 25 females with BPD, and 25 age- and gender-matched healthy subjects completed all study procedures, including evaluations and MRI protocols.
2.2. MRI data acquisition
All MRI studies were conducted on a 1.5 T (Magnetom Sonata[Maestro Class] – Siemens AG, Medical Solutions, Erlangen, Germany) with an eight-channel head coil. To minimize variation, the same investigator positioned all subjects using the orbito-metal line as landmark. The following two conventional sequences were performed: (a) Axial T2-weighted FLAIR (fluid-attenuated inversion recovery) in a plane parallel to the anterior commissure-posterior commissure (AC-PC) line [TR = 8500 ms, TE = 107 ms, IT = 2500 ms, slice thickness = 5.0 mm, slice interval = 1.5 mm, field of view = 240 mm, matrix size = 256 × 256, number of excitations = 1]; b) Sagittal T1-gradient echo volumetric acquisition for multiplanar reconstruction (TR = 2000 ms, TE = 3.42 ms, flip angle = 15 degrees, field of view = 245 mm, 1.0-mm slice thickness with no gaps, totaling 160 slices per slab, matrix size = 256 × 256, number of excitations = 1). All patients and controls included in the study had normal images on visual inspection. Scans displaying low image quality or clinical abnormalities were excluded.
2.3. Images processing
Structural images were processed using the recon-all pipeline of the Freesurfer package, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). The main steps of this pipeline are gray/white matter segmentation, pial and white matter surface modeling, transformation of the cortical surface to spherical coordinates, nonlinear surface registration based on curvature (gyrus and sulcus) allowing for analysis of multiple subjects and automated parcellation of cortical areas. Technical details of such procedures are described in the pivotal studies (Dale et al., 1999; Fischl et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004).
The following five morphometric parameters (per vertex) were extracted using Freesurfer: average convexity (depth of sulcus), mean radial curvature, metric distortion (Jacobian), cortical thickness, and surface area. The average convexity is a measure of the primary folding of a surface. Mean radial curvature is used to quantify small secondary and tertiary folds. Metric distortion measures the deformation of individual cortical surfaces when registered to the Freesurfer template (fsaverage). Cortical thickness is the distance between white matter and pial surfaces. Surface area reflects the area of the pial surface. Since this is a hypothesis-driven investigation focusing on OFC characterization, the results were masked to include only the surface vertices within the segmented lateral and medial portions of the OFC. The delineation of medial and lateral OFC of each individual was defined using the automated parcellation described in Fischl et al. (2004) using the Desikan-Killiany Atlas (Desikan et al., 2006). Additional information about Freesurfer cortical parcellation can be found at http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation. Further technical details of these parameters are also described in prior publications (Dale et al., 1999; Fischl et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004).
2.4. Statistical analyses
Clinical and demographic data were presented as mean ± standard deviation. Before analyses were performed, measures were examined for normality using the Shapiro-Wilk test. The level of significance was set at p<0.05, two-tailed. Age and handedness matching between patients and controls was evaluated using independent sample t-tests and chi-square tests. Statistical difference maps between BPD and controls were constructed, in Freesurfer, using a GLM (generalized linear model) vertex-by-vertex analysis and assuming a significance level of p ≤ 0.05; corrected for multiple comparisons using false discovery rate (FDR).
3. Results
3.1. Demographic and clinical data
Table 1 presents demographic information for the BPD (n = 25) and the healthy (n = 25) groups; the two groups showed no significant demographic differences. Among BPD patients, the mean (±SD) duration of the disorder was 16.6 ±9.5 years and the mean CGI score (an indicator of the severity of psychiatric disorders) was 3.3 ±1.8 (range 3–6). Although clinically stable and without fulfilling complete criteria for any current psychiatric comorbidity at the time of the study, all BPD patients had a past psychiatric history, mainly mood disorders (19 patients; 76%). Among them, 13 patients had the diagnosis of major depressive disorder and 6 of bipolar disorder. Other diagnoses were alcohol and drug abuse/dependence (13; 52%) and nonspecific psychotic disorders (9; 36%). Twelve BPD patients (48%) had a history of two or more psychiatric comorbidities. Regarding the number and type of psychotropic medications used, all BPD patients were taking at least one medication (antidepressant, mood stabilizer or antipsychotic) at the time of the study. Twenty-four patients were taking a mood stabilizer, while 18 were taking antidepressants and 10 were using antipsychotics. Six patients were on monotherapy, 13 were taking two medications, and six were on three medications.
