Abstract
Bartonella bacilliformis is the bacterial agent of Carrión's disease and is presumed to be transmitted between humans by phlebotomine sand flies. Carrión's disease is endemic to high-altitude valleys of the South American Andes, and the first reported outbreak (1871) resulted in over 4,000 casualties. Since then, numerous outbreaks have been documented in endemic regions, and over the last two decades, outbreaks have occurred at atypical elevations, strongly suggesting that the area of endemicity is expanding. Approximately 1.7 million South Americans are estimated to be at risk in an area covering roughly 145,000 km2 of Ecuador, Colombia, and Peru. Although disease manifestations vary, two disparate syndromes can occur independently or sequentially. The first, Oroya fever, occurs approximately 60 days following the bite of an infected sand fly, in which infection of nearly all erythrocytes results in an acute hemolytic anemia with attendant symptoms of fever, jaundice, and myalgia. This phase of Carrión's disease often includes secondary infections and is fatal in up to 88% of patients without antimicrobial intervention. The second syndrome, referred to as verruga peruana, describes the endothelialcell-derived, blood-filled tumors that develop on the surface of the skin. Verrugae are rarely fatal, but can bleed and scar the patient. Moreover, these persistently infected humans provide a reservoir for infecting sand flies and thus maintaining B. bacilliformis in nature. Here, we discuss the current state of knowledge regarding this life-threatening, neglected bacterial pathogen and review its host-cell parasitism, molecular pathogenesis, phylogeny, sand fly vectors, diagnostics, and prospects for control.
Methods
Authors were included based upon their relevant expertise in the field. Available databases were searched with key words for applicable articles in both English and Spanish. All cited work was previously peer-reviewed and published elsewhere.
Janus: The Two Faces of Carrión's Disease
A brief history
Bartonellosis, or Carrión's disease, is a remarkable bi-phasic illness that manifests as either angiogenic lesions that occur primarily on the skin or as a severe hemolytic anemia. It is caused by Bartonella bacilliformis, a bacterium presumed to be transmitted between humans through bites of infected female phlebotomine sand flies, most notably Lutzomyia verrucarum [1]–[4]. Humans are the only known reservoir. Carrión's disease is endemic to arid, high-altitude (approximately 600–3200 m) valleys in the Andes Mountains of Peru, Ecuador, and Colombia [5]–[10], coincident with the habitat of the sand fly vector [5]. Evidence for bartonellosis in the region predates Columbus. Skin lesions akin to those typical of bartonellosis are depicted on 2,000-year-old pre-Inca ceramics, and Bartonella-like bacteria have been observed in skin lesions taken from the mummified remains of a human sacrifice victim who died over 1,000 years ago [11]. Descriptions of these lesions, referred to as verruga peruana (VP), appear in the journals of Spanish conquistadors who arrived in South America during the 16th century, and in 1630, Gago de Vadillo published the first medical treatise on the disease [12].
Two events inexorably linked with the early history of bartonellosis occurred within 15 years of one another at the end of the 19th century. The first was a dramatic outbreak of hemolytic anemia among workers based in the upper Rimac valley, above Lima, during the construction of a railway line to the mining town of La Oroya in 1871. During the course of a few weeks at least 4,000 men died of what became known as Oroya Fever (OF), although the agent of the syndrome remained elusive. Second, the common etiology of OF and VP was revealed when, in 1885, a medical student named Daniel Carrión injected himself with exudate from a verruga lesion, then developed OF and died [12]. Carrión sacrificed his life to establish the common etiology of VP and OF and is one of the most heroic figures in Peruvian medical history. In honor of his contribution, South American bartonellosis is commonly known as Carrión's disease. Finally, in 1905, Alberto Barton solved the riddle of the etiological agent of Carrión's disease when he observed intracellular bacteria in blood smears from Oroya fever patients. These bacteria were subsequently named Bartonella bacilliformis in his honor.
Carrión's disease is most common in Peru, where nearly all cases have occurred over the last 70 years in the Departments of Ancash, Cajamarca, Cuzco, Lima, and Amazonas [13]. Sporadic cases may have also occurred in Chile, Bolivia, and Guatemala [14]. During the last two decades, several outbreaks have occurred at abnormal altitudes and in historically nonendemic regions of Ecuador, Colombia, and Peru (e.g., near sea level in Huaral and Ica, Peru [13], [15]; the Utcubamba River Valley in Amazonas [16]; Urubamba Valley and Sacred Valley of the Incas near Cuzco [17], [18]; and the high Amazonas jungle [19]). In addition, multiple outbreaks have occurred concurrently in endemic regions (e.g., Ancash Region, Peru; Zamora-Chinchipe Province, Ecuador) [5]–[8], [20], [21]. Children have been hardest hit by these outbreaks, with fatality rates of approximately 10% [13], [15], [22]. A 10-fold increase in cases (948 to 10,390) was reported in Peru from 1997–2005 [23], with 52 cases per 100,000 Peruvians in 1999 [13]. Reported rates are considerably higher in endemic regions of Peru, at 242 cases per 100,000 inhabitants (http://www.dge.gob.pe/). An estimate of the at-risk population for Carrión's disease is 1.7 million people in an area of 145,000 km2 [15]. In one prospective cohort study of a Peruvian community in an endemic area, an incidence rate of 12.7 per 100 person-years was estimated, reflecting a clustering of 70% of cases within 18% of homes [24].
Factors contributing to the emergence and geographical expansion of B. bacilliformis are unclear but include climatic events that favor sand fly propagation, such as increased temperature, humidity, and rainfall during El Niño cycles [21], [25], [26], and possibly additional sand fly species serving as competent vectors (see “Sand fly vectors,” below) [5], [27]–[29]. In short, Carrión's disease is a reemerging public health threat in several South American nations and to travelers who visit these countries [10], [30].
Oroya fever (OF)
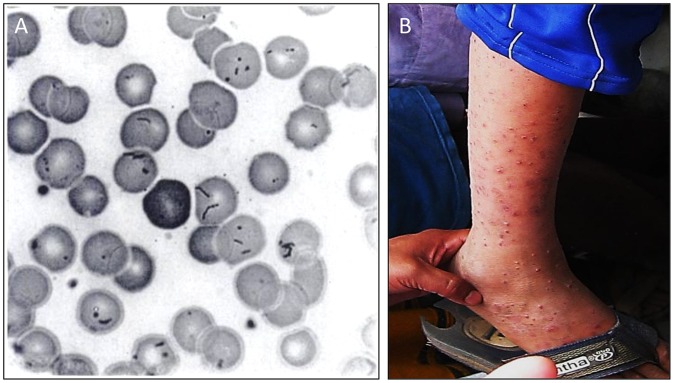
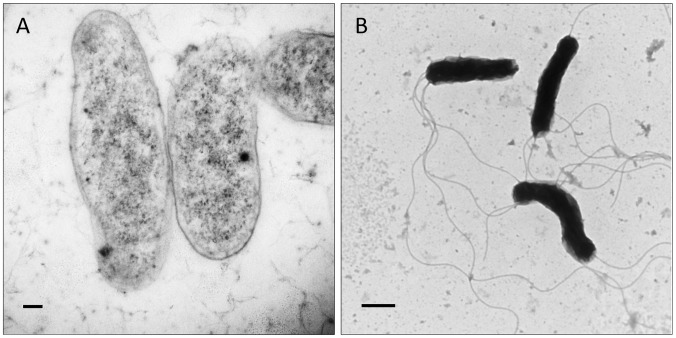
Carrión's disease manifests as two distinct syndromes that occur independently or sequentially. The first phase, OF (also known as the acute, primary or hematic phase), is more common in children (>60% of cases [13], [22], [31]) in both endemic and nonendemic areas, and it is characterized by an acute bacteremia at about 60 days (range of 10–210 days; [32]) following the bite of an infected sand fly. From the inoculation site, B. bacilliformis colonizes the entire circulatory system, and nearly every erythrocyte is infected (Figure 1A). OF involves a severe reduction in hematocrit (>80% destruction) and acute hemolytic anemia from splenic culling of infected erythrocytes [33]–[35]. Patients with OF present for 1–4 weeks with an array of symptoms, including pallor, fever, anorexia, malaise, cardiac murmur, myalgia, headache, jaundice, tachycardia, and hepatomegaly. Complications are common during OF, and include high mortality in pregnant women and their unborn children, cardiovascular and neurological problems, respiratory infections, and arthralgia [22], [36]. This phase of disease can be fatal in 40%–88% of infected patients without antimicrobial intervention [2], [5], [12], [14], [32], [36], [37] and is by far the highest reported case-fatality rate for any Bartonella species. Secondary infections are also common and potentially life-threatening, and they may arise due to an immune-compromised state that accompanies the disease. These include salmonellosis, toxoplasmosis, malaria, shigellosis, histoplasmosis, and pneumocystosis [5], [14], [37]. Even with appropriate antimicrobial therapy, fatality rates of 9%–11% are typical [13], [15], [38]. A milder form of OF has also been reported with a significantly lower case-fatality rate (0.7%), suggesting that virulence potential and associated disease severity may be strain-dependent [16]. Bartonella rochalimae, a distantly related Bartonella species isolated from a tourist who visited South America has been reported to cause a relatively mild febrile illness that could be confused with OF. Although associated symptoms are similar, no intraerythrocytic bacteria are observed in blood smears and there is only a slight drop in hematocrit and mild anemia [39].
