Abstract
Inotuzumab ozogamicin (IO) is a CD22 monoclonal antibody that targets B lymphocytes in early stages of development, successfully inducing remission in patients with relapsed acute lymphoblastic leukemia (ALL). We report our experience of 26 patients who were treated with IO followed by allogeneic stem cell transplantation (SCT).
Background
No highly effective salvage therapy exists for patients with relapsed acute lymphoblastic leukemia (ALL). Inotuzumab ozogamicin (IO) is a CD22 monoclonal antibody attached to calicheamycin that targets B lymphocytes in early stages of development, successfully inducing remission in patients with multiply relapsed ALL.
Methods
We describe our findings in 26 patients who received allogeneic hematopoietic stem cell transplantation (SCT) after treatment with IO between September 2010 and October 2011.
Results
Patients with a median age of 33 years (range, 5-70 years) received an allogeneic matched sibling donor (n = 9), matched- or 1-antigen mismatched unrelated donor (n = 16), or cord blood donor SCT (n = 1) while in complete remission (n = 23) or with active disease (n = 3). At the time of SCT, 15 patients were in complete remission without evidence of minimal residual disease (MRD) measured by multiparameter flow cytometry. Patients were heavily pretreated, including 5 patients who had received previous allogeneic SCT. Patients received a median of 3 courses of IO (range, 1-5 courses) before SCT. Seven patients are alive at a median follow-up of 13 months (range, 5-16 months), with 1-year event-free and overall survival (OS) of 22% and 20%, respectively. Patients without MRD at time of SCT had a markedly better 1-year OS of 42%. The cumulative incidence of nonrelapse mortality (NRM) at 6 months and 1 year were 40% and 60%, respectively, with 5 deaths attributed to venoocclusive disease (VOD).
Conclusions
Treatment with IO allows more patients to undergo transplantation while in remission, with favorable overall survival in patients without MRD who undergo transplantation. Reduction in hepatic toxicity is needed to improve overall results.
Keywords: Acute lymphoblastic leukemia, Allogeneic hematopoietic stem cell transplantation, Inotuzumab ozogamicin, Venoocclusive disease
Introduction
The incorporation of targeted therapies, such as antigen-specific monoclonal antibodies1-3 and tyrosine kinase inhibitors (TKIs) for Phil adelphia chromosome–positive acute lymphoblastic leukemia (ALL),4-7 into the conventional multiagent cytotoxic regimens for ALL, have dramatically improved the therapeutic landscape for patients with this disease. Adult patients can now be expected to enjoy complete response (CR) rates up to 90%, with long-term survival of 35% to 50%.8-11 Similarly, CR rates in the salvage setting are improved with the use of antigen-specific antibody therapy, such as epratuzumab, an unconju-gated monoclonal antibody directed against CD22,12 and blinatumomab, a bispecific single-chain antibody targeting CD3 and CD19,13 allowing more patients to be eligible for allogeneic hematopoietic stem cell transplantation (SCT).
After relapse, allogeneic SCT currently provides the most effective approach for inducing long-term disease control. Fielding et al reported an overall survival (OS) rate of 7% for adult patients with relapsed ALL after treatment in the Medical Research Council (MRC) UKALL12/ECOG 2993 study.14 The select few who were able to receive allogeneic SCT had a significantly better OS rate of 16% to 23% compared with 4% for those who received chemotherapy alone.14 Similarly, in a population-based study of 76 adults with relapsed ALL reported to the Swedish Adult Acute leukemia Registry, the OS rate was 22% at 3 years for those who received allogeneic SCT, compared with 0% for patients who received chemotherapy alone.15 Furthermore, the data from patients whose disease relapsed after initial treatment in the multicenter LALA-94 trial showed a clear survival advantage for transplantation in second complete remission (CR2) compared with active disease.16
The limitations of current salvage regimens include both lack of efficacy and toxicity. Upfront ALL regimens consist of dose-intense, multiagent, cytotoxic chemotherapy combinations; at the time of relapse, many patients have had previous exposure to chemotherapy drugs often used for salvage and have experienced toxicity resulting from dose-intense therapy. Thus antibody therapy, with a nonover-lapping toxicity profile and a mechanism of action distinct from cytotoxic chemotherapy drugs, is an ideal therapeutic agent for relapsed disease.