Table 1.
Demographics and global brain measurements.
| BPD (n = 25) | Control (n = 25) | Test values | Degrees of freedom |
||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P valuea | |||
| Age in years | 32.7 ± 9.1 | 32.2 ± 7.1 | tb=2.98 | 1 | 0.82 |
| Females (n, %) | 25 (100%) | 25 (100%) | χc=2.70 | 1 | – |
| Right-handed (n, %) | 21 (84%) | 20 (80) | χc=1.95 | 1 | 0.79 |
| Years of education | 9.2 ± 6.3 | 12.8 ± 5.1 | tb=3.75 | 1 | 0.12 |
| Intracranial volume (mm3) | 1456 ± 107 | 1464 ± 115 | tb=2.78 | 1 | 0.74 |
| Total brain volume (mm3) | 1301 ± 105 | 1305 ± 112 | tb=3.27 | 1 | 0.51 |
| Cortical gray matter (mm3) | 463.6 ± 44.1 | 470.2 ± 39.9 | tb=2.95 | 1 | 0.68 |
| White matter volume (mm3) | 229.9 ± 27.3 | 252.4 ± 18.3 | tb=2.80 | 1 | 0.71 |
a. Independent t-testb or Chi-square testc (2-tailed, significance set at p ≤ 0.05).
Abbreviation: BPD: borderline personality disorder; SD: standard deviation; brain measurements are provided in mm3.
3.2. Morphometrics of the OFC
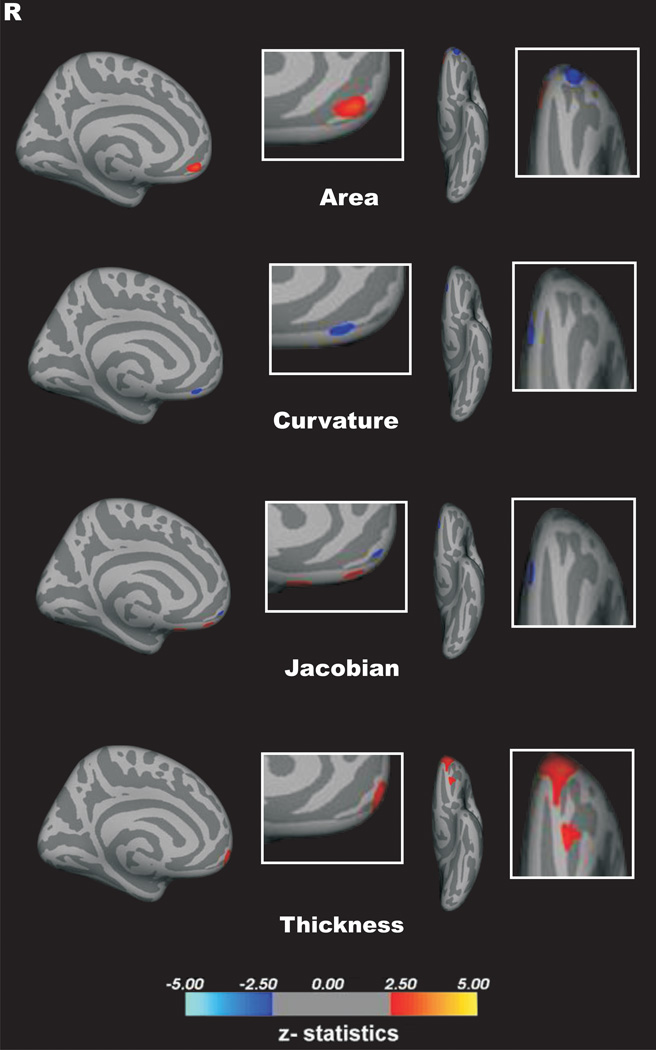
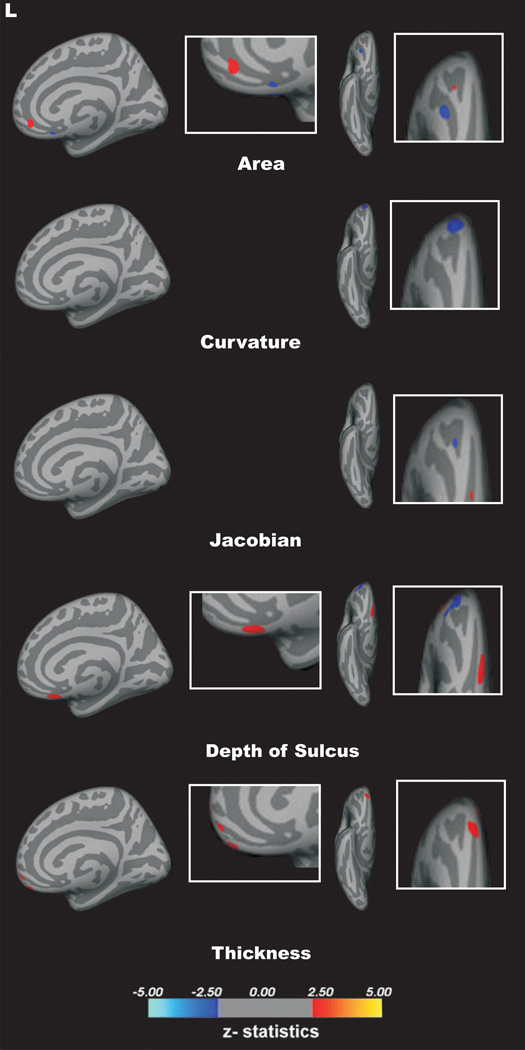
A vertex-by-vertex surface-based analysis showed that patients with BPD, compared with controls, had a significant reduction of cortical thickness and curvature, but an increase in metric distortion (p < 0.05, corrected for multiple comparisons) in the left medial OFC. In addition, a significant reduction of cortical thickness, curvature, depth of sulcus, surface area and metric distortion in the right medial OFC of BPD group as compared with healthy controls (p < 0.05, corrected for multiple comparisons; Fig. 2). There were no additional significant between-group differences in the surface area or depth of sulcus of the left medial OFC.
Figure 2.
3.3. Correlational analyses
A correlation analysis between CGI scores and the five parameters (thickness, curvature, metric distortion, surface area, and depth of sulcus) at the segmented lateral and the medial OFC was performed, but no significant correlations were found (p > 0.05).
4. Discussion
This study examined structural OFC differences between BPD patients and matched controls, using a multi-parameter approach based on geometric cortical surface characteristics. Thus, five morphological features, including volumetric (thickness and surface area) and geometric (curvature, depth of sulcus, and metric distortion) parameters were measured. Bilateral reduction in cortical thickness and mean curvature of the medial OFC in BPD patients compared with healthy controls were observed. In addition, surface area and depth of sulcus parameters were reduced in the right, but not left, medial OFC of the BPD group. Intriguingly, the metric distortion measure was lower in the right, but higher in the left, medial OFC in the BPD group as compared with the healthy control group. In contrast, there were no significant between-group differences in any of the five parameters in the lateral OFC. Finally, our correlational analysis showed no association between structural MRI measures and illness severity, as assessed by the CGI.
