Abstract
T helper type 9 (TH9) cells can mediate tumor immunity and participate in autoimmune and allergic inflammation in mice but little is known about the TH9 cells that develop in vivo in humans. We isolated T cells from human blood and tissues and found that most memory TH9 cells were skin-tropic or skin-resident. Human TH9 cells co-expressed TNFα and granzyme B, lacked coproduction of TH1/TH2/TH17 cytokines and many were specific for C. albicans. IL-9 production was transient and preceded the up-regulation of other inflammatory cytokines. Blocking studies demonstrated that IL-9 was required for maximal production of IFN-γ, IL-9, IL-13, and IL-17 by skin tropic T cells. IL-9 producing T cells were increased in the skin lesions of psoriasis, suggesting these cells may contribute to human inflammatory skin disease. Our results indicate human TH9 cells are a discrete T cell subset, many are tropic for the skin, and although they may function normally to protect against extracellular pathogens, aberrant activation of these cells may contribute to inflammatory diseases of the skin.
INTRODUCTION
IL-9 producing CD4+ TH9 cells have been proposed as a newly described pro-inflammatory subset of TH cells. In mouse models, TH9 cells enhanced immune responses to melanoma and helminth infection and contributed to pathogenicity in autoimmune and allergic animal models of colitis, uveitis, EAE and asthma (1–11). In humans, IL-9 production was increased in the asthmatic lung and in T cells from infants with atopic dermatitis (12–16). Aside from these reports, studies of human TH9 cells have focused almost exclusively on naïve or memory T cells driven to produce IL-9 in vitro by stimulation with exogenous TGF-β (6, 7, 14, 17).
Several unanswered questions remain regarding the biology of TH9 cells. First, the prevalence, characteristics and function of TH9 cells arising in vivo in humans remain unstudied. Second, TH2, TH17 and regulatory T cells can produce IL-9 after specific in vitro manipulations, raising the question as to whether IL-9 producing TH9 cells exist as a discrete T cell subset (6, 17). Third, innate lymphoid cells (ILC) were the major source of IL-9 in one mouse model and the relative importance of ILC vs. T cell-derived IL-9 has not been established (3). Lastly, IL-9 production was no longer demonstrable at sites of established tissue inflammation in several animal models, despite the requirement of IL-9 for initial development of the inflammatory state (18, 19). The eventual fate of IL-9 producing T cells in these models remain undetermined.
We report here studies of IL-9 producing T cells isolated from human blood and peripheral tissues. We find that human TH9 cells are a discrete, identifiable T cell subset, largely tropic for the skin, that have the capacity to amplify immune responses by enhancing cytokine production from TH1, TH2, TH9 and TH17 cells.
RESULTS
IL-9 is transiently and selectively produced by skin-tropic TH cells after stimulation with Candida albicans
Based on evidence that epithelial barrier tissues are progressively colonized by memory T cells responding to pathogens encountered through that tissue, (20, 21) we studied human tissue tropic T cells from peripheral blood for their capacity to make IL-9. Skin- and gut-tropic memory TH cells were isolated based on expression of the skin-homing receptor cutaneous lymphocyte antigen (CLA) or the gut-homing integrin α4β7. In order to most closely mimic pathophysiologic stimulation, we stimulated T cells with autologous monocytes pulsed with a panel of viral, bacterial, and fungal pathogens and analyzed subsequent proliferation and cytokine production. We observed enhanced proliferation of tissue tropic T cells after stimulation with pathogens commonly encountered through that respective epithelial surface (Fig. 1A, S1). CLA+ skin tropic T cells proliferated more than other populations when stimulated with the skin organism Staphylococcus epidermidis, α4β7+ gut tropic T cells preferentially proliferated to the gut pathogens Rotavirus and Salmonella typhimurium, and the non-gut/non-skin tropic T cells (which include lung homing T cells) proliferated maximally to the lung pathogens Influenza A and Mycoplasma pneumoniae. T cell responses to HSV-1 and Candida albicans were maximal in skin tropic T cells but also present in other tissue-homing populations.
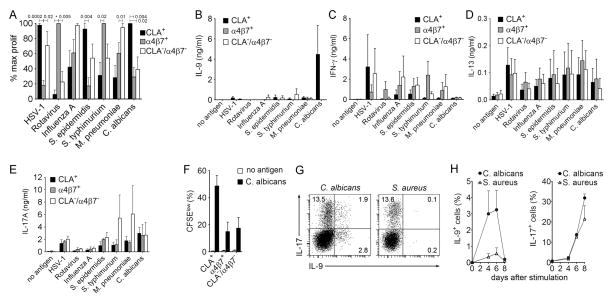
Fig. 1. IL-9 is transiently and selectively produced by skin-tropic TH cells after stimulation with Candida albicans.
(A) Tissue tropic T cells preferentially respond to pathogens encountered through that epithelial surface. The % maximal proliferation (% max prolif) of T cells tropic for skin (CLA+), gut (α4β7+) and other tissues (CLA−α4β7−) is shown. CLA+, α4β7+, and CLA−/α4β7− memory CD4+ T cells were isolated from PBMC of healthy donors and separately stimulated with autologous monocytes pulsed with a panel of viral, bacterial and fungal antigen preparations. Proliferation was assayed by CFSE dilution and maximal proliferation for each antigen was used to calculate relative responses of other T cell subsets (mean + s.e.m. of duplicates). *p=0.0001, otherwise p values are as shown. (B–E) IL-9 was produced selectively by skin tropic T cells in response to C. albicans. Cytokine production of T cell subsets following stimulation with autologous antigen-pulsed monocytes was measured in cell culture supernatants using a bead-based multiplex assay (mean + s.e.m. of duplicates). Significant IL-9 production was observed only in CLA+ skin tropic T cells stimulated with C. albicans whereas IFN-γ, IL-13 and IL-17 were produced in variable amounts by all tissue tropic subsets and in response to all antigens tested. (F) All tissue tropic T cell subsets proliferated in response to C. albicans, demonstrating that selective IL-9 production by skin tropic T cells was not the result of poor recognition of C. albicans by other T cell subsets. The % CFSElow T cells is shown (mean + s.e.m. of duplicates). (G,H) IL-9 is transiently produced after pathogen stimulation. T cells were stimulated with C. albicans and S. aureus, and IL-9 and IL-17 production was assessed at different time points by flow cytometry after stimulation with phorbol 12-myristate 13-acetate and ionomycin (PMA+I). Cytokine production of stimulated T helper cells on day 6 (G) and the kinetics of IL-9 and IL-17 production (H) are shown (mean + s.d.). Data are representative of independent experiments with 5 (A,F), 3 (B–E) or 2 (G,H) donors.