Figure 1. Clinical manifestations of Carrión's disease.
(A) Erythrocyte infection during OF, as observed in a blood smear stained with Wright's stain (reprinted by permission from [203]). (B) VP lesions on a child in Peru (reproduced from Future Microbiology 4(6): 743–758 (2009) with permission of Future Medicine, Ltd).
Verruga peruana (VP)
The second syndrome (also known as the tissue, chronic, or eruptive phase) involves blood-filled nodular hemangiomas of the skin, termed VP or Peruvian warts (Figure 1B). Tissue involvement results from bacterial invasion of capillary endothelium and generates bacteria-filled vacuoles, termed Rocha Lima inclusions, and localized cellular proliferation, leading to formation of VP [40] (see “Angiogenesis,” below). Verrugae are cutaneous and usually occur on the head and extremities, where they can persist for several weeks to months. Lesions are classified as miliary (multiple red papules of <3 mm in diameter), mular (blood-filled nodules), and diffuse (groups of subdermal nodules >5 mm in diameter) [32]. Although OF occurs at a lower frequency, the eruptive phase is a more common manifestation in inhabitants of endemic regions [16]. The higher incidence of verruga in endemic areas likely reflects the immune status of the population (see “Host immune response, protection,” below). In cases of sequential illness, patients erupt with verrugae roughly 4–8 weeks after resolving OF [41]. VP is rarely fatal, but lesions can bleed or scar the patient, and eruptions can be accompanied by fever and malaise, lymphadenopathy, acute bone and joint pains, headache, and a chronic Bartonella infection [2], [22], [32]. The recent discovery of a new Bartonella taxon, referred to as Candidatus Bartonella ancashi, as the etiological agent of persistent bacteremia and VP in a three-year-old boy living near Caraz, Peru, indicates that species other than B. bacilliformis may be responsible for some chronic bartonellosis cases in endemic areas [42]. Whether Candidatus Bartonella ancashi can cause OF is unknown.
Sand fly vectors
B. bacilliformis is believed to be transmitted by bites of phlebotomine sand flies to humans living in the high mountain valleys of Peru and neighboring countries of Andean South America. Townsend was the first to advance this view, and he identified Phlebotomus verrucarum (now L. verrucarum) as the vector of “Verruga” [43]–[45]. Experiments by Townsend and other workers provided strong circumstantial evidence of sand fly vectorship but no conclusive proof.
Having the suspicion based on circumstantial evidence that a suspected vector insect transmits a specific pathogen to man or other animals does not constitute incrimination. One must determine the role the insect plays in the natural history of the pathogen. Killick-Kendrick and Ward [46] outlined five criteria that must be fulfilled to declare with reasonable certainty that a sand fly is a vector of human disease. Applied to the transmission of B. bacilliformis, these are:
The suspected vector sand fly must bite humans and be present in a place where humans become infected with B. bacilliformis.
The distribution of the suspected vector should encompass the distribution of the disease in humans, and the sand fly must be sufficiently abundant to maintain pathogen transmission.
B. baciliformis should be isolated from wild-caught sand flies and be shown to be indistinguishable from the pathogen causing disease in humans in the same area.
It should be demonstrated that naturally or experimentally infected flies can maintain the infection through the complete extrinsic life cycle of the pathogen.
Experimental transmission of B. bacilliforms by bite or some other means would generally be considered conclusive proof that a sand fly is a vector of a given pathogen.
Of these criteria, the first two are fairly easy to satisfy through observations of sand fly behavior in the field, and numerous workers have done so. The remaining three criteria are more difficult to satisfy and must be accomplished through painstaking analysis and experimentation to conclusively incriminate a sand fly species as a vector of B. bacilliformis.
Beginning in 1926, Noguchi and associates conducted several experiments in which triturated, wild-caught sand flies from the “Verruga zone” in Peru were inoculated into monkeys to produce infection [47], [48]. Around the same time, Battistini performed a series of experiments in which sand flies collected from the Verruga zone were released into a screened cage with a rhesus monkey. Although this was the first sand fly experiment in which the disease was transmitted by means other than inoculation, the sand flies were not observed and it was not determined whether transmission was by bite [49]–[51]. Between 1937 and 1939, Hertig and associates conducted several feeding experiments in which wild-caught sand flies were either released into small cages with a rhesus monkey, or placed in small feeding cages and strapped to the monkey's shaved belly. Five monkeys became infected as determined by blood culture but no cultures were made from the sand flies [1]. Furthermore, uncertainty remained as to whether transmission was by bite or some other means such as by defecation, as “… there existed opportunities for other types of contact than that of the mouthparts between sand fly and monkey, such as the deposit of fecal matter if such there should be” [1]. Later, these scientists isolated what they said was B. bacilliformis from the proboscis of each of two “Phlebotomus verrucarum” (L. verrucarum) collected from an epidemic focus at Huinco in the Santa Eulalia Valley [1]. Because culture methods used at that time were likely not sufficiently refined to distinguish between B. bacilliformis and other Bartonella species, there is a lingering question as to whether this was the same pathogen causing disease in humans. However, it is fair to say that Hertig's team was the first to incriminate L. verrucarum as a vector of B. bacilliformis. Attempts to culture B. bacilliformis from sand fly guts were unsuccessful; hence, they were unable to demonstrate any developmental cycle in the sand fly that might explain the mechanism of transmission [1].
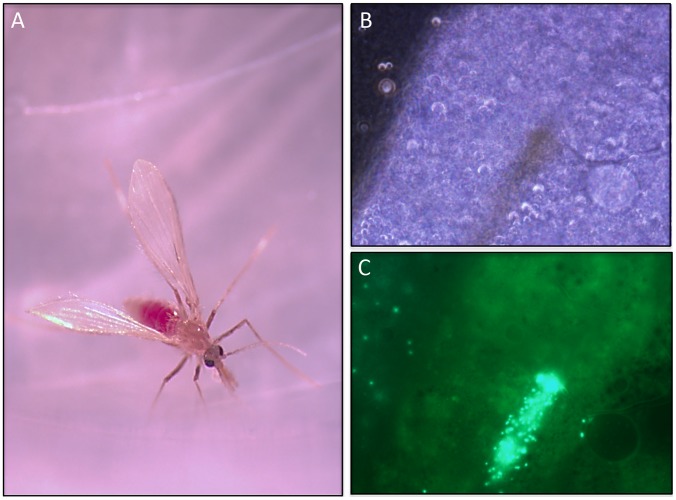
In preliminary experiments conducted recently by three of the authors (MFM, JMB, and PGL), L. verrucarum from a laboratory colony (Walter Reed Army Institute of Research) were fed through a chick-skin membrane on human blood containing green fluorescent protein (GFP)-labeled B. bacilliformis. Serial dissections of putatively infected flies and microscopic examination of their guts under UV over seven days revealed massive proliferation of the bacteria within the blood meal and invasion of red blood cells during the first three days (Figure 2). The infection persisted for at least seven days in the gut of the sand fly. Contrary to what Hertig observed [1], no B. bacilliformis were seen on the mouthparts or in any organ other than the midgut. Diuretic droplets deposited on glass cover slips following feeding contained no labeled bacteria, an indication that posterior station transmission via diuresis is unlikely. Transmission by bite during a second blood meal was not attempted, nor were fecal droplets from the digested blood meal examined for B. bacilliformis contamination. The mechanism of transmission of B. bacilliformis by sand flies is still unclear and is the subject of ongoing investigations.
Figure 2. B. bacilliformis infection of a phlebotomine sand fly.
(A) Female L. verrucarum at 16 h post-feeding with an artificial blood feeder containing human blood and GFP-expressing B. bacilliformis (low-passage strains 14866 and 14868). (B) Light micrograph of L. verrucarum midgut at five days post-feeding on human blood containing GFP+ B. bacilliformis. Central brown area is residual blood meal. (C) Corresponding UV light micrograph of (B). Note the GFP+ B. bacilliformis in residual blood meal and elsewhere in the midgut.
