Abstract
Forodesine was originally developed for T-cell leukemias and was effective in T-cell acute lymphoblastic leukemia (T-ALL). The current study was done to test its utility in B-cell ALL (B-ALL). Our preclinical investigations (lymphoblasts from pediatric patients with B-ALL [n=12]) demonstrate activity in vitro. Minimal activity in the clinic suggests that this agent should be used in combination with other established or novel ALL agents.
Background
The discovery that purine nucleoside phosphorylase (PNP) deficiency leads to T-cell lymphopenia was the basis for introducing PNP inhibitors for T-cell leukemias. Forodesine is an orally bioavailable PNP inhibitor with picomolar potency. Because T lymphoblasts and indolent chronic lymphocytic leukemia (CLL) B cells inherently elicit favorable pharmacokinetics to accumulate deoxyguanosine triphosphate (dGTP), forodesine demonstrated promising activity in preclinical and clinical settings for patients with T-cell acute lymphoblastic leukemia (T-ALL) and B-cell CLL (B-CLL). However, the use of forodesine in B-cell ALL (B-ALL) is unknown.
Patients and Methods
Leukemic blasts obtained from pediatric patients with de novo B-ALL (n=10) were incubated with forodesine and deoxyguanosine (dGuo), and the biological end points of apoptosis, intracellular dGTP accumulation, and inhibition of RNA and DNA synthesis were measured. Additionally, adult patients with B-ALL (n= 2) were intravenously infused with 80 mg/m2/d daily for 5 days. After therapy, clinical response, toxicity, laboratory biomarkers including PNP enzyme inhibition, and plasma forodesine, dGuo, and intracellular dGTP levels were analyzed.
Results
Our in vitro investigations demonstrated that forodesine treatment inhibited proliferation and induced modest apoptosis in de novo B-ALL lymphoblasts. There was time-dependent accumulation of dGTP and inhibition of RNA and DNA synthesis. During therapy, neither patient achieved a complete response (CR), but there was disease stabilization for several weeks in both patients. There was significant maintained inhibition of PNP enzyme in red blood cells, accumulation of forodesine and dGuo in plasma, and intracellular dGTP accumulation in both patients.
Conclusion
Our preclinical and clinical investigations suggest that forodesine has activity in B-ALL. However, it needs to be either infused with dGuo or combined with established chemotherapeutic agents based on mechanistic rationale.
Keywords: Apoptosis, dGTP, Forodesine, B-cell acute lymphoblastic leukemia
Introduction
The discovery of purine nucleoside phosphorylase (PNP) deficiency, a metabolic disorder that results in congenital immunodeficiency in association with severe T-cell immune depletion, and the elucidation of its pathophysiologic characteristics provided the rationale for the development of deoxyguanosine (dGuo) analogs for leukemias.1,2 Because dGuo is readily catabolized by PNP, phar-macologic inhibition of this enzyme manifests a deoxyguanosine triphosphate (dGTP)-mediated T-cell lymphopenia. Although T-cell–specific depletion was observed with dGuo, the efficient catabolism of dGuo by PNP limited its use clinically. Taken together, these data provided a rationale for the development of PNP-resistant dGuo analogs or PNP inhibitors for the treatment of leukemias.
The PNP-resistant dGuo derivative arabinosylguanine (ara-G) is toxic to T lymphoblasts and resistant to degradation by endogenous PNP.3 Once within cells, ara-G is phosphorylated by deoxycytidine (dCyd) kinase and deoxyguanosine kinase4,5 into ara-GTP. The resultant accumulation of intracellular ara-GTP inhibits DNA synthesis.6 Similar to dGuo, ara-G has shown antileukemic activity in T-lymphoblastic cell lines and in the clinic3,7,8 through a T-lymphoid lineage-specific accumulation of ara-GTP during therapy.9,10 A clinical trial conducted by the Childrens Cancer Group and the Pediatric Oncology Group of nelarabine, a prodrug of ara-G, in refractory T-cell leukemias and lymphomas11 demonstrated higher responses in T-ALL. Additionally, a phase II trial in patients with refractory T-cell malignancies demonstrated substantial single-agent activity, with an objective response rate of more than 50% in the subset of patients with T-cell leukemia.8 Of note, some activity was also seen in patients with B-lineage disease.
Forodesine (also known as BCX-1777/immucillin H) was developed as an orally bioavailable novel PNP transition-state inhibitor with a low picomolar inhibitory constant for human enzymes.12 The principal requisite for this agent to demonstrate its action relies on complete inhibition (> 95%) of the PNP enzyme and subsequent accumulation of dGTP, the intracellular metabolite of substrate dGuo. Forodesine exhibited cytotoxicity in T-cell leukemia cell lines through accumulation of large amounts of intracellular dGTP perturbing the cellular milieu.13 This in vitro activity in cell lines provided the impetus to initiate a clinical study in patients with T-cell leukemia to investigate the pharmacokinetic and pharmacodynamic profile of this inhibitor. In agreement with its preclinical activity, forodesine demonstrated promising activity in patients with T-cell leukemia through accumulation of dGuo in plasma and dGTP in leukemia cells.14 Forodesine is also effective against indolent B- and T-cell diseases (both in vitro15,16 and in clinical trials),17,18 as well as in peripheral T-cell lymphoma.19,20
With this background, we proposed to test forodesine in B-ALL based on the following rationale. First, akin to T cells, inhibition of PNP promotes accumulation of dGTP, subsequent imbalance in the deoxyribonucleotide triphosphates (dNTPs), and eventually cell death in B cells.21 Second, T-cell therapies such as nelarabine have demonstrated clinical activity in B-cell leukemias.22 Third, B cells have high levels of deoxycytidine kinase (dCK), the enzyme that facilitates the accumulation of dGTP.21 Fourth, the rate-limiting enzyme dCK that converts dGuo to dGTP is present in high levels in pediatric lymphoblasts.23 Finally, forodesine has demonstrated some activity in adult patients with relapsed or refractory B-ALL.18
The goal of the present study was to evaluate the efficacy of forodesine in B-ALL. Given that B cells contain high levels of dCK, we hypothesized that this agent could sensitize B-ALL lymphoblasts to apoptosis. Our preclinical studies with 12 pediatric patients with de novo acute leukemia (most had B-ALL [10 patients] with 1 each T-ALL and acute myelogenous leukemia [AML]) and extended studies on clinical evaluation of adult patients with B-ALL (n = 2) provide the rationale for utility of this agent in B-cell leukemias.
Patients and Methods
Drug and Other Chemicals
Forodesine for clinical use was provided by BioCryst Pharmaceuticals (Birmingham, AL). For quantitation of deoxynucleotides, dNTPs were obtained from Amersham/GE Healthcare Life Sciences (Pittsburgh, PA) and were used as standards. [3H]deoxyadenosine triphosphate (dATP) and [3H]deoxythymidine triphosphate were purchased from PerkinElmer Life Sciences (Waltham, MA) and MP Biomedicals (Santa Ana, CA), respectively.
