SUMMARY
Immune system defects are at the center of aging and a range of diseases. Here we show that prolonged fasting reduces circulating IGF-1 levels and PKA activity in various cell populations, leading to signal transduction changes in long-term hematopoietic stem cells (LT-HSC) and niche cells that promote stress resistance, self-renewal and lineage-balanced regeneration. Multiple cycles of fasting abated the immunosuppression and mortality caused by chemotherapy, and reversed age-dependent myeloid-bias in mice, in agreement with preliminary data on the protection of lymphocytes from chemotoxicity in fasting patients. The pro-regenerative effects of fasting on stem cells were recapitulated by deficiencies in either IGF-1 or PKA and blunted by exogenous IGF-1. These findings link the reduced levels of IGF-1 caused by fasting, to PKA signaling and establish their crucial role in regulating hematopoietic stem cell protection, self-renewal and regeneration.
INTRODUCTION
Prolonged fasting (PF) lasting 48–120 hours reduces pro-growth signaling and activates pathways that enhance cellular resistance to toxins and stress in mice and humans (Fontana et al., 2010b; Guevara-Aguirre et al., 2011; Holzenberger et al., 2003; Lee and Longo, 2011; Longo et al., 1997). The physiological changes caused by PF are much more pronounced than those caused by calorie restriction or overnight fast, in part because of the requirement to fully switch to a fat- and ketone bodies-based catabolism after glycogen reserves are depleted during PF (Longo and Mattson, 2014). Studies in mice indicate that PF can protect them from chemotoxicity by reducing circulating insulinlike growth factor-1 (IGF-1) (Lee et al., 2010; Raffaghello et al., 2008). A preliminary case series study also indicates that PF has the potential to ameliorate several side effects caused by chemotherapy in humans (Safdie et al., 2009). One of the side effects, myelosuppression, is often dose-limiting in chemotherapy treatment, in part because damage to adult stem/progenitor cells impairs tissue repair and regeneration (Kofman et al., 2012; Mackall et al., 1994; van Tilburg et al., 2011; Williams et al., 2004). Despite the rising interest in nutrient-dependent changes in stem cell populations, little is known about how acute or periodic dietary interventions affect the hematopoietic system.
HSPCs residing in the adult bone marrow (BM) are contained within the Lin−Sca-1+c-Kit+ (LSK) population of cells, which include the self-renewing long-term and short-term hematopoietic stem cells (LSK-CD48−CD150+, LT-HSC and LSK-CD48−CD150−, ST-HSC) and the multipotent progenitors (LSKCD48+, MPP)(Figure S1)(Challen et al., 2009; Rathinam et al., 2011). Together, these cells are responsible for adult hematopoietic regeneration. In the heterogeneous HSCs, several subtypes are identified as Lymphoid-(Ly-HSCs), balanced HSC (Bala-HSC) and Myeloid-HSCs (My-HSCs) according to their distinct mature blood cell outputs (Figure S1) (Benz et al., 2012; Challen et al., 2010; Muller-Sieburg et al., 2004). In both mice and humans, these HSC subtypes modulate hematopoietic lineage potential and play an important role in lineage-homeostasis during aging (Beerman et al., 2010; Challen et al., 2010; Cho et al., 2008; Pang et al., 2011). Here, we studied the role of multiple PF cycles on chemotherapy–induced and age-dependent immunosupression and investigated how PF affects HSC self-renewal, the Ly-, My- and Bala-HSC subtypes as well as their hematopoietic reconstitution outcomes.
RESULTS
Cycles of prolonged fasting (PF) reduce damage in bone marrow stem and progenitor cells and protect mice against chemotoxicity
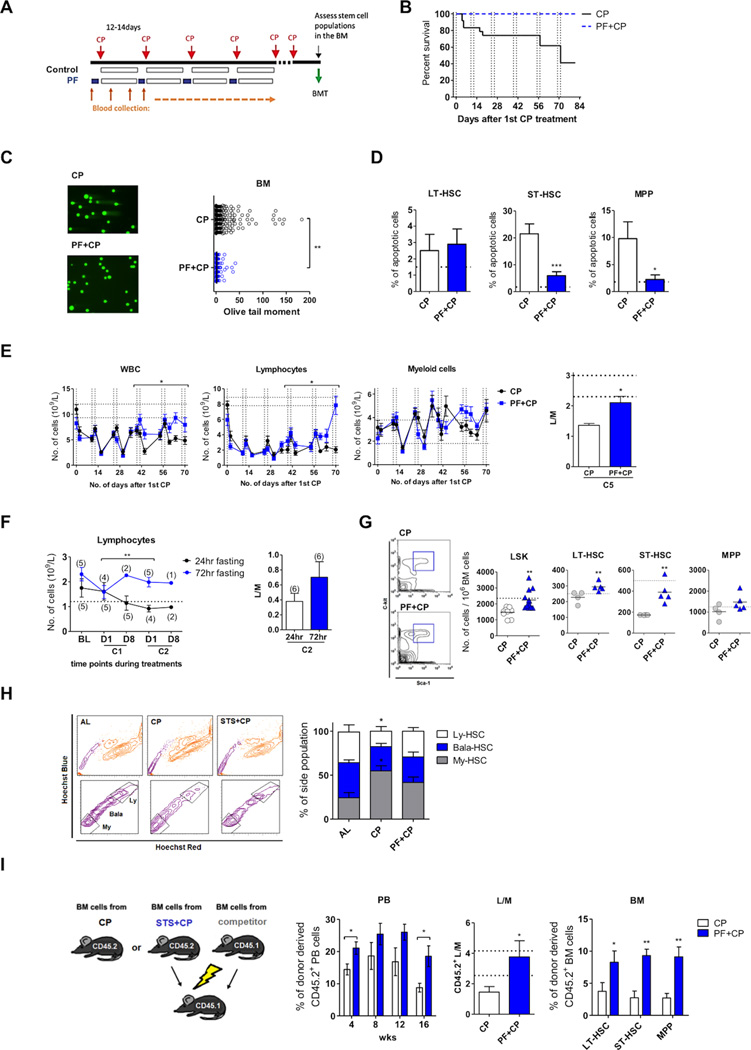
Chemotherapy drugs cause immunosuppression by inducing DNA damage and cell death in both peripheral blood (PB) and bone marrow (BM), which often results in long-term impairment of hematopoiesis (Bedford et al., 1984; Yahata et al., 2011). To test whether PF may protect the hematopoietic system against immunosuppressive toxicity, mice were fasted or fed an ad lib diet (AL) and then challenged with cyclophosphamide (CP) for multiple cycles (Figure 1A) (Adams et al., 2007). In agreement with our previous results with etoposide and doxorubicin, we observed a significant protective effect of cycles of 48-hours PF against CP-induced mortality (Figure 1B and S1A) (Raffaghello et al., 2008). The PF cycles also led to a decrease in the DNA damage caused by CP in leukocytes and BM cells (Figure 1C and S1B).
Figure 1. Prolonged fasting cycles protect the hematopoietic system and reverse the chemotherapy-induced hematopoietic suppression.
(A) Diagrammatic representation of the experimental procedure to analyze the effects of prolonged fasting (PF, 48hr) during 6 cycles of cyclophosphamide chemotherapy (CP, 200mg/kg, i.p.).
(B) Survival curve with vertical dashed lines indicating the pre-chemo starvation period; p<0.01, Log-rank (Mantel-Cox) test; n=20 (10 male and 10 female).
(C) DNA damage measurement (olive tail moment) in bone marrow (BM)cells (day 81, 6th recovery phase).
(D) Apoptosis measurement (TUNEL assay) in HSCs and MPP (day 81, 6th recovery phase).
(E) Hematological profile of mice. Total white blood cell (WBC), lymphocyte counts and lymphoid/myeloid ratio (L/M) in mice treated with 6 cycles of CP (200mg/kg, i.p.). Each point represents the mean ± s.e.m; horizontal dashed lines indicate the ranges of baseline values; * p<0.05, Two-way ANOVA, comparing CP vs. PF+CP during the recovery phase, n=12 (6 male and 6 female); L/M ratio of peripheral blood (PB) is defined as number of lymphocytes divided by number of myeloid cells (i.e. granulocytes and monocytes). See also supplementary Figure S1F and S1G.