Other vector sand flies
Owing to discrepancies between the distribution of Carrión's disease and L. verrucarum, the existence of secondary vectors was suggested [27]. Whereas L. verrucarum is limited to altitudes below approximately 3,200 m above sea level L. peruensis has been collected in human habitations in endemic villages as high as 3,740 m ASL [27; 2005, Wilfredo Arque, unpublished data, personal communication]. Since Hertig's landmark experiments, substantial evidence has accumulated in Peru to also incriminate L. peruensis, which is highly anthropophilic and abundant or dominant in bartonellosis foci in Ancash and Cusc departments, respectively. In studies conducted during an outbreak in the Urubamba Valley, L. peruensis in large numbers and a few L. pescei were the only sand fly species found. B. bacilliformis was identified using PCR in 2% of L. peruensis analyzed [17]. In two other foci in Peru, 2%–8% of pools of L. verrucarum collected from patient houses in the Caraz area, Ancash Department, and 2% of pools of L. peruensis collected from patient houses in villages 70 km south of Cusco, Cusco Department were PCR positive for B. bacilliformis (2005, Sofia Romero, unpublished data, personal communication).
Besides L. verrucarum and L. peruensis, several other sand fly species are suspected vectors of B. bacilliformis. Caceres et al., working in the Peruvian provinces of Jaen, San Ignacio and Utcubamba, found L. robusta and L. maranonensis in great abundance in intradomiciliary habitats and, because of the absence of L. verrucarum and L. peruensis, considered these as likely vectors of B. bacilliformis in associated foci [28]. Elsewhere, on the eastern slope of the Andes in what is known as “Yungas,” or high jungle, Tejada et al. found that L. serrana, an avid man-biter, comprised 93.6% of sand flies collected during an investigation of a bartonellosis outbreak in Huamalíes, Huánuco Department [52].
In neighbor Andean countries of Colombia and Ecuador, where L. verrucarum and L. peruensis are absent, other species are suspected as vectors, but insufficient evidence has been produced to implicate them with certainty. Dominant species in Colombian districts where human bartonellosis has been reported, include L. gomezi, L. panamensis, and L. serrana, all of which are anthropophilic but none of which has ever been associated with B. bacilliformis transmission [6]. L. colombiana is considered the most likely vector of B. bacilliformis in Colombia based on its highly anthropophilic behavior and occurrence in all three departments of the country from which bartonellosis outbreaks have been reported [6]. The species that transmits this pathogen in Ecuador remains unknown [6].
Spatial and temporal dynamics of vector sand flies
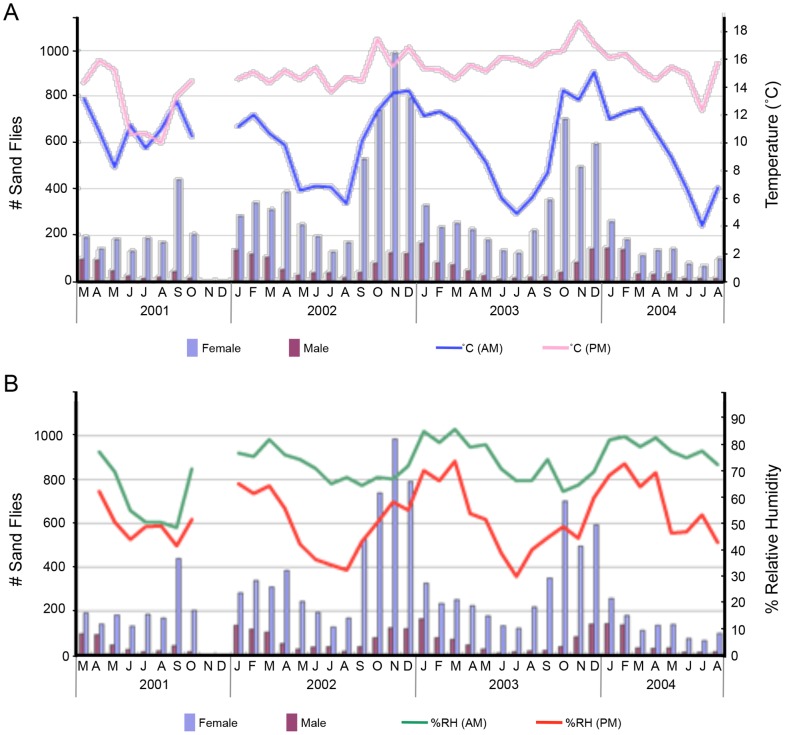
Three-year longitudinal studies of suspected vector sand flies associated with bartonellosis outbreaks in Ancash and Cusco departments of Peru revealed that both L. verrucarum and L. peruensis exhibit unimodal annual temporal distribution patterns with lowest population densities occurring in June and July, corresponding to the “winter” dry season; and highest population densities in November, prior to the onset of the “summer” rainy season in December (Figure 3) [53]. It was further determined that sand fly population densities are directly correlated with average minimum ambient temperatures and relative humidity (Figure 3) [53]. As nighttime temperatures and relative humidity increase, sand fly activity also increases (Figure 4) [53]. These findings are consistent with the notion that the height of the B. bacilliformis transmission season, March to May, [24], [26] corresponds to the descending side of the seasonal sand fly population peak, February to June, when the aggregate population is oldest and when the highest percentage of flies have had an opportunity to take at least one blood meal and are thus more likely to be infected.
Figure 3. Monthly sand fly collection results from three villages in the Cusco Region, Peru.
Results show a unimodal annual population distribution pattern with: (A) corresponding mean morning (blue line) and evening (pink line) temperatures and (B) corresponding mean morning (green line) and evening (red line) relative humidity. Collections were made during two nights per month at case homesteads from March 2001 to August 2004. The data gap between October 2001 and January 2002 is due to a cessation of activity mandated by the Peruvian Ministry of Health.
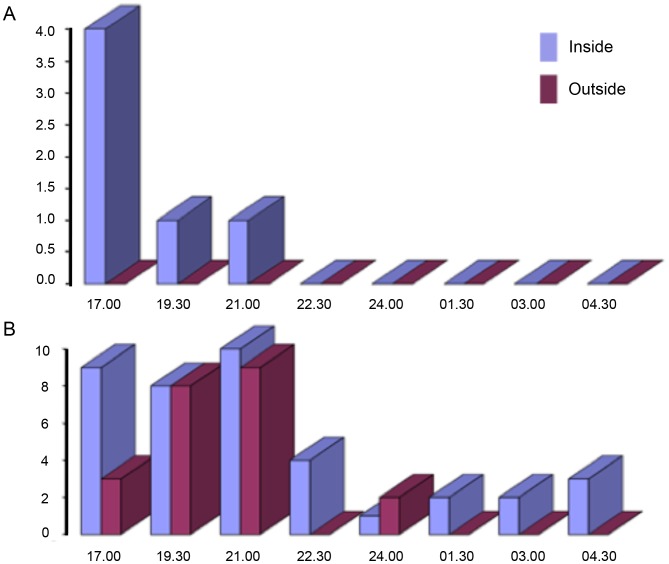
Figure 4. Results of collection-bottle-rotator (CBR) trap collections of sand flies in Peru.
Results show that: (A) nightly sand fly activity is limited to early evening (1800–2000 hrs) from March through July, the coldest part of the year, which represents the Peruvian winter, and (B) as nighttime temperatures increase in late August through November (late winter and spring), sand fly activity extends throughout the night. “Inside” and “outside” refer to trap locations within and outside a domicile, respectively.
In the Ancash and Cusco regions of Peru, approximately 80% of the flies were captured inside case houses, mostly in sleeping quarters, and 35%–40% of females were blood-fed. Being weak fliers, sand flies breed in close proximity to their blood-meal source, feeding at dusk and during the evening, when ambient temperatures drop and relative humidity rises [53]. L. verrucarum and L. peruensis both rest inside human dwellings and feed readily indoors, preferentially on humans [24], [53]. Results of bloodmeal analyses conducted in an endemic focus of cutaneous leishmaniasis in Chaute, Lima, showed that L. peruensis is more anthropophilic than is L. verrucarum [54]. It was also shown that multiple-host blood meals are common, including bloodmeals containing blood from animals only present within the houses mixed with blood from animals only present in nearby shelters, indicating movement of flies from animal shelters to the houses (or vice versa) to finish feeding. People living in endemic villages report that the sand fly bites occur indoors during the evening, directly before and during the hours of sleep, further confirming that transmission occurs inside the home during the evening and the night [24].