Patients
This project consists of reports from 2 studies: in vitro investigations in blasts from de novo pediatric acute leukemia with forodesine (n=12) and pharmacodynamic and pharmacokinetic evaluations of forodesine in adult patients with B-ALL (n=2) during therapy. Among pediatric patients, the majority were patients with active B-ALL (B-ALL [n=10]; acute myelogenous leukemia [AML] [n=1]; acute T-cell lymphoblastic leukemia [T-ALL] [n=1]).
Collection and Isolation of Lymphoblasts from Peripheral Blood Obtained from Pediatric Patients With B-ALL
Whole blood or bone marrow was collected in heparinized tubes from pediatric patients with leukemia at initial diagnosis. The specimen was diluted 1:3 with cold phosphate-buffered saline (PBS) (0.135 M NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4 [pH 7.4]) and layered onto Ficoll-Hypaque (specific gravity, 1.086; Life Technologies, Grand Island, NY). The blood was then centrifuged at 433g for 20 minutes, and mononuclear cells were removed from the interphase. Cells were washed twice with cold PBS and resuspended in 10 mL RPMI 1640 (Life Technologies), supplemented with 10% fetal bovine serum. The isolated lymphoblasts were incubated with or without 2 µM forodesine and 20 µM dGuo in a culture incubator at 37°C. These concentrations were selected based on plasma pharmacologic data obtained during a phase I study of forodesine.8 Cultures were maintained and aliquots (1×107 cells/mL) were removed at the end of incubation times. A Coulter channelyzer (Coulter Electronics) was used to determine cell number and mean cell volume. These cells were measured for intracellular dNTP, macromolecule syntheses, and apoptosis levels.
Assays
Measurement of Cell Apoptosis in Response to Forodesine and dGuo
The lymphoblasts were incubated with or without 2 µM forodesine and 20 µM dGuo. Cultures were maintained and aliquots (1×107 cells/mL) were removed at the end of incubation times (24 or 48 hours). Percent apoptosis was measured by annexin V binding assay (PharMingen, San Diego, CA) according to the manufacturer’s instructions. Briefly, fresh cells were washed with PBS and resuspended in 200 µL of 1×annexin binding buffer obtained from BD Biosciences (San Jose, CA), at a concentration of 1×10 cells/mL. Annexin V—fluorescein isothiocyanate (5 µL) was added, and the cells were incubated in the dark for 15 minutes at room temperature. Ten microliters (50 µg/mL) propidium iodide was then added to the labeled cells and analyzed immediately with a FACSCalibur cytometer (BD Biosciences). Data from at least 10,000 events per sample were recorded and processed using Cell Quest software (BD Biosciences).
Measurement of Global DNA and RNA syntheses
The lymphoblasts were incubated with or without 2 µM forodesine and 20 µM dGuo for indicated time points (24 or 48 hours), and the global DNA and RNA syntheses were measured by [3H] thymidine and [3H]uridine incorporation assays. Before removal of the aliquot, 10 µCi/mL [3H]thymidine or [3H]uridine was added to these cultures, and the incubation was continued for an additional 30 minutes. The labeled cells were then extracted using perchloric acid, and the pellets were incubated overnight with KOH at 37° C to dissolve DNA and RNA, and the radioactivity was measured by scintillation counting. [3H]thymidine and [3H]uridine were purchased from Moravek Biochemicals (Brea, CA).
Measurement of PNP Inhibition During Therapy
PNP inhibition was measured by spectrophotometric assay. Erythrocytes were separated from whole blood and PNP was extracted from red blood cells by cell hemolysis. Addition of the substrate inosine results in conversion of inosine to hypoxanthine by PNP. Hypoxanthine is catalyzed by xanthine oxidase to form a stable product, uric acid. The accumulation of uric acid is quantitatively determined by measuring the increase in absorbance at a wavelength of 293 nm. The observed data are then normalized by measuring the absorbance at a wavelength of 405 nm, which represents the number of erythrocytes used to prepare the PNP extract. The inhibitory effect was calculated using the equation [1 —(PNP activity post dose/PNP activity pre dose)] × 100.
Measurement of Plasma dGuo and Forodesine During Therapy
Blood samples (10 mL) before and after treatment at indicated days were obtained in green-stopper Vacutainer tubes containing heparin and 50 µM BCX-3424 (BioCryst Pharmaceuticals) as an internal control. The plasma was removed after centrifugation and stored at —70°C and analyzed at BioCryst Pharmaceuticals. Plasma dGuo and forodesine were analyzed as described previously14 using high-performance liquid chromatography with tandem mass spectrometry detection. Briefly, BCX-1777 was extracted from plasma using a phenylboronic acid affinity solid-phase extraction cartridge and dGuo was extracted from plasma using a Waters Oasis HLB affinity solid-phase extraction cartridge (Waters Corp, Milford, MA). The mass of BCX-1777 plus H+ (267.1 m/z) and the mass of dGuo plus H+ (268.1 m/z) are monitored in quadrupole 1. The BCX-1777 product ion 148.0 m/z and the dGuo product ion 157.0 m/z are monitored in quadrupole 3. The concentrations of forodesine and dGuo were determined by weighted (1/x) quadratic regression analysis of peak areas produced from the standard curve.
Measurement of Intracellular dNTP Pool
Cells incubated in vitro with dGuo and forodesine were collected at different time points (24 or 48 hours at 37°C). Similarly, blood samples were obtained from patients at different time points during treatment with forodesine, and leukemic blasts were isolated. The nucleotides in the blast cells were extracted by 60% methanol as described previously, and the dNTPs were quantitated in these cell extracts by DNA polymerase assay as described by Sherman and Fyfe.25
Clinical Trial Conducted in Adult Patients with B-ALL
Patient Eligibility
Patients were eligible to participate if they had failed at least 1 previous treatment regimen for B-ALL. Patients were required to have an Eastern Cooperative Oncology Group performance status of 2 or better, a life expectancy of at least 3 months, adequate organ function with aspartate transaminase and alanine transaminase levels µ≤3 times the upper limit of normal, and calculated creatinine clearance > 40 mL/min. Female patients had to have a negative serum or urine pregnancy test within 2 to 7 days before the start of the study, and all patients had to be free of active or uncontrolled infection, including infection with human immunodeficiency virus or hepatitis viruses.
Collection of Blood Samples
Patients signed an informed consent approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center before participation in the study. Blood samples were collected on the pretreatment day and on days 1 through 4 on cycles 1 to 3, and pharmacokinetic and pharmacodynamic parameters were determined.