(F) Hematological profile of human subjects. Lymphocyte counts and lymphoid/myeloid ratio (L/M) in patients undergoing two cycles (C1 and C2) of platinum-based doublet chemotherapy in combination with either 24hr or 72hr (48 before and 24 hours after chemo) prolonged fasting; D1 and D8 indicate the 1 day (before chemo) and 8 day of each chemotherapy cycle; Each point represents the mean ± s.e.m; ** p<0.01, Two-way ANOVA; Sample size is indicated in parentheses.
(G) FACS analysis of hematopoietic stem and progenitor cells (day 84, end of 6th cycle); horizontal dashed lines indicate the baseline value. (H) Proportion of the lymphoid-biased (Ly-HSC), balanced (Bala-HSC) and of the myeloid-biased (My-HSC) hematopoietic stem cells. The markers used are lower side population of LSK (lower-SPLSK) for My-HSC, middle-SPLSK for Bala-HSC and upper-SPLSK for Ly-HSC. The lower panels show a magnification of the SP population in the upper panels.*p<0.05, One-way ANOVA comparing to AL.
(I) BM cells collected from mice treated with either CP or PF+CP were transplanted into the recipient mice. The chimerism of donor-derived cells in PB and that in BM was determined 16 weeks after primary BM transplantation. The ratio of lymphocytes to myeloid cells (L/M) in the reconstituted blood was also measured. For (G and I), n= 6 to 10 per group, * p<0.05, ** p<0.01, t-test comparing the PF with the non-fasted control group both in combination with cyclophosphamide treatment.
To determine whether HSPC protection may be involved in the effects of PF on chemotherapy-induced toxicity, we collected BM cells at the end of 6 cycles of CP or PF + CP treatments and measured apoptosis. Given that the HSPCs represent a minor fraction of the total BM, we further examined apoptosis in the subpopulations of these cells (i.e. LT-HSC, ST-HSC and MPP) by performing TUNEL assay. The results indicate that without affecting BM cellularity, PF diminished CP-induced apoptosis in HSPCs (p<0.05, t-test), particularly in ST-HSCs and MPPs (Figure 1D, S1C and S1D). The PF-induced protection against CP-induced apoptosis was also confirmed by Annexin V binding assay for HSPCs (Figure S1E).
Prolonged fasting cycles promote lineage-balanced hematopoietic regeneration
To assess whether the protection of HSPCs improved the hematopoietic recovery, we compared the hematological profiles of CP and PF+CP mice at baseline (before CP treatments, after PF), at nadir (2–4 days after CP) and during the recovery phase (8–10 days after CP) for each cycle of chemotherapy. Multi-cycle CP treatments resulted in a major decline in white blood cell (WBC) counts (Figure 1E). In the control group, WBC suppression, especially the number of lymphocytes, persisted for more than 70 days (6 cycles) (Figure 1E). PF reduced WBC counts independently of chemotherapy and did not prevent the CP-induced decrease in the number of WBCs (Figure 1E, time 0). However, the beneficial effect of PF was evident starting on cycle 4 (day 39) with the return of lymphocytes to normal levels after the 5th cycle (day 56) (Figure 1E). At the end of 6-cycles of treatment, mice in the PF group also showed normal or close to normal levels of lymphoid cells and normal ratios of lymphoid and myeloid cells (L/M) (Figure 1E, right panel). This recovery was observed at similar time-points in 3 independent experiments (N=20).
To begin to determine whether PF cycles can potentially promote a similar effect in humans, we also analyzed the hematological profiles of cancer patients from a Phase I clinical trial for the feasibility and safety of a 24–72 hours PF period in combination with chemotherapy. Although 3 different platinum-based drug combinations were used (Table S1), the results from a Phase I clinical trial indicate that 72 but not 24 hours of PF in combination with chemotherapy were associated with normal lymphocyte counts and maintenance of a normal lineage balance in WBCs (Figure 1F). These encouraging preliminary results will need to be expanded and confirmed in the ongoing Phase II randomized phase of the clinical trial.
In agreement with the effect of PF on the recovery in WBC numbers and improvement in lymphoid/myeloid ratio, results of FACS analyses for stem cell populations indicated an improved preservation of LT-HSCs and ST-HSCs and the enhanced resistance to the myeloid bias in the PF group after 6-cycles of CP treatment in mice (Figure 1G and H).
To assess whether the increased HSCs in BM from PF + CP mice can enhance hematopoietic regeneration, we collected BM cells from the CP- or PF+CP-treated mice and transplanted the same number of cells into the immunocompromised (irradiated) recipient mice. Results of this competitive repopulation assay indicate that, compared to the control group fed ad libtum, the BM cells from mice exposed to 6-cycles of CP treatment preceded by PF have higher regeneration capacity leading to efficient blood reconstitution with improved lymphoid/myeloid ratio (L/M), as evident from the improved engraftment in blood and in BM (Figure 1I and S1G and S1H).
Prolonged fasting cycles regulate stem cell populations independently of chemotherapy and help reverse from immunosenescence
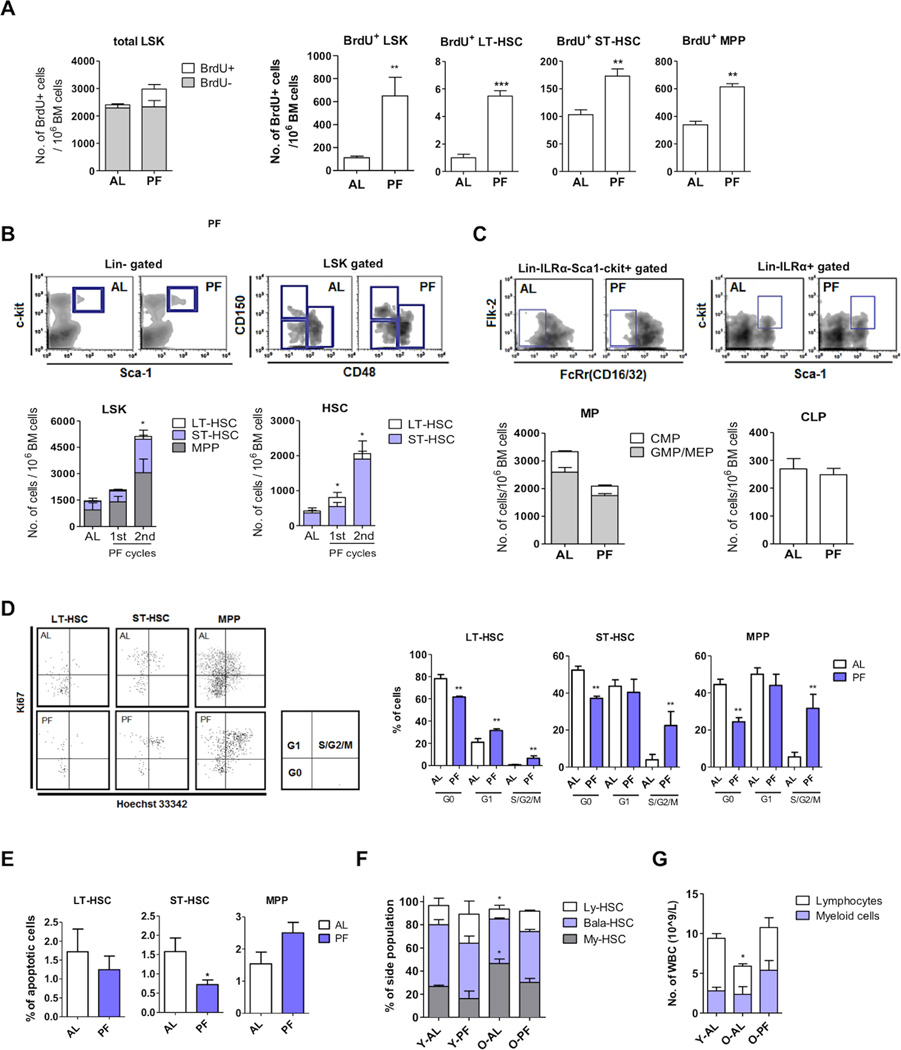
We tested whether the cycles of PF alone could also stimulate HSC self-renewal. Results using BrdU incorporation assays indicated an approximately 6-fold increase of newly generated (BrdU+) HSPCs (i.e. LT-HSC, ST-HSC and MPP) in PF mice, which represents 93.7% of the total increase in HSPCs after PF cycles (Figure 2A). We found that the increase in LSK cell number is due mainly to an increase in LT-HSCs and ST-HSCs (Figure 2B). By contrast, the number of total BM cells and that of progenitors (i.e. MPP, multipotent progenitors; CLP, common lymphoid progenitors; common myeloid progenitor, CMP) was not increased by PF, and, in fact, the number of CMP was slightly decreased during PF (Figure 2C and S2A).