As entomological field studies have shown, vectors of infectious diseases, including vectors of bartonellosis, are sensitive to temperature, humidity, wind, and rainfall patterns; therefore, their abundance is influenced by climate variability [24], [26]. During the El Niño weather event that occurred in Peru during late 1997 through mid-1998 weather cycles, the rainy season was extended, and the humidity and minimum monthly temperatures rose. This climatic anomaly allowed potential vector populations to flourish, and unusually high numbers of sand flies were collected [24], [26]. The net result was that major epidemics of bartonellosis erupted in Peru, especially in Caraz and Cusco areas. The impact of weather extremes on vector sand fly ecology and transmission of B. bacilliformis warrants thorough study [26].
Host Cell Parasitism, Angiogenesis, and Immune Response
Colonization of the host: How, when, and where
Bartonella species infect an array of mammals with species specificity for their natural reservoir host(s); humans generally serve as incidental hosts. Notable exceptions include B. bacilliformis (human reservoir) and B. quintana (found in humans and nonhuman primates [55]). Infection of reservoir mammals is generally subclinical with persistent intraerythrocytic bacteremia. Bartonella are typically inoculated directly into blood by the bite of an arthropod vector such as fleas, ticks, and sand flies or indirectly when feces of the vector are scratched into the skin. B. henselae can also be transmitted via the scratch of a contaminated cat claw. The persistence of bartonellae in the natural host facilitates uptake and transmission by hematophagous arthropod vectors. Colonization of the host's lymphatics and circulatory system may be enhanced by blood flow and perhaps motility in flagellated species. For example, B. bacilliformis are highly motile by lophotrichous flagella (Figures 5 and 6), appendages consisting of approximately 42 kDa flagellin subunits that are resistant to protease treatment [56].
Figure 5. Transmission electron micrographs showing morphology of B. bacilliformis.
Bacteria were grown three days on heart infusion agar containing 4% sheep erythrocytes and 2% sheep serum at 30°C and 100% relative humidity. Cells were subsequently fixed in 2% glutaraldehyde in cacodylate (pH 7.2), epoxy embedded by standard methods, then sectioned and stained with uranyl acetate (UA) and lead citrate stains. Micrographs show B. bacilliformis (strain KC583): (A) from a thin section; (B) applied directly to a grid stained with UA to show flagella. Scale bars represent 100 nm in (A) and 500 nm in (B).
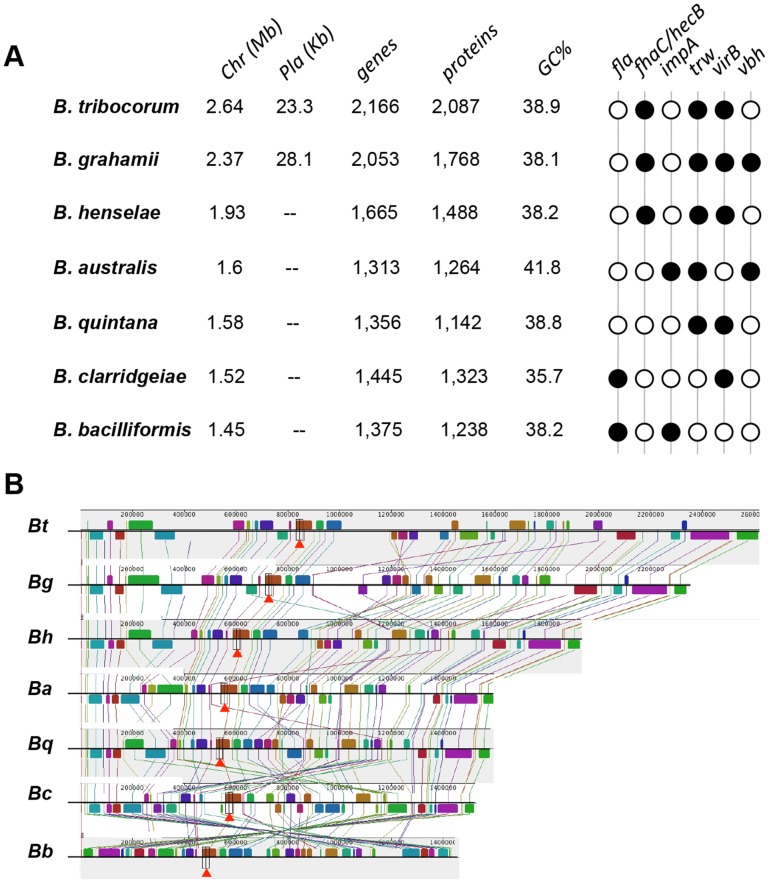
Figure 6. Genomic structure of seven Bartonella chromosomes.
(A) Chromosomes (Chr) are arranged in size from largest (B. tribocorum) to smallest (B. bacilliformis). Plasmid (Pla) sizes are listed, if present. B. quintana's genome encodes the least number of proteins (1,142), and B. clarridgeiae has the lowest GC% (35.7%). Several virulence-related ORFs have been used to infer phylogeny (fla – vbh) and black circles indicate their presence in a particular species. (B) Multiple alignment of seven complete genomes using pM. Location, orientation and position of locally collinear syntenic blocks (LCBs) shared amongst all chromosomes are color-coded and connected by lines. User can analyze location, orientation, and size of LCBs in multiple chromosomes simultaneously (red arrowheads). Local rearrangements, duplications, and inversions are easily identified. Abbreviations correspond to the Bartonella species shown in (A).
Adherence to host cells
Bartonella infections involve interactions with both nucleated cells and erythrocytes. While erythrocyte adherence by different bartonellae is known to be host-specific, adhesion to nucleated cells is considered to be relatively generic. B. bacilliformis invades a variety of human cell types, including endothelial cells, dermal fibroblasts, and laryngeal epithelium [57]. Similarly, B. henselae and B. quintana have been shown to adhere and invade diverse human cell types, including epithelial cells [58], endothelial cells [59], [60], and macrophages [61]. In each case, trimeric autotransporter adhesin proteins (TAAs) are thought to play a leading role in adherence to nucleated host cells [62], [63].
B. bacilliformis possesses three brp genes encoding Bartonella repeat proteins (Brps) [64]. These proteins, along with Bartonella adhesin A (BadA) of B. henselae and variably expressed outer membrane proteins (Vomps) of B. quintana, belong to the TAA family of type V secretion systems [62], [63]. BadA is the best-understood Bartonella adhesin and consists of surface filaments with head, neck, and repetitive stalk domains assembled on a C-terminal anchor [65]. BadA's head is crucial for host cell interactions and triggering a proangiogenic response [66]. Although Bartonella TAAs share remarkable features and functional similarities, they vary considerably in size, and multiple gene variants are often found in the genome [67]. There are at least two variants of the badA gene in B. henselae, and four genes encode the Vomps of B. quintana (vompA–vompD) [63]. B. bacilliformis Brps share common domains and structural features with TAAs (reviewed in [64]).
The role of BadA has been well studied in B. henselae, where it is essential for autoagglutination, adhesion to host cells, and extracellular matrix proteins such as fibronectin and collagens, inhibition of phagocytosis, and induction of a proangiogenic response in host cells [62], [68], [69]. The Vomps of B. quintana have also been shown to play a similar role in host cell interaction and autoagglutination [63], [70], [71]. Thus, while the biological role of B. bacilliformis Brps has not yet been investigated, it is plausible that the Brps play similar roles in pathogenesis.
Erythrocytes are another major target cell during bartonelloses. Optimal adherence to erythrocytes by B. bacilliformis occurs in vitro at approximately six hours after co-incubation [34] and is thought to be energy-dependent [72]. Studies have shown that B. bacilliformis and B. henselae recognize five or six proteins, respectively, of human erythrocyte membranes [73]. A polar tuft of fibrous projections resembling peritrichous flagella on B. bacilliformis was observed to make contact with the erythrocyte membrane during adhesion [72], and antiserum to flagella was shown to significantly reduce B. bacilliformis association with red cells as compared to controls [56]. These data suggest that flagella may possess adhesive qualities and/or increase bacteria–host cell collisions. It has been proposed that Trw T4SS and flagella expression are mutually exclusive phenotypes (Figure 6), and the Trw system replaces the erythrocyte adhesion role of flagella among non-flagellated Bartonella [74]. Specifically, TrwL and TrwJ adhesins have been shown to result in adherence to erythrocytes. TrwJ1 and TrwJ2 were shown to localize to the bacterial cell surface and serve as species-specific adhesins for erythrocytes [75], [76].