Inotuzumab ozogamicin (IO) is a CD22 monoclonal antibody bound to calicheamycin, a toxic product of Micromonospora echinospora.17 Calicheamycin causes sequence-specific breaks in double-stranded DNA, which eventually result in cell apoptosis. IO is rapidly internalized, and releases calicheamycin intracellularly. More than 80% of B-lineage ALL blasts express CD22, which is present in early stages of B-cell development. In a phase II study reported from our institution, 49 patients with refractory ALL were treated with a median of 2 courses of IO (range, 1-5).18 Each course of therapy was composed of 1.8 mg/m2 of IO infused over 1 hour administered over 3 to 4 weeks. An overall response rate of 57% was noted.18 The most frequent adverse events during the first course of treatment were mild fever, hypotension, and reversible liver enzyme elevations. Forty-five percent (n = 22) of patients were able to proceed to transplantation; reasons not to proceed with transplantation included persistent disease and lack of a suitable donor.10
Here we report on the outcomes of 26 patients who were treated with IO at any time before proceeding to allogeneic SCT.
Patients and Methods
Patients
The patient database at M.D. Anderson Center was screened for patients with the diagnosis of refractory ALL who received allogeneic SCT between September 2010 and October 2011 and were treated with IO at any time before SCT. IO was administered intravenously at 1.8 mg/m2 every 3 weeks; the first 3 adults and 3 children received a dose of 1.3 mg/m2. Details of IO treatment have been previously described.18 Patients were treated in clinical trials that were approved by the institutional review board, and written informed consent was obtained in accordance with the Declaration of Helsinki.
Conditioning Regimens
Patients received 1 of 5 regimens based on appropriate eligibility criteria and physician preference: (1) cyclophosphamide 60 mg/kg × 2 doses or single etoposide 60 mg/kg combined with 12 Gy of total body irradiation (TBI); (2) clofarabine 40 mg/m2 followed by pharmacokinetically dosed busulfan once daily for 4 days, with busulfan dose adjusted to target an average daily area under the curve (AUC) of 5500 μmol/min for patients < 60 years of age or 4000 μmol/min for patients 60 years or older; (3) clofarabine 40 mg/m2 for 4 days combined with busulfan targeted to daily AUC of 5000 μmol/min for 3 days and 1 dose of thiotepa at 5 mg/kg; (4) fludarabine 25 mg/m2 daily × 5 doses followed by 2 daily doses of melphalan at 70 mg/m2; (5) fludarabine 25 mg/m2 daily × 5 doses followed by 2 daily doses of melphalan at 70 mg/m2 + a single dose of thiotepa at 10 mg/kg.
Donors
All donors were human leukocyte antigen (HLA)-A, -B, -C, and -DR B1–compatible with recipients. HLA typing for class I antigens was performed using standard serologic or low-resolution molecular techniques, followed by confirmatory typing with high-resolution molecular typing using polymerase chain reaction (PCR) for class I and II antigens for sibling donors; high-resolution molecular typing of class I and II antigens was performed for all unrelated donors.
Peripheral blood stem cells were obtained from donors using standard mobilization protocols and apheresis techniques, with a target progenitor cell dose of 4 million CD34-positive (CD34+) cells/kg and a minimal acceptable dose of 2 million CD34+ cells/kg; bone marrow was used if peripheral blood could not be used. Stem cells from all related donors were collected at M.D. Anderson Cancer Center. Peripheral blood progenitor cells or bone marrow harvests from unrelated donors were obtained through the National Marrow Donor Program.