Figure 2. Prolonged fasting cycles promote a chemotherapy-independent hematopoietic regeneration.
Mice in the control group were fed ad libitum and those in the PF group were fasted for one or two cycles as indicated. n=4 to 12 female mice per group.
(A) BrdU incorporation assay for LSK cells. Mice undergoing 24+48hr prolonged fasting were injected (i.p.) with BrdU (0.1mg/g, twice a day, for 2 days, starting after 24hr of fasting.
(B) Number of long-term hematopoietic stem cells (LT-HSC), short-term hematopoietic stem cells (ST-HSC) and multipotent progenitors (MPP).
(C) Number of common lymphoid progenitors (CLP) and myeloid progenitors (MP)
(D) Cell cycle analysis for BM cells using Ki67 and Hoechst33342.
(E) Apoptosis analysis for BM cells using TUNEL assay.
For (A) to (E), * p<0.05, ** p<0.01, *** p<0.005, t-test comparing the AL-fed controls.
(F) Proportion of the lymphoid-biased (Ly-HSC), balanced (Bala-HSC) and the myeloid-biased (My-HSC) hematopoietic stem cells. The markers used are lower side population of LSK (lower-SPLSK) for My-HSC, middle-SPLSK for Bala-HSC and upper-SPLSK for Ly-HSC.
(G) Number of lymphocytes and myeloid cells in young (6 months, 48hrs fasting) and old (18 months, 8 cycles of fasting) mice. For (F) and (G), * p<0.05, **p<0.01 and *** p<0.005, one-way ANOVA.
Results from cell cycle analyses indicate that PF alone induced a major increase of S/G2/M phase in LT-HSCs, ST-HSCs and MPPs (Figure 2D). The significant induction in cell cycle entry could explain at least part of the PF-induced increase in HSCs. In addition to the Ki67/Hoechst33342 staining for cell cycle analysis, the PF-induced self-renewal proliferation was also confirmed by analysis using Pyronin Y/Hoechst33342 staining (Figure S2B). On the other hand, results from the TUNEL assay indicate that apoptosis was barely detectable in any subpopulation of HSPCs from either AL-fed or PF mice when no chemotherapy treatment was applied. Apoptosis analysis using Annexin V and 7AAD indicated similar results (Figure S2C). Although PF alone reduces the apoptosis rate in ST-HSCs significantly, the small reduction (from 1.57% to 0.72%) in apoptosis/cellular death could only contribute to a very small portion of the PF-induced increase in HSCs and MPP (Figure 2E). However, as studies of HSCs have shown that induction of proliferation may sometimes be accompanied by an increase of apoptosis (Nakada et al., 2010; Tothova et al., 2007), it is important to note that this was not observed in PF-induced self-renewal proliferation.
Besides the increase in the number of HSCs and MPP, we also observed a PF-dependent alteration of lymphoid-, myeloid-biased and balanced-HSCs ratio (Figure 2F, S2D and S2E). Whereas most HSCs from young mice are balanced in lymphopoiesis and myelopoiesis, the majority of HSCs from elderly mice are myeloid biased (Beerman et al., 2010; Challen et al., 2010; Cho et al., 2008; Dykstra et al., 2007; Morita et al., 2010; Muller-Sieburg et al., 2004; Pang et al., 2011). We therefore investigated if PF cycles can correct this bias in aged mice. Results from 18-month old mice indicate that 8 cycles of PF could reverse the age-dependent myeloid bias in HSC subtypes and reverse the effect of aging on WBC number in whole blood (Figure 2F and 2G), similar to the changes observed in mice and possibly patients PF in combination with chemotherapy (Figure 1E,1F and 1H). Taken together, these results suggest that PF cycles can also stimulate the HSCs in a chemotherapy-independent manner which leads to a lineage-balanced hematopoietic regeneration.
Mimicking the effects of prolonged fasting by deficiency in GH/IGF-1 signaling promotes hematopoietic recovery
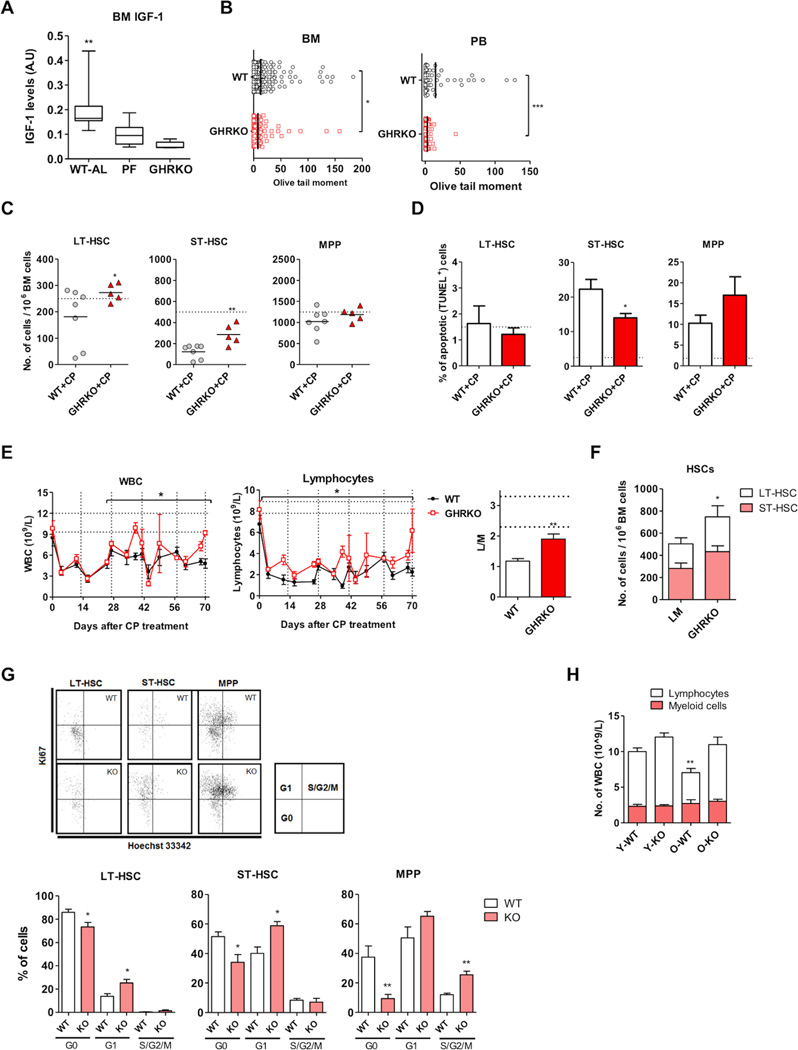
We previously showed that PF reduces circulating IGF-1 levels and that IGF-I deficiency is sufficient to protect mice against chemotherapy toxicity (Lee et al., 2010). To determine if the improved hematopoietic regeneration caused by PF in mice can be replicated by IGF-1 deficiency, we studied the hematopoietic system in growth hormone receptor knockout (GHRKO) mice, which have very low circulating and BM IGF-1 levels (Al-Regaiey et al., 2005) (Figure 3A, S3A and Table S2). We found that CP-induced DNA damage measured by the comet assay in PB and BM cells of GHRKO mice was significantly reduced compared to that in cells from wild-type littermates (Figure 3B). Similar to what was observed in mice undergoing pre-chemo PF cycles, ST-HSCs of the GHRKO mice were protected from CP-induced apoptosis (Figure 3C). Also, the number of HSCs (i.e. LT-HSCs and ST-HSCs) preserved in the BM of GHRKO mice was higher than that of the wild-type littermates (Figure 3D). An improvement in hematopoietic recovery analogous to that caused by PF, was also observed in GHRKO mice (Figure 3E).
Figure 3. Deficiency in GHR/IGF-1 signaling promotes hematopoietic regeneration in both chemo-treated and untreated mice.
Measurements were performed in GHRKO and their age matched littermates, with or without treatment with 6 cycles of CP (200mg/kg, i.p.). n=4 to 8 female mice per group.
