Abstract
Combinatorial HIV/SIV vaccine approaches targeting multiple arms of the immune system might improve protective efficacy. We compared SIV-specific humoral immunity induced in rhesus macaques by five vaccine regimens. Systemic regimens included ALVAC-SIVenv priming and Env boosting (ALVAC/Env); DNA immunization; and DNA plus Env co-immunization (DNA&Env). RepAd/Env combined mucosal replication-competent Ad-env priming with systemic Env boosting. A Peptide/Env regimen, given solely intrarectally, included HIV/SIV peptides followed by MVA-env and Env boosts. Serum antibodies mediating neutralizing, phagocytic and ADCC activities were induced by ALVAC/Env, RepAd/Env and DNA&Env vaccines. Memory B cells and plasma cells were maintained in bone marrow. RepAd/Env vaccination induced early SIV-specific IgA in rectal secretions before Env boosting, although mucosal IgA and IgG responses were readily detected at necropsy in ALVAC/Env, RepAd/Env, DNA&Env and DNA vaccinated animals. Our results suggest combined RepAd priming with ALVAC/Env or DNA&Env regimen boosting might induce potent, functional, long-lasting systemic and mucosal SIV-specific antibodies.
Keywords: Simian Immunodeficiency Virus; poxvirus-, adenovirus-, and DNA-based vaccines; mucosal and systemic humoral immunity; memory B cells; functional antibody activities
1. INTRODUCTION
Over the last few years, results from pre-clinical and clinical HIV/SIV vaccine trials have shown that no single vaccination platform is able to completely prevent HIV/SIV acquisition [1,2]. Despite this, the HIV vaccine field has made significant breakthroughs and several vaccination strategies have shown potential in preventing viral acquisition and/or controlling SIV/HIV infection. The most striking result, which has energized the field, is the modest success of the clinical HIV vaccine trial (RV144) in Thailand, which provided 31.2% protective efficacy [3]. This protective outcome has now been reproduced in the rhesus macaque SIV model (Vaccari M. et. al., manuscript in preparation), providing a benchmark for further evaluation of novel vaccine designs. Other pre-clinical advances include partial protection against SIV or SHIV acquisition by vaccine regimens including DNA priming/rAd5boosting [4]; combined DNA/MVA, MVA only, Ad26/MVA, or DNA/inactivated virus particle strategies [5–7]; replication-competent Ad priming/Env protein boosting [8] and cells secreting gp96-Ig with SIV peptides plus Env protein [9]. Additional approaches, such as live CMV vectors [10], HIV gp41 subunit virosomes [11], and alphavirus replicon priming/trimeric Env boosting strategies [12], while not preventing acquisition, have resulted in dramatic control of viral replication.
Multiple immune mechanisms have been associated with protection including systemic and mucosal cellular and humoral responses. Among cellular responses, CD8+ effector memory T cells targeting either a few defined [13] or diverse MHC-restricted T cell epitopes [10,14], have provided strong viremia control. From the humoral arm of the immune system, neutralizing antibodies induced by vaccination have been shown to protect against intrarectal challenge with SHIVSF162p4 [8,12]. Moreover, vaccine-elicited neutralizing and non-neutralizing antibodies have played a role in blocking SIV/SHIV acquisition and decreasing viremia through Fc receptor mechanisms such as antibody-dependent cell mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated viral inhibition (ADCVI) [15–18]. Mucosal immune responses have also been associated with protection. The presence of vaccine-induced humoral responses, including IgG [7] and IgA [19] in the rectal mucosa, has been correlated with delayed virus acquisition, and both vaginal IgA with transcytosis inhibiting activity and IgG with neutralizing and non-neutralizing activities have been correlated with protection against SHIVSF162P3 [11]. Results from the RV144 trial suggest an important role for HIV-specific antibodies, particularly anti-HIV-1 gp120 V1/V2 IgG, in the prevention of HIV infection [20–22]. In view of the multiplicity of potentially protective immune responses, research using combinatorial vaccine regimens capable of inducing both cellular and humoral immune responses systemically and at mucosal sites are a current priority in pre-clinical HIV vaccine design [1].
Five distinct HIV/SIV vaccination platforms are being developed at the Vaccine Branch in the National Cancer Institute. Three are administered systemically, including canary pox virus (ALVAC) vectors in combination with an envelope (Env) protein boost [23–27], and DNA vaccination given with or without Env protein [7,28–33]. Two of the vaccine regimens target mucosal inductive sites, including replication-competent adenovirus type 5 host range mutant recombinants (RepAd) as a mucosal prime followed by systemic Env protein boosting [8,19,34–37], and TLR agonist plus IL-15-adjuvanted viral-specific peptides given intrarectally in combination with modified vaccinia Ankara (MVA) vectors and Env protein [38–41]. All five regimens to varying degrees have demonstrated partial protection against viral acquisition and/or significant reductions in viremia post-challenge. The ALVAC/Env regimen has prevented CD4+ T cell depletion in vaccinated rhesus macaques [23], and more recently recreated the protective efficacy of the RV144 human trial by conferring protection from SIV acquisition in 40% of vaccinated macaques (Vaccari M. et. al., manuscript in preparation). DNA vaccination alone has induced potent immune responses and decreased acute and chronic viremia after intrarectal challenge with pathogenic SIVmac251 [29]. Moreover, further boosting of a DNA vaccine regimen with homologous virus particles provided enhanced immune responses and protected 25% of vaccinated macaques from acquisition after a heterologous repetitive intrarectal SHIVsmE660 challenge [7]. RepAd/Env vaccination has induced strong cellular and humoral immune responses, protected chimpanzees from HIV acquisition [35] and macaques from SHIVSF162P4 acquisition [8], and provided durable protection against SIVmac251 in the rhesus macaque model [36]. Intrarectal Peptide/Env vaccination has protected macaques from intrarectal SIVmac251 challenge in a vaccine- and adjuvant-dependent manner [41].
Thus, although these five vaccine regimens have demonstrated induction of robust and durable cellular and humoral immune responses associated with protective efficacy, the level of protection needed to control the global HIV epidemic has not yet been achieved individually by any of them. In view of the complexity of the immune system and the multiplicity of observed protective immune correlates, combinatorial approaches might result in improved protection. Practically, the resources necessary to conduct head-to-head comparative preclinical efficacy studies of these 5 approaches in appropriately powered studies are too great. Therefore, to first identify combination approaches that might target all arms of the immune system, eliciting enhanced immunity and ultimately greater protection, we designed an immunogenicity study for side-by-side evaluation of the humoral (presented herein) and cellular (Valentin A. et. al., manuscript in preparation) immune responses elicited by these vaccine regimens using a small number of rhesus macaques. Our results suggest testing of two combination approaches in future pre-clinical challenge studies.
2. MATERIALS AND METHODS
2.1. Animals, immunization protocols and sample collection
Twenty-two naïve Mamu-A*01-positive Indian rhesus macaques were housed and maintained at Advanced BioScience Laboratories, Inc. (ABL, Rockville, MD) according to the standards of the American Association for Accreditation of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals of the NIH. Animal protocols were reviewed and approved by the ABL Animal Care and Use Committee prior to implementation. All animals were negative for SIV, simian T-cell leukemia virus-type 1 and simian type D retrovirus. Animals in each immunization protocol (ALVAC/Env, RepAd/Env, DNA&Env, DNA, and Peptide/Env) were immunized with SIV Env-specific vaccines at the time points summarized in Fig. 1 (for detailed immunization protocols including additional non-Env vaccine components, refer to Supplementary Tables 1–5) and showed no vaccination-associated adverse effects. Briefly, animals in the ALVAC/Env protocol (n=6, Supp. Table 1) received 4 doses of a SIVM766.4 gag/pro/env ALVAC vector (VCP2432) via the intramuscular (IM) route at 1 × 108 pfu/dose at weeks 0, 4, 12 and 24. The last two immunizations also included 200 µg of each of the following proteins containing a peptide tag (gD) from herpes simplex virus [42,43]: gD-SIVM766.4 gp120 and gD-SIVCG7V gp120 (ABL) using alum as adjuvant. SIVM766 (referred to hereafter as as M766) is a transmitted founder of SIVmac251 [44,45]; SIVCG7V (CG7V) is a transmitted founder of SIVsmE660 [44].