We next evaluated cytokine production in response to pathogen exposure. Surprisingly, we found substantial IL-9 production only in cultures of skin-tropic CLA+ T cells stimulated with C. albicans-pulsed monocytes (Fig. 1B); in contrast, other TH cell cytokines (e.g., IFN-γ (TH1), IL-13 (TH2), IL-17 (TH17)) were detected at variable levels in all tissue homing subsets and with all pathogens tested (Fig. 1C–E). IFN-γ suppresses IL-9 production in mouse T cells and pathogen-induced production of IFN-γ could be masking IL-9 production (22). To evaluate this possibility, we tested pathogen responses in the presence of IFN-γ neutralizing antibody. IFN-γ blockade significantly increased IL-9 production in response to C. albicans but did not affect IL-9 production in response to other pathogens (Fig. S2A). The lack of IL-9 production in C. albicans-stimulated α4β7+ and CLA−/α4β7− TH cells was not a consequence of differential stimulation; TH cell proliferation was observed in all TH cell subsets, although it was highest in CLA+ T cells (Fig. 1F). We further studied the source and expression kinetics of IL-9 produced in response to C. albicans by stimulating PBMC with C. albicans or S. aureus, organisms known to induce strong TH17 responses (23) (Fig. 1G). Both organisms induced equivalent IL-17 production. However, IL-9 was only induced by C. albicans, and IL-9 production was unique in that it was transient, peaked at day 6, and declined rapidly thereafter (Fig. 1H). These results demonstrate that transient IL-9 expression is specifically induced in skin homing CLA+ TH cells by C. albicans.
Human TH9 cells are a distinct T cell population tropic for the skin
Our previous experiments, utilizing pathogens to stimulate the cytokine production of circulating peripheral blood effector T cells, found IL-9 producing T cells only within the skin-homing TH population. To determine if TH9 cells specific for other tissues existed but were not specific for the pathogens we tested, we isolated CLA+, α4β7+, and CLA−/α4β7− CD25− effector TH cells from the blood and polyclonally stimulated these T cells with anti-CD3/anti-CD2/anti-CD28. We analyzed cytokine production by intracellular cytokine staining (Fig. 2A,B) and multiplex bead analysis (Fig. 2C). IL-9 production was enriched in skin tropic CLA+ TH cells, although some production was also observed in the gut-tropic and non-skin non-gut CLA−/α4β7− T cells, a population that included lung homing T cells. The kinetics of IL-9 production were unique in that it was produced only transiently after activation. Mulitplex bead analysis of T cell supernatants confirmed that the majority of IL-9 was produced by CLA+ skin homing T cells (Fig. 2C). At the time of peak IL-9 production (day 2), simultaneous staining for all four cytokines demonstrated that the majority of IL-9+ skin-homing TH cells lacked co-expression of other TH lineage cytokines (IFN-γ, IL-17, IL-13) consistent with their identity as a distinct T cell subset (Fig. 2D,E). In the smaller populations of IL-9 producing T cells that did coproduce IFN-γ, IL-13, or IL-17, IL-9 was also transiently produced (Fig. S4). In contrast to CLA+ cells and in accordance with previous reports, α4β7+ TH cells highly expressed IFN-γ (24), and CLA−/α4β7− TH cells were enriched for IL-17 producing T cells (25) (Fig. 2B). The production of IL-9 by CLA+ TH cells was not affected by including anti-TGF-β neutralizing antibody, ruling out potential autocrine induction of IL-9 by endogenous TGF-β (Fig. 2F). These results demonstrate that IL-9 is selectively and transiently produced by a distinct population of TH9 cells expressing cutaneous homing receptors that suggest these cells are tropic for the skin.
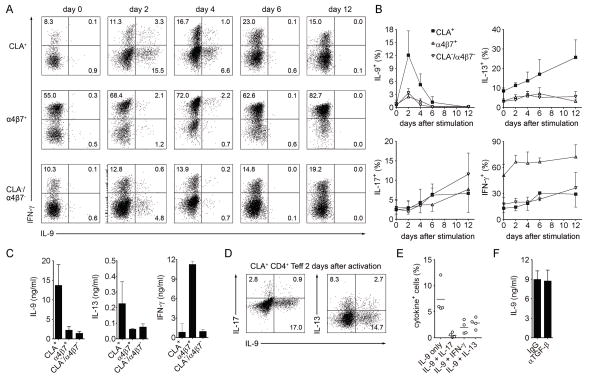
Fig. 2. Human TH9 cells are a distinct T cell population tropic for the skin.
(A, B) CLA+, α4β7+, and CLA−/α4β7− CD25− memory CD4+ T cells (TEFF) were isolated from healthy donors and polyclonally stimulated with αCD3/αCD2/αCD28. The production of IL-9, IFN-γ, IL-13, and IL-17 was assessed by flow cytometry after stimulation with PMA+I at the indicated time points. Histograms from an individual donor (A) and aggregate data are shown (B) (mean + s.d.). The majority of IL-9 production was observed in CLA+ skin tropic T cells and IL-9 was produced only transiently after activation, in contrast to other cytokines tested simultaneously (IL-13, IL-17, IFN-γ). (C) Cytokine production as measured by bead multiplex analysis of supernatants from tissue tropic subsets on day 4 after stimulation is shown (mean + s.d.); results confirmed flow cytometry studies demonstrating most IL-9 is produced by CLA+ T cells. (D,E) Most IL-9 producing T cells lacked production of other TH lineage cytokines. 2 days after stimulation, CLA+ Teff were analyzed by flow cytometry for co-production of IL-9 and IL-17, IFN-γ and IL-13. Representative histograms (D) and aggregate data (E) are shown (mean + s.d.). (F) IL-9 production was not dependent on the presence of TGF-β. IL-9 in supernatants from T cells stimulated for four days in the presence of neutralizing antibody to TGF-β or an isotype-matched control antibody is shown (mean + SD). Data are representative of independent experiments with at least 4 donors.
