Abstract
Control of interactions between nanoparticles and biosystems is essential for the effective utilization of these materials in biomedicine. A wide variety of nanoparticle surface structures have been developed for imaging, sensing, and delivery applications. In this research Highlight, we will emphasize advances in tailoring nanoparticle interfaces for implementation in nanomedicine.
Introduction
Nanoparticles exhibit unique physical properties that are couple with size range commensurate with biomolecular and cellular systems.1,2 These features make them attractive materials for therapeutic and diagnostic applications. However, utilization of these materials in biomedicine requires controlled interactions with biomacromolecules.3 For example, specifically designed nanoparticle monolayer structures can impart enhanced cellular internalization ability, non-cytotoxicity, and improved payload binding capacity necessary for effective intracellular delivery.4 Similarly, surface functionality can be tuned to provide the selective or specific recognition required for biosensing.5,6 Finally, even non-interaction can be an important attribute, with non-interacting “stealth” properties preventing particle capture by the immune system.7
While tailoring particle interfaces is a challenging task, chemists have a well-equipped toolbox to provide functionality through synthesis.8 Using different strategies, nanoparticles have been functionalized with a variety of ligands such as small molecules, surfactants, dendrimers, polymers, and biomolecules. Different multivalent surface structures fabricated with small molecules and polymeric ligands offer the capability to incorporate multiple therapeutic drugs or bio-macromolecules by covalent or non-covalent conjugation.9 Similarly, biomolecule-conjugated nanoparticles can impart desired properties such as specific recognition or biocompatibility.10 The ease of such functionalization allows chemists to create the desired functionalities for their application in clinics.
Considerable effort and innovation have been applied to the creation and study of nanoparticle interfaces. In this Highlight, we will explore just a few of the recent studies in nanoparticle surface engineering. These studies serve to underscore the importance of chemistry in the creation of nanoparticle interfaces, and the biomedical applications of these particulate systems.
Functionalization of nanoparticles using small molecule ligands
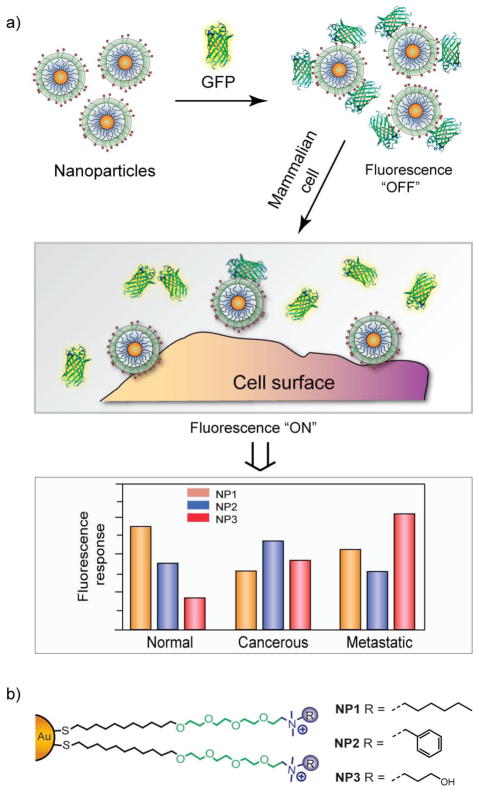
A wide variety of ligands have been incorporated onto nanoparticle surfaces, allowing them to be used in sensing of biomolecules11,12 and cells,13,14 diagnosis of diseases,15 and intracellular delivery.16 For example, nanoparticles functionalized with ligands exhibiting differential affinity towards proteins and cell surface molecules have been employed for their identification.4 In one approach, nanoparticles were electrostatically complexed with green fluorescent protein (GFP) and the resulting assemblies were used to discriminate between healthy, cancerous and metastatic cells derived from both human and murine cell lines.14 In the process of sensing, GFP was quenched while adsorbed to the particle surface. Displacement of the GFP from the gold nanoparticle (AuNP) surface upon addition of cells restored the fluorescence, providing readout [Fig. 1]. This process is driven by nanoparticle-cell affinity and can be controlled by subtle changes on the ligand headgroups.17 Using a similar strategy, seven proteins of different sizes and isoelectric points were identified employing a sensor array composed of six cationic AuNPs and one anionic poly(p-phenylene ethylene) (PPE) polymer.11 The surface charge in conjunction with hydrophobicity and aromatic stacking of nanoparticles plays a crucial role in such sensing applications.
Fig. 1.