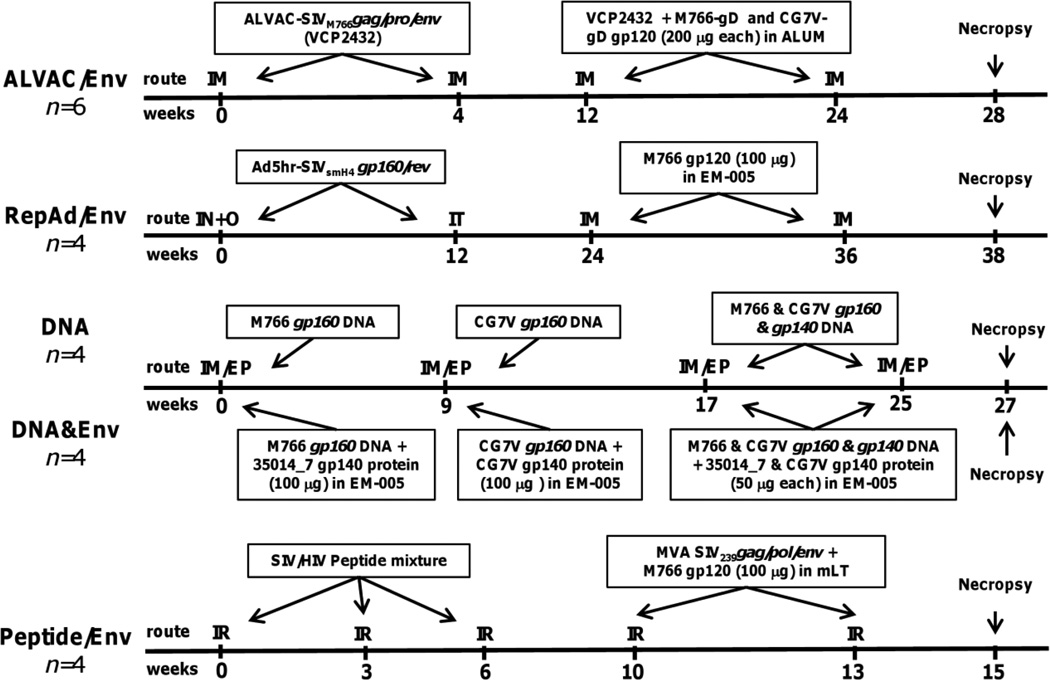
Fig. 1. Schematic representation of immunization protocols highlighting envelope-related immunogens.
Naïve Mamu A01*-positive rhesus macaques were distributed among five different vaccine protocols and immunized at the indicated weeks. Only Env immunogens are shown. More detailed descriptions of each regimen can be found in the Supplementary Information, Tables 1–5. IM, intramuscular; IN, intranasal; O, oral; IT, intratracheal; IM/EP, intramuscular injection followed by in vivo electroporation; IR, intrarectal.
Animals in the RepAd/Env protocol (n=4, Supp. Table 2) were immunized with Ad5hr-SIVsmH4env/rev and Ad5hr-SIV239gag at a dose of 5 × 108 pfu per recombinant per site intranasally plus orally at week 0 and intratracheally at week 12. RepAd/Env animals also received 2 IM protein immunizations at weeks 24 and 36 consisting of 100 µg of M766 gp120 formulated in 10 µg of EM-005 adjuvant (Infectious Disease Research Institute, Seattle, WA) [46] in 100 µl of PBS. SIVsmH4 gp120 is highly similar to SIVCG7V gp120, differing only by 3.8% in amino acid (aa) sequence. On the other hand, SIVsmH4 gp120 is less similar to M766 gp120, differing by 17.3% in aa sequence.
Animals in the DNA (n=4, Supp. Table 3) and DNA&Env (n=4, Supp. Table 4) protocols received the same DNA inoculations administered via the IM route followed by in vivo electroporation (IM/EP) using the ELGEN® adaptive constant-current electroporator (Inovio Pharmaceuticals, Inc., Blue Bell, PA) at weeks 0, 9, 17 and 25 (See Supp. Table 3 for complete list of additional DNA vaccines and adjuvants administered). The DNA vaccine mixture contained; 3 mg of M766 gp160 DNA (plasmid 221S; for EP1); CG7V gp160 DNA (plasmid 252S; for EP2) and 0.75 mg of each M766 gp160 and gp140 (plasmid 241S) and CG7V gp160 and gp140 (plasmid 255S) for EP3 and EP4. Animals in the DNA&Env co-immunization protocol additionally received 100 µg Env protein formulated in 10µg EM-005 adjuvant [46] in 200 µl of PBS administered immediately following the DNA injection into the same muscle via the IM route (4 injections) using the following proteins: SIV35014_7 gp140 at week 0; CG7V gp140 at week 9; and a mixture of 50 µg each of SIV35014_7 and CG7V gp140 at weeks 17 and 25. SIV35014_7 is another early transmitted SIVmac251 Env variant and is highly related to M766 gp120, differing by 5 aa [45].
Animals in the Peptide/Env protocol (n=4, Supp. Table 5) were vaccinated intrarectally (IR) with 3 doses of a peptide mixture (13 peptides, 0.5 mg/peptide [41]) of SIV/HIV helper and cytotoxic T cell epitopes (no SIV Env peptides were included) given at weeks 0, 3 and 6, adjuvanted with a cocktail of IL-15 (300 µg) plus TLR agonists MALP2 (10 µg), polyI:C (1 mg) and CpG (500 µg) per dose. Peptide and adjuvants were mixed in 100 µl of the liposomal transfection reagent DOTAP (Roche, Indianapolis, IN). The animals were subsequently immunized IR with 2 MVA vectors, one expressing SIVmac239 Env, Gag, and Pol, and the other expressing SIVmac239 Tat, Nef, and Rev at a dose of 5 × 108 per vector at weeks 10 and 13 using the same adjuvant cocktail described above. At weeks 10 and 13 the animals also received 100 µg M766 gp120 in mutant E. coli labile toxin R192G (mLT, 50 µg/dose, a kind gift of Dr. John Clements, Tulane University, New Orleans, LA) IR. M766 gp120 and the trimeric gp140 proteins were produced in HEK293 cells grown in serum-free media in a Hollow Fiber bioreactor as described in [45]. The gD-tagged M766 and CG7V proteins were produced in CHO cells.
Blood and bone marrow samples were collected after animal sedation and passed through Ficoll gradients to obtain single cell suspensions as previously described [47,48]. After washing and lysis of contaminating red blood cells, PBMCs and bone marrow cells were stored in FBS 10% DMSO in liquid nitrogen until used. Serum was isolated from clotted blood samples and stored at −70°C until used. Rectal secretions were collected using cotton-tipped swabs and then stored at −70°C in 1ml of 1X PBS buffer containing 0.1% BSA, 0.01% thimerosal, and 750 Kallikrein inhibitor units of aprotinin [49].
2.2. Binding titers, PEPSCAN analysis, neutralizing and non-neutralizing antibody assays
Heat-inactivated serum samples were assayed for M766 and CG7V gp120-specific IgG binding titers and SIVmac251 gp120-specific IgA binding titers as previously described [47]. Serum samples were also assayed by PEPSCAN analysis using SIVmac251 gp120 linear peptides as previously described [50]. The ability of sera to neutralize SIVmac251.6 was tested on TZM-bl cells as previously described [51]. Antibody-dependent cellular phagocytosis (ADCP) activity in necropsy serum samples was measured as previously described [52], with minor modifications. Briefly, SIVmac251 gp120 was biotinylated with the Biotin-XX Microscale Protein Labeling Kit (Life Technologies, Grand Island, NY), and 3–5 µg of gp120 was incubated with a 100-fold dilution of 1 µm Yellow-Green streptavidin-fluorescent beads (Life Technologies) for 25 min at room temperature in the dark. Serum dilutions of each sample (1:30 to 1:3,000) were added to 250,000–300,000 THP-1 cells in a 96-well U-bottom plate. The bead-gp120 mixture was further diluted 5-fold in R10 media and 50µL was added to the cells and incubated for 3 hours at 37°C. At the end of incubation, cells were washed at low speed and fixed in 2% PFA. Cells were then assayed for fluorescent bead uptake by flow cytometry using a BD Biosciences LSR II. The Phagocytic score of each sample was calculated by multiplying the percentage of bead positive cells by the MFI of the positive THP-1 cells. ADCC in serum samples collected at necropsy was measured using the RF-ADCC assay as previously described using human PBMCs as effectors and SIVmac251 gp120-coated CEM.NKr cells as targets at an E:T ratio of 50:1 [53].
2.3. Evaluation of gp120-specific circulatory B cells
Frozen PBMCs were thawed, washed, and counted. Cells (2 × 106) were blocked with unconjugated CD4 antibodies and stained with a combination of fluorochrome-conjugated monoclonal antibodies and biotinylated M766 gp120 followed by APC-conjugated Streptavidin (Life Technologies) for detection (Mohanram et al., submitted). Antibodies included: PE-Cy5-conjugated anti-CD19 (J3–119, Beckman Coulter, Fullerton, CA); PerCP-eFluor710-conjugated anti-CD27 (0323) and eFluor650NC-conjugated anti-CD20 (2H7, both from eBioscience, San Diego, CA); PE-Cy7-conjugated anti-CD21 (B-ly4, BD Biosciences, San Jose, CA); PE-Texas Red-conjugated anti-IgD (polyclonal, Southern Biotech, Birmingham, AL); QDot605-conjugated anti-CD2 (S5.5), QDot800-conjugated anti-CD14 (Tuk4) and Aqua Live/Dead viability Dye all from Life Technologies. M766 gp120 was biotinylated with the Biotin-XX Microscale Protein Labeling Kit (Life Technologies). At least 50,000 B cell events (Live CD2-CD14-CD20+CD19+ lymphocytes) were acquired on a LSRII (BD Biosciences) and analyzed using FlowJo software (TreeStar, Inc. Ashland, OR) as previously described [47]. M766 gp120-specific B cells were detected within the memory (CD27+IgD−) B cell subpopulation. Background M766 gp120-specific B cell staining of pre-immunization samples for each individual animal was subtracted from post-vaccination values (average at pre-immunization across all macaques was 0.08%).
