Abstract
The Ron receptor tyrosine kinase is overexpressed in approximately half of all human colon cancers. Increased Ron expression positively correlates with tumor progression, and reduction of Ron levels in human colon adenocarcinoma cells reverses their tumorigenic properties. Nearly all colon tumors demonstrate loss of the adenomatous polyposis coli (APC) tumor suppressor, an early initiating event, subsequently leading to β-catenin stabilization. To understand the role of Ron in early-stage intestinal tumorigenesis, we generated Apc-mutant (ApcMin/+) mice with and without Ron signaling. Interestingly, we report here that significantly more ApcMin/+ Ron-deficient mice developed higher tumor burden than ApcMin/+ mice with wild-type Ron. Even though baseline levels of intestinal crypt proliferation were increased in the ApcMin/+ Ron-deficient mice, loss of Ron did not influence tumor size or histological appearance of the ApcMin/+ adenomas, nor was β-catenin localization changed compared to ApcMin/+ mice with Ron. Together, these data suggest that Ron may be important in normal intestinal tissue homeostasis, but that the expression of this receptor is not required for the formation and growth of adenomas in ApcMin/+ mice.
Keywords: Apc, Proliferation, Adenoma
Introduction
The adenomatous polyposis coli (APC) tumor suppressor gene is mutated in the majority of sporadic human colon cancers [1]. Patients with familial adenomatous polyposis (FAP) inherit a germline mutation in APC that causes the development of hundreds to thousands of colon polyps at a young age, some of which will progress into carcinomas if not removed [1,2]. The ApcMin/+ mouse serves as a useful model of FAP; germline mutation of the mouse Apc gene results in multiple spontaneous adenomatous polyps that demonstrate loss of heterozygosity of the wild-type Apc allele [3,4]. One important difference between FAP and the ApcMin/+ model is that the adenomas in ApcMin/+ mice do not progress to carcinomas without additional chemical insults or genetic alterations, perhaps due to the short lifespan of the animals as a result of the intestinal obstruction and anemia from tumor burden [4–6]. At the molecular level, ApcMin/+ adenomas, as well as inherited and sporadic human colorectal tumors, demonstrate cytosolic and nuclear accumulation of the transcription co-activator β-catenin. β-catenin is a tightly regulated effector of APC, whose activity and the expression of its transcriptional targets contributes significantly to tumor development when Apc is inactivated [7–9]. The ApcMin/+ model has been useful in testing the importance of a variety of signaling pathways and tumor markers, such as EGFR, MMP7, and iNOS in intestinal tumorigenesis using genetic and pharmacological approaches [10–12].
Recently, studies have shown that the heterodimeric transmembrane receptor tyrosine kinase Ron, a member of the Met tyrosine kinase family, may be important in human colon cancer [13,14]. Ron is overexpressed, constitutively phosphorylated, and activated in many cancer types, including primary human sporadic colon cancer and in the colon cancer cell lines Colo201, HT-29, and HCT116 [13–17]. Activation of the Ron receptor occurs upon binding of the ligand, hepatocyte growth factor-like protein (HGFL), which induces receptor tyrosine phosphorylation and activation [18,19]. The downstream effects of Ron activation are vast and include cell proliferation, cell cycle progression, cell motility, angiogenesis, cell survival, apoptosis, cellular transformation, tumor progression, and metastasis [20]. The PI3K, MAPK, Ras, Src, Fak, and β-catenin signaling pathways have all been implicated in mediating the signal from Ron to exert these effects [13,15–17,21–24]. These features make Ron an attractive therapeutic target for cancer; however, very little is known about its role in colorectal tumorigenesis in vivo. Therefore, we sought to determine the in vivo role of Ron in intestinal tumorigenesis using the well-characterized ApcMin/+ mouse model, since APC loss is an early event in sporadic colon cancer [1,3,25,26]. In this report, we demonstrate that Ron is expressed in the non-transformed intestinal epithelium and adenomas in the ApcMin/+ mice. By generating ApcMin/+ mice that lack the tyrosine kinase domain of the Ron receptor (RonTK−/−), we show that Ron is not required for intestinal tumor initiation, and its loss leads to an increase in the number of animals exhibiting higher tumor numbers compared to control ApcMin/+ mice, perhaps due to enhanced proliferation in the morphologically normal intestine.
Materials and Methods
Animals
ApcMin/+ mice on a C57BL/6J background (The Jackson Laboratory) were crossed with Ron receptor tyrosine kinase domain knockout mice (RonTK−/−) [27]. RonTK−/− mice were generated on a Black Swiss background and backcrossed 8 generations onto the C57BL/6J background prior to mating with the ApcMin/+ mice, therefore only approximately 0.4% of the original Black Swiss background was introduced into the initial cross. ApcMin/+;RonTK+/+, ApcMin/+;RonTK+/−, and ApcMin/+;RonTK−/− mice (n=13 each) were euthanized at three months of age. At this point, their intestines were harvested, fixed in 4% paraformaldehyde overnight, and then transferred to 70% ethanol. Tumor number, size, and location along the gastrointestinal tract were determined using an Olympus dissection microscope as described previously [12]. For histopathological and immunohistochemical analyses, a minimum of 2 tumors per gastrointestinal segment (duodenum, jejunum, ileum, and colon) from each mouse (at least 4 mice per genotype) were excised and embedded in paraffin. For all analyses, ApcMin/+;RonTK+/+ mice served as controls while ApcMin/+;RonTK+/− and ApcMin/+;RonTK−/− mice were the experimental animals.
Histopathological Analysis
4-μm sections were deparaffinized, hydrated through graded ethanol washes, and stained with Mayer’s hematoxylin and eosin. The sections were dehydrated, incubated in Citrisolv, and mounted with permount. Certified pathologists analyzed tumor grade and inflammation without previous knowledge of the genotype and according to published guidelines [28].