(A) BM IGF-1 level in GHRKO mice and PF mice compared to wild type mice fed ad libitum (WT-AL), * p<0.05, ** p<0.01, one-way ANOVA.
(B) DNA damage measurement (olive tail moment) in BM cells and mononuclear peripheral blood cells (PB) from GHRKO and their littermates (WT) (day 81, 6th recovery phase).
(C) Apoptosis measurement (TUNEL assay) in hematopoietic stem and progenitor cells (day 81, 6th recovery phase).
(D) Number of hematopoietic stem and progenitor cells (day 84, end of 6th cycle); horizontal dashed lines indicate the chemo-free baseline value.
(E) Total white blood cell (WBC) and lymphocyte counts in PB of GHRKO mice and their littermates (WT); each point represents the mean ± s.e.m; vertical dashed lines indicate CP treatments; horizontal dashed lines indicate baseline value; * p<0.05, Two-way ANOVA for recovery phases; lymphoid/Myeloid ratio (L/M) after 6 cycles of CP treatments. PB L/M ratio is defined as the number of lymphocytes divided by the number of myeloid cells (i.e. granulocytes and monocytes). (F) Number of long-term hematopoietic stem cells (LT-HSC) and short-term hematopoietic stem cells (ST-HSC). (G) Cell cycle analysis using Ki67 and Hoechst33342.
(H) Number of lymphocytes and myeloid cells in young (age 6 months) and old (age 18 months) mice.
For (B to D) and (F to I) *p<0.05, **p<0.01; t-test comparing to the wild-type control.
We found that IGF-1 deficiency also caused the protective effects and the regenerative effects independently of chemotoxicity. Unlike PF mice, GHRKO mice did not have a higher frequency of total HSPCs (Figure S3B). However, similarly to what we observed after PF cycles, the frequency of HSCs (i.e. LT-HSCs plus ST-HSCs) was significantly higher in GHRKO mice compared to that in those age and sex matched littermates, with increased cell cycle entry but no detectable differences in apoptosis (Figure 3F,3G, S3C to S3E). Also, the age-dependent myeloid bias was not observed in the GHRKO mice (Figure 3H). These data suggest that the periodically reduced IGF-1 signaling caused by PF cycles may play a crucial role in the hematopoietic regeneration observed in PF mice.
Prolonged fasting promote hematopoietic regeneration in IGF-1/PKA dependent manner
To understand the molecular mechanism by which the PF and the GHR/IGF-1 deficiency promote hematopoietic recovery/regeneration, we reanalyzed two of our previously published microarray data sets and looked for genes whose expression significantly changed in response to PF with a focus on genes similarly affected by exposure of epithelial cells to IGF-1 deficient serum (Guevara-Aguirre et al., 2011; Kim and Volsky, 2005; Kirschner et al., 2009; Lee et al., 2012). In starved mice, the expression of the PKA catalytic subunit alpha (PKACα) was significantly reduced in all tissues tested (Table S3). Similarly, IGF-1 deficient serum from growth hormone receptor-deficient (GHRD) human subjects caused changes in the expression of both positive and negative regulators of PKA consistent with an inhibition of its kinase activity (Table S4).
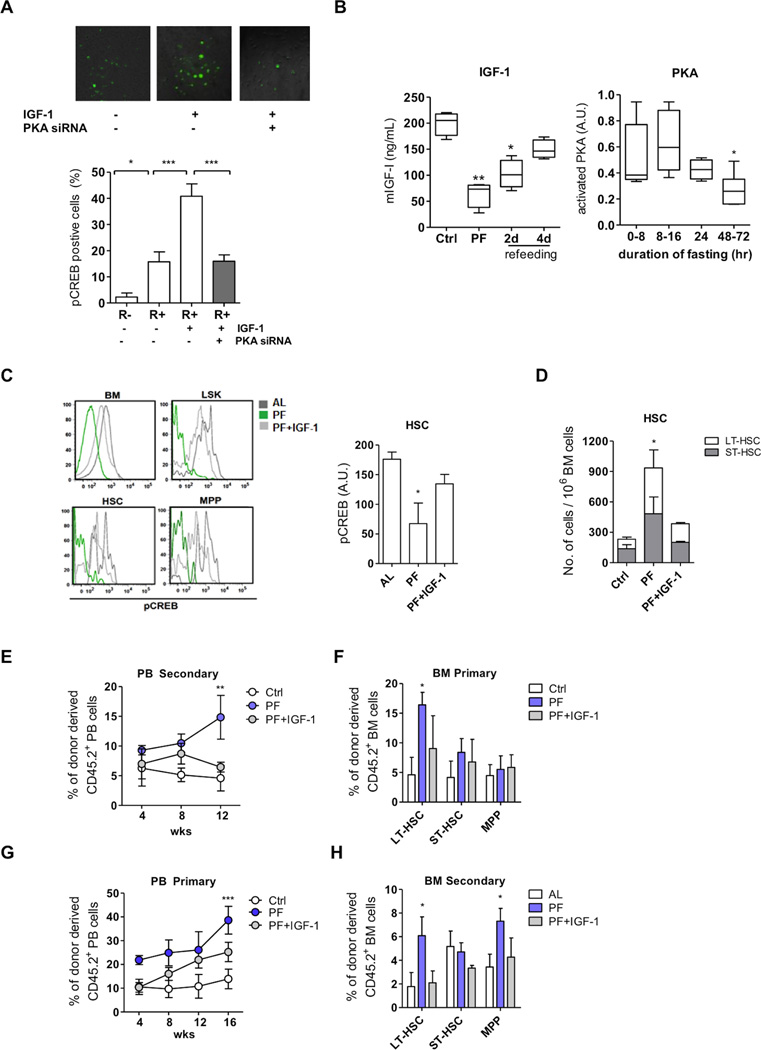
As PKA phosphorylates the cAMP response element-binding transcription factor (CREB) at Ser133, p-CREB is commonly used as an indicator of intracellular PKA activity (Gonzalez and Montminy, 1989). Using mouse embryonic fibroblasts devoid of the endogenous IGF-1 receptor (R- cells) and those overexpressing the human IGF1R (R+ cell) we showed that CREB phosphorylation is positively regulated by IGF-1/IGF-1R in a PKA-dependent manner, confirming the link between IGF-1 and PKA/CREB signaling in mammalian cells (Figure 4A). IGF-1 receptor (IGF-1R) expression, which was higher in progenitor cells compared to LT-HSCs (Venkatraman et al., 2013), was not affected by PF (Figure S4A). Taken together, our in vivo results indicate that PF reduces PKA signaling in BM cells at least in part through reduced IGF-1 levels and PKA activity, but without affecting IGF-1R expression (Figure 4B).
Figure 4. Prolonged fasting promotes IGF-1/PKA dependent hematopoietic regeneration.
(A) PKA-dependent phosphorylation of CREB visualized by ICC in mouse embryonic fibroblast (MEFs) devoid of endogenous IGF-1R (R- cells) or overexpressing human IGF1R (R+ cells). R+ cells were treated with IGF-1 and compared to cells transfected with PKACα siRNA.
(B) Prolonged fasting (PF) reduces both circulating IGF-1 levels and PKA activity in BM cells in mice.
IGF-1 injection blunted the PF-induced (C) reduction of PKA/pCREB,(D) increase in hematopoietic stem cells, (E-H) The chimerism of donor-derived cells in PB and that in the BM was determined 16 weeks after primary and secondary BM transplantation.. n=4 to 8 female mice per group, * p<0.05, **p<0.01 and *** p<0.005, one-way ANOVA.
To demonstrate that IGF-1 is a mediator of PF-dependent effects on HSCs, we tested whether exogenous IGF-1 can blunt the effect of PF on HSC number and PKA activity. Fasted mice were injected with IGF-1 (200ug/kg) to reverse the reduction of IGF-1 during PF. Results indicate that IGF-1 administration significantly blunted PF-induced reduction of PKA/pCREB in the LSK population, particularly in HSCs (Figure 4C). It also blunted the PF-induced increase in HSCs but not in MPPs (Figure 4D and Table S4). We further investigated whether the induction in HSCs can lead to enhanced engraftment and whether this effect is IGF-1/PKA-dependent. Results of competitive repopulation assays indicate the PF improved hematopoietic reconstitution in PB and in BM. This effect was blocked by exogenous IGF-1 (Figure 4E, 4F and S4B and S4C). Results of secondary transplantation further confirmed the effects in long-term repopulation capacity (Figure 4G and H). Overall, these results strongly support a role for lower IGF-1 and the consequent reduced activity of PKA in PF-dependent stimulation of HSC self-renewal and the improvement in both short- and long-term hematopoietic repopulation capacities (Figure 4E to 4H).