2.4. Evaluation of gp120-specific antibody secreting bone marrow cells
M766 gp120-specific antibody secreting cells (ASC) were assayed in bone marrow by ELISPOT as previously described [54]. Briefly, bone marrow cells were thawed and either used immediately without stimulation (Day 0) or were first polyclonally-stimulated for three days (Day 3) in the presence of 1µg/ml CpG (ODN-2006, MGW Operon, Huntsville, AL), 50 ng/ml IL-21 (Peprotech, Rocky Hill, NJ), and 0.5 µg/ml anti-human sCD40L (Peprotech). Env-specific IgA and IgG ASC were normalized to the total number of IgG and IgA ASC and reported as percentage IgA and IgG Env-specific activity [47,54].
2.5. Quantification of SIV-specific IgA and IgG antibodies in rectal secretions
Rectal swabs were thawed and the recovered solution was passed through a 5 µm PVDF microcentrifugal filter unit (Millipore, Billerica, MA) in order to remove contaminating particles. The buffer flow-through was collected and stored at −20°C until analysis. SIV gp120-specific IgA and IgG antibodies were measured by ELISA using recombinant M766 and CG7V gp120 protein as previously described [49,55]. Plates were loaded with serial dilutions of secretions as well as dilutions of Env-specific IgA and IgG standards derived from IgG-depleted pooled serum or purified serum IgG, respectively, obtained from SIVmac251- infected macaques and quantified as previously described [56]. To account for varying amounts of total immunoglobulin in rectal secretions, total concentrations of IgA and IgG antibodies were measured in each sample by ELISA and used to normalize gp120-specific IgA and IgG antibody concentrations. SIV gp120-specific IgA/G antibodies are expressed as ng of gp120-specific IgA/G per µg of total IgA/G.
2.6. Statistical Analysis
Data were analyzed using Prism (v5.03, GraphPad Software) and SAS/STAT software (Version 9.3 for Windows, SAS Institute Inc., Cary, NC). Within each panel of Figures 2–6, a Kruskal-Wallis test of the global null hypothesis of equal means in all groups (or over all times) was performed, and when the null hypothesis was rejected at the p <0.05 level, differences between individual groups (or times) were tested as exploratory analyses to assess the sources of the rejection. A p value < 0.05 from the Mann-Whitney test without correction for multiple comparisons was thus considered statistically significant. Correlation results are from Spearman rank correlation analysis. PEPSCAN data were summarized by calculating the mean absorbance for each peptide over the animals within a group and using the Mann-Whitney test to analyze these values for the peptides spanning each variable loop or constant region.
3. RESULTS
3.1. Immunization platforms used in the current study
Figure 1 summarizes only the SIV envelope immunogens encoded within vectors or delivered as soluble proteins in each immunization regimen. For a complete list of vaccine components in each regimen, see Supplementary Tables 1–5. The individual protocols were designed based on previous vaccine strategies that successfully achieved partial protection against viral challenge. Therefore, the envelope components administered across the five protocols were similar but not identical, as detailed in Materials and Methods. Key protocol differences included the following: 1) The ALVAC/Env and DNA&Env protocols utilized matched envelopes for priming and boosting, and additionally, the ALVAC/Env protocol included a heterologous Env boost (CG7V). The RepAd/Env protocol used a heterologous Env prime (SIVsmH4env) and boost (M766 gp120), differing by 17% in aa sequence. Although the Peptide/Env protocol lacked SIV Env priming in the peptide immunizations, it administered highly related Env components at the last two immunizations. 2) While almost all protocols administered 100 µg of total Env (one or two proteins) per immunization, the ALVAC/Env animals were immunized with 200 µg of each Env (400 ug of protein) per immunization. 3) The RepAd/Env and DNA&Env protocols used EM-005, an oil-in-water emulsion containing a TLR-4 agonist as adjuvant [46], whereas the ALVAC/Env protocol used ALUM and the Peptide/Env protocol used mLT. 4) While the recombinant protein used by the RepAd/Env and Peptide/Env protocols was M766 gp120, the proteins used by the DNA&Env protocol were recombinant 35014_7 (highly related to M766) and CG7V gp140s. Furthermore, both recombinant gp120 proteins used by the ALVAC/Env protocol contained the gD peptide tag from herpes simplex virus. In addition to these similarities and differences in the Env immunizations, the time of collection of necropsy samples differed. Animals in all protocols were euthanized 2 weeks after the last immunization except for the ALVAC/Env protocol in which euthanasia was performed 4 weeks after the last immunization.
3.2. SIV-specific binding antibodies induced by five different immunization strategies
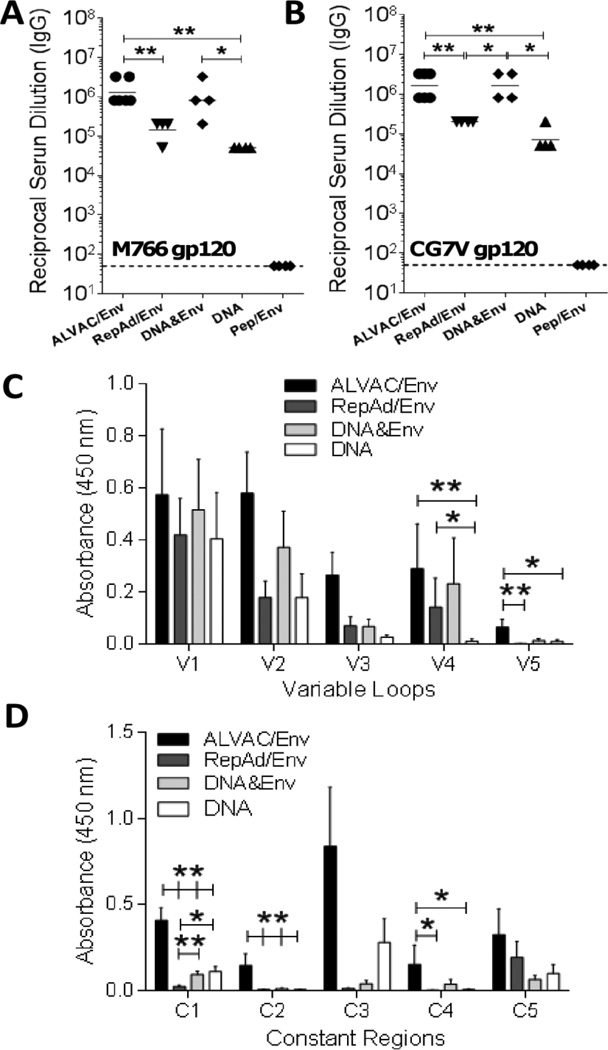
To evaluate SIV-specific antibodies induced by each immunization platform (Fig. 1), we initially tested IgG binding activity of serum samples collected at necropsy. All immunization regimens with the exception of the mucosally delivered Peptide/Env regimen induced M766 (Fig. 2A) and CG7V (Fig. 2B) gp120-specific serum IgG antibodies with differing titers across the four groups (p<0.0001 by the exact Kruskal-Wallis test). Higher titers were observed in macaques immunized with the ALVAC/Env and DNA&Env platforms compared to the RepAd/Env and DNA platforms (Fig. 2A–B). Given the lack of SIV Env-specific binding activity in sera of the Peptide/Env vaccinated animals, no further assessments of humoral responses were conducted for this vaccine regimen.
Fig. 2. SIV-specific binding titer and PEPSCAN analysis at necropsy.
(A–B) Serum IgG binding titers at necropsy for animals in each immunization protocol were determined for M766 (A) and CG7V (B) gp120. (C–D) Serum SIVmac251 native gp120-specific IgG PEPSCAN analysis for Variable (C) and Constant (D) regions performed in the 4 vaccination groups that showed positive binding. Data are shown as geometric means (A–B) and means ± SEM (C–D). *, p<0.05 and **, p<0.01 indicate statistically significant differences between the indicated groups by the Mann-Whitney test.