Clinical Trial
The clinical trial was a phase I/II open-label multicenter study of forodesine hydrochloride in patients with relapsed and refractory B-cell ALL, with the primary end point of assessing the safety of repeated intravenous doses of forodesine hydrochloride. Secondary end points included assessment of the efficacy of the drug in this disease, as well as evaluation of the steady-state pharmacokinetics and correlating pharmacodynamic parameters with any potential response. Patients were to receive 4 weeks of treatment with 5 days of intravenous dosing each week. Patients having clinical response (defined as achieving complete response [CR], CR without platelet recovery, partial response, or stable disease) could receive an additional 4 weeks of treatment with forodesine. Patients were evaluated for grades 3 and 4 non-hematologic toxicity and grade 4 hematologic toxicity. Forodesine treatment would be delayed or dosage reduced depending on the recovery from toxicity.
Statistical Analysis
All data were graphed using GraphPad Prism (GraphPad Software, San Diego, CA). Statistical significance (a value of .05) was determined by the 2-tailed paired Student t test.
Results
In Vitro Study of Forodesine in Pediatric B-ALL Lymphoblasts
Forodesine in Presence of Exogenous dGuo-Induced Cytotoxicity in Pediatric B-ALL Lymphoblasts
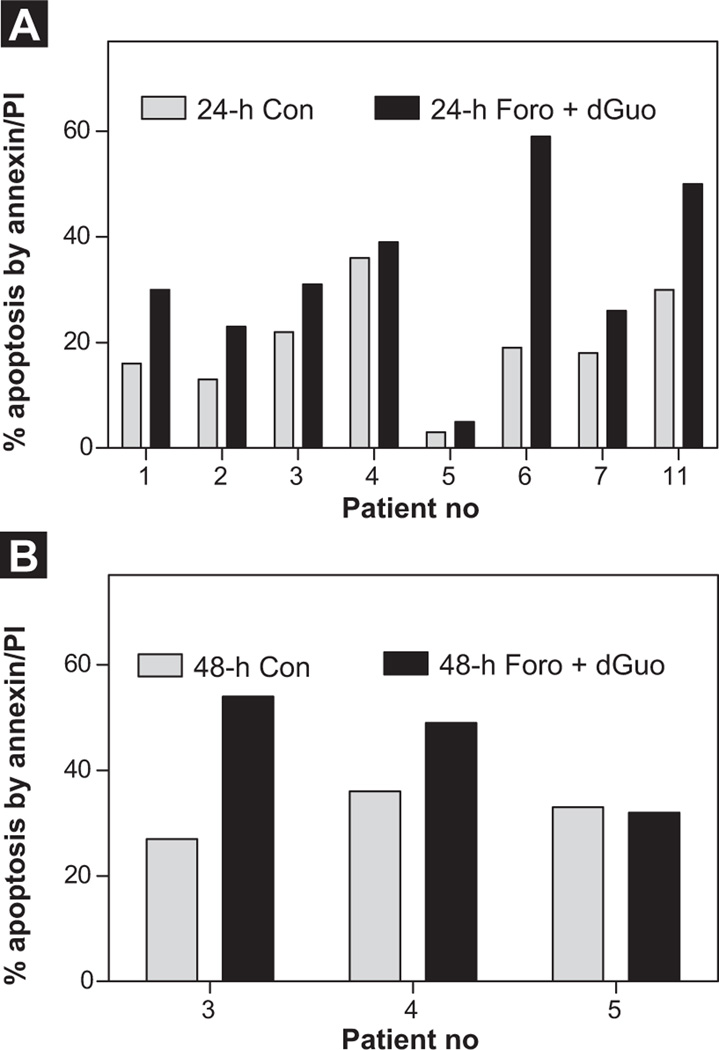
Characteristics of patients enrolled in the in vitro study are provided in Table 1 (pediatric B-ALL [n = 10]; T-ALL [n = 1]; AML [n = 1]; adult B-ALL [n = 2; No. 9001, No. 9002]). When measured by annexin/propidium iodide binding assay (Figure 1), in samples from 8 patients tested, there was heterogeneity in response to forodesine ranging from 2% to 40% apoptosis (normalized to time-matched control), with median 10% apoptosis. Statistical analysis demonstrated significant induction of apoptosis in these cells compared with untreated controls (Figure 1A, Figure 24 hours; P = .018; Figure 1B, Figure 48 hours; P = .041). There was no significant amount of apoptosis in the sample from patient 3, a patient with AML, compared with other patients with B-ALL. We do not have cytotoxicity data for patient 8, a patient with T-ALL.
Table 1.
Patient Characteristics and Forodesine and dGuo-lnduced Apoptosis
| % Viabilitya | ||||||
|---|---|---|---|---|---|---|
| Patient No. | Age (years) | Sex | Diagnosis | WBC 103/µL | Untreated | Treated |
| 1 | 11 | M | Pre–B-ALL | 10 | 84 | 70 |
| 2 | 11 | F | Pre–B-ALL | 2.4 | 87 | 77 |
| 3 | 2 | M | AML | 28.9 | 78 | 69 |
| 4 | 19 | M | Pre–B-ALL | 2.5 | 64 | 61 |
| 5 | 15 | M | Pre–B-ALL | 0.6 | 97 | 95 |
| 6 | 21 | M | Pre–B-ALL | 75.9 | 81 | 41 |
| 7 | 5 | M | Pre–B-ALL | 11.1 | 82 | 74 |
| 8 | 25 | M | T-ALL | 14.1 | – | – |
| 9 | 7 mo | M | Pre–B-ALL | 34.7 | – | – |
| 10 | 19 | M | Pre–B-ALL | 26 | – | – |
| 11 | 1 | M | Pre–B-ALL | 207 | 70 | 50 |
| 12 | 15 | M | Pre–B-ALL | 116 | – | – |
Two adult patients with B-ALL for clinical and pharmacology study on forodesine at a dose of 80 mg/m2/day (30 min infusion daily x 5).
Patient 1 = No. 9001.
Patient 2 = No. 9002.
Abbreviations: B-ALL = B-cell acute lymphoblastic leukemia; WBC = white blood cell.
Leukemia blasts were incubated in vitro without (untreated) or with 2 µM forodesine and 20 µM dGuo and % viability was measured by annexin/propidium iodide binding assay.
Figure 1.
Forodesine (Foro) in Presence of Deoxyguanosine (dGuo) Induces Apoptosis in Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL) Lymphoblasts. Leukemia Blast Cells Isolated From Peripheral Blood Were Incubated With Forodesine (2 µM) and dGuo (20 µM) for 24 Hours (n = 8) (A) or 48 Hours (n = 3) (B). The Percent Apoptosis was Measured by Annexin/ Propidium Iodide (PI) Binding Assay. Gray Bars Denote the Time-Matched Controls and Black Bars Denote Cells Incubated With Forodesine and dGuo for Respective Time Points. The P value is Obtained From Paired 2-TailedtTest
Abbreviation: Con = control.