Immunohistochemistry
4-μm-thick sections were deparaffinized and hydrated as above. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide and non-specific immunoreactivity was blocked using 5% goat serum in phosphate-buffered saline. The tissues were incubated with a rabbit polyclonal anti-Ron β C-20 antibody (Santa Cruz), rabbit polyclonal anti-β-catenin antibody (NeoMarkers), or equivalent concentration of rabbit IgG (Sigma) at a concentration of 2μg/ml. Primary antibodies were detected using 0.75μg/ml goat anti-rabbit biotinylated secondary antibody (Vector Labs). Antibody immunoreactivity was amplified using the VECTASTAIN ABC kit (Vector Labs), and visualized using DAB substrate (Vector Labs). The sections were counterstained in hematoxylin, dehydrated, incubated with Citrisolv, and mounted. All images were captured using a Nikon FX-35DX camera attached to the Nikon Microphot microscope and Spotcam Advanced software (Nikon).
Proliferation Assay
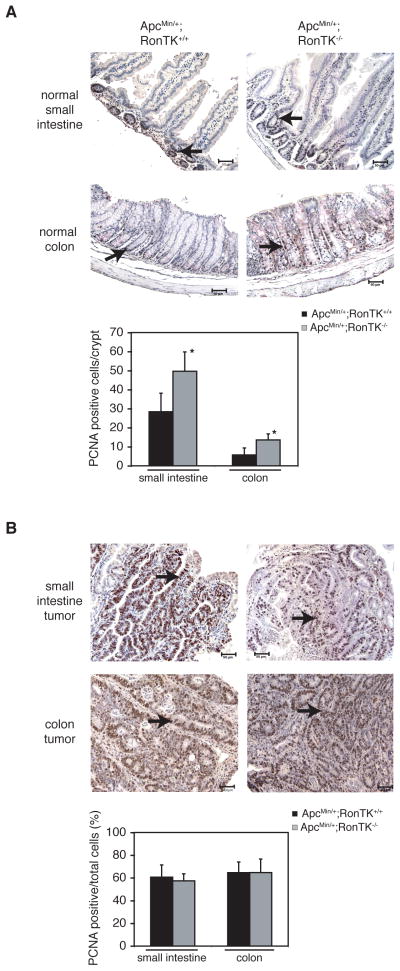
Immunohistochemistry was performed with an anti-PCNA mouse monoclonal antibody (BD Biosciences; 1 μg/ml), and goat anti-mouse biotinylated secondary antibody (Vector Labs; 0.75 μg/ml) as described above. The proliferation index was measured by quantification of PCNA-positive nuclei. Three fields of intact crypts were counted on a Nikon Microphot-FXA EP1-FL3 microscope at 400X magnification. Quantification of PCNA-positive cells in intestinal crypts from four different ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice were used to calculate the average number of proliferating cells per crypt. In the size-matched adenomas, the percentage of proliferating cells was determined by quantifying the number of PCNA-positive cells out of at least 600 total cells per mouse at 400X magnification from four different ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice.
Apoptosis Assay
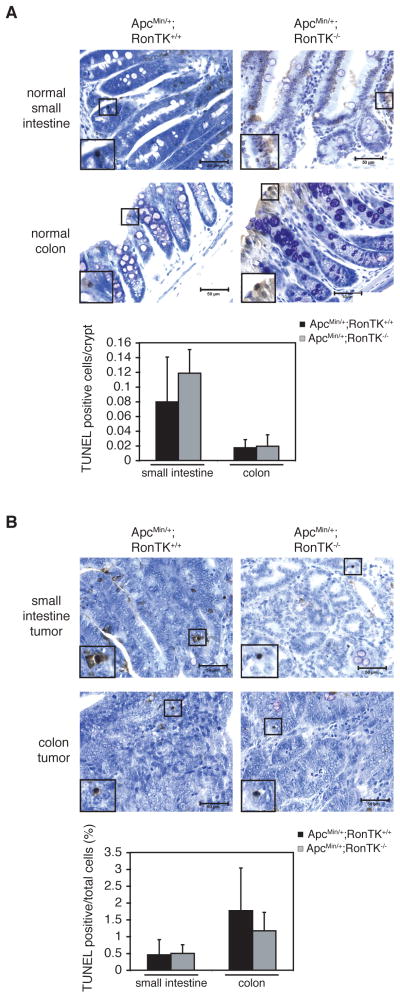
Tissues were analyzed for apoptosis using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International) according to the manufacturer’s instructions. Apoptosis in the normal small intestine and colon was quantified by counting the number of TUNEL-positive nuclei in intact crypts at 400X magnification in tissues from three different ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/−mice. In the size-matched adenomas, the number of TUNEL-positive nuclei out of at least 600 total nuclei was quantified per tumor at 400X magnification from three different ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice.
Real-time PCR Analyses
Duodenal and colon tissues were harvested from 3 month-old C57BL/6J wild-type or RonTK−/− mice on the same background (n=6 each), snap frozen in liquid nitrogen, and stored at -80°C. RNA was isolated from frozen tissues using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was generated from 2 μg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR analysis was then performed using the Applied Biosystems 7300 Real Time PCR System and Sequence Detection Software Version 1.3.1 (Applied Biosystems). Each cDNA sample was run in duplicate and amplified by Power SYBR Green PCR Master Mix (Applied Biosystems) using the following primers: cyclin D1 forward 5′-ccatgaactacctggaccg-3′ and reverse 5′-cacaaacctctgtgcatgc-3′; c-myc forward 5′-ttccacggccttctctcctt-3′ and reverse 5′-tcaatttcttcctcatcttcttgct-3′; Cox-2 forward 5′-cctgccccacagcaaact-3′ and reverse 5′-ccttcctcccgtagcagatg-3′; Gapdh forward 5′-aatggtgaaggtcggtgtg-3′ and reverse 5′-gaagatggtgatgggcttcc-3′. Table 1 presents the data as the average relative gene expression normalized to Gapdh for each gene and shown as a fold change relative to the wild-type control tissues. Each value represents 6 tissues per genotype from individual mice analyzed in duplicate from one experiment; three experimental replicates were performed with similar results.