Schematic representation of competitive binding between the quenched nanoparticle-GFP complexes and the cell surface (a), using array of nanoparticles functionalized with different ligands (b).
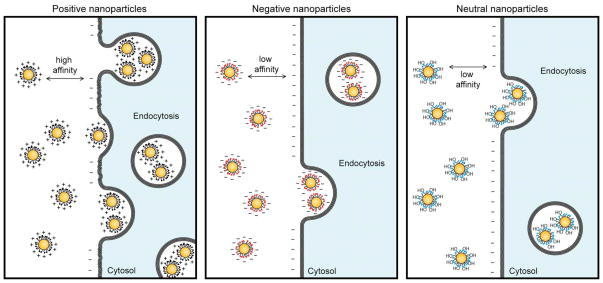
The surface charge of small molecule coated nanoparticles likewise dictates their interaction with cell surface molecules and hence their cellular uptake.18 In general, nanoparticles functionalized with positively charged ligands exhibit higher internalization into cells compared to the neutral and negatively charged particles [Fig. 2].19 In one study, positively charged magnetic iron oxide (Fe3O4) nanoparticles functionalized with dendritic guanidine headgroups were employed to investigate their cell penetration capability.20 These particles were found to be as efficient as human immunodeficiency virus-1 transactivator (HIV-TAT) peptide. In some cases, however, negatively charged nanoparticles such as carboxylate-functionalized iron oxide nanoparticles were efficiently uptaken into cells, presumably through pinocytosis or direct diffusion through the cell membrane.21 The study on the role of charge functionality of nanoparticles in delivering covalently attached drug molecules into tumor tissues showed intriguing results: positive particles were more effective in delivering drugs into proliferating peripheral cells because of their enhanced uptake, however negative particles that diffuse more quickly performed better while delivering deep into tissues. 22 Neutral functional groups are likewise important, preventing non-specific nanoparticle-biological interactions.23
Fig. 2.
Schematic illustration of surface charge dependent nanoparticle internalization by cells. Positively charged particles have high internalization efficiency as compared to negative and neutral particles.
Entry into cells is just first hurdle in terms of therapeutic delivery. Intracellular delivery, in particular release into the cytosol poses another challenge that needs to be addressed. Nanomaterials internalized by cells are often entrapped in the endosome, preventing access to the cytosol and limiting their utility. To overcome this issue, signaling peptides have been coated onto the surface of nanoparticles. For example, AuNPs coated with nuclear localizing signal (NLS) were able to escape the endosome and penetrate the nucleus of cancer cells to induce DNA damage.24 In another study, the endosomal escape of two AuNPs was compared: one coated with sub-nanometer striations of alternating anionic (sulfonate head group) and hydrophobic groups, and the other with a random distribution of ligands.25 While the former particles were found in the cytosol, the latter ones were mostly entrapped in endosomes.
While most nanoparticle based drug delivery systems attach the payload on their surfaces, mesoporous silica nanoparticles (MSNs) provide an alternative mechanism. Through appropriate functionalization various guest molecules such as drugs can be accommodated into their pores and biomacromolecules such as genes on their surface.26,27 For instance, amine-functionalized MSNs have been used as a gene delivery platform both in vitro and in vivo.28,29 Amine terminated tetra(ethylene glycol) functionalized honeycomb MSNs transported DNA and chemicals into plant cells and intact leaves.30 In addition, the pores on MSNs can efficiently be capped by appropriate ligands to prevent uncontrolled leakage and deliver in both a time and location dependent way.
Polymer functionalized nanoparticles
Polymer coatings provide an alternative to small molecule ligands, imparting the properties macromolecular systems to the particle surface. Coating nanoparticles with polymers such as polyethylene glycol (PEG) facilitates passive targeting of tumor tissue through EPR effect.31,32 This stealth coating with PEG and other polymers prevents adsorption of blood serum proteins, increases circulation time, and enhances the probability of particle permeation into tumor tissues.33,34,35 Attaching targeting moieties on PEGylated nanoparticles further enhances their targeting of tumors.36 Recently, PEGylated AuNPs were decorated with various amounts of human transferrin (Tf), generating a series of Tf-coated particles with near-constant size and electrokinetic potentials, and then delivered into mice bearing Neuro2A tumors.37,38 PEGylation demonstrated enhanced stabilization in salt solution compared to unmodified particles. A Tf content dependent particle accumulation in solid tumor was clearly visualized in vivo.