Cortical morphology is of great interest in both normal development and a wide variety of brain disorders (Huttenlocker, 1990; Sowell et al., 2004; Pannizon et al., 2009; Ecker et al., 2010; Pereira et al., 2011). Emerging evidence suggests that alterations of cortical thickness and surface area may reflect underlying genetic and neurobiological processes. Hence, approaches based on cortical morphology may provide valuable information to better understand the underpinnings of a number of neurodegenerative and psychiatric disorders (Huttenlocker, 1990; Sowell et al., 2004; Pannizon et al., 2009; Ecker et al., 2010; Pereira et al., 2011). Previous studies have highlighted that cortical thickness is likely to reflect dendritic arborization or changing myelination at the gray/white matter interface (Rakic, 1988; Armstrong et al., 1995; Van Essen, 1997). On the other hand, surface area is influenced by the division of progenitor cells in the embryological periventricular area, and is associated with the number of minicolumns (Rakic, 1988; Sowell et al., 2004; Pannizon et al., 2009; Ecker et al., 2010; Pereira et al., 2011). Finally, geometric differences (depth of sulcus, curvature, and metric distortion) are predominantly linked with the development of neuronal connections and cortical pattern of connectivity, and are thus a marker for cerebral development (Armstrong et al., 1995; Van Essen, 1997). Therefore, it is likely that the reported maps of cortical morphology reflect multiple genetic and/or neurobiological etiologies, which need further investigation (Pannizon et al., 2009; Ecker et al., 2010; Pereira et al., 2011)
Studies in the literature have suggested that components of the frontolimbic network, such as the anterior cingulate cortex (ACC), the OFC, the dorsolateral prefrontal cortex and the amygdala-hippocampus complex, are potentially involved in BPD pathophysiology. There is also rising evidence that patients with BPD exhibit deficits in the structure and function of the ACC, OFC and amygdala-hippocampus complex. Furthermore, studies have also observed structural alteration of the parietal cortex in BPD patients, suggesting a possible role of parietal structures in dissociative symptoms. As such areas are associated with affective regulation, such reductions might be biological substrates of BPD symptomatology. In a recent neuroimaging study using the same approach, we observed significant morphological abnormalities of cortical thickness, volume, mean curvature, metric distortion, area and depth of sulcus in such areas among BPD patients (Araujo et al., 2014). Such findings are in agreement with previous structural neuroimaging studies involving BPD patients, and highlight the involvement of these areas in the regulation of mood reactivity, impulsivity and social behavior, considered dysfunctional in these patients (Araujo et al., 2014).
A main finding of the current study is the extensive alteration of the OFC in BPD patients, affecting nearly all measured structural parameters bilaterally. In addition, it is noteworthy that the structural disruption was limited to the medial, but not lateral, OFC. These findings are consistent with positron emission tomography studies of BPD patients, which consistently showed bilateral hypometabolism in the OFC (Soloff et al., 2000; Soloff et al., 2003). In particular, the medial OFC showed the highest reduction of glucose metabolism (Soloff et al., 2005). In addition, the current findings are in agreement with previous studies that highlight the important role of the OFC in regulating mood reactivity, impulsivity and social behavior, functions considered dysfunctional in patients with BPD and considered the core symptoms of this disorder (Lyoo et al., 1998; Lacerda et al., 2003; Schmahl et al., 2003; Lis et al., 2007; Minzenberg et al., 2007; Silbersweig et al., 2007; Chanen et al., 2008; Minzenberg et al., 2008; Woodward et al., 2009; Gutiérrez-Galve et al., 2010; Lehmann et al., 2010). Moreover, to the extent that the structural disruption is an indicator of functional impairment, our findings provide supportive evidence to previous functional studies that showed OFC-limbic disconnection in BPD patients (New et al., 2007).