Table 1.
Fold-change of β-catenin/Tcf target gene mRNA expression in RonTK−/− intestines relative to RonTK+/+ controls.
| Cyclin D1 | c-Myc | Cox2 | |
|---|---|---|---|
| Small Intestine | 1.09 ± 0.13 | 1.08 ± 0.35 | 1.12 ± 0.19 |
| Colon | 1.23 ± 0.15 | 1.44 ± 0.54 | 1.41 ± 0.29 |
p > 0.05 for all genes.
Statistical Analyses
To assess tumor burden, a comparison of the mean tumor numbers of ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− was performed using the non-parametric Wilcoxon rank-sum test. Evaluation of the number of animals with tumor multiplicities greater than the median (29 tumors) of the ApcMin/+;RonTK+/+ mice was performed using a binomial test. Statistical analyses of proliferation, apoptosis, and real-time PCR analyses were performed using a Student’s t-test.
Results
Ron is Expressed in the Intestine of Apc-mutant Mice
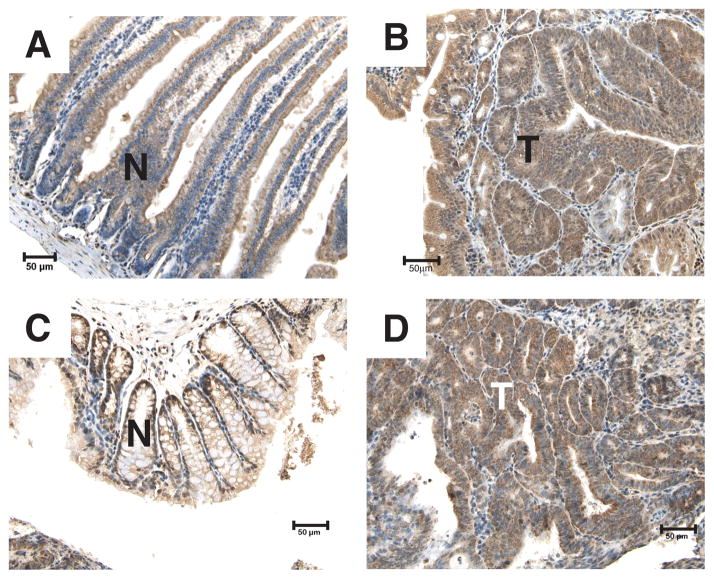
In the human adult small intestine and colon, Ron expression is localized to the crypt cells with a granular cytosolic subcellular localization [29]. To determine the expression pattern of Ron in the non-transformed intestine and adenomas from ApcMin/+ mice, immunohistochemistry was performed with an anti-Ron antibody (Figure 1). Ron was homogeneously expressed in epithelial cells of the small intestine. In addition, Ron was also localized to the crypt cells in the colon, but was expressed at a low level in other areas. In adenomas from the ApcMin/+ mice, Ron expression was diffuse. Quantitative real-time PCR analysis of RNA isolated from ApcMin/+ tumors compared to Apc+/+ normal intestinal tissue showed similar Ron expression levels (data not shown). From these data, we conclude that Ron is expressed in normal intestinal epithelium, particularly in areas (i.e. crypts) thought to give rise to tumors [30–32], and its expression is maintained in intestinal adenomas from ApcMin/+ mice.
FIGURE 1.
Ron is expressed in the non-transformed intestinal epithelium and adenomas of ApcMin/+ mice. Immunohistochemistry on tissue sections from the small intestine (A and B) and colon (C and D) of three month-old ApcMin/+ mice was performed using an anti-Ron antibody to detect Ron expression. No staining was observed using an isotype-matched IgG control (data not shown). Non-transformed epithelial and tumor areas are designated by N (non-transformed) and T (tumor). 200X Magnification. Scale bar; 50 μm.
Tumor Formation in ApcMin/+ Mice Lacking the Ron Receptor Tyrosine Kinase
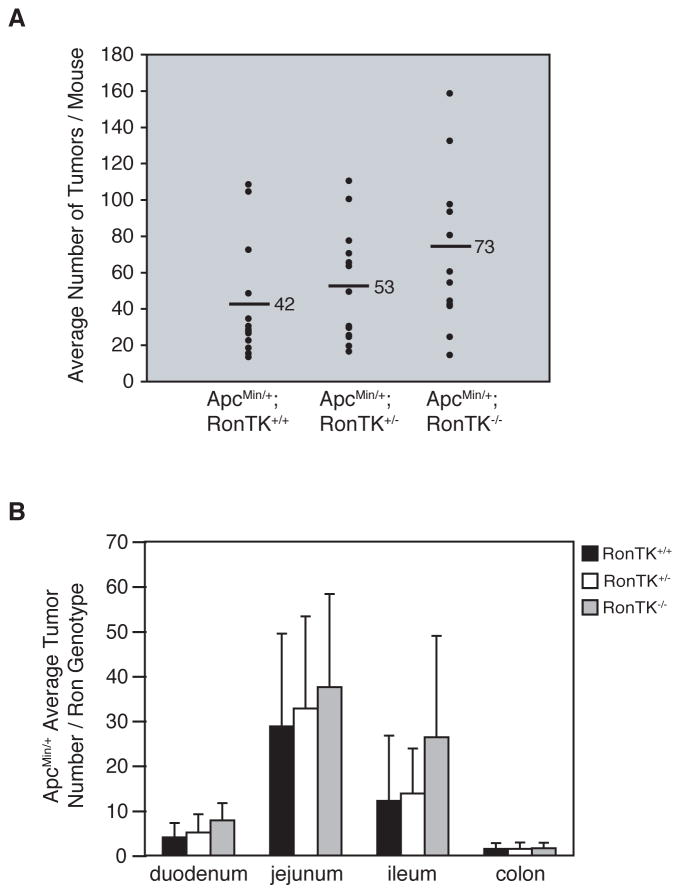
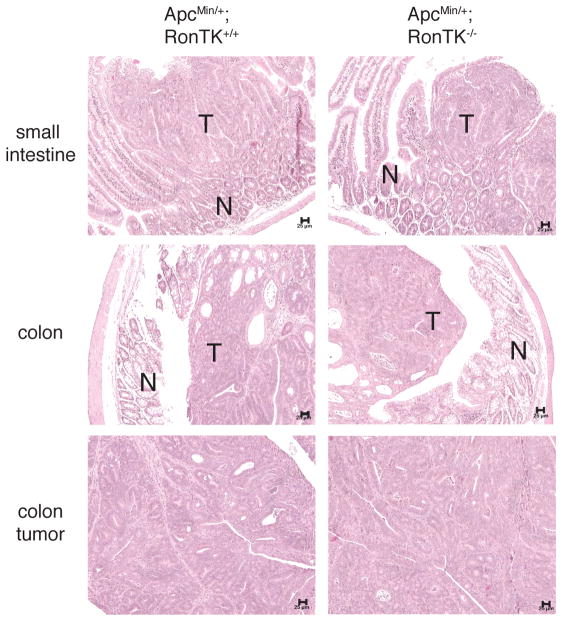
Given that Ron is expressed in normal and transformed intestinal tissue from ApcMin/+ mice, we next sought to determine the impact of Ron on intestinal tumor formation in these mice. Germline deletion of the tyrosine kinase domain of Ron in mice was reported previously, and results in the production of a truncated, non-functional receptor when stimulated with HGFL [27]. The Ron tyrosine kinase domain null (RonTK−/−) mice do not have any overt intestinal defects, nor do they develop spontaneous intestinal tumors. RonTK−/−and ApcMin/+ mice, both on the C57BL/6J background, were bred to generate mice that carried both mutant alleles. Three month-old ApcMin/+;RonTK−/− and ApcMin/+;RonTK+/+ controls were euthanized and analyzed for intestinal tumorigenesis. Even though there was not a significant difference in the mean number of tumors along the entire gastrointestinal tract or in individual regions in ApcMin/+ mice with and without Ron (Figure 2A), we found that significantly more ApcMin/+;RonTK−/− mice (11/13) had higher tumor numbers than the median (29 tumors) for the ApcMin/+;RonTK+/+ control mice (p=0.0017 by binomial test; Figure 2B). Mean tumor size was also unchanged between ApcMin/+;RonTK−/− (1.54 ± 0.04 mm) and ApcMin/+;RonTK+/+ (1.49 ± 0.04 mm) mice. Histopathological examination of tumor and surrounding