Invasion of host cells
Bartonella species are facultative intracellular bacteria, and growth within host cells is typical. Virulence studies with B. bacilliformis and cultured epithelial or endothelial cell monolayers demonstrated that the bacterium can induce host cells to reconfigure the cytoskeleton and enhance uptake. Internalization is significantly reduced (approximately 30% of untreated controls) if actin filament formation is inhibited with cytochalasin D, or if bacteria are pre-treated with anti-B. bacilliformis antibodies [57]. These inhibition studies indicate that the bacterium plays an active role during internalization and the process involves a surface-exposed molecule(s) that is accessible to antibody.
Parasitism of erythrocytes (hemotrophy) is an uncommon strategy practiced by a handful of bacterial pathogens, including Bartonella and Anaplasma species and Mycoplasma haemofelis [77]. Hemotrophy is thought to satisfy the absolute requirement of bartonellae for hemin. Erythrocyte invasion by bartonellae is markedly different than entry into other cell types, because red cells are passive, non-endocytotic, and cannot contribute to internalization. Early work showed that three determinants were important for erythrocyte invasion by B. bacilliformis, including deformin, flagella, and proteins encoded by an invasion-associated locus (ialAB).
Deformin is an extracellular factor that generates trenches and pits in erythrocyte membranes (Figure 7) that resemble those seen on infected red cells [34]. The invaginations are thought to provide entry portals for bacterial colonization. However, even with deformin, bacteria must be motile to access the cytosol [78]. Initial studies showed that deformin was a secreted, homodimeric protein (130 kDa in its native state). Activity was enhanced by pretreatment of erythrocytes with trypsin or neuraminidase and abrogated if cells were pretreated with phospholipase D. Deformin-induced invaginations were reversible by treatment with vanadate or dilauroyl phosphatidylcholine (DLPC), or if intracellular Ca2+ levels were increased with ionophores [78], [79]. Subsequent work contradicted earlier results and showed that deformin was a small, hydrophobic molecule of approximately 1400 Da with high affinity for albumin [80]. Finally, a third study implicated a set of 36 kDa proteins in the culture supernatant as being required for maximal activity [81]. Despite these discrepant observations, the nature of deformin and its role in hemotrophy warrant further investigation, especially considering the marked morphological changes the factor produces in erythrocyte membranes.
Figure 7. Scanning electron micrographs of deformin-induced invaginations and pits on erythrocyte membranes.
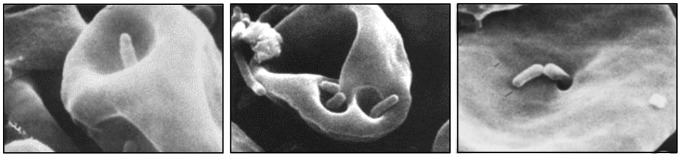
B. bacilliformis colonization of cell membrane deformations is readily apparent. Reprinted by permission from [34].
Flagellum-mediated motility also appears to play an important role in hemotropy. If B. bacilliformis is treated with antiflagellin antibodies, in vitro invasiveness for human erythrocytes is nearly abrogated [56]. B. bacilliformis also possesses an invasion-associated locus comprised of two genes, ialA and ialB, that can confer an invasive phenotype on Escherichia coli for human red cells in vitro [82]. The ialA gene codes for a (di)nucleoside polyphosphate hydrolase that may function to reduce stress-induced dinucleotide “alarmones” encountered in the host cell and therefore enhance pathogen survival [83]. The ialB gene codes for a protein with similar molecular mass and considerable amino acid sequence similarity to the adhesion and invasion locus (Ail) protein of Yersinia enterocolitica [84] and the resistance to complement killing (Rck) protein of Salmonella typhimurium [85]. Both Ail and Rck are implicated in host cell attachment, invasion, and serum resistance. Whether IalA and IalB mediate or facilitate invasion of other host cell types is unknown. A signature-tagged mutagenesis study using B. birtlesii identified ialB as a gene necessary to establish bacteremia in a mouse model. Moreover, the ialB mutant strain displayed a reduced ability to invade erythrocytes in vitro [75]. These results suggest that the ialAB locus may be required for optimal intracellular invasion by many Bartonella species.
A major difference between B. bacilliformis and other common, pathogenic Bartonella species, such as B. henselae and B. quintana, is the absence of VirB T4SS (Figure 6). VirB and the coupling protein VirD4 constitute a machine that delivers T4SS effectors (Beps) to the host cell's cytosol. In turn, translocated Beps subvert cellular functions, including: (1) rearrangement of the actin cytoskeleton, leading to formation of and internalization by invasomes [59], [86]; (2) activation of a pro-inflammatory response via NF-κB [87], [88]; (3) inhibition of apoptosis [88]; and (4) capillary sprout formation in endothelial cells cultured in a collagen matrix [89]. During invasome formation, B. henselae are contacted and moved rearward to form an aggregate on the leading lamella of the endothelial cell. The bacterial mass is subsequently internalized by membrane protrusions that are rich in cortical F-actin, ICAM1, and phosphotyrosine. Chemical inhibition studies suggest that invasome activity is actin-dependent and microtubule-independent. Not surprisingly, B. bacilliformis cannot trigger invasome formation because it lacks a VirB T4SS.
Intracellular growth
Bartonellae invade and multiply within cells of their natural hosts. It is thought that seeding of the bloodstream occurs from a primary niche, including the endothelium and migratory cells, such as lymphocytes and phagocytes, or perhaps hematopoietic progenitor cells [74], [90]. Regardless, it appears that these bacteria have increased intracellular growth rates [91]. This is puzzling, considering the absolute requirement of bartonellae for heme and the iron-limiting nature of the intracellular niche. Two independent systems have been described whereby bartonellae bind and transport heme. The hemin-binding proteins (Hbp's) are a group of porin-like, beta-barrel proteins that are surface-exposed [92], [93]. Hbp's are encoded by paralagous gene families [94], and the number of genes varies among species (ranges from three hbp genes in B. bacilliformis to eight in B. australis). B. bacilliformis encodes three closely linked, tandem hbp genes (e.g., BARBAKC583_1213-1215 and BbINS_05732, 05727, and 05722). Several roles for Hbps have been proposed, including: (1) coating of the bacterium with heme to serve as a nutritive reservoir [93], (2) providing a heme-based antioxidant barrier against reactive oxygen species [93], (3) decreasing oxygen levels around the bacterium to provide a more favorable microaerophilic environment [93], [95], and (4) serving as an adhesin to bind fibronectin, heparin, and HUVECs [96].
Bartonella species also employ a more typical hemin utilization (Hut) system consisting of a hemin receptor, an ABC transporter (including a permease, ATPase, and a periplasmic heme-binding protein) and a cytosolic heme-degrading/storage protein [97]. In B. quintana and B. henselae, the tonB gene is located immediately upstream and in opposite orientation to the hut locus and encodes TonB, a protein that energizes outer membrane processes, presumably including Hut-mediated heme transport. Interestingly, B. bacilliformis lacks an obvious TonB homologue, begging the question as to the nature of its functional surrogate (note: ExbB and ExbD are found in all three bacteria). Regulation of the hut locus is accomplished through the iron response regulator (Irr) and promoter regions containing an H-box, a motif known to interact with Irr [97]. Other than Irr's involvement, the hemin transport system in Bartonella species is remarkably similar to those of other gram-negative bacteria.
B. bacilliformis is generally accepted as nonhemolytic on blood agar. However, incomplete β-hemolysis can be observed on thin blood agar plates that are cultured for at least four days. The hemolysin is extracellular and can pass through 0.2 µm filters into underlying medium, where it produces a hemolytic zone that silhouettes the colony's shape [98]. A similar, incomplete β hemolytic activity with a delayed appearance (four days' growth) was also observed with B. elizabethae [99]. Finally, a contact-dependent hemolysin has been described from B. bacilliformis that is proteinaceous, requires prolonged incubation periods, acts independently of deformin, and is most active at 37°C [100]. The molecular nature of the hemolysins and their potential role in virulence have not been investigated thoroughly, to date. It is tempting to speculate that hemolysins participate in escape from vacuoles and cytosolic membranes of host cells.
Angiogenesis
The clinical manifestation and histological appearance of VP are similar to bacillary angiomatosis (BA), a pathology that can occur in immunocompromised individuals infected with B. henselae or B. quintana [101]–[103]. VP and BA are characterized by tumor-like lesions or nodules filled with immature capillaries and swollen endothelium due to chronic colonization by bacteria and infiltration of polymorphonuclear leukocytes and macrophages [104], [105]. BA caused by B. henselae has been observed primarily on skin, although the bacteria can disseminate and affect other organs such as eyes, liver, and heart [102], [106]. In contrast, VP lesions are rarely observed in organs other than skin and subcutaneous tissue [10]. As in tumor angiogenesis, Bartonella-triggered neovascularization follows a series of steps that involve disruption of the normal pattern of the extracellular matrix and basal membrane, endothelial cell migration and proliferation at the site of angiogenic stimuli [107].