Forodesine in the Presence of dGuo-Enhanced Accumulation of dGTP in Pediatric B-ALL Lymphoblasts
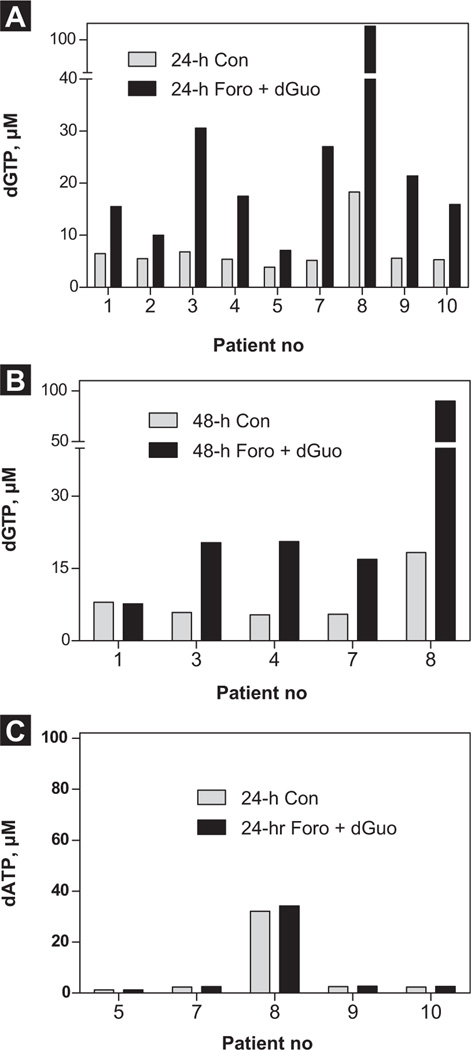
There was significant accumulation of dGTP in these cells, ranging between 3 and 90 µM, with a median 16 µM (24 hours; P = .045; n = 9; Figure 2A) after forodesine and dGuo treatment compared with time-matched controls (Figure 2B; Figure 48 hours). In particular, patient 8, who was diagnosed with T-ALL, showed 3-fold higher accumulation of dGTP levels compared with patients with B-ALL. When measured for dATP levels, there was no difference in accumulation between controls and treated samples (Figure 2C), which is consistent with our previous studies in T-cell leukemias.
Figure 2.
Incubation With Forodesine (Foro) and Deoxyguanosine (dGuo) Promoted the Accumulation of Deoxyguanosine Triphosphate (dGTP) but not [3H] Deoxyadenosine Triphosphate (dATP) in Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL) Lymphoblasts. Leukemic Blast Cells Isolated From Peripheral Blood Were Incubated With Forodesine (2 µM) and dGuo (20 µM) for 24 Hours (n = 9) (A) or for 48 Hours (n = 5) (B). The Cells Were Extracted With 60% Methanol, and the Intracellular Accumulation of dGTP (A and B) and dATP (n = 5; 24 hours) (C) was Measured by DNA Polymerase Assay as Described in the Patients and Methods Section
Abbreviation: Con = control.
Forodesine in the Presence of dGuo-Inhibited Global RNA and DNA Synthesis in Pediatric B-ALL Lymphoblasts
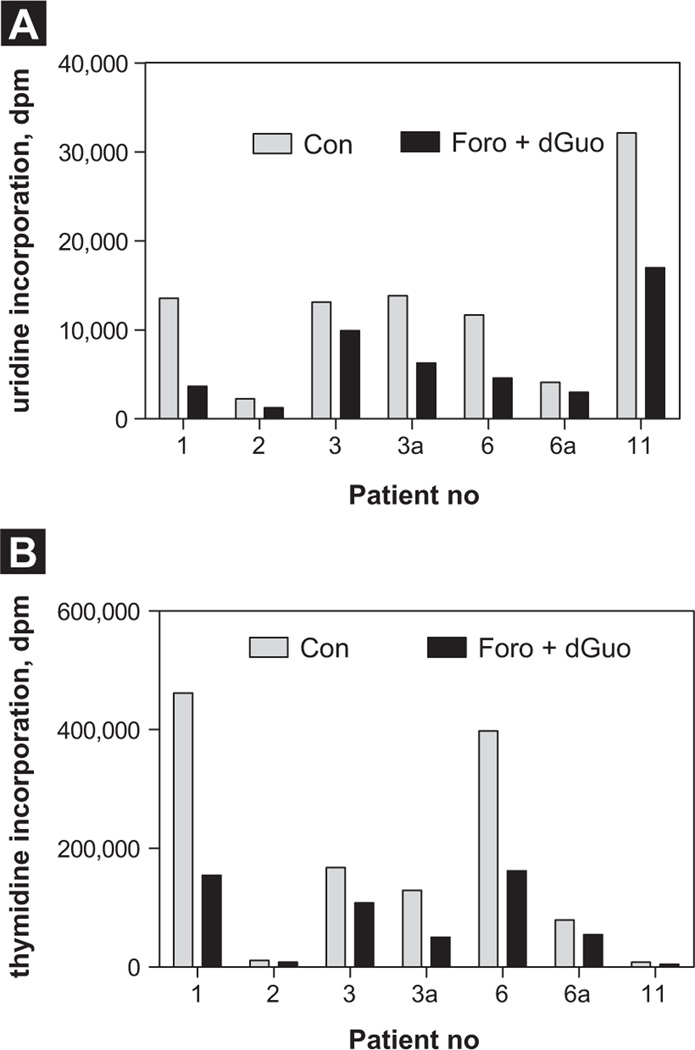
In general, cancer cells, including leukemic cells, are known to sustain their function through active DNA, RNA, and protein synthesis. To test if for-odesine can potentially inhibit these macromolecule syntheses in these lymphoblasts as a result of perturbation of the dGTP pool, we performed [3H]uridine and [3H] thymidine incorporation assays to measure their levels of inhibition. Although samples varied in response to forodesine, there was significant inhibition of RNA synthesis at 24 hours (Figure 3A; n = 5; P = .015). For DNA synthesis, there was inhibition observed in 3 of 5 patients; however, the statistical analysis by 2-tailed t test did not show significance (Figure 3B; P = .0675). Samples 3a and 6a were collected at 48 hours for these patients. Of note, the values given are absolute disintegrations per minute values, and one can observe the variations in the basal levels of macromolecule synthesis between different patient samples.