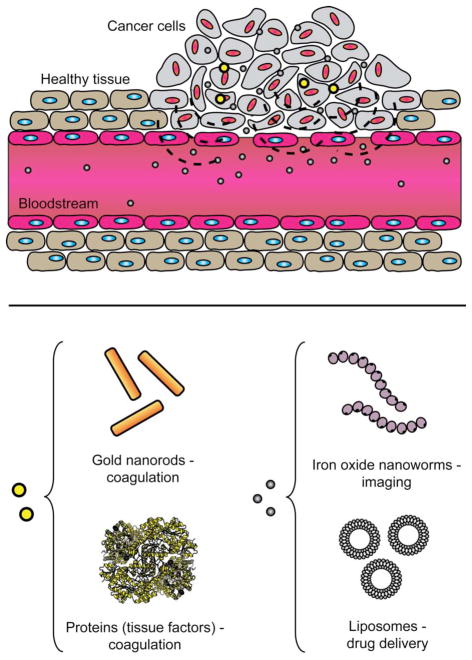
Polymer coating is a versatile process, allowing the core properties of different nanomaterials to be applied. In one example, a cooperative nanosystem consisting of two discrete nanomaterials was shown to be a potential therapeutic system.39 The first component was a PEG coated gold nanorod (AuNR) that accumulated in murine tumor tissues by the passive targeting mechanism. These nanorods were used as photothermal antennas to heat the tumor tissues via remote infrared irradiation.40 Local tumor heating accelerated the recruitment of the second component: a targeted nanoparticle consisting of either magnetic nanoworms (NWs) or doxorubicin loaded liposomes. The targeting species was a cyclic peptide that binds to stress related protein p32 that is upregulated in tumor cells upon thermal treatment. Treatment of mice containing xenografted tumors with the combined nanoparticle cooperative systems resulted in significant reduction of tumor volume than with individual nanoparticles. An extended study was carried out using a similar PEG-AuNRs but with different targeting nanoparticles which exhibited improved in vivo tumor targeting efficiency.41 AuNRs were pre-delivered to tumor site through passive targeting and subsequently irradiated with a near infrared laser to damage blood vessels that activated coagulating proteins [Fig. 3]. By functionalizing the surfaces of the NWs and liposomes with coagulating cascade targeting peptides, an increase in particle accumulation in tumors was observed.
Fig. 3.
Schematic illustration of tumor targeting by cooperative nanomaterials. Pre-delivered gold nanorods can guide the therapeutic (drug loaded liposomes) and diagnostic (iron oxide nanoworms) payloads to the tumor site.
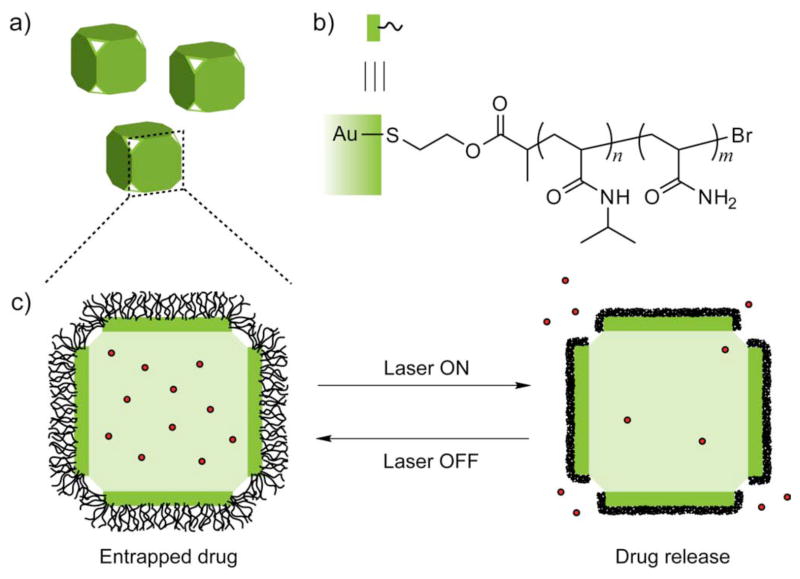
Polymer functionalized nanoparticles can facilitate controlled release of drugs as well. For example, gold nanocages were functionalized with poly(N-isopropylacrylamide) (pNIPAAm) and derivatives that change conformation in response to small variations in temperature.42 When exposed to near infrared, the temperature increase caused the polymer chains to collapse, exposing the pores of nanocages and thereby releasing anti-cancer drugs. When the heating was turned off, the drop in temperature brought the polymer back to its original conformation, closing the pores and hence stopping the release [Fig. 4]. These nanocages were used to control the dosage release by regulating the power density and irradiation time.