In an effort to study angiogenesis induced by B. bacilliformis, early studies employed nonhuman primate models, including rhesus monkeys [108]. Resulting angiogenic lesions varied depending on the susceptibility of the monkeys as well as the virulence capacity of the bacteria. Intradermal injection of young rhesus monkeys with B. bacilliformis obtained from the blood of a patient with a fatal case of OF gave rise to granulomatous nodules filled with new capillaries similar to VP [47]. Nodules varied in size (0.5–2 cm in diameter and 2–10 mm high), appeared at the site of inoculation within three weeks, and increased in size until 60 days post-inoculation, where they remained stationary before starting to regress [47]. The pathogen was readily found inside angioblasts and endothelial cells of nascent capillaries. Noguchi demonstrated that B. bacilliformis that had been passaged in rhesus monkeys, as well as those that were taken from VP tissues led to more severe clinical manifestations of infection [109]. In those test animals, numerous miliary, cherry-red verrugae appeared within one week around the site of inoculation (eyebrows) and were scattered over remote areas such as the groin and legs.
Despite these initial studies, there are few reports describing the molecular basis of B. bacilliformis–induced angiogenesis. This is largely due to the lack of an inexpensive in vivo animal model. Although BALB/c mice resist infection by B. bacilliformis [110], a SCID/BEIGE murine model for studying Bartonella infection may prove useful, but has not been tested in this regard [111]. Garcia et al. investigated the effect of B. bacilliformis extracts on human endothelial cells in vitro as well as in an in vivo model of angiogenesis consisting of subcutaneous implantation of sponge discs containing bacterial extracts into adult Sprague-Dawley rats [103]. The study revealed that a mitogenic factor in bacterial extracts caused vascular proliferation in a dose-dependent manner. However, this factor did not induce a pro-inflammatory response as it did not lead to an increase in the number of phagocytes and lymphocytes. A subsequent report found that B. bacilliformis GroEL had mitogenic activity toward endothelial cells undergoing serum starvation-induced apoptosis [112], but it did not affect caspase 3 activity. B. henselae was also shown to stimulate endothelial cell proliferation, but to a much lesser extent than B. bacilliformis extracts [112]. Subsequently, GroEL of B. henselae was found in a secreted fraction of the bacterium and caused endothelial cell proliferation and acted by triggering release of intracellular calcium stores [113]. It is possible that GroEL of both species functions as a chaperonin to stabilize a yet unidentified mitogenic factor. However, in contrast to B. bacilliformis, an extract from B henselae was shown to have an anti-apoptotic factor that reduced apoptosis of endothelial cells by inhibiting caspase 3 and caspase 8 activities [114]. Interestingly, the anti-apoptotic activity was enhanced in the presence of live bacteria.
Our current understanding of Bartonella-induced angiogenesis suggests the process involves at least three mechanisms that work synergistically: (1) mitogenic triggering of endothelial cell proliferation, (2) inhibition of apoptosis (contact-dependent or independent), and (3) angiogenic reprogramming of infected host cells (with pro-inflammatory activation of cytokines) [67], [103]. Analysis of VP lesions revealed that infection induces production of VEGF, VEGFR1, VEGFR2, and angiopoietin-2 transcripts [115]. Interestingly, endothelium was shown to produce very little VEGF, whereas overlying epidermis was the major source of VEGF. To our knowledge, the mechanism whereby B. bacilliformis induces production of these pro-angiogenic factors has not been investigated, to date. Expression of hypoxia-inducible factor 1 (HIF-1) and subsequent induction of VEGF have been well documented in tumor angiogenesis [116], [117].
B. henselae infection studies using in vitro cell culture models and BA tissues showed that bacteria induced HIF-1 activation and VEGF secretion [68], [69]. This angiogenic response was shown to be dependent on expression of BadA and its interaction with β1-integrin mediated by fibronectin binding [62]. Moreover, B. henselae infection was shown to trigger a VirB/Bep-dependent angiogenic phenotype in human endothelial cell models. This includes inhibition of endothelial cell apoptosis and a robust induction of capillary sprout formation mediated by BepA [89], [118], and NF-κB-dependent stimulation of a pro-inflammatory response leading to production of IL-8, expression of adhesion molecules such as E-selectin and ICAM-1, and up-regulation of macrophage chemoattractant protein-1 (MCP-1) [105], [119]. It is reasonable to speculate that B. bacilliformis does not display effector protein-dependent cell proliferation or anti-apoptotic activity, because it lacks a T4SS. Nevertheless, it may be of interest to determine if B. bacilliformis Brps are involved in interaction with β1-integrin and elucidate what role they might play in inducing a pro-angiogenic response.
Host immune response, protection
Lymphadenopathy is a frequent complication. Immunosuppression is a common manifestation of B. bacilliformis infection and probably results from acute anemia and leukocytosis. In fact, a compromised immune state is thought to contribute to the increased incidence of secondary infections seen in infected individuals.
Lifelong humoral immunity apparently results from a B. bacilliformis infection [2], [120]. In endemic areas, up to approximately 60% of the population can be seropositive (mainly IgM isotype), and many of these individuals are healthy [121]. Nevertheless, seropositive people who are asymptomatic or post-eruptive for verrugae are often blood culture–positive for B. bacilliformis [122]. An extraordinary carrier rate, up to 15%, has been reported in some areas of Peru [123], and it is this infected population that presumably serves as the reservoir [1], [3], [77]. An inverse correlation between patients' chronological age and incidence or disease severity in endemic regions suggests that humoral immunity, as a result of recurring or chronic infections, confers partial immunological protection [24], [36]. Because bartonellae are found in various body fluids and cells, participation by both humoral and cellular arms of the immune system would undoubtedly need to be invoked to resolve the infection. However, for unknown reasons, this does not occur in many afflicted individuals, and the infection is persistent.
Phylogeny and Genomics: Clues to the Evolution of B. bacilliformis
Bartonella comparative genomics
Bartonella genomes consist of single, circular chromosomes ranging in size from 1.45 Mb in B. bacilliformis to 2.64 Mb in B. tribocorum, and are largely collinear with a Guanine+Cytosine (GC) content of 38%–42% [124]. Adenine+Thymidine (AT)-richness of these genomes is a universal attribute of host-associated bacteria. Several Bartonella species also harbor circular plasmids [125]–[128], although none have been reported for B. bacilliformis. The relatively small size of Bartonella genomes is a common attribute among endosymbionts and host-associated bacteria, and possibly reflects genome reduction as a result of decreased selection pressure in the intracellular niche and in the context of a narrower range of hosts [129]. It appears that the number of vertically inherited genes within Bartonella genomes is relatively consistent (approximately 1,000), but the number of horizontally acquired (imported) genes varies significantly; thus the genomes of species such as B. tribocorum and B. grahamii, which both exceed 2 Mb, contain more than 400 imported genes, whereas those of species such as B. bacilliformis and B. quintana, which are 1.45 and 1.6 Mb respectively, contain 150 or fewer imported genes [127].
Comparative genomics has been used to survey the distribution of coding sequences in extant Bartonella species, then correlate this distribution to the phylogenetic juxtapositions of these species as a means to infer patterns of gene acquisition and loss during evolutionary radiation of the genus [124], [130]. The focus of considerable interest is on genes encoding virulence factors that mediate interactions with the host, such as the virB and trw gene clusters and numerous others described elsewhere in this review, as they are likely key determinants of both host specificity and divergent modes of parasitism that characterize the genus (Figure 6).
Taxonomy/phylogeny of the genus
The genus Bartonella was proposed almost a century ago by Strong to accommodate B. bacilliformis, following its description in red blood cells of OF patients by Alberto Barton in 1909 [131]. The advent of 16S rDNA sequencing in the late 1980s suggested that B. bacilliformis was genetically similar to the trench fever agent, then called Rochalimaea quintana [132], and also a newly emerging pathogen that was subsequently named Rochalimaea henselae [133], [134]. The genera Bartonella and Rochalimaea were later unified with one another [135] and with the genus Grahamella [136], taking the name of the longest established taxon, Bartonella. Over the past 13 years, the genus has gradually expanded, such that it now includes 31 “validated” species and subspecies (http://www.bacterio.net/index.html) along with many more informal taxa and partially characterized strains. B. bacilliformis is the type species of the genus.