normal tissue from ApcMin/+;RonTK−/− and ApcMin/+;RonTK+/+ mice revealed that the tumors were indistinguishable from one another and considered benign adenomas without prominent inflammation in either the tumor or surrounding non-transformed tissue (Figure 3). Furthermore, there were no significant differences in the tumor number, size, or histopathology of ApcMin/+;RonTK+/− mice compared to the ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− animals (data not shown). Together, these results demonstrate that the Ron receptor is not required for formation of intestinal adenomas in ApcMin/+ mice.
FIGURE 2.
Ron deletion significantly increases the number of ApcMin/+ mice with high tumor burden, but does not impact mean tumor number or distribution. ApcMin/+ mice were crossed with RonTK+/− mice to generate ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/−mice. Intestinal tumors were analyzed at three months of age. A) The mean number of tumors per mouse and within each segment of the gastrointestinal tract (duodenum, jejunum, ileum, and colon) per genotype (n=13). p>0.05 for all comparisons between genotypes, Wilcoxon rank-sum test. B) The mean number of tumors per mouse is plotted per genotype (n=13). Each data point represents a single mouse; the median tumor number in ApcMin/+;RonTK+/+ controls is denoted (29, black line). p=0.0017, binomial test.
FIGURE 3.
Adenomas from ApcMin/+;RonTK−/− mice are histologically similar to those from ApcMin/+;RonTK+/+ mice. Tissues from three-month old ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice were stained with hematoxylin and eosin and examined histologically. No differences were detected between genotypes in the appearance of non-transformed (N) and tumor (T) tissues in the small intestine and colon. 100X magnification. Scale bar; 25 μm.
ApcMin/+ Ron-deficient Mice Exhibit Increased Proliferation of the Non-transformed Intestinal Epithelium
Ron is a multifunctional protein that has been shown to regulate proliferation and cell death in several in vitro and in vivo models [21,24,33–36]. To determine if Ron inactivation affected these processes in ApcMin/+ intestinal tissues, proliferation and cell death were quantified in intestinal tissues from ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− using the immunohistochemical detection of proliferating cell nuclear antigen (PCNA), and the TUNEL assay (Figures 4 and 5). The ApcMin/+;RonTK−/− mice exhibited significantly more PCNA-positive cells in the non-transformed crypt epithelium of the small intestine and colon than ApcMin/+;RonTK+/+ tissues (Figure 4A), although analysis of adenomas from these mice showed no significant differences (Figure 4B). Ki67 immunohistochemistry was also performed on the same tissues and verified this finding (data not shown). In contrast, there were no significant differences in the number of TUNEL-positive cells in neither the non-transformed crypt epithelium nor the adenomas from ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice (Figures 5A and 5B). Together, these results suggest that Ron loss does not affect the growth of ApcMin/+ adenomas, despite an increase in proliferation of the non-transformed epithelium in ApcMin/+;RonTK−/− mice.
FIGURE 4.
Proliferation in the ApcMin/+;RonTK−/− non-transformed intestinal epithelium is significantly increased compared to ApcMin/+;RonTK+/+ mice. Tissues from three month-old ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice were analyzed for proliferation by staining with an anti-PCNA antibody. Proliferating cell nuclei are dark brown (black arrows). 200X magnification. Scale bar; 50 μm. Quantification of PCNA staining is graphed as the number of PCNA-positive cells per crypt in the normal small intestine (n=4) and colon (n=4) (A) and the percentage of proliferating cells in adenomas (B). *p<0.02, Student’s t-test.