Supportive Care
Graft-versus-host disease (GVHD) prophylaxis consisted of a combination of tacrolimus and methotrexate 5 mg/m2 intravenously on days 1, 3, 6, and 11 after transplantation for matched related and matched unrelated donor transplants. Patients with matched unrelated donors additionally received antithymocyte globulin at a total dose of 4 mg/kg infused over 3 days. Patients with adult mismatched unrelated donors received tacrolimus and mycophenolate mofetil 2 g/d plus 2 doses of cyclophosphamide at 50 mg/kg infused on days 2 and 3 after transplantation. Finally, recipients of cord blood units received tacrolimus and mycophenolate mofetil. Patients who experienced grade ≥ 2 acute GVHD received intravenous methylprednisolone at a dosage of at least 0.5 mg/kg every 6 hours and, if possible, were enrolled in treatment protocols for GVHD. Institutional transplantation guidelines for antimicrobial, antifungal, and antiviral prophylaxis were followed as previously reported.19 Patients with a previous history of central nervous system involvement received intrathecal prophylaxis after transplantation as feasible.
Definitions and Clinical Outcome Variables
Criteria for CR included normal cytogenetic findings, the absence of circulating blasts, < 5% marrow blasts, and a platelet count of 100 × 109/L or higher. Standard morphologic criteria were used todiagnose recurrent disease. The disease stage at transplantation was defined using established criteria. Minimal residual disease (MRD) was uniformly assessed in patients using multiparameter flow cytometry methods, published previously.6 Response was documented as the best response occurring after 30 days from SCT. Hematologic recovery was defined as the date that the patient had an absolute neutrophil count of 0.5 × 109/L or higher for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count of 20 × 109/L or higher without transfusion support. Failure to engraft by day 30 after transplantation was considered primary engraftment failure. Hematopoietic chimerism was evaluated in bone marrow (using unsorted cells) or peripheral blood (with myeloid and T-lineage sorting) by restriction fragment length polymorphisms using PCR methods to determine donor engraftment. Mixed chimerism was defined as the presence of any detectable (> 1%) recipient DNA in addition to donor-derived DNA in myeloid or T-lineage cells.
OS was estimated from the time of SCT until death from any cause, and patients still alive at last follow-up were considered censored. Event-free survival (EFS) was estimated from SCT until the date of relapse or death from any cause. Patients alive and disease free at last follow-up were considered censored. Nonrelapse mortality (NRM) was defined as death from any cause other than disease progression or relapse. The diagnosis of GVHD was confirmed by biopsy when feasible but was ultimately determined by clinical presentation. Acute GVHD was clinically graded as 0 to IV based on standard criteria.20 Hepatic venoocclusive disease (VOD) was diagnosed by previously established clinical criteria of painful hepatomegaly, jaundice, and fluid retention, and/or characteristic histologic changes consistent with sinusoidal injury.21
Statistical Methods
The primary endpoints of this analysis were OS, EFS, and NRM. The Kaplan-Meier method was used to estimate OS and progression-free survival.22 The cumulative incidence of NRM was estimated considering disease progression as a competing risk. The cumulative incidence of GVHD was estimated considering death in the absence of GVHD as a competing risk. All P values presented are 2-sided and statistical significance was determined at the 5% level. Descriptive statistics were used to summarize patient demographics.
Results
Patient and Treatment Characteristics
Patient demographics and baseline disease characteristics are listed in Table 1. Twenty-four adults and 2 children, with a median age of 33 years (range, 5-70 years) were evaluated in this study. Among 20 patients with available cytogenetic data at diagnosis, 9 (45%) were considered high risk defined by the presence of the t(9;22), t(4;11), hypodiploid or complex cytogenetics, and 11 patients (55%) were classified as intermediate risk based on the absence of high-risk or good-risk (hyperdiploidy) cytogenetic features. Specifically, 4 patients had the t(9;22) translocation, and none received TKI maintenance after transplantation because of poor count recovery. All the patients had primary refractory or multiply relapsed disease at time of transplantation with a median of 3 lines of salvage therapy (range, 2-6 lines), with 5 patients having undergone previous allogeneic SCT. At time of SCT, 58% (n = 15) did not have MRD after salvage therapy. The salvage therapy immediately preceding SCT was IO in 24 patients: 20 patients received intravenous IO at 1.8 mg/m2 weekly every 3 to 4 weeks; 4 patients (children and adults treated early in the phase II study) received 1.3 mg/m2 for the first cycle. Patients received a median of 3 courses of IO (range, 1-5 courses) at a median of 36 days (range, 13-85 days) before starting the SCT preparative regimen. Two patients who did not respond to IO received other chemotherapy immediately before SCT. The majority of patients received a matched related (n = 8) or matched unrelated (n = 12) donor transplant (77%). The majority of patients received a myeloablative preparative regimen (85%). Finally, the majority of patients received tacrolimus and minidose methotrexate for GVHD prophylaxis (85%).