Figure 3. Forodesine in Presence of Deoxyguanosine (dGuo) Inhibits Macromolecule Syntheses in Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL) Lymphoblasts. Leukemic Blast Cells Isolated From Peripheral Blood Were Incubated With Forodesine (2 mM) and dGuo (20 µM) at 24 Hours (A). Global RNA Synthesis (n = 5) and DNA Synthesis (B) Were Measured by [3H]uridine and [3H]thymidine Incorporation Assays, Respectively. Samples 3a and 6a Were Collected at 48 Hours for These Patients, Respectively. Given are the Absolute Disintegrations per Minute Values for Time-Matched Controls and Treated Samples.
Abbreviation: Con = control.
Clinical Response in Adult Patients With B-ALL During Therapy
Two patients were treated with forodesine at 80 mg/m2/day daily for 5 days through 3 cycles. The study was closed early for slow accrual, and as such no further patients with B-ALL were treated. The first patient, a 61-year-old woman with precursor B-ALL, received initial therapy with the hyperCVAD (cyclophosphamide, vincristine [Oncovin], doxorubicin [Adriamycin], dexamethasone) regimen plus rituximab and achieved a CR after 2 courses of treatment but had disease relapse within 5 months. She went on to receive multiple salvage regimens, including augmented hyper-CVAD, liposomal vincristine, liposomal annamycin, combination high-dose cytarabine and methotrexate with alemtuzumab and rituximab, without achieving a response. She was then enrolled in the study and received 3 cycles of forodesine, with disease progression on day 8 of the third cycle, and treatment was discontinued. Five days later, she was hospitalized for progressive shortness of breath and right-sided pleuritic pain and died of the complications of her disease and infection.
The second patient was a 23-year-old woman with precursor B-ALL and t(4;11) who achieved CR after receiving 2 courses of the hyperCVAD regimen. She then underwent allogeneic stem cell transplantation from an HIA-matched sibling after a preparative regimen of total body irradiation, etoposide, and rituximab. Her disease relapsed after transplantation and she received salvage therapy with liposomal vincristine and dexamethasone without a response. She completed 1 cycle of forodesine but experienced intracranial hemorrhage shortly after the initiation of cycle 2 and died of the associated complications. In summary, neither patient achieved a CR, but there was disease stabilization for several weeks in both patients.
Inhibition of PNP Enzyme in Red Blood Cells During Therapy in Adult Patients With B-ALL
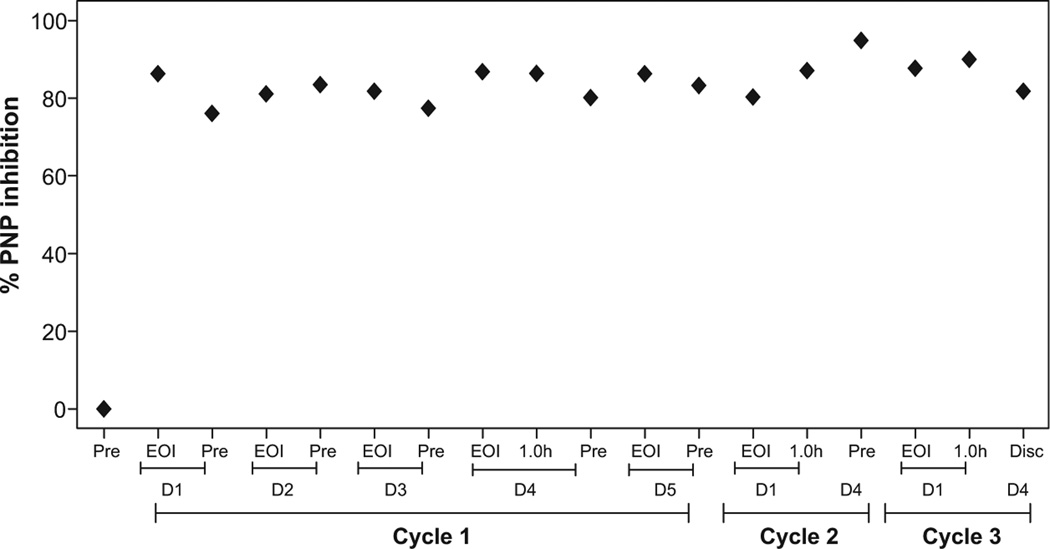
The pretreatment and end of infusion samples were collected between days 1 and 5 of week 1 and on days 1 and 4 of week 2 and week 3. The red blood cells were isolated from whole blood, and the cells were processed to determine percent PNP inhibition in these samples. The inhibition of PNP for patient No. 9001 before therapy was 0% and increased to a median of 84% of inhibition (range 76%–95%) (Figure 4). Because accumulation of dGTP is promoted by PNP inhibition, > 90% PNP inhibition is required for this agent to be efficacious. Assessment of PNP inhibition for patient No. 9002 was not performed.
Figure 4.
Measurement of Inhibition of Purine Nucleoside Phosphorylase (PNP) Enzyme in Red Blood Cells During Therapy in Adult I Patients With B-Cell Acute Lymphoblastic Leukemia (B-ALL). Red blood Cells From Adult Patients With B-Cell Acute Lymphoblastic Leukemia (B-ALL) (n = 2) Were Isolated From Whole Blood Starting Days 0 to 6 of Week 1 Until Days 1 to 4 of I Week 3, and the Cells Were Processed to Determine Level of PNP and its Inhibition After Start of Therapy, as Described in I Patients and Methods Section. The Average Specific Activity of PNP was 3.7 mU/min, Where 1 mU is Defined as Amount of PNP Crude Lysate That Catalyzes the Phosphorolysis of 1 µmol of Inosine per Minute Under Standard Assay Conditions
Abbreviations: D = day; Disc = discontinued; EOI = end of infusion; Pre = pre-treatment.