FIGURE 5.
Cell death in ApcMin/+;RonTK−/− mice is not altered compared to ApcMin/+;RonTK+/+ mice. Tissues from three month-old ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice were analyzed for cell death using the TUNEL assay (n=3). TUNEL-positive cells are dark brown and have been magnified to show their histological appearance (black box). 400X magnification. Scale bar; 50 μm. Quantification of TUNEL staining is graphed as the number of TUNEL-positive cells/crypt in the normal small intestine (n=4) and colon (n=4) (A) and as the percentage of TUNEL-positive cells in adenomas of the small intestine and colon (B). p>0.05, Student’s t-test.
Ron Deficiency Does Not Alter β-Catenin Localization or Target Gene Expression in ApcMin/+ Mice
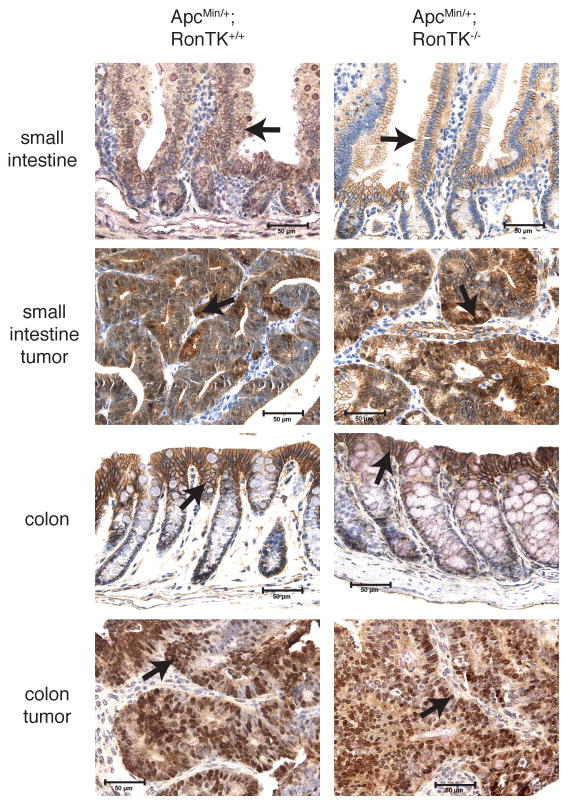
Loss of Apc is associated with increased β-catenin transcriptional activity that is sufficient for tumorigenesis in the mouse gut [7,37]. Stabilization and nuclear accumulation of β-catenin is a very common feature of adenomas from ApcMin/+ mice [38], and moreover, tyrosine phosphorylation and nuclear localization of β-catenin has been observed in mouse mammary tumors driven by Ron overexpression [23]. Therefore, one possible mechanism by which Ron loss may influence adenoma formation in ApcMin/+ mice is by alteration of β-catenin expression or activity. To determine if Ron inactivation overtly alters the localization of β-catenin in this model, we performed immunohistochemistry with an anti-β-catenin antibody. There was abundant cytosolic and nuclear β-catenin in adenomas from ApcMin/+;RonTK+/+ mice and ApcMin/+ Ron-deficient mice (Figure 6). Prominent staining at cell-cell contacts was observed in the non-transformed intestinal epithelium of ApcMin/+ mice with and without Ron signaling (Figure 6). Moreover, no significant changes in the expression of several β-catenin/Tcf target genes associated with intestinal tumorigenesis, including cyclin D1, c-myc, and Cox-2, were observed (Table 1). These data suggest that the mechanism by which Ron loss influences adenoma formation in this model is not likely to be through further modulation of β-catenin localization and transcriptional activity.
FIGURE 6.
β-catenin localization is similar in intestinal tissue from ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice. Tissues from three month-old ApcMin/+;RonTK+/+ and ApcMin/+;RonTK−/− mice were analyzed for β-catenin localization by immunohistochemistry using an anti-β-catenin antibody (brown). Non-transformed epithelium demonstrates predominantly membranous staining (arrows), and adenomas exhibit intense nuclear (arrows) and cytosolic staining in the small intestine and colon from both genotypes. 400X magnification. Scale bar; 50 μm
Discussion
Despite the numerous reports describing Ron overexpression and activation in various cancers including breast, lung, pancreas, and colon, little is known about the cooperation of Ron with other pathways deregulated in cancer [13–15]. Ron is a valid therapeutic target for cancer as antibody inhibition can reduce tumor cell growth in xenograft models [39,40]. Therefore, it is important to understand the consequence of Ron inactivation in normal tissues as well as during tumorigenesis in vivo. The ApcMin/+ model of murine intestinal tumorigenesis resembles the majority of human inherited and sporadic colon cancers, which contain aberrant APC/β-catenin signaling [26,30,41–43]. Using this paradigm for intestinal tumorigenesis, we show here that the loss of Ron in ApcMin/+ mice is not required for intestinal tumorigenesis. Moreover, our results show that germline loss of Ron in this model allows for an increase in the number of ApcMin/+ mice with high tumor burden compared to control ApcMin/+ mice with wild-type Ron, but there was no overall affect on tumor progression. While the rates of cell death in both non-transformed and adenoma tissues and the localization of β-catenin remained constant, Ron loss increased proliferation in the normal intestinal crypt epithelium. This study is significant as it indicates that Ron expression in ApcMin/+ mice is not required for tumorigenic transformation or the progression to adenomas.