Next, given the correlation uncovered in the RV144 trial between protective efficacy and IgG antibodies directed to the V1/V2 regions of HIV-1 envelope [21], we conducted a SIVmac251 Env-specific PEPSCAN analysis of serum collected at necropsy. The 4 regimens that induced SIV Env-specific binding antibodies all elicited V1/V2-specific antibodies. In fact, SIV-specific binding activity was comparable among immunization groups for the V1, V2 and V3 variable loop regions. An overall difference in the levels of binding activity in the four tested groups (p<0.05 by the exact Kruskal-Wallis test) was observed in both the V4 and V5 regions. The ALVAC/Env, DNA&Env and RepAd/Env immunization protocols induced higher V4 binding activity compared to the DNA alone protocol (Fig. 2C), whereas the ALVAC/Env protocol displayed higher binding activity to the V5 region compared to the RepAd/Env and DNA protocols and marginally non-significantly higher binding (p=0.06) compared to the DNA&Env protocol. When analyzing the constant regions, overall differences in the binding activity amongst the four tested groups were detected in the C1, C2 and C4 regions (p<0.0001, p<0.01, and p<0.05 for C1, C2, and C4, respectively by the exact Kruskal-Wallis test). Animals in the ALVAC/Env protocol had higher binding activity against the conserved C1 and C2 envelope regions compared to all other tested protocols, and against the C4 region compared to RepAd/Env and DNA regimens (Fig. 2D). Animals in the RepAd/Env protocol had lower binding activity against C1 envelope peptides compared to the DNA&Env and DNA regimens (Fig. 2D).
3.3 Vaccination-induced development of B cell memory in the bone marrow
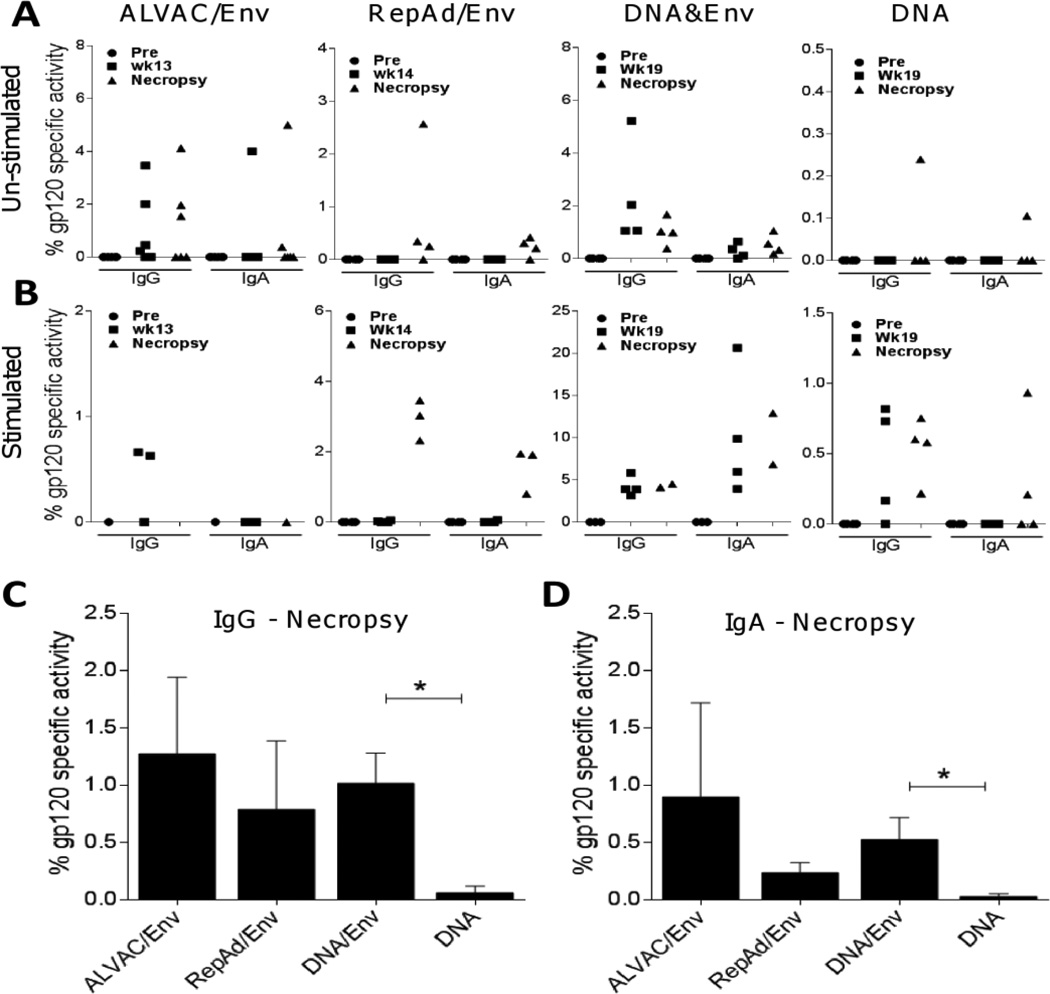
Given that vaccine-mediated exposure to antigen can induce the generation of long-lived bone marrow-resident plasma cells and memory B cells [57], we measured the abundance of M766 gp120-specific IgA- and IgG-producing ASC in the bone marrow of vaccinated animals. Measurement of M766 gp120-specific ASC on un-stimulated (Fig. 3A) bone marrow cells reflects the abundance of long-lived plasma cells, which continuously secrete immunoglobulins without needing stimulation. As shown in Fig. 3A, detectable levels of IgG gp120-specific plasma cells were seen in animals in the ALVAC/Env, and DNA&Env groups both at intermediate, as well as necropsy time points. For animals in the RepAd/Env group, IgG gp120-specific plasma cells were only detectable at necropsy (Fig. 3A), indicating that IgG-secreting plasma cell responses were directly associated with Env immunization. They were absent at Wk 14 for RepAd/Env (before the Env boost) and also absent in the DNA only vaccinated animals. The percentage of IgA gp120-specific activity was very low overall, but was seen in the majority of animals in the RepAd/Env and DNA&Env immunization groups (Fig. 3A). Only one animal in the DNA immunization group showed detectable levels of IgG or IgA gp120-specific plasma cells in bone marrow (note scale difference on the Y axis for the DNA regimen), supporting the notion that inclusion of protein provides improved humoral immunity [32] and Jalah R. et al. (submitted).
Fig. 3. SIV-specific ASC in the bone marrow are induced by systemic administration of gp120 protein.
M766 gp120-specific IgG and IgA activity (percent of total IgG and IgA responses) were evaluated in bone marrow at the indicated time points in each of the immunization protocols. (A) gp120-specific IgG and IgA secreting plasma cells in un-stimulated bone marrow. (B) gp120-specific IgG and IgA secreting memory B cells in bone marrow after 3 days of polyclonal stimulation. (C–D) Comparative IgG (C) and IgA (D) gp120-specific plasma cells at necropsy among immunization groups. Data are shown as means ± SEM (C–D). *, p<0.05 indicates statistically significant differences between the indicated groups by the Mann-Whitney test.
Although long-lived plasma cells produce antibody continuously, memory B cells require stimulation in order to differentiate into plasmablasts and actively secrete immunoglobulins [54]. Therefore to measure bone marrow-resident SIV-specific memory B cells induced by the different immunization platforms we stimulated bone marrow cells polyclonally for 3 days prior to ELISPOT assay. Because assay of plasma cells was given priority, insufficient cells remained for assessment of memory B cells on all animals. Despite this, following stimulation we observed induction of IgG gp120-specific memory B cells in the ALVAC/Env (Wk13, 2/3 tested animals), RepAd/Env (necropsy, 3/3 tested animals), DNA&Env (Wk19, 4/4 tested animals; necropsy, 2/2 tested animals) and DNA (Wk19, 3/4 tested animals; necropsy, 4/4 tested animals) immunization protocols (Fig. 3B, left sides). Similarly, IgA gp120-specific memory B cells were induced by the RepAd/Env (necropsy, 3/3 animals tested), DNA&Env (Wk19 and necropsy, 4/4 and 2/2 animals tested, respectively) and DNA (necropsy, 2/4 animals tested) vaccination platforms (Fig. 3B, right sides). The lack of memory B cells in the RepAd/Env group at the wk 14 time point likely reflects that the macaques were not yet exposed to the M766 test antigen, unlike macaques of the other immunization groups at the intermediate time points. Overall, the highest levels of IgG and IgA gp120-specific memory B cells were observed in animals immunized with the DNA&Env vaccine platform.
Cross-group comparisons at both the intermediate and necropsy time-points were performed for the un-stimulated IgG and IgA gp120-specific ASC in bone marrow (Fig. 3A), where complete sets of data obtained from all animals in each group were available. Significant differences across the four immunization protocols were observed only at the intermediate time-point for IgG (p=0.0023) and at necropsy for IgA (p=0.031) by the exact Kruskal-Wallis test. A comparison of mean levels of bone marrow-resident plasma cells elicited by the different vaccination platforms at necropsy is shown in Fig. 3C–D. Immunization with ALVAC/Env, RepAd/Env and DNA&Env induced comparable levels of SIV-specific IgG ASC plasma cells, contrasting with the very low levels induced by DNA immunization (Fig. 3C). Similarly, SIV-specific IgA ASC plasma cells were observed at low levels in animals immunized with ALVAC/Env, RepAd/Env, and DNA&Env (Fig. 3D). Due to animal variability, statistically significant differences in IgG and IgA ASC levels at necropsy were only observed when comparing DNA&Env immunized animals with animals in the DNA immunization group (Fig. 3C and 3D).