Fig. 4.
Schematics showing gold nanocages (a) functionalized with poly(N-isopropylacrylamide) (pNIPAAm) polymer (b) that carries drug molecules. On exposure to near-infrared laser, the polymer collapse and thus release the pre-loaded drug. When the laser is turned off, the polymer chains relax back to the extended conformation and terminate the release (c).
Biomolecule functionalized nanoparticles
Biomolecule coated nanoparticles provide specific attributes that are difficult or impossible to achieve using synthetic materials,43 such as providing efficient delivery of biomacromolecules with minimal cytotoxicity.44 In one of the early examples of bionanotechnology, Mirkin and Alivisatos independently reported the creation of oligonucleotide-AuNP conjugates through gold-thiol bond formation.45,46 Later studies demostrated that DNA-AuNP complexes were capable of inducing gene silencing via an antisense mechanism.47 A notable feature of DNA-AuNPs compared to free DNA molecules is their efficient internalization into cells. This rather surprising uptake of anionic nanomaterials is apparantly mediated by scavenger receptors.48 Several research groups have also reported functionalized AuNPs as siRNA delivery agents. Gene regulation with polyvalent siRNA-AuNP conjugates was reported, where siRNA was covalently coupled with AuNP through an ethylene glycol spacer and an alkylthiol.49 This functionalization resulted in a serum stable conjugate making it useful material for in vivo gene regulation. Another advantage of attaching siRNA to AuNPs is that the resulting particles provoke less innate immune response compared to commercial transfection agents such as Lipofectamine.50
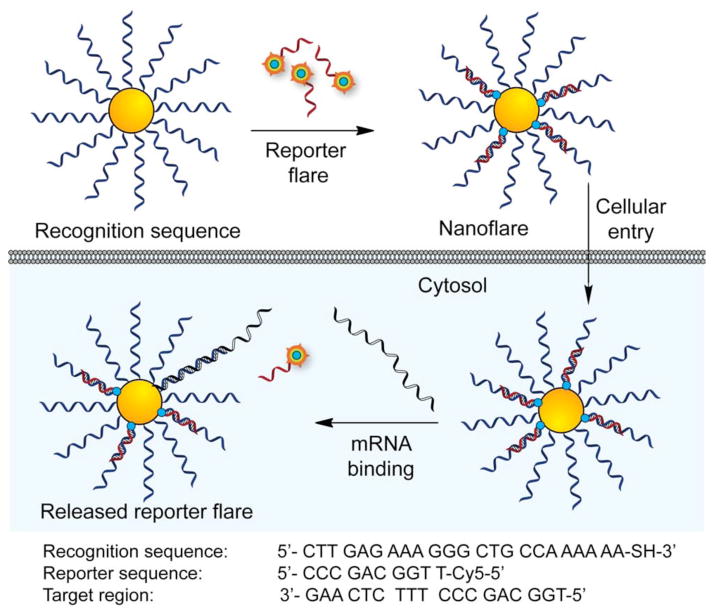
The functionalization of nanoparticles with oligonucleotides opened up a variety of new opportunities in molecular diagnostics, including detection of nucleic acids51,52 and intracellular biomolecules like ATP.53 In another example, novel oligonucleotide-AuNP probes were developed by hybridizing fluorophore-labeled complementary sequence and used as cellular ‘nanoflare’ for visualizing and quantifying mRNA in living cells.52 In this design, AuNPs were functionalized with thiolated oligonucleotides complementary to a specific mRNA transcript and hybridized with short cyanine (Cy5) dye-terminated reporter sequence. When the reporter flare sequence was bound to the nanoparticle, the Cy5 fluorescence was quenched due to the proximity to the AuNP surface. Once the nanoflare is delivered into cells, the target RNA displaces the reporter flare strand, generating a ‘turn on’ fluorescence signal [Fig. 5]. These probes do not require any microinjection or additional transfecting reagents to enter the cells and are highly resistant to enzymatic degradation as well as nontoxic under physiological conditions. In another approach, AuNP functionalized with fluorophore labeled aptamers were used to determine the level of intracellular ATP by a similar mechanism.53
Fig. 5.
Schematic showing the fabrication of ‘nanoflares’ for intracellular mRNA quantification. Fluorescent dye labeled reporter flare, which is hybridized to DNA-AuNPs, gets replaced by target mRNA inside cell and thus reports the quantity of intracellular mRNA.