Our studies demonstrate that Ron is expressed in the normal small intestine and colonic epithelium and adenomas of ApcMin/+ mice. Specifically, expression of Ron in the colonic crypt epithelium correlates with the location in which adenomas arise in the intestine of these animals [30–32]. Previously, Boon et al., 2006 [44] published immunohistochemical staining of Ron in ApcMin/+ mice suggesting that Ron was expressed in the non-transformed epithelium. In this report, the investigators were unable to detect Ron expression at appreciable levels in the ApcMin/+ adenomas using non-quantitative methods [44]; however, this does not preclude the validity of our data, which demonstrates that Ron expression is maintained in the ApcMin/+ adenomas. Our results are also consistent with the findings in human tissue showing that Ron is expressed in the normal intestinal epithelium with continued expression, or overexpression, observed in colorectal cancer [14,17,29]. Thus, our studies extend upon those in the literature and show that Ron is expressed in the small intestine and colon of non-transformed epithelium and adenomas of ApcMin/+ mice.
To determine the functional significance of Ron expression in the gut, we monitored adenoma formation in Ron tyrosine kinase-proficient and -deficient ApcMin/+ mice. Interestingly, we found that significantly more ApcMin/+;RonTK−/− mice developed a greater number of tumors than ApcMin/+;RonTK+/+ mice, although these tumors were not different in size or histology. These data indicate that Ron signaling is not required for intestinal tumorigenesis in the presence of an Apc mutation. These results were unexpected given that Ron overexpression has been shown to promote the tumorigenic phenotype in vitro [15,16,45]. In contrast to other studies wherein silencing of Ron overexpression in immortalized human colon carcinoma cell lines reversed their tumorigenic properties and had dramatic consequences with respect to β-catenin activation, our study shows that basal Ron expression is not required for cellular transformation or adenoma formation in the presence of an Apc mutation in vivo [17,21,46]. Our data do not, preclude, however, the importance of Ron overexpression observed in human colorectal cancer, where studies have suggested that inhibiting this overexpressed receptor may have important anti-tumor effects [15,16,39,40,45]. In addition to focusing on early-stage tumor progression, unique to our studies is that we have examined the effect of de novo Ron loss in all cell types on intestinal tumorigenesis in vivo, where other factors, such as the tumor microenvironment, play a role. It is also possible that there are species differences and differences in the absolute levels of Ron produced in these models that might contribute to these apparently conflicting data. However, our result is consistent with a previous study that showed stable knockdown of Ron in metastatic SW620 colon cancer cells did not prevent tumor formation in nude mice [21], and another study that observed increased benign tumor formation in an in vivo skin tumorigenesis model in mice deficient for Ron [47]. There is also precedent for other tumor-associated molecules, such as telomerase, to have different functions in normal tissue and at various stages of tumorigenesis [48]. It is noteworthy that the variability in mean tumor number per animal was greater than what we have observed and published using the ApcMin/+ model previously [12,49,50]. Given that the RonTK−/− mice were backcrossed 8 generations onto the C57BL/6J genetic background prior to mating with ApcMin/+ mice, and that no identified ApcMin/+ modifying loci are on the same chromosome as Ron (chromosome 9), we have no evidence to suggest that an ApcMin/+ modifying locus is present in our model [4,51–53]. However, we cannot discount the possibility that a novel modifier might be present in the region directly around the Ron gene.
Using markers of proliferation, we found that Ron inactivation in ApcMin/+ mice leads to significantly greater numbers of intestinal crypt cells undergoing proliferation, which may make the cells more susceptible to Apc loss of heterozygosity and transformation. It is interesting, however, that the tumors themselves failed to show enhanced proliferation in spite of Ron loss, which is consistent with the in vitro studies described above [21]. It is well documented that 100% of intestinal adenomas demonstrate loss of the wild-type copy of Apc [3], and we confirmed that Apc was similarly lost in adenomas from the ApcMin/+;RonTK−/− mice, suggesting that Apc inactivation is likely still required for driving adenoma formation (S.M., K.G., unpublished data). As RonTK−/− mice do not develop spontaneous gastrointestinal tumors, it is clear that Ron loss is not sufficient to initiate tumor formation. Finally, although Ron loss did not significantly affect apoptosis in the non-tumor or adenoma tissues, we cannot rule out a role for Ron in tumor cell survival as has been shown in other tumor types [54].
We investigated whether modulation of β-catenin signaling may be a mechanism by which Ron loss increased tumor load in ApcMin/+ mice. Our results suggest that Ron loss does not profoundly influence β-catenin nuclear localization in this model given that there were no differences in β-catenin localization between ApcMin/+;RonTK−/− and ApcMin/+;RonTK+/+ control mice in normal and adenoma small intestine and colon tissues. Additionally, quantitative real-time PCR analyses on normal intestinal tissue from RonTK+/+ and RonTK−/− mice showed that transcript levels of β-catenin target genes c-myc and cyclin-D1 [55,56] were unchanged with respect to Ron status. Nevertheless, it remains possible, that Ron may alter β-catenin activity in a way that was not measured in our experiments or captured in the time frame of our analyses.
As a second potential mechanism, we hypothesized that Ron may protect against tumor formation through modulating stromal-epithelium interactions in the intestine, for example, by regulating inflammation through cytokine production or the production of free oxygen radicals [27,57]. However, there were no apparent differences in inflammatory cell recruitment in the intestines of ApcMin/+;RonTK−/− tissues by histopathological analysis compared to controls. Cox-2, also considered a β-catenin transcriptional target, was examined because Ron inactivation can inhibit its expression in macrophages [58–61], and pharmacological or genetic Cox-2 inhibition can significantly decrease tumor numbers in ApcMin/+ mice [62]. However, we did not find a statistically significant change in Cox-2 expression in RonTK−/− intestinal tissues compared to wild-type controls. While Cox-2 may not be the major molecular target of Ron in this model, it is possible that other factors may modulate inflammation in the gut downstream of Ron that could impact intestinal tumor formation.
In summary, our studies demonstrate that Ron is not required for intestinal tumorigenesis in the ApcMin/+ model. Interestingly, our studies also suggest that while Ron targeted therapies may be effective for the treatment of advance-stage cancers that exhibit overexpression of this receptor [39,40], blocking Ron in normal tissue may have an unfavorable outcome in the context of other alterations that promote tumor formation.