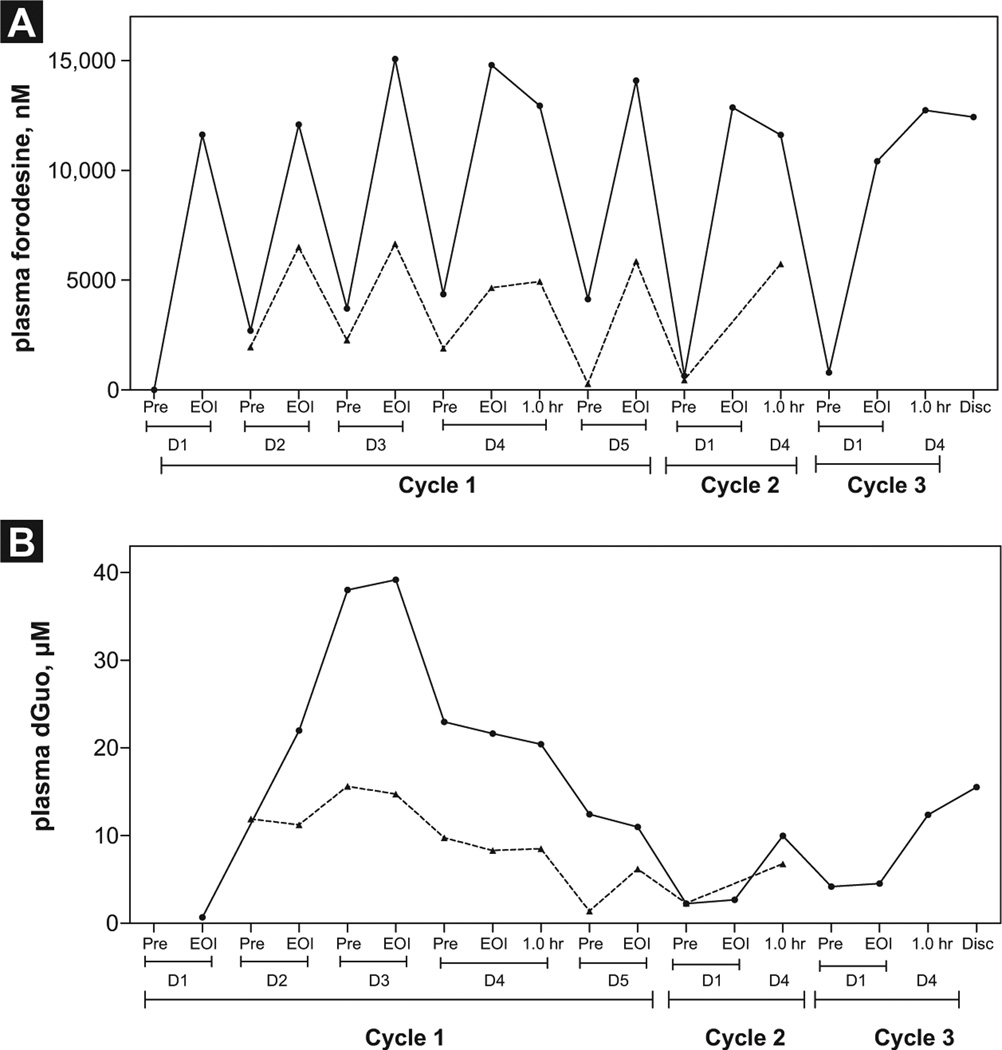
Measurement of Plasma Forodesine and dGuo Levels During Therapy in Adult Patients With B-ALL
To determine if forodesine was at steady-state level, we next measured the plasma forodesine levels during therapy in these 2 patients. Forodesine levels were quantitated on day 0 and at the end of infusion between days 1 and 5 of week 1 and on days 1 and 4 of week 2 and week 3 (n = 2). For patient No. 9001 (Figure 5A; solid line), on day 0 the forodesine level was 0 µM and increased to 12 µM on day 1. By day 2, the level dropped to 2 µM before infusion and increased to 12 µM at the end of infusion. The pattern was very similar between pretreatment and end of infusion. For patient No. 9002 (Figure 5A; dashed line) the pattern was similar; however, the levels were almost half that of the other patient. The levels increased to 6 µM (range 4.7–6.7 µM) and dropped to 1 µM (range 0.21–2.3 µM) after treatment. These forodesine levels were comparatively higher than those in the samples of the patient with B-CLL, in which the maximum was 1.3 µM.17
Figure 5.
Measurement of Plasma Forodesine and Deoxyguanosine (dGuo) Levels During Forodesine Therapy in Adult Patients With B-Cell Acute Lymphoblastic Leukemia (B-ALL). Whole Blood was Collected From Adult Patients With B-ALL at Indicated Time Points During Therapy, and Plasma was Separated. Plasma Forodesine Levels (A) and Plasma dGuo Levels (B) in Each nt Sample Were Determined Using Tandem Mass Spectrometry Liquid Chromatography as Described Under Patients and Methods Section (No. 9001, Solid Line; No. 9002, Dashed Line)
Abbreviations: D = day; Disc = discontinued; EOI = end of infusion; Pre = pre-treatment.
With these concentrations of plasma forodesine and levels of PNP inhibition, an increase in endogenous dGuo was expected. The level of dGuo in pretreatment samples for patient No. 9001 was less than the level of detection and increased to 22 µM and then further to 38 µM and was maintained at 12 µM on day 4 of week 3 (Figure 5B; solid line). For patient No. 9002, the levels were again lower, ranging from 7 to 12 µM (Figure 5B; dashed line).
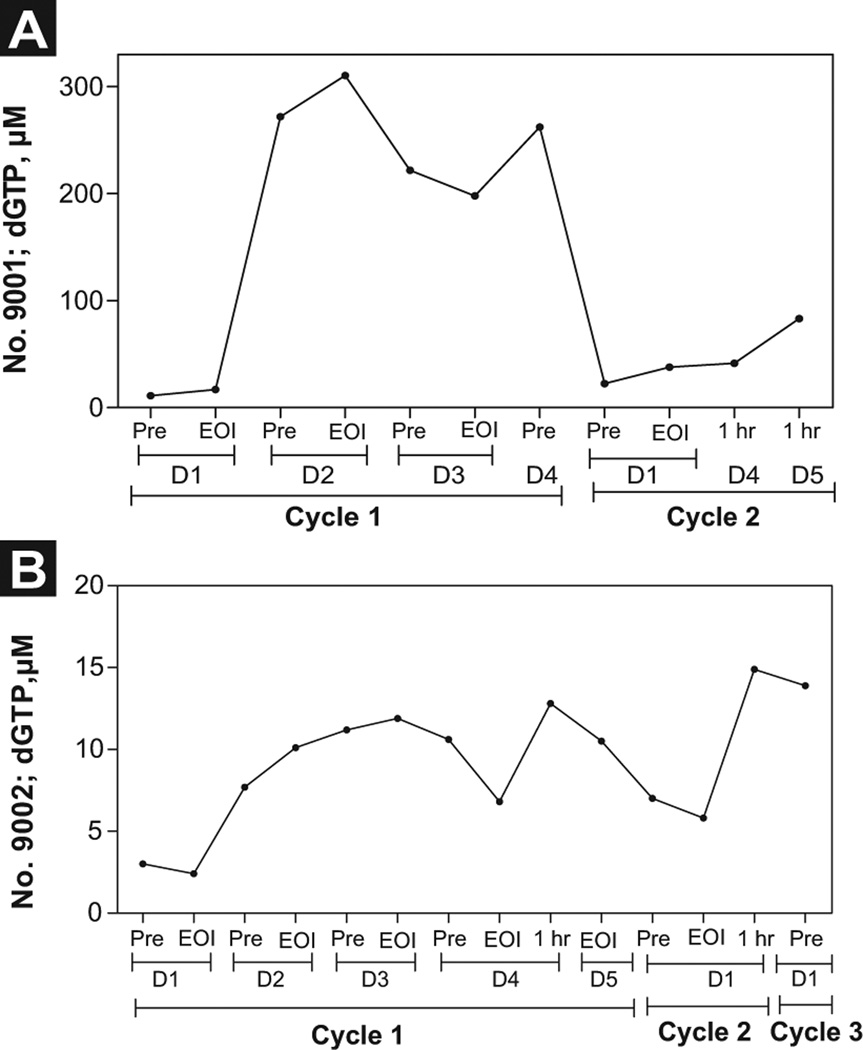
Cellular Pharmacology During Therapy
The starting level of intracellular dGTP for patient No. 9001 on day 0 of cycle 1 was 11 µM and increased to 18 µM by the end of infusion. On day 2, there was an increase to 270 µM, with further elevation to 310 µM at the end of infusion. This level was maintained until day 4 of cycle 1. By cycle 3, the levels dropped to 37 µM and were maintained through day 4 (Figure 6A). For patient No. 9002, the intracellular dGTP levels were 10-fold less, with a maximum level of 15 µM (Figure 6B), suggesting heterogeneity in the accumulation of dGTP between patients.