An intriguing preliminary finding in the current study is the differential effect of BPD on metric distortion, showing reduction in the right, but increase in the left, spherical distortion of the OFC. Metric distortion has been previously proposed as a measure of overall cortical folding, providing cortical shape information not otherwise captured by mean curvature or depth of sulcus (Ecker et al., 2010). In that context, our data support bilateral alteration in cortical folding in the BPD group. In addition, it suggests a potential hemispheric lateralization effect, which may reflect the difference in function between the right and left medial OFC. For example, in a landmark study, O’Doherty et al. (2001) showed that the right OFC is especially responsive to punishment, whereas the left OFC is relatively more activated during reward. They also found that dominant activity in the medial OFC is associated with reward processing (O’Doherty et al., 2001). Conversely, punishment processing is correlated with the lateral OFC dominance. These data are consistent with a large body of literature on brain asymmetry in emotional processing, which associates the right hemisphere with the processing of negative affect and the left hemisphere with the processing of positive affect (Alves et al., 2008). Interestingly, a large longitudinal study found a reduction of cortical thickness in the right hemisphere of healthy subjects at increased familial risk for major depression, a psychiatric disorder highly comorbid with BPD (Peterson et al., 2009). Therefore, the metric distortion asymmetry and the more pronounced disruption in the right medial OFC in our cohort suggest a potential role for functional brain asymmetry in the pathophysiology of BPD. However, future multimodal neuroimaging approaches are needed to further investigate these preliminary findings.
The strengths of the current pilot study are a hypothesis-driven multi-parameter approach, a homogenous group of BPD patients with no psychiatric comorbidity at the time of observation, and a sample size that is adequate, given the restricted selection criteria and as compared with prior MRI studies in BPD. Limitations include the fact that the cohort of patients was medicated, and thus the effect of psychotropic medications cannot be ruled out. Findings cannot be generalized to males or to BPD patients with comorbidities. The lack of correlation between the morphometric findings and the CGI scores, although of importance, could be explained by the nonspecific nature of the CGI. Moreover, since the majority of patients presented mild to moderate severity at the time of the study (CGI scores of 3 and 4), the statistical power of the correlational analysis was significantly reduced. Due to operational limitations, we could not assess specific aspects of BPD patients, such as impulsivity, aggression and suicidality. The BPD population enrolled in the study is consistent with clinical observations of such patients, as 2/3 had a history of mood disorder and 1/2 had two or more psychiatric comorbidities. However, since we chose to enroll only BPD patients with no current psychiatric comorbidity in order to refine our analysis, we do not know whether and how these psychiatric comorbidities might impact on brain morphology; nevertheless, the sample is too small to assess this important point. Therefore, our final results should be interpreted with caution. Finally, additional insight would have been possible if other aspects of BPD, such as impulsivity, aggression and suicidality, had been systematically evaluated with structured psychometric scales.
In summary, this pilot study showed significant morphological alterations in the medial OFC of female BPD patients in comparison with a well-matched healthy control group. In addition, we demonstrated the utility of surface-based analysis in structural studies of BPD. This multi-parameter approach offered a detailed description of volumetric and geometric features of the OFC in BPD. Finally, we found preliminary evidence of hemispheric asymmetry, a finding that awaits further investigation using multi-parameter neuroimaging paradigms.
Figure 1.
Highlights.
We use MRI to compare the orbitofrontal cortex (OFC) in borderline personality disorder vs healthy controls.
We correlate MRI findings with symptom severity of symptoms measured by the GGI.
We found suggestive evidence of hemispheric asymmetry that should be examined in future studies.
We demonstrated the utility of surface-based analysis in structural studies of BPD.