Phylogeny of the genus remains somewhat unresolved. The evolutionary relationship of Bartonella to Brucella and other genera within the Agrobacterium/Rhizobium group of α-proteobacteria is well established, but delineation of clusters of species within the genus remains somewhat uncertain. The value of intra-genus phylogenetic inference derived from 16S rDNA alignments was limited due to its conservation amongst Bartonella species [137], [138], thus alignments of other loci have been more frequently exploited. The first of these to be widely adopted was gltA [139], and phylogenetic inference derived from this locus indicated that B. bacilliformis was the most outlying species, in a lineage deeply divergent from other members of the genus. Phylogenies derived from ftsZ, groEL, and rpoB genes also inferred this peripheral position for B. bacilliformis [140]. However, the advent of phylogenies derived from alignment of concatenated sequence data from multiple loci cast doubt on B. bacilliformis' position as an outlier. Reconstructions by Gundi et al. [141] strongly supported a specific evolutionary relationship with Bartonella clarridgeiae, a position that was reaffirmed by Inoue and colleagues in 2010 [142]. This study also inferred that B. bacilliformis and B. clarridgeiae lay within a well-supported cluster that also contains B. birtlesii, B. bovis, B. capreoli, B. chomelii, and B. schoenbuchensis.
Whole genome sequence (WGS) data of several Bartonella species have now been released, and those of many more are either in draft form or in progress at this writing. Among these, a complete genome is available for one B. bacilliformis strain (KC583), whereas data in the form of contigs have been released for a second strain (INS), and projects on nine other strains are currently in progress (http://www.ncbi.nlm.nih.gov/genome/genomes/524). WGS data are being increasingly used to infer a robust phylogeny amongst Bartonella species.
Engel and colleagues [130] generated concatenated sequences from 478 loci within genomes of representatives of eight Bartonella species (B. bacilliformis, B. clarridgeiae, B. grahamii, B. henselae, B. quintana, B. rochalimae, B. shoenbuchensis, B. tribocorum, and two unnamed taxa), and from their alignment inferred a phylogeny with four lineages, where B. bacilliformis is in a lineage separate from all other Bartonella. Most recently, Guy and colleagues [124] described two new, fully resolved genomes (B. australis and B. vinsonii subspecies berkhoffii) and four near-complete genomes (B. bovis [2 isolates], B. vinsonii subspecies berkhoffii, and B. schoenbuchensis). The group then compared 428 orthologous core genes amongst the 16 Bartonella taxa described above. This phylogeny conflicted with that proposed by Engel et al. [130], in that B. australis rather than B. bacilliformis, was the earliest diverging species and that B. bacilliformis clustered with B. bovis and B. schoenbuchensis. Subsequent addition of WGS data for an isolate of an informal taxon referred to as “Bartonella tamiae” to this dataset and a revision of the genes included did not result in a repositioning of B. bacilliformis within the inferred phylogeny, although “B. tamiae” replaced B. australis as the earliest diverging species [124].
Placement of B. bacilliformis within a cluster containing B. bovis and B. schoenbuchensis, rather than the earliest diverging species of the genus, has led to reevaluation of hypothetical evolutionary processes that have shaped Bartonella genomes. The small genome size of B. bacilliformis could be explained by reductive genome evolution following a recent host switch; a model akin to that proposed for B. quintana [143]. As all other members of the cluster containing B. bacilliformis are ruminant-associated, it has been proposed that B. bacilliformis descended from an ancestral strain that successfully made a host shift from ruminants to humans [124]. The alpaca (Vicugna pacos) and the lama (Lama glama), the two most common domesticated ruminant species in the endemic region, are perhaps a source of this ancestral strain. Domestication of these ruminants began some 6,000 years ago, providing a time frame for a hypothetical B. bacilliformis host-shift.
Genetic diversity among B. bacilliformis strains
Few studies exploring the genetic diversity of B. bacilliformis have been published [144], [145]. These studies employed a variety of different typing methods, including pan-genomic approaches such as amplified fragment-length polymorphism and infrequent restriction site PCR, and/or comparison of nucleotide sequences at loci including gltA, ialB, and, most frequently, the 16S–23S rDNA internal transcribed spacer (ITS). All these approaches have delineated genotypes within the species and have been useful in characterizing the molecular epidemiology of bartonellosis [144], [145]. Most recently, a multilocus sequence typing (MLST) scheme was developed for B. bacilliformis [146]. Application of this procedure to 46 isolates collected in Peru yielded interesting findings. Perhaps the most significant was that B. bacilliformis may not be a single species; the MLST data provided clear evidence that two isolates recovered from patients with OF had diverged from other B. bacilliformis isolates. These isolates are therefore likely members of a novel Bartonella genospecies. The two divergent isolates were not epidemiologically linked (i.e., they were obtained nine years apart and from locations that lie 150 km from one another). Furthermore, two additional isolates that are also potential members of this new genospecies have been encountered elsewhere [10]. Taken together, results suggest that novel B. bacilliformis-like genospecies have a wide distribution in Peru. The study by Chaloner et al. [146] delineated eight genetic lineages among the isolates surveyed and, in line with many previous studies, these lineages correlated to some degree with the epidemiological provenance of isolates. Thus, strains associated with new foci of Carrión's disease clustered together and were distinct from the most common genotypes, which were encountered across endemic regions of Peru. However, extrapolation of these data to explore the general epidemiology of Carrión's disease may be somewhat premature given the relatively small size and opportunistic nature of the collection examined. This shortfall can only be accurately addressed with systematic surveys, although MLST appears to be an appropriate genotyping method to employ if such studies can be instigated.
In an effort to explore more comprehensively the extent of genetic variation among B. bacilliformis strains, we compared the KC583 WGS with genome sequence data available in the form of contigs for the INS isolate (the only genomic data currently available for another B. bacilliformis strain [147]), using progressive Mauve (pM). A total of 20 contigs, containing approximately 1,444,079 base pairs of data, were analyzed. Remarkably, our efforts revealed the two datasets differed by only 27 single-nucleotide polymorphisms (SNPs), with no evidence of any indels or repeat motifs. We mapped the position of these 27 SNPs, finding that 19 lay in putative open reading frames. Of these, 13 were nonsynonymous and two introduced a stop codon. Taken together, these observations indicate that the two B. bacilliformis strains, despite sharing no specific epidemiological relationship (being isolated roughly 50 years apart from patients inhabiting geographically remote locations) possess, for all intents and purposes, indistinguishable genomes. Such is the extraordinary nature of these findings that their credibility must be questioned; the upcoming release of genomic data from an additional nine B. bacilliformis isolates will provide a broader insight into intraspecies genomic diversity and put the results described above into better context.
Diagnostics and Identification
Diagnostic symptoms of OF include an extraordinarily low erythrocyte count (approximately 500,000/mm3) [33], [77], [148] and numerous infected erythrocytes in blood smears prepared with Wright's, Giemsa, or eosin/thiazine stains [32], [121] (Figure 1A). Blood smears have served as a standard diagnostic tool for Carrión's disease for decades, due to low cost and ease of use. However, the test suffers from low sensitivity, despite its high specificity [4]. Diagnoses can also be made based on blood culture, PCR, immunological tests, or a combination thereof (see below). Diagnosis of VP is made by the occurrence of cutaneous angiomatous skin lesions. As in BA, the histopathology of verrugae reveal bacteria, albeit with difficulty [40], when sectioned and stained with Warthin-Starry silver stain [149] or Giemsa stain [32]. A patient's age (especially <5 years old) and whether bartonellosis has recently occurred within the family are also useful predictors of a B. bacilliformis infection [24].
Growth requirements and physiology
Bartonellae can be isolated from patient blood by preparing samples with a lysis-centrifugation method [150] or a freeze-thaw protocol [151]. Primary isolates of Bartonella species have also been obtained using a BACTEC blood culture system (Becton Dickinson) and Bartonella alphaproteobacteria growth medium (BAPGM) [152], [153]. Homogenized tissues may also be used as an inoculum for blood plates or endothelial cell monolayers [154].
The slow growth rate of B. bacilliformis (approximately7-hour generation time in vitro [34]) can complicate identification and requires that isolates be cultured several days to visualize colonies. Most bartonellae are microaerophilic, capnophilic (5% CO2), and grown at approximately 37°C, whereas B. bacilliformis requires cooler temperatures (25°C–30°C) and ambient CO2 for culture. In addition, humidity of the incubator should be near 100%. Like other Bartonella species, B. bacilliformis cannot synthesize protoporphyrin IX (PPIX) or heme (Fe2+-PPIX), and the essential nutrient is obtained from hemin (Fe3+-PPIX), hemoglobin, and host erythrocytes. Interestingly, Bartonella cannot utilize heme-rich scavenger molecules of the host, such as lactoferrin or transferrin, unlike many pathogens. Commonly used growth media for Bartonella species include chocolate agar, hemin agar, or various blood agars (for recipes, see [155]). The basal medium of these formulations varies and includes Brucella medium, trypticase soy, GC, heart infusion, or Columbia agars. Media supplements often include erythrocytes (sheep, rabbit, etc.), hemoglobin, IsoVitalex (Becton Dickinson), and serum (sheep). The medium should be pH 7.0–7.5 for optimal growth.