Table 1.
Patient Characteristics (N = 26)
| Characteristic | No. (%) |
|---|---|
| Median Age, y (Range) | 33 (5-70) |
| Sex | |
| Male | 17 (65) |
| Female | 9 (35) |
| Disease Histologic Features | |
| B lineage | 24 (92) |
| Mixed lineage | 2 (8) |
| Cytogenetic Risk Diagnosis | |
| High risk | 9 (35) |
| Intermediate risk | 11 (42) |
| Unknown | 6 (23) |
| Disease Status at Transplantation | |
| Active disease | 3 (12) |
| Remission | 23 (88) |
| MRD present | 11 |
| MRD absent | 15 |
|
Median Lines of Chemotherapy Before SCT (range),
5 Previous Allogeneic SCT |
3 (2-6) |
| No. Courses IO | |
| 1 | 1 |
| 2 | 12 |
| 3 | 7 |
| 4 | 5 |
| 5 | 1 |
| Median Mo to SCT (Range) | 19 (6-50) |
| Transplantation Preparative Regimen | |
| Busulfan-clofarabine | 11 (42) |
| Busulfan-clofarabine-thiotepa | 4 (15) |
| Fludarabine-melphalan | 4 (15) |
| Fludarabine-melphalan-thiotepa | 4 (15) |
| Cyclophosphamide or etoposide/TBI | 3 (13) |
| Allotype | |
| Matched-related donor | 9 (35) |
| Matched-unrelated or 1 Ag mismatched | 16 (62) |
| Mismatched cord | 1 (3) |
Abbreviations: Ag = antigen; IO = inotuzumab ozogamicin; MRD = minimal residual disease; SCT = stem cell transplantation; TBI = total body irradiation.
Response, Relapse, EFS, and OS
Transplantation outcomes are listed in Table 2. Twenty patients were evaluable for response; 5 patients died before day 30 (disease progression [n = 1], organ failure [n = 4]), and 1 patient had severe GVHD and was not assessed before his death at day 132 after SCT. The overall CR rate was 90% (n = 18). Engraftment was prompt in all except 1 patient having 100% donor chimerism by day 30; 1 patient with active disease at time of transplantation remained a mixed chimera and subsequently had relapsed disease. The median days to absolute neutrophil concentration (ANC) recovery to > 0.5 × 109/L and platelet count > 20 × 109/L were 14 (range, 10-37 days) and 20 days (range, 12-35 days), respectively (Table 2).
Table 2.
Transplantation Outcomes of Evaluable Patients (n = 21)
|
Days to ANC Recovery > 0.5 × 109/L, Median
(Range) |
14 (10-37) |
|
Days to Platelet Count > 20 × 109/L, Median
(Range) |
20 (12-35) |
| Full Donor Chimerism Day 30 (%) | 95 |
| Complete Remission (%) | 95 |
| Remission | 53 |
| Remission without platelet recovery | 42 |
| Acute GVHD, Cumulative Incidence (%) | |
| Grades II-IV | 48 |
| Grades III-IV | 14 |
Abbreviations: ANC = absolute neutrophil concentration; GVHD = graft versus host disease.
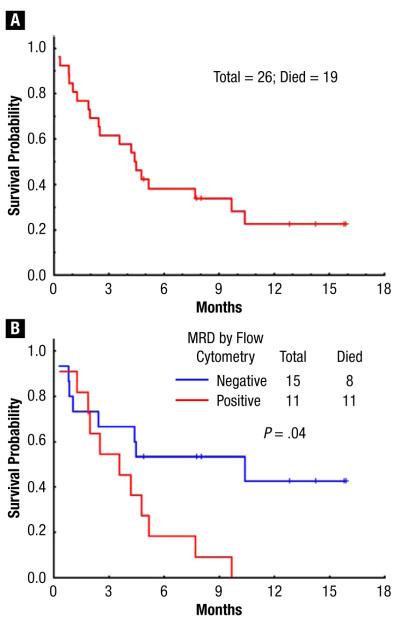
Eight patients had relapsed disease at a median of 3 months after SCT (range, 1-4 months). EFS rates at 6 months and 1 year were 30% and 22%, respectively (Figure 1). Seven patients are alive at a median follow-up of 13 months (range, 5-16 months), with OS 38% and 20% at 6 months and 1 year, respectively (Figure 2A). Of note, patients without MRD at time of SCT had a significantly better OS of 42% at 1 year; there were no survivors at 1 year in patients with MRD present before SCT (Figure 2B).