Nanoparticles coated with proteins and antibodies can bind to cell surface receptors, providing targeted delivery. For example, multivalent gold and silver nanoparticles were engineered by loading Herceptin, an antibody to the Her-2 receptor overexpressed in ovarian and breast tumor cells.54 The avidity between the nanoparticles coated with Herceptin and its receptor Her-2 targeted these particles into tumors. Antibody conjugated AuNPs also provide a useful tool for targeting particles for imaging and photothermal therapy. Light scattering by the gold core conjugated with anti-epidermal growth factor receptor (anti-EGFR) was used for imaging cancer cells.55 The nanoconjugates bound specifically to the cancerous cells with six-fold greater affinity than to non-cancerous control cells. When the anti-EGFR conjugated NPs (~40 nm size) were incubated with carcinoma cells and irradiated with laser, induced local heating was observed with subsequent cell death.56 Protein coated magnetic nanoparticles (MNPs) have also been used as contrast agents for imaging.57 The targeting protein moiety helps localize these particles near specific tumor tissues. Different targeting biomolecules including EGF58 (epidermal growth factor), Herceptin,59 and CEA (carcinoembryonic antigen)60 enable specific detection of a wide variety of cancer types. Successful development of such ultra-sensitive molecular imaging MNP nanoprobes could enhance the ability to visualize biological events critical to diagnostic and therapeutic purposes.
Summary and Outlook
The tunable surfaces of nanoparticles provide versatile scaffolds for numerous applications in biomedicine. In this Highlight, we have focused on how different chemical modalities (synthetic and natural) can be displayed on on nanoparticle surfaces, and how these functionalities can be utilized to address a variety of biomedical applications.
Several important aspects of particle surface functionalization, however, need to be addressed during translation from experimental success to clinical practice. On the synthetic front, the surface functionalities should be fabricated to endow biomimetic properties such as stability in complex biological media, non-cytotoxicity, and specificity for particular cells/tissues. The knowledge of structure-activity relationship of nanoparticle surfaces to predict their impacts in biological contexts would help in designing effective therapeutic materials. Likewise, detailed knowledge of cellular uptake mechanism of a functionalized nanoparticle is central to improving its theragnostic abilities. Taken together, continued effort to understand the complex interaction of nanoparticles with biosystems on the basis of surface functionalities would establish nanoparticles as efficient tool for diagnosis and treatment of diseases.
Acknowledgments
Funding from the National Institutes of Health (GM077173) is gratefully acknowledged.
References
- 1.Daniel MC, Astruc D. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 2.Redl FX, Black CT, Papaefthymiou GC, Sandstrom RL, Yin M, Zeng H, Murray CB, O’Brien SP. J Am Chem Soc. 2004;126:14583–14599. doi: 10.1021/ja046808r. [DOI] [PubMed] [Google Scholar]
- 3.Chou LY, Ming K, Chan WC. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 4.Saha K, Bajaj A, Duncan B, Rotello VM. Small. 2011;7:1903–1918. doi: 10.1002/smll.201100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 6.De M, Rana S, Akpinar H, Miranda OR, Arvizo RR, Bunz UH, Rotello VM. Nat Chem. 2009;1:461–465. doi: 10.1038/nchem.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman AS. J Control Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Boisselier E, Astruc D. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 9.Moyano DF, Rotello VM. Langmuir. 2011;27:10376–10385. doi: 10.1021/la2004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rana S, Bajaj A, Mout R, Rotello VM. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You CC, Miranda OR, Gider B, Ghosh PS, Kim IB, Erdogan B, Krovi SA, Bunz UH, Rotello VM. Nat Nanotechnol. 2007;2:318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Zhao H, Lin Y, Zhu N, Ma Y, Mao L. Angew Chem Int Ed Engl. 2010;49:4800–4804. doi: 10.1002/anie.201001057. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj A, Miranda OR, Kim IB, Phillips RL, Jerry DJ, Bunz UH, Rotello VM. Proc Natl Acad Sci U S A. 2009;106:10912–10916. doi: 10.1073/pnas.0900975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj A, Rana S, Miranda OR, Yawe JC, Jerry DJ, Bunz UHF, Rotello VM. Chem Sci. 2010;1:134–138. [Google Scholar]
- 15.El-Boubbou K, Zhu DC, Vasileiou C, Borhan B, Prosperi D, Li W, Huang X. J Am Chem Soc. 2010;132:4490–4499. doi: 10.1021/ja100455c. [DOI] [PubMed] [Google Scholar]