Acknowledgments
Grant information:
ACS RSG CCG-105987 (Goss)
NCI T32 CA 59268 (Meyer)
NIH NCI CA 100002 (Waltz)
The authors thank Drs. Laura James from the Department of Surgery at the University of Cincinnati and Dingcai Cao from the Department of Surgery at the University of Chicago for statistical analyses. We also thank Dr. Gregory Boivin from the Department of Pathology at the University of Cincinnati for histopathological evaluation and Rita Angel from the Department of Pathology at the University of Cincinnati for Ki67 immunohistochemistry.
Abbreviations
- Apc
adenomatous polyposis coli
- Cox2
cyclooxygenase-2
- EGFR
epidermal growth factor receptor
- Fak
focal adhesion kinase
- FAP
familial adenomatous polyposis
- HGFL
hepatocyte growth factor-like protein
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- Min
multiple intestinal neoplasia
- MMP7
matrix metalloproteinase 7
- PCNA
proliferating cell nuclear antigen
- PI3K
phosphatidylinositol-3-kinase
- TK
tyrosine kinase domain
- TUNEL
terminal deoxynucleotidyl transfer mediated dUTP nick end labeling
References
- 1.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 2.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 3.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54(22):5947–5952. [PubMed] [Google Scholar]
- 4.Moser AR, Dove WF, Roth KA, Gordon JI. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992;116(6):1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodir NM, Chen X, Park R, et al. Smad3 Deficiency Promotes Tumorigenesis in the Distal Colon of ApcMin/+ Mice. Cancer Res. 2006;66(17):8430–8438. doi: 10.1158/0008-5472.CAN-06-1437. [DOI] [PubMed] [Google Scholar]
- 6.Suzui M, Okuno M, Tanaka T, Nakagama H, Moriwaki H. Enhanced colon carcinogenesis induced by azoxymethane in min mice occurs via a mechanism independent of beta-catenin mutation. Cancer Lett. 2002;183(1):31–41. doi: 10.1016/s0304-3835(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 7.Sheng H, Shao J, Williams CS, et al. Nuclear translocation of beta-catenin in hereditary and carcinogen-induced intestinal adenomas. Carcinogenesis. 1998;19(4):543–549. doi: 10.1093/carcin/19.4.543. [DOI] [PubMed] [Google Scholar]
- 8.Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC. Reciprocity between membranous and nuclear expression of beta-catenin in colorectal tumours. Virchows Arch. 1997;431(3):167–172. doi: 10.1007/s004280050084. [DOI] [PubMed] [Google Scholar]
- 9.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 10.Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61(23):8357–8360. [PubMed] [Google Scholar]
- 11.Baker SM, Harris AC, Tsao JL, et al. Enhanced intestinal adenomatous polyp formation in Pms2−/−;Min mice. Cancer Res. 1998;58(6):1087–1089. [PubMed] [Google Scholar]
- 12.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997;94(4):1402–1407. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res. 2000;261(1):229–238. doi: 10.1006/excr.2000.5012. [DOI] [PubMed] [Google Scholar]
- 14.Okino T, Egami H, Ohmachi H, et al. Presence of RON receptor tyrosine kinase and its splicing variant in malignant and non-malignant human colonic mucosa. Int J Oncol. 1999;15(4):709–714. doi: 10.3892/ijo.15.4.709. [DOI] [PubMed] [Google Scholar]
- 15.Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol. 2005;12(4):273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Wang MH, Yao HP, Zhou YQ. Oncogenesis of RON receptor tyrosine kinase: a molecular target for malignant epithelial cancers. Acta Pharmacol Sin. 2006;27(6):641–650. doi: 10.1111/j.1745-7254.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22(2):186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 18.Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8(5):1195–1202. [PubMed] [Google Scholar]
- 19.Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155(3):357–371. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 20.Danilkovitch-Miagkova A. Oncogenic signaling pathways activated by RON receptor tyrosine kinase. Curr Cancer Drug Targets. 2003;3(1):31–40. doi: 10.2174/1568009033333745. [DOI] [PubMed] [Google Scholar]
- 21.Xu XM, Wang D, Shen Q, Chen YQ, Wang MH. RNA-mediated gene silencing of the RON receptor tyrosine kinase alters oncogenic phenotypes of human colorectal carcinoma cells. Oncogene. 2004;23(52):8464–8474. doi: 10.1038/sj.onc.1207907. [DOI] [PubMed] [Google Scholar]
- 22.Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage-stimulating protein receptor, the RON receptor tyrosine kinase. Carcinogenesis. 2003;24(8):1291–1300. doi: 10.1093/carcin/bgg089. [DOI] [PubMed] [Google Scholar]
- 23.Zinser GM, Leonis MA, Toney K, et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66(24):11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
- 24.Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65(4):1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]
- 25.Ichii S, Horii A, Nakatsuru S, Furuyama J, Utsunomiya J, Nakamura Y. Inactivation of both APC alleles in an early stage of colon adenomas in a patient with familial adenomatous polyposis (FAP) Hum Mol Genet. 1992;1(6):387–390. doi: 10.1093/hmg/1.6.387. [DOI] [PubMed] [Google Scholar]
- 26.Levy DB, Smith KJ, Beazer-Barclay Y, Hamilton SR, Vogelstein B, Kinzler KW. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994;54(22):5953–5958. [PubMed] [Google Scholar]