References
- Alves NT, Fukusima SS, Aznar-Casanova JA. Models of brain asymmetry in emotional processing. Psychology & Neuroscience. 2008;1:63–66. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. 4th ed. revised. Washington, DC: American Psychiatric Press; 2004. [Google Scholar]
- Araujo TB, de Araujo Filho GM, Sato JR, de Araujo CM, Lisondo CM, Carrete H, Jr, Ancona AF, Lin K, Bressan RA, Ramalho JFS, Jackowski AP. Cortical morphological changes in female Borderline Personality Disorder patients: a multimodal approach. Revista Brasileira de Psiquiatria. 2014;36(1):32–38. doi: 10.1590/1516-4446-2013-1120. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Brunner R, Henze R, Parzer P, Kramer J, Feigl N, Lutz K, Essig M, Resch F, Stieltjes B. Reduced prefrontal and orbitofrontal gray matter in female adolescents with borderline personality disorder: is it disorder specific? Neuroimage. 2010;49:114–120. doi: 10.1016/j.neuroimage.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Chanen AM, Velakoulis D, Carison K, Gaunson K, Wood SJ, Yuen HP, Yucel M, Jackson HJ, McGorry PD, Pantelis C. Orbitofrontal amygdala and hipocampal volumes in teenagers with first-presentation borderline personality disorder. Psychiatry Research: Neuroimaging. 2008;163:116–125. doi: 10.1016/j.pscychresns.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ecker C, Marquand A, Mourão-Miranda J, Johnston P, Daly EM, Brammer MJ, Maltezos S, Murphy CM, Robertson D, Williams SC, Murphy DGM. Describing the brain in autism in five dimensions: magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. Journal of Neuroscience. 2010;30:10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer PL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV axis I Disorders – Patient Edition (SCID-I/P, Version 2.0. 9/98 revision) New York, NY: Biometrics Research Department, New York State Research Institute; 1998. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Goodman M, Hazlett EA, Avedon JB, Siever DR, Chu KW, New AS. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid major depression. Journal of Psychiatric Research. 2011;45:803–807. doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Guy W. Assessment Manual for Psychopharmacology. Rockville, MD: Department of Health, Education and Welfare, Alcohol, Drug Abuse and Mental Health Administration; 1976. [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological Psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Lis E, Greenfield B, Henry M, Guilé JM, Dougherty G. Neuroimaging and genetics of borderline personality disorder: a review. Journal of Psychiatry and Neuroscience. 2007;32:162–173. [PMC free article] [PubMed] [Google Scholar]
- Malloy P, Bihrle A, Duffy J, Cimino C. The orbitomedial frontal syndrome. Archives of Clinical Neuropsycholoy. 1993;8:185–201. [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic structural changes in borderline personality disorder. Journal of Psychiatric Research. 2008;42:727–733. doi: 10.1016/j.jpsychires.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Journal of Psychiatric Research. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biological Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–1640. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:208–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Ibarrexte-Bilbao N, Marti MJ, Compta Y, Junqué C, Bargallo N, Tolosa E. Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding and cortical thickness. Human Brain Mapping. 2011;11:2521–2534. doi: 10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramartine P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Progress in Brain Research. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rüsch N, van Elst LT, Ludaescher P, Wilke M, Huppertz HJ, Thiel T, Schmahl C, Bohus M, Lieb K, Hesslinger B, Hennig J, Ebert D. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20:385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Brendel G, Pan H, Beutel M, Pavony MT, Epstein J, Lenzenweger MF, Thomas KM, Posner MI, Stern E. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164:1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Constantine D. Gender differences in a fenfluramine-activated FDG PET study of borderline personality disorder. Psychiatry Research: Neuroimaging. 2005;138:183–195. doi: 10.1016/j.pscychresns.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatry Research: Neuroimaging. 2003;123:153–163. doi: 10.1016/s0925-4927(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biological Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer PL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Axis II Disorders (SCID-II, Version 9/89) New York, NY: Biometrics Research Department, New York State Research Institute; 1989. [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Zhao L, Richardson P, Clark L, Deakin JF, Williams S, Dolan MC. A voxel-based morphometric MRI study in men with borderline personality disorder: preliminary findings. Criminal Behavior and Mental Health. 2009;19:64–72. doi: 10.1002/cbm.716. [DOI] [PubMed] [Google Scholar]