3.4 Changes in phenotype and antigen specificity of circulatory B cells
To better understand the effects of each immunization platform on induction of antibody and maturation of SIV-specific B cells, we evaluated the phenotype and antigen specificity of circulatory memory B cells. The percentages of total circulatory B cells (CD2−CD14−CD19+CD20+), as well as percentages of B cell subsets (naïve cells and resting, activated and tissue-like memory cells) were comparable at the pre, intermediate and necropsy time points in macaques of each immunization protocol (data not shown). Next, using a biotinylated M766 gp120 protein, we determined the abundance of SIV-specific circulatory memory B cells (CD27+IgD− B cells) in the 4 immunization groups. SIV gp120-specific memory B cells in PBMC were only detected following vaccination with the RepAd/Env, DNA&Env and DNA immunization platforms (Fig. 4A) at the intermediate time point and at necropsy for RepAd/Env and DNA&Env. The highest percentage of gp120+ memory B cells was observed in the DNA&Env vaccinated animals at Wk19 (geometric mean of 0.8%) after 3 DNA&Env immunizations, followed by animals in the RepAd/Env and DNA&Env groups at necropsy (geometric means of 0.2% and 0.3%, respectively). The small number of animals in each group precluded us from reaching statistically significant differences.
Fig. 4. Adenovirus- and DNA-based immunization regimens induce gp120+ circulatory memory B cells.
PBMCs were stained with fluorochome-conjugated monoclonal antibodies and analyzed by Flow Cytometry. (A) M766 gp120+ B cells were analyzed in the memory (CD27+IgD−) B cell compartment. Correlations between IgG (B) and IgA (C) gp120-specific circulatory memory B cells in PBMC and their respective gp120 memory B cells (polyclonally stimulated) as measured in bone marrow (Fig. 3B). Data shown (n=21) correspond to samples with measurable gp120-specific activity by both assays at both the intermediate and necropsy time points (RepAd/Env, DNA&Env and DNA groups). Correlation r and p values are from Spearman’s rank analysis.
To better understand the distribution of vaccine-elicited memory B cells and plasma cells, we performed correlation analyses of circulatory and bone marrow-resident M766 gp120-specific memory B cells and plasma cells. The percentage of gp120+ memory B cells present in PBMCs directly correlated with the gp120-specific IgG (Fig. 4B) and IgA (Fig. 4C) memory B cells in bone marrow detected as ASC following 3 days of polyclonal stimulation. Long-lived gp120-specific IgG and IgA plasma cells in the bone marrow did not correlate, however, with circulatory gp120+ memory B cells (data not shown). Our data suggest that vaccine-elicited memory B cells freely circulate between blood and bone marrow, but SIV-specific plasma cells, as expected, home to bone marrow and perhaps other tissue sites for maintenance and long-term antibody secretion.
3.5. Induction of SIV-specific antibodies in rectal secretions
We next investigated levels of SIV-specific IgA and IgG antibodies in rectal secretions. As shown in Fig. 5A, M766 gp120-specific IgA was not greatly induced post-prime or at necropsy by any immunization platform. On the other hand, CG7V gp120-specific IgA was induced post-prime (Wk14) by the RepAd immunization in the absence of a systemic protein boost when compared to the other immunization protocols (Fig. 5B, post-prime, p <0.05). At necropsy time points, CG7V gp120-specific IgA was detectable in at least 50% of animals immunized with ALVAC/Env, RepAd/Env and DNA&Env, and in 25% of animals immunized with DNA (Fig. 5B, Necropsy). Evaluation of M766 gp120-specific IgG showed comparable levels of these antibodies at necropsy in animals vaccinated with ALVAC/Env, RepAd/Env and DNA&Env; however, only the DNA&Env immunization induced these antibodies post-prime (Fig. 5C). With regard to CG7V gp120-specific IgG responses, while the ALVAC/Env protocol did not induce elevated levels post-prime, the RepAd/Env, DNA&Env and DNA immunization protocols induced responses in ≥50% of animals (Fig. 5D, post-prime). At necropsy, higher levels of CG7V gp120-specific IgG were detectable in animals vaccinated with DNA&Env compared to the other vaccination platforms (Fig. 5D, necropsy, p <0.01).
Fig. 5. Dynamics of SIV-specific IgA and IgG antibodies in rectal secretions induced by different immunization platforms.
M766 and CG7V gp120-specific IgG and IgA antibodies (expressed as ng of gp120-specific IgA/G per µg of total IgA/G) in rectal secretions were measured at the indicated time points by ELISA. (A–D) Summary of mucosal antibodies at post-prime and necropsy time points: M766 gp120-specific IgA (A) and IgG (C), and CG7V gp120-specific IgA (B) and IgG (D) observed for each animal. Post-prime time points are as follows: Wk13 for ALVAC/Env, Wk14 for RepAd/Env and Wk11 for DNA&Env and DNA. (E–F) Dynamics of CG7V gp120-specific IgA (E) and IgG (F) induced after each immunization by the RepAd/Env (left) and DNA&Env (right) immunization protocols. Data are shown as means ± SEM following subtraction of pre-vaccination values. *, p<0.05 and **, p<0.01 indicate statistically significant differences between the indicated groups (B and D) and between the indicated times and week 1 (E and F) by the Mann-Whitney test. Diagonal double lines in E and F indicate a 10 week interval between the observed values. Identification codes for each macaque are included in E and F.
As rectal secretions were available from multiple time points over the course of RepAd/Env and DNA&Env immunizations, we evaluated the kinetics of Env-specific IgA and IgG induction. For both vaccine regimens, significant differences were seen in the IgG and IgA measurements across the time points tested (p<0.0001 and p=0.030 for RepAd/Env and DNA&Env IgA values, respectively, Fig 5E; and p <0.0001 and p=0.0006 for RepAd/Env and DNA&Env IgG values, respectively, Fig. 5F). RepAd/Env immunization induced substantial amounts of CG7V gp120-specific IgA in all vaccinated macaques after the second RepAd prime (Fig. 5E, RepAd/Env, Wk14). Interestingly, the levels of gp120-specific IgA remained positive until the first protein boost (Wk24) and transiently increased thereafter (Wk25 and 26; Fig. 5E). On the other hand, in the DNA&Env vaccination group CG7V gp120-specific rectal IgA became detectable in 50% of the animals only after the third immunization (Fig. 5E, DNA&Env Wk25). Unlike IgA, CG7V gp120-specific rectal IgG was observed in the majority of macaques only after the first systemic protein boost in animals vaccinated with RepAd/Env (Fig. 5F, RepAd/Env, Wk25 onwards). In contrast, DNA&Env immunization induced high levels of CG7V gp120-specific IgG antibodies that were readily detectable after the second DNA&Env immunization and peaked after the third immunization (Fig. 5F, DNA&Env Wk11 and 19).