TH9 cells are selectively found in human skin and are independent of TGF-β and IL-2
Memory T cells are frequent in human peripheral tissues; there are approximately 20 billion antigen-experienced memory T cells in the skin of a healthy adult, twice the number of total circulating T cells (26). Analogous populations of memory T cells have been found in human lung and gut (27, 28). Studies in mice have shown that pathogen-specific T cells accumulate in epithelial barrier tissues such as the skin following infection and are protective against pathogen re-exposure, even in the absence of circulating T cells (21, 29–32). In both mice and humans, a subpopulation of these resident memory T cells (TRM) are sessile and non-recirculating (20, 21, 31). It is therefore critical to study the T cells resident within peripheral tissues to gain a true understanding of effector memory T cell function. We isolated tissue resident memory T cells (TRM) from healthy human skin, small intestine and lung. TH cells transiently producing IL-9 after activation were found exclusively in skin and were largely absent from gut or lung (Fig. 3A). Similar findings were observed in T cells isolated from skin by collagenase digestion (Fig. 3A) and from short term explant cultures (Fig. S3). Inclusion of neutralizing antibody to TGF-β, and the addition of endogenous IL-2 or neutralizing anti-IL-2 antibodies to cultures did not alter IL-9 production (Fig 3B, S2C), demonstrating that skin resident TH9 cells were not dependent on TGF-β or IL-2 for survival or cytokine production.
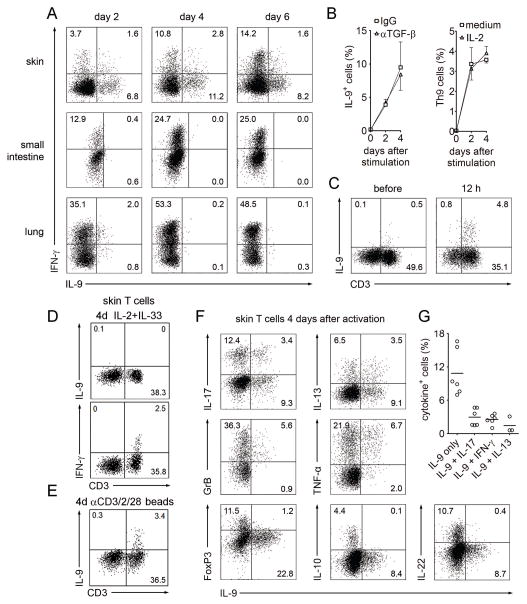
Fig. 3. TH9 cells are selectively found in human skin, constitute a distinct T cell population and are independent of TGF-β and IL-2.
(A) TH9 cells are resident in human skin but not small intestine or lung. T cells were isolated from healthy human skin, small intestine and lung, stimulated with αCD3/αCD2/αCD28 and production of IL-9 and IFN-γ was assessed by flow cytometry at the indicated time points after stimulation with PMA+I. (B) Skin TH9 cells are independent of TGF-β and IL-2. T cells freshly isolated from healthy skin were stimulated as in (A) and cultured in the presence or absence of neutralizing antibody to TGF-β or isotype matched control antibody (left) or exogenous IL-2 (right). Percentage of IL-9+ Th cells was assessed by flow cytometry at the indicated time points after stimulation with PMA+I (mean + s.d.). (C) CD3+ T cells are the major source of IL-9 in human skin. Cell suspensions from human skin were simulated with PMA+I before or after 12 hours of stimulation with αCD3/αCD2/αCD28 beads. CD3+ T cells were the major source of IL-9 both before and after T cell stimulation. (D,E) Innate lymphoid cells (ILC) were not an appreciable source of IL-9 in healthy human skin. Cell suspensions from human skin were stimulated for four days (4d) with either (D) IL-2 (10 U/ml)+IL-33 (50 ng/ml) (to stimulate ILC) or (E) αCD3/αCD2/αCD28 beads (to stimulate T cells), then treated with PMA+I. IL-9 was not produced by CD3− cells after IL-2+IL-33 but was produced by CD3+ cells after bead treatment. CD3+ T cells produced IFN-γ without bead stimulation but IL-9 production was not observed unless T cells were first bead stimulated. (F) TH9 cells co-produced granzyme B and TNF-α but lacked FoxP3 and production of other TH lineage cytokines. Four days after stimulation, skin resident T cells were analyzed for co-expression of IL-9 with various cytokines, granzyme B and FoxP3. (G) The frequency of skin resident T cells producing IL-9 alone or in combination with IL-17, IFN-γ, or IL-13, 4 days after stimulation is shown. Data are representative of independent experiments with at least 3 (A, D, E) or 6 donors (B, C, F, G). Panels C–E are gated to show all viable cells as determined by forward/side scatter, panels A and F show viable CD3+/CD8− lymphocytes.
In mice, it has been suggested that innate lymphoid cells (ILC), not T cells, are the main source of IL-9 produced in vivo (3). IL-33 and IL-2 can induce production of IL-9 from human ILC (33). We observed no IL-9 production after treatment of healthy skin cells with IL-33 and IL-2 but CD3+ T cells were a major source of IL-9, both before and after polyclonal T cell stimulation (Fig. 3C–E).
The majority of IL-9+ skin-resident TH cells lacked co-expression of other TH lineage signature cytokines, although minor subsets of IFN-γ+, IL-17+ or IL-13+ TH cells did co-express IL-9 (Fig 3F,G), as revealed by combined intracellular FACS staining for all four cytokines. IL-9 production was transient as observed in CLA+ TH cells from blood, regardless of co-production of other cytokines. TH cells co-producing IL-9 with two or more TH cytokines were not observed, neither in CLA+ skin homing nor in skin resident TH cells (Fig. S4). IL-9+ skin-resident TH cells also lacked co-expression of IL-10, IL-22, and the Treg marker FoxP3 but co-expressed TNFα and the cytotoxic molecule granzyme B (Fig. 3F), further supporting the notion that TH9 cells are a distinct subset with proinflammatory and cytotoxic properties (4).