Figure 1.
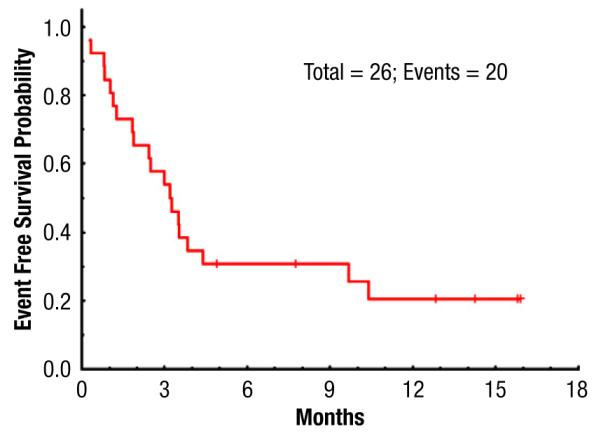
Event-Free Survival for Study Population
Figure 2.
(A) Overall Survival for Study Population. (B) Overall Survival by Minimal Residual Disease (MRD) status
Treatment Toxicity
Observed regimen-related toxicities are listed in Table 3 and appear within an expected range of transplant-related complications. However there was marked hepatic toxicity, with 5 patients having fatal hepatic VOD at a median of 23 days after SCT (range, 3-55 days). These 5 patients had received a median of 2 courses of IO (range, 1-5) administered at a median of 40 days (range, 27-68 days) before the start of the SCT preparative regimen; 2 patients had received previous allogeneic SCT. The transplantation preparative regimens were busulfan combined with clofarabine (n = 1), busulfan/clofarabine combined with thiotepa (n = 2), and fludarabine, melphalan, and thiotepa (n = 2). Diffuse alveolar hemorrhage (DAH) developed in 4 patients. Among these, 1 patient also had VOD; presumably the DAH was a component of the generalized sinusoidal endothelial damage. The remaining 3 patients had infectious pneumonia that became complicated by DAH. The element of renal dysfunction associated with VOD likely contributed to the relatively high rate of grade 3/4 elevated creatinine levels reported for the group (35%). The rate of grade 3/4 mucositis (19%) was within the expected range for this group, among whom 43% received myeloablative SCT conditioning.
Table 3.
Regimen-Related Toxicities
| Toxicity | Grade, n (%) |
|
|---|---|---|
| All Grades | Grade 3/4 | |
| Liver | ||
| Transaminitis | 24 (92) | 7 (27) |
| Bilirubin elevation | 17 (65) | 10 (38) |
| Venoocclusive Disease | 5 (19) | |
| Gastrointestinal Tract | ||
| Diarrhea | 12 (47) | 2 (8) |
| Mucositis | 24 (92) | 5 (19) |
| Nausea/vomiting | 21 (82) | 1 (4) |
| Urinary Tract/Kidney | ||
| Creatinine rise | 15 (58) | 9 (35) |
| Hemorrhagic cystitis | 6 (23) | 4 (15) |
| Lung | ||
| Diffuse alveolar hemorrhage | — | 4 (15) |
The cumulative incidence of NRM at 100 days, 6 months, and 1 year was 32%, 40%, and 60%, respectively (Figure 3). There were 11 nonrelapse deaths caused by the following: pneumonia (n = 3), GVHD (n = 2), and multiorgan failure, including VOD (n = 6). Disease recurrence accounted for 8 deaths. The cumulative incidence of acute GVHD grades II to IV and III to IV were 38% and 7%, respectively (Table 2).
Figure 3.