3.7. Anti-SIV functional properties of antibodies induced by the different immunization protocols
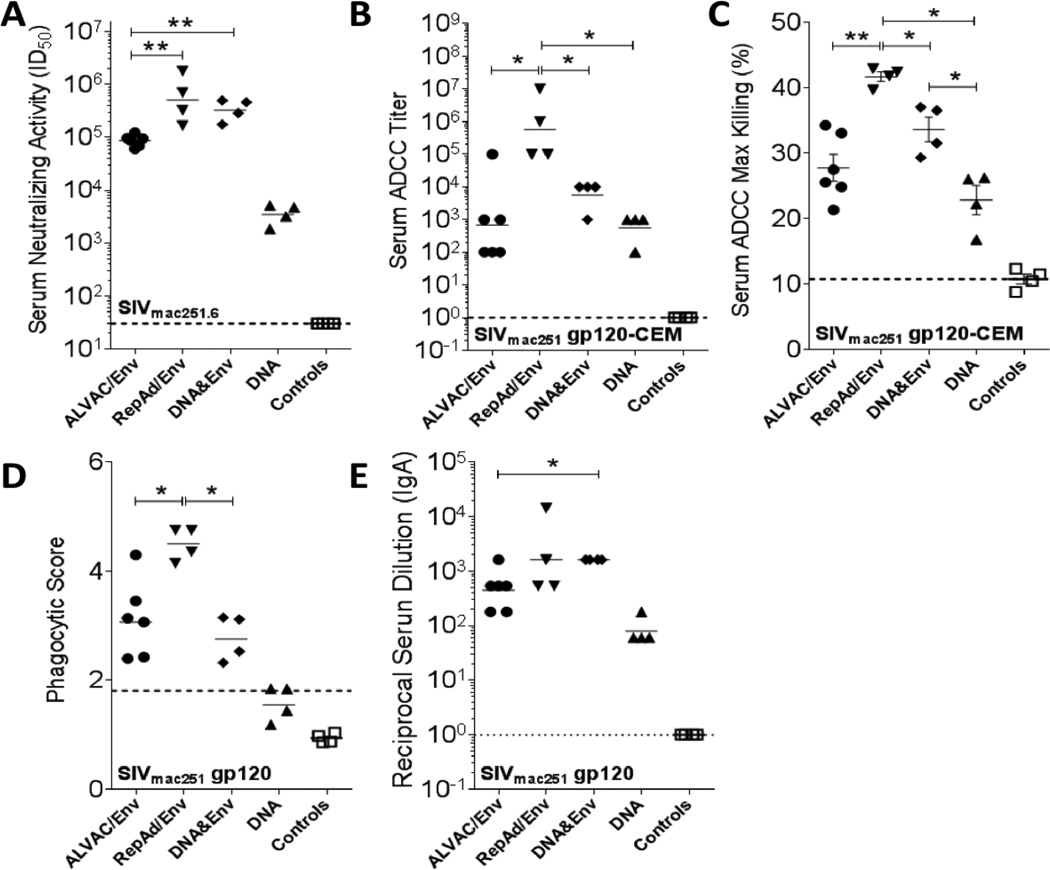
To determine if the vaccine-induced serum binding antibodies were functional, we tested them for both neutralizing and non-neutralizing activity. Serum samples collected at necropsy were able to neutralize SIVmac251.6, a tier 1 virus. Titers induced by ALVAC/Env, RepAd/Env and DNA&Env immunization were higher than those induced by DNA immunization (p<0.05, Fig. 6A). Further, titers elicited by the RepAd/Env and DNA&Env protocols were higher than those of the ALVAC/Env protocol (p<0.01, Fig. 6A). No neutralization of a Tier 2 virus (SIVmac251,30) or a SIVmac251 challenge stock was detected for sera from any immunization protocol (data not shown).
Fig. 6. Functional properties of SIV-specific antibodies in serum of immunized macaques.
Sera collected at necropsy in the different immunization protocols were tested for SIV-specific functional activity. (A) Neutralization in TZM-bl cells of Tier 1 SIVmac251.6. (B–C) ADCC activity in serum was measured against SIVmac251 gp120-coated CEM.NKr target cells and is presented as ADCC titer (B) and as percent maximum killing (C). (D) SIVmac251-specific antibody-dependent cellular phagocytosis activity in serum. (E) SIVmac251 gp120-specific serum IgA binding titers. Data are shown as geometric mean (A, B, D and E) and as means ± SEM (C). Dotted lines represent cutoff points for each assay. Sera collected before vaccination (pre-immune) were used as controls. *, p<0.05 and **, p<0.01 indicate statistically significant differences between the indicated groups by the Mann-Whitney test.
Next we assayed the same serum samples collected at necropsy for non-neutralizing function by measuring ADCC and ADCP [52,53]. The vaccination platforms tested induced high ADCC titers against SIVmac251 gp120-coated CEM-NKr target cells (Fig. 6B). ADCC titers in RepAd/Env vaccinated animals were significantly higher (p<0.05) than those of the ALVAC/Env, DNA&Env and DNA vaccinated animals. Measured concurrently, the ADCC maximum killing achieved by serum from RepAd/Env vaccinated animals was higher (p<0.05) when compared to the other vaccination groups (Fig. 6C). ADCP activity in serum was only induced by vaccination with RepAd/Env, ALVAC/Env and DNA&Env platforms, with the highest activity observed in the RepAd/Env vaccinated animals (p<0.05, Fig. 6D).
High serum titers of Env-specific IgA have been correlated with risk of HIV acquisition in the RV144 vaccine trial [20] and were shown to compete with IgG-mediated effector functions [71]. Therefore, we evaluated SIVmac251 gp120-specific serum IgA in each immunization group (Fig. 6E). Env-specific IgA titers in the ALVAC/Env, RepAd/Env and DNA&Env groups were all higher (p<0.05) than those of the DNA group, which also showed detectable IgA levels. Serum IgA titers in the DNA&Env group were significantly higher than those observed in the ALVAC/Env group (Fig. 6E). Overall, a high serum IgA anti-Env titer was not associated with lesser functional activity of serum antibodies elicited by the ALVAC/Env regimen.
4. DISCUSSION
Due to the great impact on public health caused by HIV-1 infection worldwide, the generation of a prophylactic HIV-1 vaccine is urgently needed to control the HIV-1/AIDS pandemic [58]. The only partially protective clinical vaccine trial to date, RV144, combined two vaccination platforms (ALVAC and AIDSVAX) believed to provide insufficient protection when used individually [59]. The successful outcome of that joint strategy encourages further development of combined vaccine approaches that individually excel at inducing specific types of anti-HIV/SIV immune responses. Together they might achieve enhanced protective efficacy. Therefore, in the present study we evaluated in depth the humoral immunogenicity of five different SIV-specific vaccination regimens targeting systemic and/or mucosal compartments with the aim of selecting combinatorial prime-boost approaches for future challenge studies.
The initial evaluation of SIV-specific binding antibodies showed that four vaccine regimens elicited serum antibody titers at necropsy ranging from 104 to 106 (Fig. 2A, B). The significantly higher titers observed in the ALVAC/Env and DNA&Env groups, as well as the broader recognition of variable and conserved peptide regions by sera of the ALVAC/Env group (Fig. 2C,D), may be related to the number of immunizations and amount of Env proteins administered. The ALVAC/Env and DNA&Env regimens used two protein components (M766 and CG7V Env) at a higher dose for the former (200 ug each gp120 protein/dose, given twice), and more frequently for the latter (one or two protein components at a total dose of 100 ug given in each of 4 immunizations). In contrast, the RepAd/Env strategy used only a single protein component (M766 gp120) at low dose (100 ug) on two occasions, and the DNA regimen lacked Env protein as vaccine component. In this regard, co-immunization with Env elicited significantly higher humoral responses, indicating that the responses elicited by the DNA only regimen could be further improved, in agreement with previous observations [7,32,60] and Jalah et al. (submitted).
The envelope proteins used in the ALVAC/Env protocol included the gD peptide tag which has been reported to contribute minimally to increasing immunogenicity [18]. However, the gD-envelope proteins were produced in CHO cells and administered in alum, unlike the other Env components produced in HEK293 cells and administered in EM-005 adjuvant, which may have altered their immunogenicity. Additionally, sera of the ALVAC/Env animals were collected four weeks after the last immunization rather than two weeks for the other groups. Collectively, the longer period post-vaccination, the amount and type of protein, and the use of alum may have enhanced titers and influenced the breadth of epitopes recognized by ALVAC/Env-vaccinated animals. Surprisingly, despite the high serum binding titers exhibited at necropsy in the ALVAC/Env group, Env-specific memory B cells were not detected in PBMCs of these animals (Fig. 4A). It is possible that by this 4 week post-immunization time point, the memory B cells had homed to the bone marrow or to mucosal and secondary lymphoid tissues [61]. In fact, ALVAC/Env immunization induced both IgG and IgA Env-specific memory B cells (Fig. 3B) and plasma cells (Fig. 3A) in the bone marrow of some vaccinated animals.
The highest levels of Env-specific memory B cells were among animals in the DNA&Env group (Fig. 4A), which may reflect the greater number of protein immunizations and the use of two different envelope variants (Supp. Table 4). The disappearance of memory B cells from peripheral blood at necropsy in the DNA and DNA&Env groups following a peak response at week 19 suggested the cells may have migrated to the bone marrow. In fact, at necropsy, the DNA&Env group had high levels of memory B cells in the bone marrow, while lower levels were detected in the DNA only group (Fig. 3B; note Y axis scales). Overall, the correlation of Env-specific memory B cells in bone marrow with those in peripheral blood (Fig. 4B,C) highlights the role played by mature bone marrow B cells as quick responders to both vaccination and infection [62]. Further, our results suggest that a strong and durable induction of memory B cells in bone marrow niches guarantees the circulatory presence of Env-specific memory B cells.