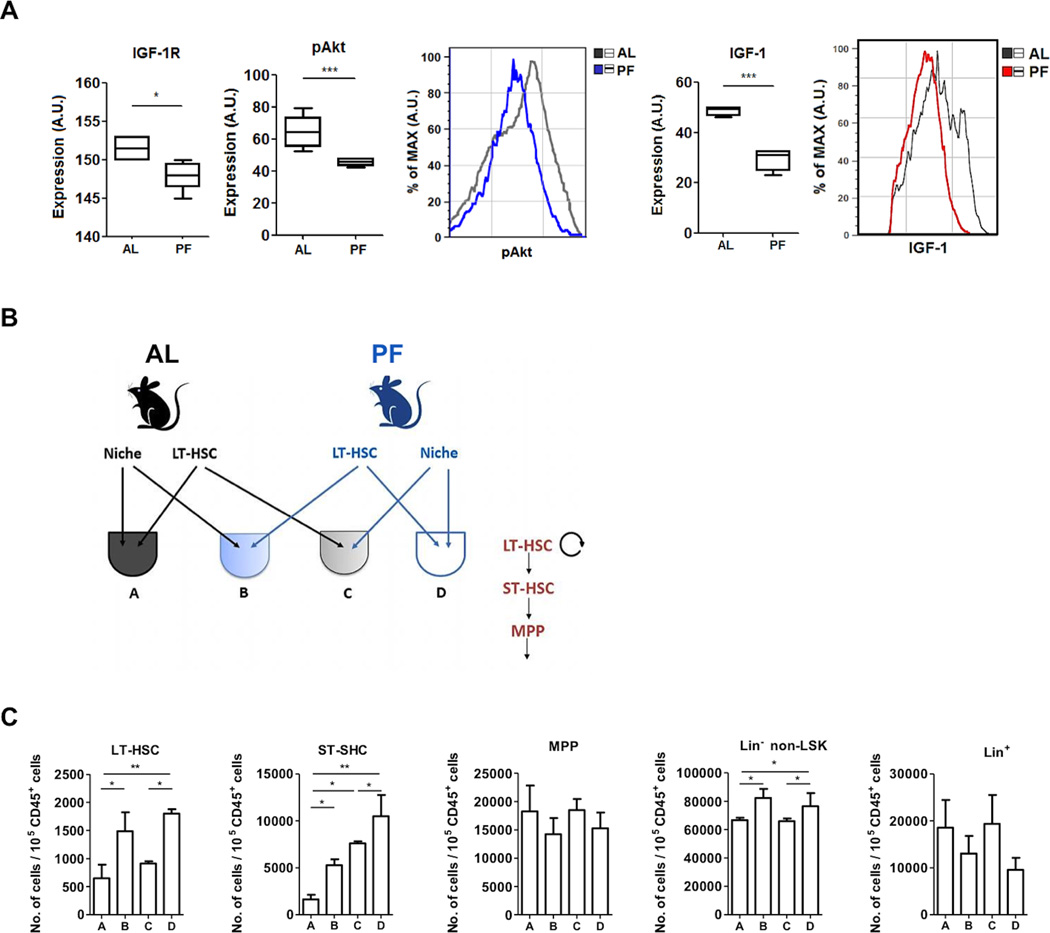
As IGF-1R signaling and IGF-1 expression were both reduced in the BM stromal niche cells (Lin−CD45−) from fasted mice (Figure 5A), we investigated whether the stromal niche could play a role in promoting PF-induced HSC self-renewal by reducing IGF-1 levels in the microenvironment (as previously shown in Figure 3A). To test this, LT-HSCs were purified (CD45+LSK CD150+CD48−) from mice on either PF or the control diet and then cross-exposed to the stromal niche cells (CD45−Lin− fraction) from mice on either PF or the ad lib diet using co-culture systems (Figure 5B). Notably, LT-HSCs are unable to survive in the absence of niche cells, so the isolated LT-HSCs were not studied alone. Results indicate that the effect of PF on LT-HSC is sufficient to promote the self-renewal of LT-HSC and its capacity to generate ST-HSC and non-LSK progenitors (Lin- non-LSK)(Figure 5C, comparing A to B, C to D). Also, the PF-treated niche cells could increase the generation of ST-HSCs from ad lib diet LT-HSCs (comparing A to C) and increases further the ST-HSC number generated by PF-treated LT-HSCs (comparing B to D). These results confirm the role of LT-HSCs in mediating PF-dependent hematopoietic regeneration but also indicate that niche cells exposed to PF can contribute to the ST-HSC component of this regeneration in vitro.
Figure 5. The role of stromal niche in PF-induced HSC self-renewal.
(A) Levels of the indicated proteins in BM stromal niche cells (Lin-CD45-).
(B) Diagrammatic representation of the co-culture experiment.
(C) Number of CD45+ progenies generated by the purified LT-HSCs exposed to the indicated niche cells.
* p<0.05, **p<0.01 and ***p<0.005, t-test for (A) and one-way ANOVA for (C).
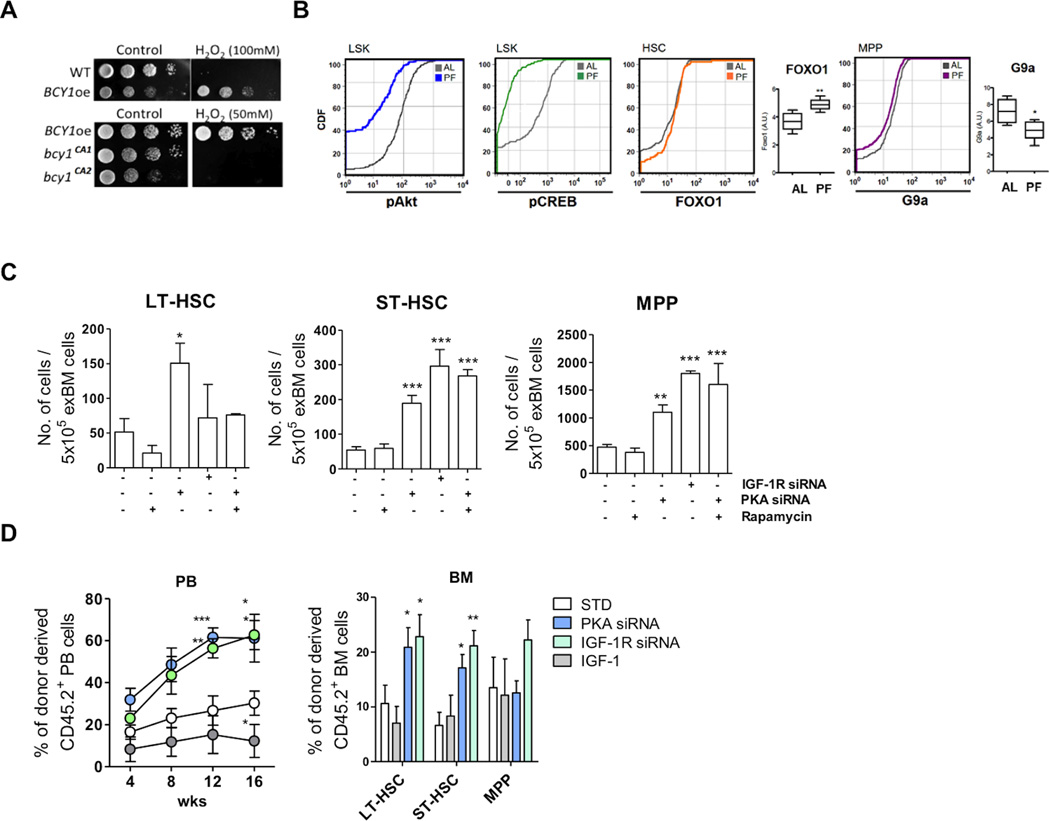
Reduction of IGF-1 or PKA signaling promotes HSC self-renewal
PKA has conserved pro-aging roles in yeast and mammals (Fabrizio et al., 2001; Rinaldi et al., 2010). In yeast, integration of an extra copy of the regulatory and inhibitory subunit of PKA, BCY1, (BCY1oe) enhanced whereas mutations in BCY1 that activate PKA decreased, cellular resistance to H2O2-induced oxidative stress (Figure 6A) in agreement with our previous results with RAS2- and adenylate cyclase deficient mutants (Fabrizio et al., 2003; Fabrizio et al., 2001). In mammalian cells, it was confirmed by us and others that disruption of PKA signaling protects against stress (Figure S5A to S5C) (Yan et al., 2007).