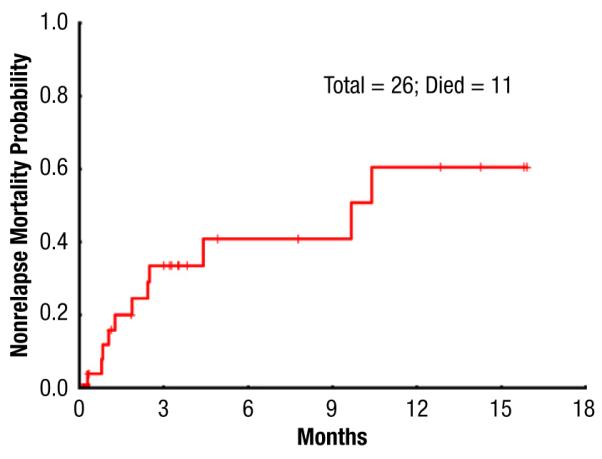
Nonrelapse Mortality for Study Group
Discussion
Our findings demonstrate that patients with multiply relapsed ALL are able to achieve a CR with IO, with nearly half of the patients experiencing eradication of MRD. This has allowed for (1) more patients to be eligible for transplantation and (2) better transplantation results compared with historical data in patients who had no MRD at the time of SCT, with an OS rate of 42% at 1 year (Figure 2B). Indeed, in a previous study of 49 patients with refractory relapsed ALL treated with IO, CR or marrow CR was noted in more than 50% of patients.18 Importantly, CR was achieved while the patient maintained a good performance status because of the favorable toxicity profile of the antibody therapy. This allowed for 45% of these patients to subsequently receive SCT, in contrast to our historical data of proceeding to allogeneic SCT in only 17% of patients achieving CR after disease relapse (5% of the total salvage population).23 Although this increase in transplantation is partially attributed to more available donors through successful haplotype mismatch and cord blood transplantation, the patients’ preserved performance status remains a significant variable in allowing for increased numbers of successful transplantations.
The rate of NRM at 6 months was 32%, with 5 deaths attributed to VOD. The rates for VOD after transplantation vary widely in the literature, ranging from 0% to 38% based on the intensity of the transplantation preparative regimen.24,25 TBI regimens have historically been associated with higher rates of VOD, up to 54%,26 and thiotepa combined with other alkylating agents have historically been associated with high rates of VOD.27 Because these patients were heavily pretreated, and the majority of patients received a myeloablative regimen, including 3 patients who received TBI-based preparative regimens, the VOD rate of 19% observed in this study was not entirely surprising. Still, the rate was higher than what we have typically noted at our center for SCT in ALL.28,29 Two of the patients with VOD had undergone a second allogeneic SCT, and 4 patients had received a preparative regimen containing 2 alkylating agents—thiotepa combined with busulfan or melphalan, and clofarabine, which is also associated with hepatotoxicity, albeit commonly mild and reversible. The interval between IO administration and the start of the transplantation preparative regimen did not appear to influence the risk for VOD developing: median, 40 days in the VOD group vs. 36 days in the non-VOD group. There does not appear to be a correlation between the courses of IO administered and the risk for GVHD developing; the median number of IO courses administered2 was the same in the VOD and non-VOD groups. However, notably, VOD developed in the only patient who received 5 courses of IO. In the nontransplantation setting, IO therapy has been associated with liver function abnormalities, which were usually mild to moderate and transient in nature.18,30 Similar findings were noted after therapy with gemtuzumab ozogamicin, an antibody that is also conjugated with calicheamycin and directed against CD33; it is used for acute myeloid leukemia. When gemtuzumab was used as monotherapy, the incidence of VOD was reported to range from 3% to 15% by investigators associated with the RADAR (Research on Adverse Drug Events and Reports) project.31 When gemtuzumab was used within 3 months of SCT, the rates for VOD ranged from 15% to 40%.30
In conclusion, the use of IO allowed for greater and deeper remissions in patients with multiply relapsed ALL, allowing for greater numbers of transplants in the relapsed setting. Within the limitations of a small sample size, OS is favorable in the patients who underwent transplantation while in remission. However hepatic toxicity needs to be better controlled to improve OS for the entire group. Great care needs to be taken in selecting the transplantation preparative regimen and avoiding myeloablative double-alkylator combinations that have historically been associated with hepatic injury. Furthermore, avoiding other hepatotoxic drugs peritransplantation, such as azole anti-fungal agents, and using ursodial in an effort to prevent hepatic injury32 may also help.