With regard to induction of mucosal humoral immunity, only animals vaccinated with RepAd/Env exhibited CG7V gp120-specific IgA responses in rectal secretions prior to any Env booster immunization (Fig. 5B, post-prime), reflecting mucosal priming by the Ad5hr-SIVsmH4gp160 recombinant. Expression of inserted genes in the replication-competent Ad5hr vector persists at least 25 weeks in macrophages and myeloid dendritic cells in the rectal mucosa of macaques [63]. Here a significant CG7V gp120-specific IgA response was maintained at least 24 weeks prior to Env protein boosting (Fig. 5E). The SIVsmH4gp160 insert of the RepAd vector is closely related (96.2 % identity) to CG7V gp120, explaining the strong antibody response. The heterologous priming potential of the Ad5hr vector is highlighted by the boosting of anti-Env responses following SIV M766 gp120 protein administration (Fig. 5E, RepAd/Env Wk25) and overall maintenance of antibody responses to both proteins (Fig. 5E and data not shown). The mucosal priming by the RepAd vector is unique. A comparable response in the DNA&Env group required four DNA plus homologous protein co-immunizations to efficiently induce mucosal CG7V–specific IgA (Fig. 5E, DNA&Env). Nevertheless, vaccination with DNA&Env induced Env-specific IgG rectal antibodies that were measurable after 2 immunizations (Fig. 5C–D) and peaked after the third DNA&Env immunization (Fig. 5F, DNA&Env Wk19). Overall, at the time of necropsy, Env-specific IgG responses in rectal secretions were detected in macaques vaccinated with ALVAC/Env, RepAd/Env, DNA&Env and DNA.
IgA is the most predominant Ig at mucosal sites [64] and can protect against HIV/SIV acquisition by transcytosis inhibition [65] or by trapping viral particles in mucus [66]. We have previously correlated vaccine-elicited rectal IgA with delayed SIV acquisition [19]. Although rectal Env-specific IgG antibodies are present at much lower concentrations than IgA, they can also mediate anti-viral activities including neutralization, ADCC and antibody-dependent cell-mediated viral inhibition locally at mucosal sites [11,19]. Unlike mucosal IgA, which is generated in highly specialized gut associated lymphoid tissues [67], mucosal IgG can arise by transudation or local synthesis [68]. Given the protective potential of both mucosal IgA and IgG, their induction by vaccination is a key aim in HIV vaccine design.
With regard to induction of systemic humoral immunity, four of the vaccine platforms elicited serum antibodies with protective potential including both neutralizing [69] and non-neutralizing [70] activities (Fig. 6A–D). Unexpectedly, however, the pattern of functional antibody responses did not match that of the serum binding antibody titers (Fig. 2A,B). The RepAd/Env protocol exhibited the highest levels of functional antibodies which likely reflects both the Ad-recombinant priming as well as Env boosting in EM-005 adjuvant. However, this protocol did not elicit the highest binding antibody titers. Although serum Env-specific IgA was implicated in blocking of binding and effector function of Env-specific IgG in the RV144 trial [20,71], no evidence of this effect was observed here. It will be important to determine in future experiments if differences in vaccine-induced SIV-specific IgG subtypes influenced the pattern of binding versus functional Fc-mediated antibody activities. At present, only SIV Env-specific IgG1 can be reliably measured in macaque sera.
Collectively, our results have highlighted the induction of potent, persistent mucosal immunity by a mucosally delivered vector and the induction of potent systemic antibody and B cell memory responses by systemic vector plus Env immmunization. Our data confirm the unique mucosal priming capacity of RepAd vaccination illustrated here and previously [19,63], the systemic potency shown here and the demonstrated longevity of responses induced by DNA&Env immunization [7] and Jalah R. et al. (submitted), and the magnitude and breadth of antibody responses elicited by ALVAC/Env immunization together with a level of protective efficacy in macaques mimicking the RV144 trial results [26] and Vaccari et al. (in preparation). In view of these findings, future prime-boost strategies incorporating RepAd followed by DNA&Env; and RepAd followed by ALVAC/Env are planned to test their ability to enhance SIV-specific protective responses.
Supplementary Material
Highlights.
Systemic ALVAC/Env and DNA&Env SIV vaccines induced highest serum antibody titers
Mucosal replicating Ad/Env SIV vaccine induced earliest and persistent rectal IgA
Env co-administration with DNA enhanced serum antibody titer and B cell maturation
RepAd/Env elicited the highest serum antibody responses with functional activities
Combined mucosal/systemic regimens should enhance immunity and protective efficacy
ACKNOWLEDGMENTS
We gratefully acknowledge N. Miller (NIAID, NIH) for help with protein expression, J. Tartaglia (Sanofi Pasteur) for the ALVAC vectors, the animal caretakers and research team at Advanced BioScience Laboratories, and B. Chowdhury for technical assistance.
DISCLOSURE
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) (B.K.F., G.N.P., J.A.B., G.F., M.R.G.); National Institute of Allergy and Infectious Diseases-NIH Contract HHSN27201100016C (D. C. M.). G.N.P. and B.K.F. are inventors on US Government-owned patents and patent applications related to DNA vaccines and gene expression optimization. G.F. is an inventor on a US Government patent filed jointly with Sanofi Pasteur on the use of the ALVAC vector as a platform for an HIV vaccine. N.Y.S. is a full time employee of Inovio Pharmaceuticals and as such receives compensation in the form of salary and stock options. S.G.R. is a full time employee of Infectious Diseases Research Institute and as such receives compensation in the form of salary and stock options. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Excler JL, Tomaras GD, Russell ND. Novel directions in HIV-1 vaccines revealed from clinical trials. Curr Opin HIV AIDS. 2013;8:420–430. doi: 10.1097/COH.0b013e3283632c26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, Johnson WE, Ferrari G, Hirsch VM, Felber BK, Pavlakis GN, Earl PL, Moss B, Amara RR, Robinson HL. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, Bess JW, Jr., Piatak M, Jr., Lifson JD, Williams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon DJ, Hirsch VM, Felber BK, Pavlakis GN. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci U S A. 2013;110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogers WM, Davis D, Baak I, Kan E, Hofman S, Sun Y, Mortier D, Lian Y, Oostermeijer H, Fagrouch Z, Dubbes R, van der Maas M, Mooij P, Koopman G, Verschoor E, Langedijk JP, Zhao J, Brocca-Cofano E, Robert-Guroff M, Srivastava I, Barnett S, Heeney JL. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology. 2008;382:217–225. doi: 10.1016/j.virol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strbo N, Vaccari M, Pahwa S, Kolber MA, Doster MN, Fisher E, Gonzalez L, Stablein D, Franchini G, Podack ER. Cutting edge: novel vaccination modality provides significant protection against mucosal infection by highly pathogenic simian immunodeficiency virus. J Immunol. 2013;190:2495–2499. doi: 10.4049/jimmunol.1202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr., Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Barnett SW, Burke B, Sun Y, Kan E, Legg H, Lian Y, Bost K, Zhou F, Goodsell A, Zur Megede J, Polo J, Donnelly J, Ulmer J, Otten GR, Miller CJ, Vajdy M, Srivastava IK. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol. 2010;84:5975–5985. doi: 10.1128/JVI.02533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr., Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Science. Vol. 340. New York, N Y: Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms; p. 1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas-Inchaustegui DA, Robert-Guroff M. Fc Receptor-Mediated Immune Responses: New Tools but Increased Complexity in HIV Prevention. Curr HIV Res. 2013;11:407–420. doi: 10.2174/1570162x113116660063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Jr., Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog. 2012;8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhavi V, Kent SJ, Stratov I. HIV-specific antibody-dependent cellular cytotoxicity: a novel vaccine modality. Expert Rev Clin Immunol. 2012;8:767–774. doi: 10.1586/eci.12.74. [DOI] [PubMed] [Google Scholar]
- 18.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Jr., Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol. 2013;87:1554–1568. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012;28:1444–1457. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O’Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O’Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal R, Venzon D, Santra S, Kalyanaraman VS, Montefiori DC, Hocker L, Hudacik L, Rose N, Nacsa J, Edghill-Smith Y, Moniuszko M, Hel Z, Belyakov IM, Berzofsky JA, Parks RW, Markham PD, Letvin NL, Tartaglia J, Franchini G. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J Virol. 2006;80:3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, Tryniszewska E, Lewis MG, VanCott TC, Hirsch V, Woodward R, Gibson A, Grace M, Dobratz E, Markham PD, Hel Z, Nacsa J, Klein M, Tartaglia J, Franchini G. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hel Z, Nacsa J, Tsai WP, Thornton A, Giuliani L, Tartaglia J, Franchini G. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology. 2002;304:125–134. doi: 10.1006/viro.2002.1722. [DOI] [PubMed] [Google Scholar]