Figure 6. Reduction of IGF-1-PKA signaling promotes hematopoietic stem cell self-renewal.
(A) Yeast cells (DBY746 background) overexpressing BCY1 (BCY1oe), which reduces PKA activity, or cells carrying mutations that activate PKA activity (bcy1CA1 and bcy1CA2) were grown in SDC for 3 days and treated with H2O2 (50 or 100mM) for 30min at 30°C. Cells were serially diluted and plated onto YPD plates.
(B) PKA-regulated self-renewal pathways in PF mice. The levels of phosphorylation or expression of intracellular proteins in the indicated cellular populations and expression of indicated genes in total BM cells. BM cells were collected from mice with or without 48hr starvation (AL and PF). n=4 female mice per group, **p<0.01, *p<0.05, t-test.
(C) Number of hematopoietic stem cells (per 5×105 total BM) and progenitor cells (LT-HSC, ST-HSC and MPP) under the indicated treatments. * p<0.05, **p<0.01 and *** p<0.005, one-way ANOVA. See also Figure S7F and S7G.
BM cells treated with PKA siRNA, IGF-1R siRNA or IGF-1 (versus non-treated cells) were transplanted into immuno-compromised recipient mice.
(D) The engraftment in PB was measured at indicated time point after primary transplantation and the engraftment in BM was measured at the end of the 16 weeks after primary transplantation. n=4 to 8 female mice per group, *p<0.05 **p<0.01 and *** p<0.005, one-way ANOVA.
The role of PKA in hematopoietic regeneration, however, is poorly understood. It is known that PKA negatively regulates Foxo1 and positively regulates CREB and G9a (Chen et al., 2008; Gonzalez and Montminy, 1989; Lee et al., 2011; Yamamizu et al., 2012a). FoxOs maintain hematopoietic stress-resistance, self-renewal and lineage homeostasis (Tothova et al., 2007), while CREB and G9a promote hematopoietic lineage-commitment and differentiation (Chen et al., 2012; Yamamizu et al., 2012b). We found that in PF mice, the reduction of IGF-1/pAkt and PKA/pCREB signaling was associated with an induction of Foxo1 expression and a reduction of G9a (Figure 6B and Figure S5E), but it did not affect the expressions of Foxo3a and Foxo4 (Figure S5F to S5H). Also, the results indicated that the numbers of ST-HSC and MPP were significantly increased after treatment with PKA siRNA as well as after treatment with IGF-1 siRNA (Figure 6C, Figure S5D and Table S5), in agreement with the finding that inhibition of G9a increases primitive HSCs (Chen et al., 2012).
Given that inhibition of mTOR, another key effector of nutrient signaling, is known to enhance HSC self-renewal and maintenance autonomously and non-autonomously, we examined the crosstalk between mTOR and PKA in HSCs and MPPs (Chen et al., 2009; Huang et al., 2012). Ex vivo rapamycin (an mTOR inhibitor) treatment alone did not cause an induction in the number of HSCs as expected based upon previous studies with in vivo treatments (Figure 6C) (Nakada et al., 2010; Yilmaz et al., 2012). This could be due to the need for a longer period of mTor inhibition to achieve HSC induction (Nakada et al., 2010). In fact, when co-treated with PKA siRNA, rapamycin caused an additional induction in ST-HSC and MPP, compared to that caused by PKA-knockdown alone suggesting that diminished PKA signaling promotes the induction of HSCs, which can be further potentiated by mTor inhibition in certain stem and progenitor cell sub-populations (Figure 6C). Notably, the double inhibition of PKA and mTOR resulted in the synergistic induction in ST-HSC and MPP but blunted the induction in LT-HSC caused by PKA knock-down alone, which is similar to what was caused by IGF-R knock-down (Figure 6C), and in agreement with the potential role for IGF-1 in the regulation of both PKA and mTOR in HSCs (Fontana et al., 2010a; Longo and Fabrizio, 2002).
The BM cells treated with IGF-1R siRNA or PKA siRNA ex vivo (exBM) were further transplanted into the irradiated recipient mice to examine for their hematopoietic reconstitution capacity. In agreement with the effects observed in PF mice, the PKA or IGF-1R deficient BM cells caused a significant improvement in engraftment in PB and in BM compared to untreated BM cells (Std)(Figure 6D). The long-term repopulation capacity was also confirmed by secondary transplantation (Figure S5I).
DISCUSSION
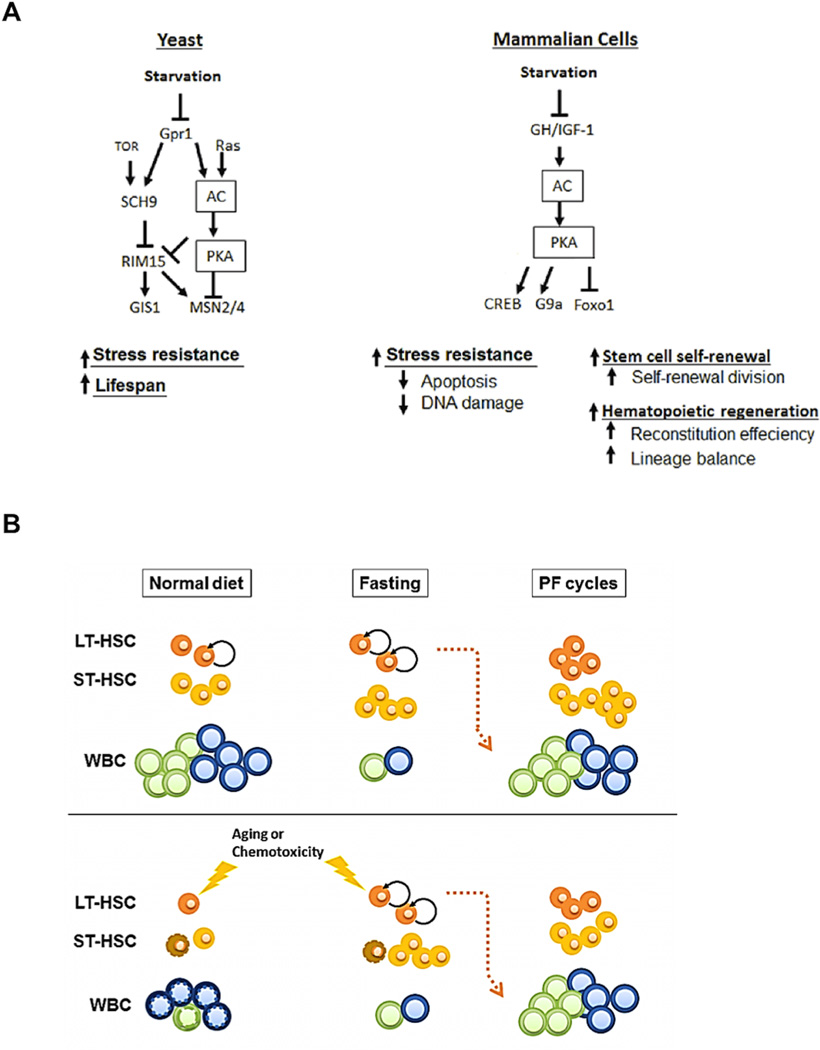
When considering changes in gene expression and metabolism, as well as the levels of various hormones, PF promotes coordinated effects that would be difficult to achieve with any pharmacological or other dietary intervention. In yeast, the key changes responsible for these protective effects of starvation are the down-regulation of the glucose-sensing Ras/adenylate cyclase/PKA and of the amino acid-sensing Tor/Sch9 (S6K) pathways (Figure 7A)(Fontana et al., 2010b). When mutations in both pathways are combined, cells are extremely resistant to a wide variety of toxins and can live up to 5-fold longer than normal (Fabrizio et al., 2001; Kaeberlein et al., 2005; Kenyon, 2001; Longo and Finch, 2003; Wei et al., 2009). In mammals, mutations that cause deficiency in the GHR/IGF-1 axis promote a range of phenotypes that overlap with those in the highly protected yeast with deficiencies in nutrient signaling pathways including dwarfism, stress resistance, and longevity extension (Figure 7A)(Lee and Longo, 2011). In fact, cells from GHR/IGF-1 deficient mice are protected from multiple forms of stress (Brown-Borg et al., 2009; Salmon et al., 2005) and the IGF-1 deficient (LID) mice, with an over 70% reduction in circulating IGF-1, are resistant to several chemotherapy drugs (Lee et al., 2010). Here we connect the GHR-IGF-1 and the PKA pro-aging pathways by showing that PKA functions downstream of IGF-1 to sensitize BM cells in agreement with results in yeast and with the previously established connection between IGF-1 and PKA in mammalian neuronal cells (Subramaniam et al., 2005).