Clinical Practice Points.
Treatment options for patients with refractory relapsed ALL are limited.
IO is effective in inducing response in patients with multiply relapsed ALL, which enables patients to be considered for allogeneic SCT.
Allogeneic SCT is feasible in this patient population, resulting in an OS of 20% at 1 year for all patients and 42% for patients who undergo transplantation with no MRD. However significant hepatic toxicity, specifically fatal VOD (19%), was noted.
The toxicity profile of the transplantation conditioning regimen needs to be carefully considered when proceeding with transplantation in this population in an effort to minimize toxicity and improve survival.
Acknowledgments
The authors thank the clinical and laboratory staff for the care of the patients and laboratory processing of patient samples.
Footnotes
Disclosure Drs Thomas and Kantarjian received funding from Pfizer. All other authors have stated that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–80. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–9. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoelzer D, Gökbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26:25–32. doi: 10.1016/j.blre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–8. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 5.Ohanian M, Cortes J, Kantarjian H, et al. Tyrosine kinase inhibitors in acute and chronic leukemias. Expert Opin Pharmacother. 2012;13:927–38. doi: 10.1517/14656566.2012.672974. [DOI] [PubMed] [Google Scholar]
- 6.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–7. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 8.Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–25. ix. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 9.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–43. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 10.Faderl S, O’Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010;116:1165–76. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin Hematol. 2009;46:64–75. doi: 10.1053/j.seminhematol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group pilot study. J Clin Oncol. 2008;26:3756–62. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 14.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 15.Kozlowski P, Åström M, Ahlberg L, et al. High curability via intensive reinduction chemotherapy and stem cell transplantation in young adults with relapsed acute lymphoblastic leukemia in Sweden 2003-2007. Haematologica. 2012;97:1414–21. doi: 10.3324/haematol.2011.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–14. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 17.Thorson JS, Sievers EL, Ahlert J, et al. Understanding and exploiting nature’s chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des. 2000;6:1841–79. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13:403–11. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 19.Kebriaei P, Saliba R, Rondon G, et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: impact of tyrosine kinase inhibitors on treatment outcomes. Biol Blood Marrow Transplant. 2012;18:584–92. doi: 10.1016/j.bbmt.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;1995:825–8. [PubMed] [Google Scholar]
- 21.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 23.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–30. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DB, Savani BN. How can we reduce hepatic veno-occlusive disease-related deaths after allogeneic stem cell transplantation? Exp Hematol. 2012;40:513–7. doi: 10.1016/j.exphem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic venoocclusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia working party. Blood. 1998;92:3599–604. [PubMed] [Google Scholar]
- 26.Ganem G, Saint-Marc Girardin MF, Kuentz M, et al. Venocclusive disease of the liver after allogeneic bone marrow transplantation in man. Int J Radiat Oncol Biol Phys. 1988;14:879–84. doi: 10.1016/0360-3016(88)90009-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee JL, Gooley T, Bensinger W, et al. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 1999;5:306–15. doi: 10.1016/s1083-8791(99)70006-6. [DOI] [PubMed] [Google Scholar]
- 28.Kebriaei P, Saliba RM, Ma C, et al. Allogeneic hematopoietic stem cell transplantation after rituximab-containing myeloablative preparative regimen for acute lymphoblastic leukemia. Bone Marrow Transplant. 2006;38:203–9. doi: 10.1038/sj.bmt.1705425. [DOI] [PubMed] [Google Scholar]
- 29.Kebriaei P, Basset R, Ledesma C, et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1819–26. doi: 10.1016/j.bbmt.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol. 2010;28:2085–93. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 31.McKoy JM, Angelotta C, Bennett CL, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Essell JH, Schroeder MT, Harman GS, et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975–81. doi: 10.7326/0003-4819-128-12_part_1-199806150-00002. [DOI] [PubMed] [Google Scholar]