Human TH9 cells have autocrine and paracrine activities
In TH9 cells isolated from human blood and skin, we observed that peak IL-9 production preceded maximal activation-induced up-regulation of IFN-γ, IL-17, and IL-13 in TH cells (Fig 4A,B). We next investigated if IL-9 could induce or enhance production of other effector cytokines in skin-tropic TH cells. Blocking IL-9 with neutralizing antibody at the initiation of in vitro culture strongly inhibited subsequent up-regulation of IL-9, IFN-γ, IL-13, and IL-17 in CLA+ TH cells (Fig. 4C). IL-9 neutralizing antibody also reduced T cell proliferation at early (day 4) but not late time points (Fig. 4E) and had no significant effects on cell viability (Fig. S2D). In parallel, we found that IL-9 receptor (IL-9R) expression was highly enriched in activated CLA+ TH cells and increased after activation (Fig. 4D).
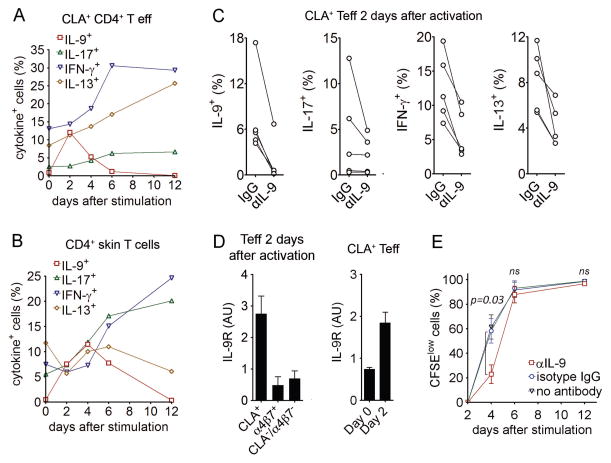
Fig. 4. Human TH9 cells have autocrine and paracrine pro-inflammatory activity.
(A,B) IL-9 production is transient and precedes the up-regulation of other inflammatory cytokines. T cells isolated from blood (A) and skin (B) were stimulated with αCD3/αCD2/αCD28 and production of IL-9, IFN-γ, IL-17, and IL-13 was assessed at the indicated time points by flow cytometry after stimulation with PMA+I. (C) IL-9 production is required for maximal production of IL-9 itself as well for as the production of other inflammatory cytokines. CLA+ TEFF were stimulated for 2 days with αCD3/αCD2/αCD28 in the presence of neutralizing antibody to IL-9 or isotype matched control antibody. Cytokine production was assessed by flow cytometry after stimulation with PMA+I. (D) Expression of the IL-9 receptor is enriched in activated skin-tropic CLA+ TEFF. IL-9 receptor (IL-9R) mRNA was measured by real-time quantitative PCR in CLA+, α4β7+, and CLA−/α4β7− TEFF after 2 days of stimulation with αCD3/αCD2/αCD28 (mean + s.e.m., 3 donors with triplicates). Baseline expression (Day 0) vs. expression after activation (Day 2) of IL-9R by CLA+ TEFF cells is shown in the right panel. (E) IL-9 enhances cellular proliferation at early timepoints. TEFF were labeled with CFSE, stimulated with αCD3/2/28 with anti-IL-9 (αIL-9) or isotype matched control antibody and proliferative cells were identified (CFSElow). Proliferation was significantly decreased at day four but was unchanged at later time points. The mean and SEM of four donors are shown. Data are representative of independent experiments with 6 (A,B), 5 (C, E), 3 (D) donors.
Human TH9 cells are increased in psoriatic skin lesions
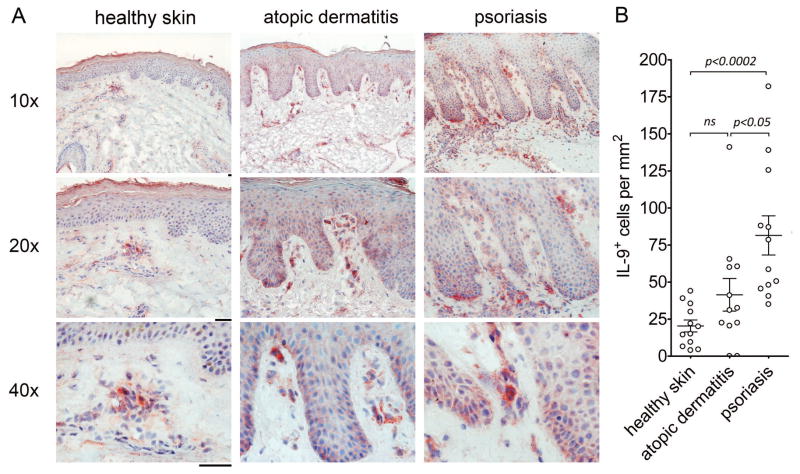
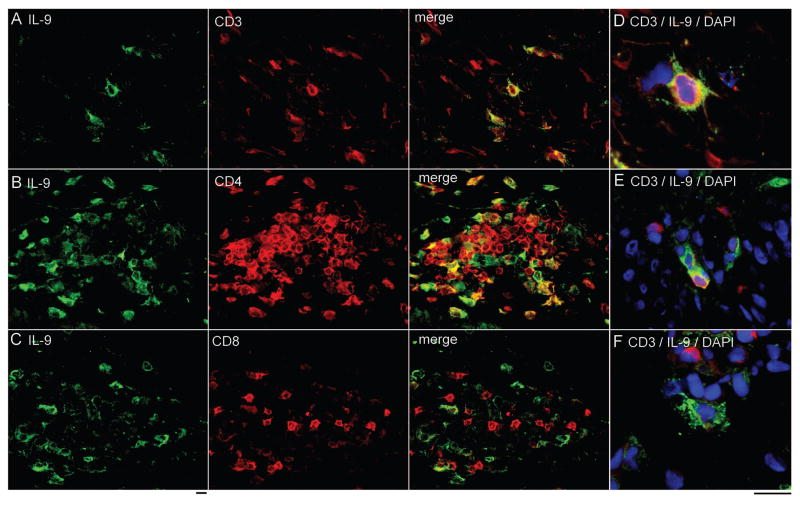
Given the ability of TH9 cells to enhance the production of proinflammatory cytokines by other T cells, we carried out immunohistochemical studies on the skin lesions of TH1/TH17-mediated (psoriasis) and TH2-mediated (atopic dermatitis) human skin diseases. IL-9 producing T cells were evident within the skin lesions of both diseases (Fig. 5). The number of IL-9 producing cells was significantly higher in psoriatic skin lesions as compared to healthy skin (Fig. 5B). Although atopic dermatitis skin lesions also tended to have increased numbers of IL-9 producing cells, this increase was not statistically significant. To establish that IL-9 in psoriatic skin lesions was produced by T cells, double color immunofluorescence stains of psoriatic skin lesions were performed. A population of IL-9 producing cells expressing CD3, CD4 but not CD8 was identified (Fig. 6A–C). Interestingly, a proportion of IL-9 producing T cells were observed to be in the process of cell division (Fig. 6D,E), consistent with our in vitro observations that IL-9 is produced transiently and only by activated cells. A subset of CD3− IL-9 producing cells were also demonstrable in psoriatic skin lesions, suggesting the possibility that ILC or other IL-9 producing cell types may contribute to IL-9 production (Fig. 6F).