- 26.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, Venzon D, Fenizia C, Morgan T, Montefiori D, Lifson JD, Miller CJ, Silvestri G, Rosati M, Felber BK, Pavlakis GN, Tartaglia J, Franchini G. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol. 2013;87:3538–3548. doi: 10.1128/JVI.02863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, von Gegerfelt A, Huang W, Guan Y, Broderick KE, Sardesai NY, LaBranche C, Montefiori DC, Pavlakis GN, Felber BK. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. 2012;8:1620–1629. doi: 10.4161/hv.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Alicea C, Patel V, von Gegerfelt AS, Montefiori DC, Venzon DJ, Khan AS, Draghia-Akli R, Van Rompay KK, Felber BK, Pavlakis GN. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A. 2009;106:15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, Alicea C, Weiss D, Treece J, Pal R, Markham PD, Marques ET, August JT, Khan A, Draghia-Akli R, Felber BK, Pavlakis GN. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni V, Rosati M, Valentin A, Ganneru B, Singh AK, Yan J, Rolland M, Alicea C, Beach RK, Zhang GM, Le Gall S, Broderick KE, Sardesai NY, Heckerman D, Mothe B, Brander C, Weiner DB, Mullins JI, Pavlakis GN, Felber BK. HIV-1 p24(gag) derived conserved element DNA vaccine increases the breadth of immune response in mice. PLoS One. 2013;8:e60245. doi: 10.1371/journal.pone.0060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Hannaman D, Reed SG, Felber BK, Pavlakis GN. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013;31:3747–3755. doi: 10.1016/j.vaccine.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, Alicea C, Minang JT, Trivett MT, Ohlen C, Zhao J, Robert-Guroff M, Khan AS, Draghia-Akli R, Felber BK, Pavlakis GN. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine. 2010;28:4827–4836. doi: 10.1016/j.vaccine.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson LJ, Robert-Guroff M. Replicating adenovirus vector prime/protein boost strategies for HIV vaccine development. Expert Opin Biol Ther. 2008;8:1347–1363. doi: 10.1517/14712598.8.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubeck MD, Natuk R, Myagkikh M, Kalyan N, Aldrich K, Sinangil F, Alipanah S, Murthy SC, Chanda PK, Nigida SM, Jr., Markham PD, Zolla-Pazner S, Steimer K, Wade M, Reitz MS, Jr., Arthur LO, Mizutani S, Davis A, Hung PP, Gallo RC, Eichberg J, Robert-Guroff M. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat Med. 1997;3:651–865. doi: 10.1038/nm0697-651. [DOI] [PubMed] [Google Scholar]
- 36.Malkevitch NV, Patterson LJ, Aldrich MK, Wu Y, Venzon D, Florese RH, Kalyanaraman VS, Pal R, Lee EM, Zhao J, Cristillo A, Robert-Guroff M. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIVmac251 rectal challenge: role of SIV-specific CD8+ T cell responses. Virology. 2006;353:83–98. doi: 10.1016/j.virol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Demberg T, Florese RH, Heath MJ, Larsen K, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Venzon D, Grant R, Patterson LJ, Korioth-Schmitz B, Buzby A, Dombagoda D, Montefiori DC, Letvin NL, Cafaro A, Ensoli B, Robert-Guroff M. A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2007;81:3414–3427. doi: 10.1128/JVI.02453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sui Y, Gagnon S, Dzutsev A, Zhu Q, Yu H, Hogg A, Wang Y, Xia Z, Belyakov IM, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Berzofsky JA. TLR agonists and/or IL-15 adjuvanted mucosal SIV vaccine reduced gut CD4(+) memory T cell loss in SIVmac251-challenged rhesus macaques. Vaccine. 2011;30:59–68. doi: 10.1016/j.vaccine.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belyakov IM, Kuznetsov VA, Kelsall B, Klinman D, Moniuszko M, Lemon M, Markham PD, Pal R, Clements JD, Lewis MG, Strober W, Franchini G, Berzofsky JA. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 41.Sui Y, Zhu Q, Gagnon S, Dzutsev A, Terabe M, Vaccari M, Venzon D, Klinman D, Strober W, Kelsall B, Franchini G, Belyakov IM, Berzofsky JA. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107:9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DH, Winters-Digiacinto P, Mitiku M, O’Rourke S, Sinangil F, Wrin T, Montefiori DC, Berman PW. Comparative immunogenicity of HIV-1 clade C envelope proteins for prime/boost studies. PLoS One. 2010;5:e12076. doi: 10.1371/journal.pone.0012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasky LA, Groopman JE, Fennie CW, Benz PM, Capon DJ, Dowbenko DJ, Nakamura GR, Nunes WM, Renz ME, Berman PW. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986;233:209–212. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- 44.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni V, Rosati M, Bear J, Pilkington GR, Jalah R, Bergamaschi C, Singh AK, Alicea C, Chowdhury B, Zhang GM, Kim EY, Wolinsky SM, Huang W, Guan Y, Labranche C, Montefiori DC, Broderick KE, Sardesai NY, Valentin A, Felber BK, Pavlakis GN. Comparison of intradermal and intramuscular delivery followed in vivo electroporation of SIV Env DNA in macaques. Hum Vaccin Immunother. 2013;9:2081–2094. doi: 10.4161/hv.25473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inchaustegui D, Lee EM, Kalisz I, Kalyanaraman VS, Dipasquale J, McKinnon K, Robert-Guroff M. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–12604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargas-Inchaustegui DA, Demberg T, Robert-Guroff M. A CD8alpha(-) subpopulation of macaque circulatory natural killer cells can mediate both antibody-dependent and antibody-independent cytotoxic activities. Immunology. 2011;134:326–340. doi: 10.1111/j.1365-2567.2011.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson LJ, Daltabuit-Test M, Xiao P, Zhao J, Hu W, Wille-Reece U, Brocca-Cofano E, Kalyanaraman VS, Kalisz I, Whitney S, Lee EM, Pal R, Montefiori DC, Dandekar S, Seder R, Roederer M, Wiseman RW, Hirsch V, Robert-Guroff M. Rapid SIV Env-specific mucosal and serum antibody induction augments cellular immunity in protecting immunized, elite-controller macaques against high dose heterologous SIV challenge. Virology. 2011;411:87–102. doi: 10.1016/j.virol.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demberg T, Brocca-Cofano E, Kuate S, Aladi S, Vargas-Inchaustegui DA, Venzon D, Kalisz I, Kalyanaraman VS, Lee EM, Pal R, Dipasquale J, Ruprecht RM, Montefiori DC, Srivastava I, Barnett SW, Robert-Guroff M. Impact of antibody quality and anamnestic response on viremia control post-challenge in a combined Tat/Env vaccine regimen in rhesus macaques. Virology. 2013;440:210–221. doi: 10.1016/j.virol.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter. 2005;12 doi: 10.1002/0471142735.im1211s64. Unit 12 11. [DOI] [PubMed] [Google Scholar]
- 52.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G, A robust. high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Brocca-Cofano E, McKinnon K, Demberg T, Venzon D, Hidajat R, Xiao P, Daltabuit-Test M, Patterson LJ, Robert-Guroff M. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine. 2011;29:3310–3319. doi: 10.1016/j.vaccine.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertley FM, Kozlowski PA, Wang SW, Chappelle J, Patel J, Sonuyi O, Mazzara G, Montefiori D, Carville A, Mansfield KG, Aldovini A. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 56.Manrique M, Kozlowski PA, Wang SW, Wilson RL, Micewicz E, Montefiori DC, Mansfield KG, Carville A, Aldovini A. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol. 2009;2:536–550. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- 57.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piot P, Quinn TC. Response to the AIDS pandemic--a global health model. N Engl J Med. 2013;368:2210–2218. doi: 10.1056/NEJMra1201533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lakhashe SK, Silvestri G, Ruprecht RM. Ruprecht, No acquisition: a new ambition for HIV vaccine development? Curr Opin Virol. 2011;1:246–253. doi: 10.1016/j.coviro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaworski JP, Krebs SJ, Trovato M, Kovarik DN, Brower Z, Sutton WF, Waagmeester G, Sartorius R, D’Apice L, Caivano A, Doria-Rose NA, Malherbe D, Montefiori DC, Barnett S, De Berardinis P, Haigwood NL. Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells. PLoS One. 2012;7:e31464. doi: 10.1371/journal.pone.0031464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q, Iida R, Shimazu T, Kincade PW. Replenishing B lymphocytes in health and disease. Curr Opin Immunol. 2012;24:196–203. doi: 10.1016/j.coi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patterson LJ, Kuate S, Daltabuit-Test M, Li Q, Xiao P, McKinnon K, DiPasquale J, Cristillo A, Venzon D, Haase A, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin Vaccine Immunol. 2012;19:629–637. doi: 10.1128/CVI.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 65.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 66.Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr Opin HIV AIDS. 2013;8:392–400. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutherland DB, Fagarasan S. IgA synthesis: a form of functional immune adaptation extending beyond gut. Curr Opin Immunol. 2012;24:261–268. doi: 10.1016/j.coi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Wu X, Jackson S. Plasma and salivary IgG subclasses in HIV type 1 infection: evidence of both transudation and local synthesis of IgG in parotid saliva. AIDS Res Hum Retroviruses. 2000;16:1423–1431. doi: 10.1089/08892220050140973. [DOI] [PubMed] [Google Scholar]
- 69.McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med. 2013;210:209–223. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson HL. Non-neutralizing antibodies in prevention of HIV infection. Expert Opin Biol Ther. 2013;13:197–207. doi: 10.1517/14712598.2012.743527. [DOI] [PubMed] [Google Scholar]
- 71.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. PNAS USA. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.