- 16.Dreaden EC, Mackey MA, Huang X, Kang B, El-Sayed MA. Chem Soc Rev. 2011;40:3391–3404. doi: 10.1039/c0cs00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyano DF, Rana S, Bunz UHF, Rotello VM. Faraday Disc. 2011 doi: 10.1039/C1FD00024A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 19.Cho EC, Xie J, Wurm PA, Xia Y. Nano Lett. 2009;9:1080–1084. doi: 10.1021/nl803487r. [DOI] [PubMed] [Google Scholar]
- 20.Martin AL, Bernas LM, Rutt BK, Foster PJ, Gillies ER. Bioconjug Chem. 2008;19:2375–2384. doi: 10.1021/bc800209u. [DOI] [PubMed] [Google Scholar]
- 21.Shi XY, Thomas TP, Myc LA, Kotlyar A, Baker JR. Phys Chem Chem Phys. 2007;9:5712–5720. doi: 10.1039/b709147h. [DOI] [PubMed] [Google Scholar]
- 22.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Nat Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma A, Stellacci F. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 24.Kang B, Mackey MA, El-Sayed MA. J Am Chem Soc. 2010;132:1517–1519. doi: 10.1021/ja9102698. [DOI] [PubMed] [Google Scholar]
- 25.Verma A, Uzun O, Hu Y, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Nat Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han YJ, Stucky GD, Butler A. J Am Chem Soc. 1999;121:9897. [Google Scholar]
- 27.Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN. Proc Natl Acad Sci U S A. 2005;102:279–284. doi: 10.1073/pnas.0408039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Proc Natl Acad Sci U S A. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VS. J Am Chem Soc. 2004;126:13216–13217. doi: 10.1021/ja046275m. [DOI] [PubMed] [Google Scholar]
- 30.Torney F, Trewyn BG, Lin VS, Wang K. Nat Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 32.Petros RA, DeSimone JM. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 33.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 34.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. J Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Kommareddy S, Amiji M. Chapter 10. Humana Press, Inc; Totowa, NJ: 2004. pp. 181–215. [Google Scholar]
- 36.Wang Y, Brown P, Xia Y. Nat Mater. 2011;10:482–483. doi: 10.1038/nmat3060. [DOI] [PubMed] [Google Scholar]
- 37.Choi CH, Alabi CA, Webster P, Davis ME. Proc Natl Acad Sci U S A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi CH, Zuckerman JE, Webster P, Davis ME. Proc Natl Acad Sci U S A. 2011;108:6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Proc Natl Acad Sci U S A. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao W, Karp JM. Nat Mater. 2009;8:453–454. doi: 10.1038/nmat2463. [DOI] [PubMed] [Google Scholar]
- 41.von Maltzahn G, Park JH, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nat Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Nat Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 44.Lytton-Jean AK, Langer R, Anderson DG. Small. 2011;7:1932–1937. doi: 10.1002/smll.201100761. [DOI] [PubMed] [Google Scholar]
- 45.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 46.Alivisatos AP, Johnsson KP, Peng XG, Wilson TE, Loweth CJ, Bruchez MP, Schultz PG. Nature. 1996;382:609–611. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- 47.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 48.Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Bioconjug Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. J Am Chem Soc. 2009;131:2072–2073. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Mol Pharmaceut. 2009;6:1934–1940. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S, Taton TA, Mirkin CA. Science. 2002;295:1503–1506. doi: 10.1126/science.1067003. [DOI] [PubMed] [Google Scholar]
- 52.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. J Am Chem Soc. 2007;129:15477–15479. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Nano Lett. 2009;9:3258–3261. doi: 10.1021/nl901517b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang W, Kim BY, Rutka JT, Chan WC. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 55.El-Sayed IH, Huang XH, El-Sayed MA. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 56.Link S, Ei-Sayed MA. Annu Rev Phys Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 57.Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S. Adv Mater. 2010;22:2729–2742. doi: 10.1002/adma.201000260. [DOI] [PubMed] [Google Scholar]
- 58.Larson TA, Bankson J, Aaron J, Sokolov K. Nanotechnology. 2007;18:325101. [Google Scholar]
- 59.Huh YM, Jun YW, Song HT, Kim S, Choi JS, Lee JH, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J Am Chem Soc. 2005;127:12387–12391. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 60.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Adv Mater. 2006;18:2553–2556. [Google Scholar]