Fig. 5. IL-9 producing cells are increased in the skin lesions of psoriasis and atopic dermatitis.
(A) Healthy skin and lesional skin samples from patients with psoriasis and atopic dermatitis were immunohistochemically stained for IL-9. Positive cells appear red. (B) TH9 cells are significantly increased in the skin lesions of psoriasis but not atopic dermatitis. The number of IL-9+ cells per mm2 in immunohistochemically stained sections of healthy, atopic dermatitis and psoriasis skin are shown. The mean and SEM of 2 independent experiments with 12 donors each per condition are shown. Scale bars=50 μM.
Fig. 6. TH9 cells are evident in human psoriatic lesional skin.
(A–C) Two color immunofluorescence staining was performed on lesional psoriatic skin. A population of IL-9 producing cells expressed (A) CD3, (B) CD4, and (C) lacked expression of CD8. (D, E) IL-9 production was observed in cells undergoing cell division. (F) A population of CD3− IL-9 producing cells was also present in psoriatic skin lesions. Scale bars=10 μM.
DISCUSSION
We report here a comprehensive characterization of human IL-9 producing T cells. We found that IL-9 was primarily produced by a discrete and stable population of T cells, strongly suggesting that TH9 cells do indeed exist in humans. Unlike human and mouse T cells generated by differentiation from naïve cells in vitro, human TH9 cells isolated from human blood and tissues lacked production of other TH lineage cytokines, co-produced TNF-α and granzyme B, lacked FOXP3 expression and were not dependent on the presence of TGF-β or exogenous IL-2. Both the naturally occurring, in vivo differentiated TH9 cells studied in this report and Th9 cells generated by in vitro differentiation (7, 17) lack IL-10 production, a feature that distinguishes them from mouse TH9 cells.
Human TH9 cells had unique features that distinguished them from other TH lineages. In healthy adults, TH9 cells were found primarily among the CLA+ skin-homing effector T cell population and were present in healthy human skin, but were effectively absent from human small intestine and lung. Remarkably, a demonstrable proportion of CLA+ TH9 cells were specific for C. albicans, suggesting that under normal conditions, TH9 cells may play a role in protecting against certain extracellular pathogens. In contrast to the production of other inflammatory cytokines, production of IL-9 by TH9 cells was transient, peaking at day two after stimulation in circulating TH9 cells and at day four after stimulation in skin resident TH9 cells. Transient production of IL-9 with subsequent down-regulation is a pattern that has also been observed in mouse in vitro differentiated TH9 cells (34). The transient nature of IL-9 production may explain why in mouse models of TH9-mediated inflammation, IL-9 producing T cells are often not readily demonstrable at the affected sites (18, 19). What becomes of TH9 cells after they stop producing IL-9 is not clear; possibilities include cessation of cytokine production, apoptopic cell death or differentiation into other TH cell subsets.
In both circulating and skin resident TH9 cells, maximal IL-9 production preceded the activation-induced up-regulation of other TH inflammatory cytokines including IFN-γ, IL-17, and IL-13. We found that IL-9 receptor (IL-9R) expression was highly enriched in activated CLA+ TH cells, suggesting skin-homing TH cells may be both a source and target of IL-9. Indeed, blocking IL-9 in vitro strongly inhibited the up-regulation of not only IL-9, but also IFN-γ, IL-13, and IL-17 in CLA+ TH cells and reduced early T cell proliferation. It has been proposed previously that IL-9 functions indirectly via the induction of IL-13 and IL-5 and therefore, IL-9 has a regulatory role on cells that produce TH2 cytokines (3). Our results, however, indicate that human IL-9 induces the production of cytokines from multiple T cell subsets, at least in skin homing T cells. Our results suggest that TH9 cell activation in tissues could initiate inflammation via both TNF-α production and via their autocrine and paracrine activity, leading to enhanced activation and cytokine production by TH1, TH2, TH9 and TH17 cells. Such a role for TH9 cells in the initiation of inflammation is consistent with findings in mouse models where dermal injection of IL-9 enhanced Th17-related psoriasiform inflammation in K5.hTGF-β1 transgenic mice (35), blockade of IL-9 suppressed both production of IL-17 by autoreactive T cells and their ability to initiate disease in adoptive transfer EAE (36), and in models in which TH9 cells were found to be critical for the recruitment and activation of IFN-γ producing anti-melanoma specific T cells (5). Consistent with a possible role in initiating and enhancing skin inflammation, we found that IL-9 producing TH9 were significantly increased in the skin lesions of psoriasis.