Figure 6.
Measurement of Accumulation of Deoxyribonucleotide Triphosphates (dNTPs) in Adult B-Cell Acute Lymphoblastic Leukemia (B-ALL) Lymphoblasts During Forodesine Therapy. Leukemia Blast Cells Were Isolated From Peripheral Blood of Adult Patients With B-ALL at Indicated Time Points During Forodesine Therapy, and dGTP Accumulation (Patient No. 9001, [A]; Patient No. 9002 [B]) was Measured by DNA Polymerase Assay as Described Previously
Abbreviations: EOI = end of infusion; Pre = pre-treatment.
Discussion
ALL is the most common malignancy of childhood. Although the cure rates for childhood ALL are currently about 80%,26–29 the outcome of relapsed ALL remains dismal, with an overall survival of only 10% for patients with early bone marrow relapse or a second or subsequent relapse.30
Allogeneic hematopoietic stem cell transplantation (HSCT) remains the mainstay of treatment for relapsed ALL. However, recurrence rates are as high as 90% for patients with morphologic evidence of residual disease going into HSCT. 31–33 In addition, higher levels of pretransplantation minimal residual disease have been shown to predict relapse after HSCT. 34–36 To that end, novel agents with the ability to induce a minimal residual disease—negative remission are needed.
Forodesine may also play an important role in the setting of recurrent T-cell ALL now that nelarabine is moving to frontline regimens for those with high-risk disease. However, the role of forodesine in B-cell ALL is not clear. Preclinical investigations in primary T-ALL and B-ALL cells from pediatric patients have demonstrated activity of forodesine and dGuo in B lymphoblasts.21 The lower activity in B-ALL was associated with less dGTP accumulation, which may result from a lower level of expression of nucleoside transporters in B cells and a higher level of 5’-nucleotidase.
In contrast to other nucleoside analogs, dGuo, as well as ara-G, are phosphorylated by dGuo kinase as well as dCyd kinase. Furthermore, both dGuo and ara-G are preferred substrates for dGuo kinase compared with dCyd kinase. At the mRNA level, both these kinases are present at similar levels in T lymphoblasts and B lymphoblasts from children. However, levels of proteins and the activity of enzymes will also affect the level of accumulation of triphosphate. This may explain heterogeneity among patients to accumulate dGTP after 24 and 48 hours of incubation with for-odesine and dGuo (Figure 2 A and B).
Although there was only a modest effect on cell death of B-leukemic lymphoblasts (Figure 1), there was inhibition of DNA synthesis, suggesting there could be an impact on the cell proliferative index (Figure 3B). Similar results were observed in a B-lymphoblastic cell line with ara-G,3 in which some S-phase cells were killed but then cells were blocked in S-phase of the cell cycle. This is in contrast to T cells, in which a majority of cells in S phase were killed; this was followed by cell death in other phases of the cell cycle by activation of Fas-mediated apoptosis. Partial inhibition of proliferation with limited apoptosis has also been observed in myeloma cell lines— another B-cell neoplasm albeit mature plasma B cells.37
Forodesine is a potent inhibitor of PNP, the mRNA of which is present at slightly higher levels in B cells.21 Most of the PNP is in large body organs and red blood cells. At the physiologic dose in patients with B-ALL, there was more than 85% inhibition of this enzyme in red blood cells (Figure 4). This inhibition resulted in a peak of at least 10 mM of dGuo in plasma (Figure 5B), suggesting that the desired concentration of dGuo could be achieved in patients with B-ALL. However, only higher levels of dGuo (patient No. 9001) resulted in more than 200 µM dGTP in B lymphoblasts. Lower dGuo resulted in only 10 µM of dGTP (Figure 6).
Collectively, these data suggest that forodesine will need to be infused with dGuo or combined with chemotherapeutic agents based on the mechanistic rationale to have clinically meaningful activity in B-ALL.
Clinical Practice Points.
Despite advances in the treatment of adult and pediatric patients with ALL, disease relapses in a significant majority of the former and a minority of the latter. New agents are needed to improve the outcome of these patients.
Inhibition of PNP leading to depletion of T cells has been demonstrated with forodesine, an orally bioavailable PNP inhibitor that has been previously evaluated for its efficacy in patients with T-ALL. Aside from T-ALL, forodesine has also demonstrated preclinical and clinical activity in indolent lymphoid neoplasms and chronic lymphocytic leukemia.
The current study was conducted to evaluate the activity of this agent in B-ALL, using lymphoblasts from pediatric patients with B-ALL for in vitro analysis. Concurrently, a phase II study examined the role of this agent in various leukemias, including 2 adult patients with B-ALL.
Although the limited data prohibit definitive conclusions, this study suggests that forodesine may be a useful agent for B-ALL only when used in combination with other agents. The exact role of the drug, alone or in combination with other agents, and the setting in which it may be used (such as initial therapy vs. maintenance) will require future trials.
Acknowledgment
This work was supported in part by grants CA136411 (Lym-phoma SPORE) and P30–16672 Cancer Center Support Grant from the National Cancer Institute.
The authors thank Yuling Chen for obtaining blood samples from patients for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Shanta Bantia is an employee of Biocryst; other authors do not have any conflicts of interest.