Figure 7. PF reduce IGF-1/PKA to promote lineage-balanced hematopoietic regeneration.
(A) A simplified model for a partially conserved nutrient signaling PKA pathway in yeast and mammalian cells. Arrows show promotion actions and horizontal bars indicate inhibitory actions. GH, growth hormone; AC, adenylate cyclase; PKA, protein kinase A; CREB, cAMP response element-binding protein; Foxo1, Forkhead box protein O1; G9a, H3 Lys-9 methyltransferase.
(B) A simplified model for PF-induced effects on WBC and HSCs. Fasting causes a major reduction in WBCs followed by their replenishment after re-feeding, based on effects on HSCs self-renewal resulting in increased progenitor and immune cells. These effects of PF can result in reversal of chemotherapy-based immunosuppression but also in the rejuvenation of the immune cell profile in old mice.
However, the studies of growth deficient yeast and mice could not have predicted the remarkable effect of PF cycles in promoting stem cell-based regeneration of the hematopoietic system. Calorie intake was previously shown to affect the balance of stem cell self-renewal and differentiation, which is important for somatic maintenance and long-term survival (Bondolfi et al., 2004; Chen et al., 2003; Ertl et al., 2008; Jasper and Jones, 2010; Rafalski and Brunet, 2011; Rando and Chang, 2012). In mice, calorie restriction (CR) promotes the self-renewal of intestinal stem cells, muscle stem cell engraftment and neural regeneration, preserves the long-term regenerative capacity of HSC and prevents the decline of HSC number during aging in certain mouse strains (Bondolfi et al., 2004; Cerletti et al., 2012; Chen et al., 2003; Ertl et al., 2008; Rafalski and Brunet, 2011; Yilmaz et al., 2012). Reduction of mTOR signaling has been implicated as one of the major molecular mechanisms responsible for the effects of CR on enhanced stem cell function (Huang et al., 2012; Rafalski and Brunet, 2011; Yilmaz et al., 2012). However, neither CR nor other dietary intervention had previously been shown to promote a coordinated effect leading to the regeneration and/or rejuvenation of a major portion of a system or organ.
Because during PF mammalian organisms minimize energy expenditure in part by rapidly reducing the size of a wide range of tissues, organs, and cellular populations including blood cells, the reversal of this effect during re-feeding represents one of the most potent strategies to regenerate the hematopoietic and possibly other systems and organs in a coordinated manner. Here we show that PF causes a major reduction in WBC number, followed, during re-feeding, by a coordinated process able to regenerate this immune system deficiency by changes beginning during the fasting period, which include a major increase in LT-HSC and ST-HSC and redirection of the frequency of Ly-HSC/Bala-HSC/My-HSC leading to a lineage-balanced mode. In fact, we show that PF alone causes a 28% decrease WBC number, which is fully reversed after re-feeding (Fig. 7b). Even after WBCs are severely suppressed or damaged as a consequence of chemotherapy or aging, cycles of PF are able to restore the normal WBC number and lineage balance, suggesting that the organism may be able to exploit its ability to regenerate the hematopoietic system after periods of starvation, independently of the cause of the deficiency (Figure 7B).
In agreement with our results, starvation protects germline stem cells (GSC) and extends reproductive longevity in C. elegans through an adaptive energy shift toward the less committed cells (Angelo and Van Gilst, 2009). In contrast, short-term fasting (≤ 24hr) in Drosophila, promotes the differentiation of hematopoietic progenitors to mature blood cells (Shim et al., 2012). It will be important to determine whether the coordinated regenerative changes observed during PF and re-feeding may resemble at least in part the sophisticated program responsible for the generation of the hematopoietic system during development.
Recent studies revealed that HSCs rely heavily on the metabolic programs that prevent aerobic metabolism to maintain their quiescent state and self-renewal capacity (Ito et al., 2012; Takubo et al., 2013; Yu et al., 2013). In the case of PF, the energy metabolism is switched progressively from a carbohydrate-based to a fat- and ketone body-based catabolism, which could contribute to HSC self-renewal, in agreement with findings that fatty-acid-oxidation promotes HSC asymmetric self-renewal over the symmetric commitment (Ito et al., 2012).
PKA is known to promote lineage specification of HSC through CREB and G9a (Chen et al., 2012; Yamamizu et al., 2012b). As inhibition of G9a has been a key strategy to promote reprogramming (Huangfu et al., 2008; Shi et al., 2008), the PF-induced down-regulation of G9a shown here may redirect cell fate through a similar process causing the induction in HSCs, analogously to that caused by G9a inhibition (Figure 5B)(Chen et al., 2012). Recent studies also indicate that PKA can directly phosphorylate and negatively regulate FoxO1 (Chen et al., 2008; Lee et al., 2011), which has a profound role in stem cell stress resistance, self-renewal and pluripotency maintenance (Tothova et al., 2007; Zhang et al., 2011). Whereas PKA is implicated in stem cell differentiation, our study suggests that cycles of PF down-regulate IGF-1 and PKA to promote stem cell self-renewal.
A therapeutic challenge of hematopoietic regeneration is to stimulate stem cell production for immediate tissue repair while avoiding stem cell depletion under stress (Pang et al., 2011). Our results indicate that cycles of an extreme dietary intervention represent a powerful mean to modulate key regulators of cellular protection and tissue regeneration but also provide a potential therapy to reverse or alleviate the immunosuppression or immunosenescence caused by chemotherapy treatment and aging, respectively, and possibly by a variety of diseases affecting the hematopoietic system and other systems and organs. The clinical data shown here provide preliminary results supporting the possibility that these effects can also be translated into effective clinical applications.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J mice (Jackson laboratory) were used in this study. Mice are either fasted for 48hr or fed ad libitum before chemotherapy treatment. Cyclophosphamide (CP) were administered intraperitoneally (i.p.) at the dose of 200mg/kg every 12–14 days (6 cycles total). IGF-1 was injected (i.p.) at the dose of 100ug/kg, twice a day. 6 to 8weeks old B6.SJL mice (Taconic) were used as recipient mice in the competitive repopulation assay. Genotyping for GHRKO mice was performed as shown in Figure S3A. All animal experiments were done in accordance with the USC Institutional Animal Care and Use Committee and NIH guidelines.
Comet assay
DNA damage (including ssDNA and dsDNA breaks) in freshly collected blood and bone marrow (BM) cells was assessed by CometAssay (Trevigen, Inc, Gaithersburg, MD) with a Nikon Eclipse TE300 fluorescent microscope and analyzed with the Comet Score (TriTek Corp., ver1.5). 100–200 cells were scored per experimental sample.
Complete blood count (CBC)
Peripheral blood (PB) was collected via tail bleeds into heparinized micro-hematocrit capillary tubes (FisherScitific) was and analyzed using BC-2800 Auto Hematology Analyzer (Mindray). CBC profiles from clinical trial were obtained from phase I clinical trial NCT00936364 (http://clinicaltrials.gov/show/NCT00936364).
Competitive repopulation assay
BM collection and transplantation were performed as previously described (Adams et al., 2007). Briefly, BM cells were collected from mice (C57B/6J) treated with 6-cycle CP. 2.5×105 BM cells from CP-treated mice were mixed with an equal number of those from a wild-type competitor mouse (B6.SJL), and injected into recipient B6.SJL, lethally irradiated 24 h previously with 10 Gy of radiation. The relative contribution of engraftment from the different cell sources was assessed by flow cytometry of the PB with CD45.2 (C57B/6) and CD45.1 (B6.SJL) antigens.