Limitations of this study include an inability to track human TH9 cells, as is possible in transgenic mice, in order to determine the eventual fate of IL-9 producing T cells. Also, the presence of IL-9 producing TH9 cells in human psoriatic skin lesions, their ability to amplify T cell responses and a role for IL-9 in one mouse model of psoriasiform dermatitis is intriguing but our results do not constitute proof that TH9 cells play a causative pathogenic role in human psoriasis. Lastly, the panel of bacterial and viral antigens we used to examine T cell reactivity was diverse but not comprehensive, and it is likely that TH9 cells reactive to multiple other pathogens also exist in vivo in humans.
In summary, we find that memory TH9 cells can be readily isolated from human blood and tissue, are preferentially skin-tropic or skin-resident, and are present in both healthy and diseased human skin. TH9 cells isolated from humans were a discrete T cell subset that showed no dependence on the presence of TGF-β, lacked IL-10 production but co-produced TNFα and granzyme B, and were essential for maximal inflammatory cytokine production of CLA+ TH1, TH2, TH9 and TH17 cells. The specificity of many memory TH9 cells for C. albicans suggests that in addition to anti-tumor effects, TH9 cells may play a critical role in healthy individuals in the defense against extracellular pathogens. The ability of TH9 cells to enhance proliferation and the production of inflammatory cytokines from other T cell subsets and their increased presence in psoriasis skin lesions suggests TH9 cells may also participate in initiating and maintaining cutaneous inflammation.
MATERIALS AND METHODS
Study design / Experimental design
This is an experimental laboratory study performed on human tissue samples. All studies were performed in accordance with the Declaration of Helsinki. Blood from healthy individuals was obtained after leukapheresis, skin was obtained from healthy patients undergoing cosmetic surgery procedures, lung and intestinal specimens were obtained as discarded tissues following resection of small tumors distant from the provided specimens; all tissues were collected with prior approval from the Partners Institutional Review Board. Skin samples for immunohistochemistry and immunofluorescence studies were obtained from patients seen at the Department of Dermatology, Inselspital/University of Bern, Bern, Switzerland. The study on human patients samples was approved by the Medical Ethics Committee of the Canton of Bern, Switzerland (approval-# 35/08). Written informed consent was obtained from all patients, Enumeration of IL-9 producing cells in immunofluorescence and immunohistochemical studies was done in an investigator blinded fashion. Mechanistic studies on cells derived from blood and human tissues were performed using in vitro assays without blinding or randomization. Study components were not predefined.
Antibodies and flow cytometry
All antibodies used in this study are listed in Table S1. For analysis of cytokine production, T cells were stimulated with either control medium or 50 ng/ml PMA and 750 ng/ml ionomycin plus 10 μg/ml Brefeldin A (Calbiochem) for four hours. Cells were then surface stained, fixed, permeabilized, stained with anti-cytokine antibodies, and examined by flow cytometry. Analysis of flow cytometry samples was performed on Becton Dickinson FACSCanto instruments and data was analyzed using FACSDiva software (V5.1). To set gates, cells were stained with isotype control antibodies and gates were set such that <1% of cells were present in all three positive staining quadrants.
Culture medium
Cell culture medium consisted of X-VIVO 15 (Lonza) supplemented with 2% (vol/vol) human serum type AB (Sigma). Where indicated, recombinant human IL-2 was added at 50 IU/ml.
Cell sorting
Peripheral blood mononuclear cells (PBMC) from healthy donors were isolated using Histopaque-1077 (Sigma). CD14+ monocytes were isolated by positive selection using magnetic microbeads (Miltenyi). Total CD4+ memory T cells were isolated by negative selection using the memory CD4+ T cell isolation kit (Miltenyi) and stained for fluorescence-activated cell sorting (FACS) with anti-α4β7, followed by anti-mouse IgG, then with anti-CLA and anti-CD25. Treg-depleted (CD25− TEFF) CLA+, α4β7+, and CLA−/α4β7− memory T cell subsets were sorted on a FACS Aria (BD Biosciences). Sorting purity of T cell subsets was typically over 95% in post sort analysis, with the exception of the CLA− subset, where a purity of >85% was achieved.
T cell stimulation with pathogen-pulsed monocytes
Commercially available antigen preparations from Herpes simplex virus-1, Rotavirus, Influenza A (H3N2), and Mycoplasma pneumoniae were obtained from Microbix. Antigen preparations from Staphylococcus epidermidis, Salmonella typhimurium, Staphylococcus aureus, and Candida albicans were generated by heat-killing microbes at 65°C for 1 h (all four pathogens), followed by three freeze-thaw cycles (bacteria only). Pathogen concentrations were determined by optical density measurements (Bio-Rad) according to the manufacturer’s instructions. All antigen preparations were used at the concentration that resulted in maximal proliferation in titration experiments using CFSE dilution. Monocytes (2.5×104) were pre-incubated with antigen preparations for 6 h at 37°C. CFSE-labeled purified CD4+ memory T cell subsets (5×104) were then co-cultured with the antigen-pulsed autologous monocytes for 7 days before analysis of CFSE dilution on a FACS Canto (BD Bioscience). CFSE labeling was performed according to standard protocols. On day 5 of co-culture, supernatants were saved and cytokine concentrations were measured using a custom-made Luminex bead assay, as previously described (37). For analysis of pathogen-induced IL-9 expression kinetics, PBMC (4×105) were cultured with C. albicans or S. aureus and analyzed for the expression of IL-9 and IL-17 at different time points by intracellular FACS staining after stimulation for 5 h with phorbol 12-myristate 13-acetate (PMA) and ionomycin (PMA+I). For experiments measuring IL-9 production in the presence or absence of IFN-γ blockade (Fig. S2A), CFSE labeled PBMC (2×105) were cultured in the presence of anti IFN-γ (10 ug/ml) or mouse IgG (10 ug/ml). On day 5 of culture, supernatants were harvested and IL-9 concentrations were measured using an IL-9 ELISA kit (ELISA MAX™, Biolegend) according to manufacturer’s instructions.