References
- 1.Giblett ER, Ammann AJ, Wara DW, et al. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975;1:1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- 2.Cohen A, Gudas LJ, Ammann AJ, et al. Deoxyguanosine triphosphate as a possible toxic metabolite in the immunodeficiency associated with purine nucleoside phosphorylase deficiency. J Clin Invest. 1978;61:1405–149. doi: 10.1172/JCI109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez CO, Jr, Stellrecht CM, Gandhi V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood. 2003;102:1842–1848. doi: 10.1182/blood-2003-01-0317. [DOI] [PubMed] [Google Scholar]
- 4.Ullman B, Martin DW., Jr Specific cytotoxicity of arabinosylguanine toward cultured T lymphoblasts. J Clin Invest. 1984;74:951–955. doi: 10.1172/JCI111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez CO, Jr, Mitchell BS, Ayres M, et al. Arabinosylguanine is phosphory-lated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Res. 2002;62:3100–3105. [PubMed] [Google Scholar]
- 6.Cohen A, Lee JW. Gelfand EW. Selective toxicity of deoxyguanosine and arabi-nosyl guanine for T-leukemic cells. Blood. 1983;61:660–666. [PubMed] [Google Scholar]
- 7.Kisor DF, Plunkett W, Kurtzberg J, et al. Pharmacokinetics of nelarabine and 9-beta-D-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18:995–1003. doi: 10.1200/JCO.2000.18.5.995. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi V, Plunkett W, Rodriguez CO, Jr, et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacoki-netics and clinical response. J Clin Oncol. 1998;16:3607–3615. doi: 10.1200/JCO.1998.16.11.3607. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi V, Plunkett W, Weller S, et al. Evaluation of the combination of nelar-abine, fludarabine in leukemias: clinical response, pharmacokinetics and pharmacodynamics in leukemia cells. J Clin Oncol. 2001;19:2142–2152. doi: 10.1200/JCO.2001.19.8.2142. [DOI] [PubMed] [Google Scholar]
- 11.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 12.Schramm VL. Development of transition state analogues of purine nucleoside phosphorylase as anti-T-cell agents. Biochim Biophys Acta. 2002;1587:107–117. doi: 10.1016/s0925-4439(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 13.Kicska GA, Long L, Horig H, et al. Immucillin H, a powerful transition-state analog inhibitor of purine nucleoside phosphorylase selectively inhibits human T lymphocytes. Proc Natl Acad Sci U S A. 2001;98:4593–4598. doi: 10.1073/pnas.071050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi V, Kilpatrick JM, Plunkett W, et al. A proof-of-principle pharmacokinetic, pharmacodynamic, clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777 forodesine) Blood. 2005;106:4253–4260. doi: 10.1182/blood-2005-03-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balakrishnan K, Burger JA, Quiroga MP, et al. Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood. 2010;116:1083–1091. doi: 10.1182/blood-2009-10-246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balakrishnan K, Nimmanapalli R, Ravandi F, et al. Forodesine, an inhibitor of purine nucleoside phosphorylase induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2006;108:2392–2398. doi: 10.1182/blood-2006-03-007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishnan K, Verma D, O’Brien S, et al. Phase 2 pharmacodynamic study of oral forodesine in patients with advanced fludarabine-treated chronic lymphocytic leukemia. Blood. 2010;116:886–892. doi: 10.1182/blood-2010-02-272039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman RR, Ghandi VV, Bennett JC, et al. Intravenous forodesine (bcx-1777), a novel purine nucleoside phosphorylase (PNP) inhibitor, demonstrates clinical activity in phase I/II studies in patients with B-cell acute lymphoblastic leukemia. Blood (ASH Annual Meeting Abstracts) 2004;104:2743. [Google Scholar]
- 19.Ogura M, Tsukasaki K, Nagai H, et al. Phase I study of BCX1777 (forodesine) in patients with relapsed or refractory peripheral T/natural killer-cell malignancies. Cancer Sci. 2012;103:1290–1295. doi: 10.1111/j.1349-7006.2012.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duvic M, Foss FM. Mycosis fungoides: pathophysiology and emerging therapies. Semin Oncol. 2007;346 suppl 5:S21–28. doi: 10.1053/j.seminoncol.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Homminga I, Zwaan CM, Manz CY, et al. In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood. 2011;118:2184–2190. doi: 10.1182/blood-2011-02-337840. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi V, Tam C, O’Brien S, et al. Phase I trial of nelarabine in indolent leukemias. J Clin Oncol. 2008;26:1098–1105. doi: 10.1200/JCO.2007.14.1986. [DOI] [PubMed] [Google Scholar]
- 23.Mansson E, Liliemark E, Soderhall S, et al. Real-time quantitative PCRassays for deoxycytidine kinase deoxyguanosine kinase and 5’-nucleotidase mRNA measurement in cell lines and in patients with leukemia. Leukemia. 2002;16:386–392. doi: 10.1038/sj.leu.2402388. [DOI] [PubMed] [Google Scholar]
- 24.Conry RM, Bantia S, Turner HS, et al. Effects of a novel purine nucleoside phosphorylase inhibitor, BCX-34 on activation and proliferation of normal human lymphoid cells. Immunopharmacology. 1998;40:1–9. doi: 10.1016/s0162-3109(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 25.Sherman PA. Fyfe JA. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem. 1989;180:222–226. doi: 10.1016/0003-2697(89)90420-x. [DOI] [PubMed] [Google Scholar]
- 26.Landrigan PJChildhood leukemias. N Engl J Med. 1995;333:1286. doi: 10.1056/NEJM199511093331912. [DOI] [PubMed] [Google Scholar]
- 27.Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997;90:4243–4251. [PubMed] [Google Scholar]
- 28.Henze G, Fengler R, Hartmann R, et al. Six-year experience with a comprehensive approach to the treatment of recurrent childhood acute lymphoblastic leukemia (ALL-REZ BFM 85). A relapse study of the BFM group. Blood. 1991;78:1166–1172. [PubMed] [Google Scholar]
- 29.Sadowitz PD, Smith SD, Shuster J, et al. Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1993;81:602–609. [PubMed] [Google Scholar]
- 30.Harned TM. Gaynon P. Relapsed acute lymphoblastic leukemia: current status and future opportunities. Curr Oncol Rep. 2008;10:453–458. doi: 10.1007/s11912-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 31.Balduzzi A, Gooley T, Anasetti C, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86:3247–3256. [PubMed] [Google Scholar]
- 32.Mehta J, Powles R, Horton C, et al. Bone marrow transplantation for primary refractory acute leukaemia. Bone Marrow Transplant. 1994;14:415–418. [PubMed] [Google Scholar]
- 33.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 34.Bader P, Hancock J, Kreyenberg H, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668–1672. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- 35.Knechtli CJ, Goulden NJ, Hancock JP, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072–4079. [PubMed] [Google Scholar]
- 36.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 37.Bieghs L, Caers J, De Bruyne E, et al. The effects of forodesine in murine and human multiple myeloma cells. Adv Hematol. 2010;2010:131895. doi: 10.1155/2010/131895. [DOI] [PMC free article] [PubMed] [Google Scholar]