- 27.Waltz SE, Eaton L, Toney-Earley K, et al. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest. 2001;108(4):567–576. doi: 10.1172/JCI11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124(3):762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 29.Okino T, Egami H, Ohmachi H, et al. Immunohistochemical analysis of distribution of RON receptor tyrosine kinase in human digestive organs. Dig Dis Sci. 2001;46(2):424–429. doi: 10.1023/a:1005673420464. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Roth KA, Moser AR, Gordon JI. Transgenic mouse models that explore the multistep hypothesis of intestinal neoplasia. J Cell Biol. 1993;123(4):877–893. doi: 10.1083/jcb.123.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen JE, Namork E, Steffensen IL, Eide TJ, Alexander J. Identification and quantification of aberrant crypt foci in the colon of Min mice--a murine model of familial adenomatous polyposis. Scand J Gastroenterol. 2000;35(5):534–539. doi: 10.1080/003655200750023813. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen JE, Steffensen IL, Loberg EM, Husoy T, Namork E, Alexander J. Qualitative and quantitative relationship between dysplastic aberrant crypt foci and tumorigenesis in the Min/+ mouse colon. Cancer Res. 2001;61(13):5010–5015. [PubMed] [Google Scholar]
- 33.Wang D, Shen Q, Xu XM, Chen YQ, Wang MH. Activation of the RON receptor tyrosine kinase attenuates transforming growth factor-beta1-induced apoptotic death and promotes phenotypic changes in mouse intestinal epithelial cells. Carcinogenesis. 2005;26(1):27–36. doi: 10.1093/carcin/bgh284. [DOI] [PubMed] [Google Scholar]
- 34.Leonis MA, Toney-Earley K, Degen SJ, Waltz SE. Deletion of the Ron receptor tyrosine kinase domain in mice provides protection from endotoxin-induced acute liver failure. Hepatology. 2002;36(5):1053–1060. doi: 10.1053/jhep.2002.36822. [DOI] [PubMed] [Google Scholar]
- 35.Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the beta-catenin pathway. Mol Cell Biol. 2001;21(17):5857–5868. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. Embo J. 1996;15(21):5866–5875. [PMC free article] [PubMed] [Google Scholar]
- 37.Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18(18):2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 39.O’Toole JM, Rabenau KE, Burns K, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66(18):9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Kaplan-Lefko PJ, Rex K, et al. Identification of a novel recepteur d’origine nantais/c-met small-molecule kinase inhibitor with antitumor activity in vivo. Cancer Res. 2008;68(16):6680–6687. doi: 10.1158/0008-5472.CAN-07-6782. [DOI] [PubMed] [Google Scholar]
- 41.Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur J Cancer. 1995;31A(7–8):1061–1064. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 42.Kartheuser A, West S, Walon C, et al. The genetic background of familial adenomatous polyposis. Linkage analysis, the APC gene identification and mutation screening. Acta Gastroenterol Belg. 1995;58(5–6):433–451. [PubMed] [Google Scholar]
- 43.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 44.Boon EM, Pouwels W, Redeker S, et al. Activation of Wnt signaling in the intestinal mucosa of Apc +/min mice does not cause overexpression of the receptor tyrosine kinase Met. Cancer Sci. 2006;97(8):710–715. doi: 10.1111/j.1349-7006.2006.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonis MA, Thobe MN, Waltz SE. Ron-receptor tyrosine kinase in tumorigenesis and metastasis. Future Oncol. 2007;3(4):441–448. doi: 10.2217/14796694.3.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213(4):402–411. doi: 10.1002/path.2245. [DOI] [PubMed] [Google Scholar]
- 47.Chan EL, Peace BE, Collins MH, Toney-Earley K, Waltz SE. Ron tyrosine kinase receptor regulates papilloma growth and malignant conversion in a murine model of skin carcinogenesis. Oncogene. 2005;24(3):479–488. doi: 10.1038/sj.onc.1208231. [DOI] [PubMed] [Google Scholar]
- 48.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28(2):155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 49.Goss KH, Risinger MA, Kordich JJ, et al. Enhanced tumor formation in mice heterozygous for Blm mutation. Science. 2002;297(5589):2051–2053. doi: 10.1126/science.1074340. [DOI] [PubMed] [Google Scholar]
- 50.Goss KJ, Brown PD, Matrisian LM. Differing effects of endogenous and synthetic inhibitors of metalloproteinases on intestinal tumorigenesis. Int J Cancer. 1998;78(5):629–635. doi: 10.1002/(sici)1097-0215(19981123)78:5<629::aid-ijc17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Silverman KA, Koratkar R, Siracusa LD, Buchberg AM. Identification of the modifier of Min 2 (Mom2) locus, a new mutation that influences Apc-induced intestinal neoplasia. Genome Res. 2002;12(1):88–97. doi: 10.1101/gr.206002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gould KA, Dove WF. Analysis of the Mom1 modifier of intestinal neoplasia in mice. Exp Lung Res. 1998;24(4):437–453. doi: 10.3109/01902149809087379. [DOI] [PubMed] [Google Scholar]
- 53.Kwong LN, Shedlovsky A, Biehl BS, Clipson L, Pasch CA, Dove WF. Identification of Mom7, a novel modifier of Apc(Min/+) on mouse chromosome 18. Genetics. 2007;176(2):1237–1244. doi: 10.1534/genetics.107.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas RM, Toney K, Fenoglio-Preiser C, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67(13):6075–6082. doi: 10.1158/0008-5472.CAN-06-4128. [DOI] [PubMed] [Google Scholar]
- 55.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 56.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 57.Correll PH, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct. 1997;1(1):69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhou YQ, Chen YQ, Fisher JH, Wang MH. Activation of the RON receptor tyrosine kinase by macrophage-stimulating protein inhibits inducible cyclooxygenase-2 expression in murine macrophages. J Biol Chem. 2002;277(41):38104–38110. doi: 10.1074/jbc.M206167200. [DOI] [PubMed] [Google Scholar]
- 59.Hull MA, Booth JK, Tisbury A, et al. Cyclooxygenase 2 is up-regulated and localized to macrophages in the intestine of Min mice. Br J Cancer. 1999;79(9–10):1399–1405. doi: 10.1038/sj.bjc.6690224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen LC, Hao CY, Chiu YS, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64(10):3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 61.Williams CS, Luongo C, Radhika A, et al. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111(4):1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 62.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]