Polyclonal activation of TEFF and cytokine expression kinetics
FACS-sorted TEFF subsets (2×104) were polyclonally activated using beads coated with antibodies against CD3, CD2, and CD28 (T cell:bead=2:1, Miltenyi). Before activation and at different time points thereafter, T cells were additionally stimulated for 5 h with PMA+I in the presence of brefeldin A and then intracellularly stained for IL-9, IFN-γ, IL-13, and IL-17 for FACS analysis according to standard protocols. Supernatants were collected at day 4 after polyclonal stimulation and cytokines were measured as described above. Where indicated, the following neutralizing antibodies were added at the start of the culture (all at 10 μg/ml): anti-TGF-β (clone 1D11, R&D systems), anti-IL-9 (MH9D1, Biolegend), IgG1 control antibody (MG1-45, Biolegend), anti IL-2 (4 ug/ml, polyclonal goat IgG; R&D systems), or polyclonal goat IgG (4 ug/ml, R&D systems).
Quantitative real-time PCR for IL-9 receptor
Total RNA was isolated from FACS-sorted T cell subsets 2 days after polyclonal activation (as described above) using the RNeasy Plus Mini kit (Qiagen), according to the manufacturer’s instructions. Complementary DNA was generated using the using the SuperScript Vilo cDNA synthesiskit (Lifetechnologies) and quantitative real-time PCR was performed using the ABI StepONE plus instrument and the Fast SYBR green master mix (Applied Biosystems). Expression of each ligand transcript was determined relative to the reference gene transcript (β-actin) and calculated as 2^−(Ct, ligand−Ct, β-actin). Data are presented as arbitrary units (AU). The primers used to detect the transcripts were purchased from Integrated DNA Technologies and were as follows: IL-9R (F-5′-GGGTGACAAATCACCTCCAG-3′; R-5′-GCCTCACTCTCCAAGGTCC-3′) and β-actin (F-5′-TCACCCACACTGTGCCCATCTACGA-3′; R-5′-CAGCGGAACCGCTCATTGCCAATGG-3′).
Isolation and polyclonal activation of tissue-resident T cells
Skin was obtained from healthy subjects undergoing cosmetic surgery procedures. Lung and small intestine samples were obtained from resection margins distant from the pathology of patients undergoing lung or gut surgery for various reasons. Tissue samples were extensively minced and then incubated for 2 h at 37°C in RPMI-1640 containing 0.2% collagenase type I (Invitrogen) and 30 Kunitz Units/ml DNAse I (Sigma Aldrich). Thereafter, cells were collected by filtering the collagenase-treated tissue through a 40μm cell strainer (Fisher Scientific). For experiments shown in Fig. S3, T cells were isolated from three week explant cultures maintained in IL-2 and IL-15 as previously described (38). After washing, tissue-derived T cells were then activated polyclonally using anti-CD3/anti-CD2/anti-CD28 coated beads, analogous to the activation of blood-derived T cells described above. Analysis of cytokine expression in tissue-resident T cells (TRM) before and at different time points after polyclonal activation and experiments with neutralizing antibody to TGF-β were performed as described above for blood-derived T cells.
Isolation and activation of skin resident Innate Lymphoid Cells (ILCs)
Cells isolated from healthy skin were plated at a density of 5×104/ml in 96 well plates and stimulated for 4 days with IL-2 (10ug/ml; NCI) plus IL-33 (50 ng/ml; R&D Systems) or with anti-CD3/anti-CD2/anti-CD28 coated beads. On day 4 of culture, cells from all wells were stimulated for 5 h with PMA+I. ILCs (Lin−; CD11b− CD3−, CD56−) and T cells (CD3+) were examined for IL-9 production by flow cytometry.
Immunohistochemical staining
Patients with chronic plaque psoriasis (3 women and 10 men) with median age 54 years (SD +/− 11y) and patients with atopic dermatitis (3 women and 7 men) with median age 45 years (SD +/− 20y) were included. Diagnosis was based on typical clinical and histopathological criteria. The patients did not receive systemic or local therapy at the site where biopsy specimens were obtained for at least 3 weeks prior to the investigation. Punch biopsies (5 mm) were taken from lesional skin. Healthy skin was obtained from X healthy control subjects undergoing reconstructive surgery for aesthetical reasons. Skin samples were immediately embedded in optimal cutting temperature (OCT) compound, snap frozen and stored at −70 °C until sectioning. Immunostaining was performed using the streptavidin-biotin complex/alkaline phosphatase method, as previously described (39). Briefly, cryostat-cut tissue sections were air dried, fixed for 10 minutes in 4% ice-cold acetone and rehydrated in TRIS-buffered saline with 0.1% saponin. The sections were incubated with the primary antibody for 1 hour at room temperature, followed by a biotinylated secondary antibody and thereafter with streptavidin-biotin complex/alkaline phosphatase method (K0376; DakoCytomation). Finally, all sections were developed in new fuchsin-naphthol (KO624, DakoCytomation) and counterstained with hematoxylin. All skin sections were quantitatively analyzed by using the digital image acquisition and analysis system NIS-Elements Software BR 2.30 (Nikon, Tokyo, Japan), in an investigator blinded fashion, as previously described (39, 40).
Immunofluorescence double staining
Double immunofluorescence was performed as previously described (39). Briefly, tissue sections were serially incubated with the primary antibody (anti-IL-9) followed by incubation with Alexa fluor 488 labeled goat-anti-rabbit IgG for 1 hour each. Sections were then incubated with one of the following mouse-anti-human antibodies for 1 hour:(1) anti-CD3, (2) anti-CD4, (3) anti-CD8. Sections were then treated with Alexa fluor 594 labelled goat-anti-mouse IgG1 (Invitrogen). The specificity of the reaction was confirmed by omitting the primary or secondary antibodies as well as by using irrelevant isotype-matched antibodies as negative controls.
Statistical analyses
Primary methods of data analysis included descriptive statistics (means, medians and s.d.). Differences between two sample groups were detected using the one tailed Wilcoxon–Mann–Whitney test, α=0.05. For comparisons of multiple groups, a Kruskal–Wallis one-way analysis of variance with a Bonferroni-Dunn’s post test for multiple means test was used, α=0.05.