FACS analysis
FACS analyses for LT-HSC(LSK-CD48−CD150+), ST-HSC(LSK-CD48−CD150−) and MPP(LSK CD48+CD150−) in BM were performed as previously described (Figure S1) (Adams et al., 2007; Challen et al., 2010;). Freshly harvested BM cells were stained with lineage, stem, and progenitor markers, followed by Annexin-V/7-AAD staining and TUNEL assay for apoptosis analysis or stained with PY/Hoechst33342 or Ki67/Hoechst33342 for cell cycle analysis. For competitive repopulation analysis, PB was collected from tail vein. 50–100ul of blood was diluted 1:1 with PBS and incubated with anti-CD45.1, anti-CD45.2 antibodies and anti-CD11b (BD Biosciences). Analysis was performed with BD FACS diva on LSR II.
BrdU incorporation
For detecting cell genesis, mice were injected (i.p.) with the filter sterilized BrdU 2.0% solution (Sigma) at 0.1 mg/g body weight in PBS, twice a day, for 2 days, starting after 24hr of prolonged fasting (PF mice). BM cells were collected and stained with anti-BrdU combining with the plasma membrane marker antibodies as mentioned above and analyzed on BD FACS diva on an LSR II, according to the manufacturer’s protocol (BD Biosciences).
Oxidative Stress Assay for yeast
Day 3 cells were diluted to an OD600 of 1 in K-phosphate buffer (pH 6) and treated with 50 or 100 mM hydrogen peroxide for 30 min. Serial dilutions of untreated and treated cells were spotted onto YPD plates and incubated at 30°C for 2–3 days.
Cell culture and treatments
Cell lines and primary cells used in this study were cultured at 37°C and 5% CO2. Mouse embryonic fibroblast with overexpressed human IGF1R (R+ cells) were derived from IGF1R knockout mice (obtained from Dr. Baserga) and cultured in DMEM/F12 supplemented with 10% FBS. Cells were seeded at 80% (R+ cells) or 50% (exBM or hAFSCs) confluence for IGF-1R and PKACα siRNA transfection (100nM, with 1% X-tremeGENE transfection reagents, Roche) and/or rapamycin treatment (5nM) and the inhibition efficiencies of the of the target proteins was shown as Table SThe IGF-1 induction (10nM, 15min) was performed at 24hr after standard incubation. CREB phosphorylation was measured by immunocytochemistry (ICC) with the pCREB-AF488 antibody (cell signaling, 1:200, overnight at 4°C). Explanted BM cells, isolated HSCs and BM stromal cells were incubated with alpha-MEM+10%FBS. Cell contents were analysis by FACS as described above.
Statistical analysis
The significance of the differences in mouse survival curves was determined by Log-rank (Mantel-Cox). Unless otherwise indicated in Figure legends, data are presented as means±sem. Student’s t-tests for two groups and ANOVA for multiple groups were used to assess statistical significance (*p<0.05, **p<0.01, ***p<0.005).
Supplementary Material
Highlights.
Prolonged fasting down-regulates a novel IGF-1/PKA pathway in stem cells
Prolong fasting protects hematopoietic cells from chemotoxicity
Prolonged fasting cycles promote HSC self-renewal to reverse immunosuppression
Inhibition of IGF-1 or PKA signaling mimics the effects of prolonged fasting
ACKNOWLEDGMENTS
We thank Dr. Lora Barsky (USC Flow Cytometry Core Facility) for assistance in the FACS analysis, and Dr. R. Baserga (Thomas Jefferson University) for providing R+ and R- cells. This study was funded in part by NIH/NIA grants AG20642, AG025135 and P01 AG034906. V.D.L. has equity interest in L-Nutra, a company that develops medical food.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. doi: 10.1126/science.1178343. [DOI] [PubMed] [Google Scholar]
- Bedford P, Berger MR, Eisenbrand G, Schmahl D. The level of DNA interstrand crosslinking in bone marrow parallels the extent of myelosuppression in mice treated with four chloroethylnitrosoureas. J Cancer Res Clin Oncol. 1984;108:141–147. doi: 10.1007/BF00390986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiology of Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp Gerontol. 2009;44:10–19. doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Boles N, Lin KKY, Goodell MA. Mouse Hematopoietic Stem Cell Identification and Analysis. Cytom Part A. 2009;75A:14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2 doi: 10.1126/scisignal.2000559. ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. Journal of Biological Chemistry. 2008;283:29228–29238. doi: 10.1074/jbc.M802906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Experimental Hematology. 2003;31:1097–1103. doi: 10.1016/s0301-472x(03)00238-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, Loeb K, Wood B, Georges G, Torok-Storb B, et al. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev. 2012;26:2499–2511. doi: 10.1101/gad.200329.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Ertl RP, Chen J, Astle CM, Duffy TM, Harrison DE. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood. 2008;111:1709–1716. doi: 10.1182/blood-2007-01-069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Liou LL, Moy VN, Diaspro A, SelverstoneValentine J, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010a;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending Healthy Life Span-From Yeast to Humans. Science. 2010b;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, et al. Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling, Cancer, and Diabetes in Humans. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778–1785. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 2010;12:561–565. doi: 10.1016/j.cmet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: Parametric analysis of gene set enrichment. Bmc Bioinformatics. 2005;6 doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Yin Z, Jones GN, Mahoney E. Mouse models of altered protein kinase A signaling. Endocr Relat Cancer. 2009;16:773–793. doi: 10.1677/ERC-09-0068. [DOI] [PubMed] [Google Scholar]
- Kofman AE, McGraw MR, Payne CJ. Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging (Albany NY) 2012;4:279–289. doi: 10.18632/aging.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Translational Medicine. 2012;4 doi: 10.1126/scitranslmed.3003293. 124ra127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Chen H, Pullikotil P, Quon MJ. Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA. Journal of Biological Chemistry. 2011;286:6423–6432. doi: 10.1074/jbc.M110.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. Journal of Cell Biology. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, Mcclure LL, Gress RE. Lymphocyte Depletion during Treatment with Intensive Chemotherapy for Cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Progress in Neurobiology. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer COS against high-dose chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Matesic LE, Flavell RA. The E3 ligase Itch is a negative regulator of the homeostasis and function of hematopoietic stem cells. Nat Immunol. 2011;12:399–U352. doi: 10.1038/ni.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi J, Wu JA, Yang J, Ralston CY, Sankaran B, Moreno S, Taylor SS. Structure of Yeast Regulatory Subunit: A Glimpse into the Evolution of PKA Signaling. Structure. 2010;18:1471–1482. doi: 10.1016/j.str.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 2009;1:988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. American Journal of Physiology-Endocrinology and Metabolism. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, Hsu H, Wang H, Tropepe V, Ward M, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145–153. doi: 10.1016/j.mcn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Tilburg CM, van Gent R, Bierings MB, Otto SA, Sanders EAM, Nibbelke EE, Gaiser JF, Janssens-Korpela PL, Wolfs TFW, Bloem AC, et al. Immune reconstitution in children following chemotherapy for haematological malignancies: a long-term follow-up. British Journal of Haematology. 2011;152:201–210. doi: 10.1111/j.1365-2141.2010.08478.x. [DOI] [PubMed] [Google Scholar]
- Venkatraman A, He XC, Thorvaldsen JL, Sugimura R, Perry JM, Tao F, Zhao M, Christenson MK, Sanchez R, Yu JY, et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–349. doi: 10.1038/nature12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Madia F, Hu J, Ge HY, Li LM, Longo VD. Tor1/Sch9-Regulated Carbon Source Substitution Is as Effective as Calorie Restriction in Life Span Extension. Plos Genetics. 2009;5 doi: 10.1371/journal.pgen.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Critical Care. 2004;8:R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Fujihara M, Tachibana M, Katayama S, Takahashi A, Hara E, Imai H, Shinkai Y, Yamashita JK. Protein kinase A determines timing of early differentiation through epigenetic regulation with G9a. Cell Stem Cell. 2012a;10:759–770. doi: 10.1016/j.stem.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Matsunaga T, Katayama S, Kataoka H, Takayama N, Eto K, Nishikawa S, Yamashita JK. PKA/CREB signaling triggers initiation of endothelial and hematopoietic cell differentiation via Etv2 induction. Stem Cells. 2012b;30:687–696. doi: 10.1002/stem.1041. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu CK. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12:62–74. doi: 10.1016/j.stem.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmele P, Kennedy M, Sellers R, et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13:1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.