Supplementary Material
Fig. S1. Tissue tropic TH cells proliferate preferentially to pathogens commonly encountered through that epithelial tissue
Fig. S2. Additional effects of IL-2, IFN-γ and IL-9 antibody blockade on human TH9 cells
Fig. S3. TH9 cells are enriched in human skin and IL-9 is transiently produced
Fig. S4. TH9 cell co-production of other cytokines
Table S1. Antibodies used in this study
Acknowledgments
We would like to thank E. Butcher for anti-α4β7 (ACT-1), and Bill Richards (BWH tissue bank), Drs. Bohdan Pomahac, Simon Talbot and Elof Eriksson of BWH and Dr. Thomas Cochran of the Boston Center for generously providing access to human tissue samples. We also thank F. Sallusto and J. Lederer for technical support. Funding: Supported by a Damon Runyon Clinical Investigator Award (to R.A.C.), R01 AR056720 (to R.A.C.), R01 AR063962 (to R.A.C.), R03 MH095529 (to R.A.C.), the SPORE in Skin Cancer P50 CA9368305 NIH/NCI (to T.S.K.), R01 A1025082 NIH/NIAID (to T.S.K), R01 AI097128 (to T.S.K and R.A.C), the Swiss National Science Foundation and the Fondation René Touraine (to C.S.), a Special Fellow Award from the Leukemia & Lymphoma Society (to R.W.), and a grant from the German Research Foundation (to E.G.).
Footnotes
AUTHOR CONTRIBUTIONS
C.S. and A.G. carried out most of the experiments, drafted figures and participated in writing the manuscript; C.Y., R.W., L.C., E.G. and J.T. assisted in carrying out experiments and helped edit the manuscript. N.Y. carried out immunohistochemical studies of psoriatic and atopic dermatitis skin. T.K. provided advice on approaches and edited the manuscript. R.C. supervised studies, drafted figures, and together with C.S., wrote, edited and revised the manuscript.
CONFLICT OF INTEREST
RAC and TSK previously had an equity interest in TremRX, a start-up company that seeks as a long-term business plan to improve vaccine formulation and delivery. During the period RAC and TSK held the equity, the interest was deemed to create a financial conflict of interest (as defined by the specific Public Health Serivce regulations) with the research discussed in this article. To resolve this matter, RAC and TSK divested themselves of the equity interest in this company, so this financial conflict of interest no longer exists. RAC has served as a consultant for Novartis, Dermira and Stiefel. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nature immunology. 2012;13:637–641. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 4.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, Kupper TS. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nature medicine. 2012 doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, Yang J, Qian J, Yi Q. Th9 cells promote antitumor immune responses in vivo. The Journal of clinical investigation. 2012 doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, Hafler DA. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, Utz PJ. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunology and cell biology. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uyttenhove C, Brombacher F, Van Snick J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. European journal of immunology. 2010;40:2230–2235. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]
- 9.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Current opinion in immunology. 2012;24:303–307. doi: 10.1016/j.coi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infection and immunity. 1998;66:3832–3840. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, Krug N. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J Allergy Clin Immunol. 2003;111:1319–1327. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 13.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, Wallaert B, Hamid QA. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 14.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature immunology. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011;128:1357–1360. e1355. doi: 10.1016/j.jaci.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullens DM, Kasran A, Dilissen E, De Swert K, Coorevits L, Van Snick J, Ceuppens JL. In vivo maturation of T(H) cells in relation to atopy. J Allergy Clin Immunol. 2011;128:234–237. e237. doi: 10.1016/j.jaci.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PloS one. 2010;5:e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, Gery I. Antigen-Specific Th9 Cells Exhibit Uniqueness in Their Kinetics of Cytokine Production and Short Retention at the Inflammatory Site. The Journal of Immunology. 2010;185:6795–6801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, Carter JB, Fisher DC, Kupper TS. Skin Effector Memory T Cells Do Not Recirculate and Provide Immune Protection in Alemtuzumab-Treated CTCL Patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, Rüde E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. The Journal of Immunology. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 23.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 24.Abramson O, Qiu S, Erle DJ. Preferential production of interferon-gamma by CD4+ T cells expressing the homing receptor integrin alpha4/beta7. Immunology. 2001;103:155–163. doi: 10.1046/j.0019-2805.2001.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 26.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald TT, Pender SL. Lamina propria T cells. Chemical immunology. 1998;71:103–117. doi: 10.1159/000058721. [DOI] [PubMed] [Google Scholar]
- 28.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident Memory T Cells (T(RM)) Are Abundant in Human Lung: Diversity, Function, and Antigen Specificity. PloS one. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nature medicine. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 32.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 33.Mjösberg J, Bernink J, Golebski K, Karrich Julien J, Peters Charlotte P, Blom B, te Velde Anje A, Fokkens Wytske J, van Drunen Cornelis M, Spits H. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Tan C, Gery I. The unique features of Th9 cells and their products. Critical reviews in immunology. 2012;32:1–10. doi: 10.1615/critrevimmunol.v32.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh TP, Schon MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, Wolf P. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PloS one. 2013;8:e51752. doi: 10.1371/journal.pone.0051752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–4100. doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Leary FM, Tajima G, Delisle AJ, Ikeda K, Dolan SM, Hanschen M, Mannick JA, Lederer JA. Injury-induced GR-1+ macrophage expansion and activation occurs independently of CD4 T-cell influence. Shock. 2011;36:162–169. doi: 10.1097/SHK.0b013e31821af669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. The Journal of investigative dermatology. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 39.Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. Journal of the American Academy of Dermatology. 2011;65:790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Schlapbach C, Yawalkar N, Hunger RE. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. Journal of the American Academy of Dermatology. 2009;61:58–65. doi: 10.1016/j.jaad.2008.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Tissue tropic TH cells proliferate preferentially to pathogens commonly encountered through that epithelial tissue
Fig. S2. Additional effects of IL-2, IFN-γ and IL-9 antibody blockade on human TH9 cells
Fig. S3. TH9 cells are enriched in human skin and IL-9 is transiently produced
Fig. S4. TH9 cell co-production of other cytokines
Table S1. Antibodies used in this study