Colony and cellular morphology, structure
B. bacilliformis is a gram-negative, non–acid fast, pleomorphic coccobacillus. Bacteria stain well with Giemsa or Gimenez stains. Most cells measure 0.5 µm wide by 1.0 µm long. Electron micrographs of B. bacilliformis are shown in Figure 5. B. bacilliformis colonies are typically very small (initially pinpoint but growing to 0.2–1 mm in diameter after several days), round, and lenticular with an even margin and a raised center in older colonies, and range from translucent to opaque. Although colonies from primary isolates can take weeks to become visible, growth is much more rapid (three to seven days to maximal size) in subsequent passages. Colony color can vary on different media but is typically clear to creamy white. B. bacilliformis is highly motile by unipolar, lophotrichous flagella, a phenotype shared with B. clarridgeiae and B. rochalimae [39], [56], [156]. Twitching motility, as observed in low-passage B. henselae and B. quintana [58], [134], has not been reported. Capsules and endospores are not present in B. bacilliformis, in keeping with other Bartonella species.
On the whole, the cellular architecture and composition of bartonellae are typical of gram-negative bacteria [157]. Notable exceptions include the unusual cellular fatty acids (CFAs) (see “Cellular Fatty Acid Analysis,” below) and architecture and immunogenicity of its lipopolysaccharide (LPS). A “deep rough” LPS chemotype (little if any O-chain polysaccharide is present), was first reported for B. bacilliformis [158], [159]. Later, similar chemotypes were reported for B. quintana [160] and B. henselae [161]. Further investigation of B. henselae's LPS showed that it contains a unique inner oligosaccharide core of two keto-deoxyoctulosonic acids (KDOs) and one glucose molecule [161]. Its pentaacyl lipid A backbone is also unusual; instead of the typical glucosamine sugars, it contains 2,3-diamino-2,3-dideoxy-glucose dissacharide (GlcN3N) with both sugars phosphorylated. Interestingly, lipid A of B. henselae's LPS possesses a long-chain fatty acid (C26:0 or C28:0) similar to other intracellular pathogens, including Legionella and Chlamydia species [161]. It is tempting to speculate that B. henselae's LPS is representative of all Bartonella, especially considering the uniform LPS chemotype for the genus. Finally, work has shown that Bartonella endotoxin is considerably less toxic relative to LPS from other gram-negative bacteria, and its activation of TLR-4 pathways is significantly (1,000–10,000-fold) lower than Salmonella's LPS [161]. In fact, recent work has shown that B. quintana LPS is a potent antagonist of TLR-4-mediated activation of human monocytes by E. coli LPS [162]. Whether a relatively low level of endotoxicity and TLR-4 antagonist activity holds for B. bacilliformis' LPS is unknown, but would not be surprising.
The cellular and subcellular protein content of Bartonella species has been analyzed by several groups over the years. Early work showed that the B. bacilliformis outer membrane fraction contains 14 proteins of 11.2 to 75.3 kDa in mass [159], as compared to nine surface-exposed proteins in B. henselae [163]. The proteomes of whole cells and subcellular fractions of B. quintana and B. henselae have been analyzed by two-dimensional gel electrophoresis and MALDI-TOF-MS of the protein spots. For example, two studies identified 44 [164] and 53 [165] proteins associated with the B. henselae outer membrane fractions. A third study analyzed the B. quintana membrane subproteome and found 60 proteins [166]. Finally, analysis of B. henselae's total proteome identified 191 different proteins (431 spots) [167]. Unfortunately, a comprehensive analysis of the B. bacilliformis proteome has not been done, to date.
Biochemical tests
Bartonellae are nonfermentative, aerobic (microaerophilic) bacteria with a relatively unremarkable physiology. Thus, conventional biochemical tests are not particularly helpful in presumptive identification of species. One problem with using standard biochemical tests on Bartonella species is that hemin is normally not included in the medium, and results must be judged cautiously. Notwithstanding this caveat, standard biochemical tests show that B. bacilliformis is strictly aerobic and does not utilize carbohydrates by preformed or de novo enzymes. Tests for oxidase, Voges-Proskauer, catalase, indole production, nitrate reduction, urease activity, hippurate hydrolysis, succinate, alkaline phosphatase, tetrathionate reductase, pyrazinamidase, tributyrin, o-nitrophenyl-β-D-galactoside, esculin hydrolysis, and arginine dihydrolase are all negative [136], [156], [168], [169]. However, tests for peptidase activity on L-proline and L-lysine (acid) are useful in presumptive, differential identification of B. bacilliformis, which is negative for proline and positive for lysine utilization [136], [156], [168], [170]. Screens are typically done in multitest formats designed for anaerobes, such as the MicroScan Rapid Anaerobe identification panel (Dade Behring).
Polymerase chain reaction (PCR)
PCR is sensitive and specific for identifying Bartonella species and can be used to confirm a presumptive identification. PCR can be done directly on affected tissues and/or blood or on enrichment cultures [171], [172]. In early work, PCR was used to amplify the 16S rRNA gene or fragments to generate a sequencing template. Resulting data were compared to known 16S rRNA gene sequences [154], [173]–[175]. However, the utility of the 16S RNA gene as a means of differentiating Bartonella species was limited due to conservation [137], [138]. More useful PCR-based strategies for genus and species identification have been described for serine protease (htrA) [176], gltA [139], [177], divisome protein (ftsZ) [178], riboflavin synthase (ribC) [179], NADH dehydrogenase gamma subunit (nuoG) [180], heme-binding protein A/Pap31 (hbpA/pap31) [181], tmRNA (ssrA) [182], RNA polymerase beta subunit (rpoB) [183], and the 16S–23S rRNA ITS [138], [184]. One recent report employed PCR, with rpoB as the target, and pyrosequencing of the resulting amplicons for rapid species identification, including B. bacilliformis [185]. Another used ialB as a PCR target and showed high sensitivity and specificity in 15 patients with OF [186]. Finally, a recent study demonstrated the utility of collecting dried-blood spots from patients in endemic areas, followed by quantitative PCR (qPCR). Results showed that 24.6% of 65 children studied were positive by qPCR on spots, whereas only 3% were blood-culture positive [187].
Cellular Fatty Acid (CFA) analysis
CFA analysis by gas chromatography has been used to identify Bartonella to the genus level. This is made possible by the group's atypical fatty acid composition. For example, predominant fatty acids of bartonellae include cis-11-octadecanoic acid (C18:1ω7c) (approximately 54%) and hexadecanoic acid (C16:0) (approximately 20%). However, B. bacilliformis is unusual in that it contains little octadecanoic acid (C18:0) (approximately 2%) compared to B. elizabethae, B. henselae, B. clarridgeiae, and B. quintana (approximately 20%). B. bacilliformis also has an unusually high amount (approximately 20%) of cis-11-hexadecanoic acid (C16:1ω7c) relative to other Bartonella species (typically <1%) [99], [150], [156], [188], [189].
Immunological tests
Immunological tests are, for the most part, sensitive and specific for Carrión's disease. However, positive results must be judged cautiously, as they may not indicate an active infection, but rather a persistent humoral immune response. The indirect fluorescence antibody (IFA) test has been used as a specific and sensitive diagnostic tool [190]. In addition, recent work with ELISAs containing recombinant LppB (43-kDa lipoprotein) showed a high degree of sensitivity and specificity in 27 confirmed patients with either chronic or acute disease [191]. Finally, an immunoblot-based protocol incorporating proteins from sonicated B. bacilliformis showed good sensitivity with sera from ten cases of acute disease and very high sensitivity using sera from 32 cases of chronic disease [192]. Likewise, work by Maguiña et al. showed a high degree of sensitivity in immunoblots probed with nine and 11 sera from patients with acute and chronic disease, respectively [193], [194]. It is clear that immunological tests are useful diagnostic tools for Carrión's disease, especially when combined with other techniques such as PCR and blood culture. However, immunodiagnostics for this disease are relatively underdeveloped.
Current Status of Treatment, Prevention and Control
Syndrome-dependent courses of therapy
Analysis of antimicrobial susceptibilities of four strains of B. bacilliformis showed that the bacterium is extremely sensitive to a wide array of drugs in vitro, including aminoglycosides, beta-lactams, macrolides, tetracyclines, fluoroquinolones, chloramphenicol, clorimoxazole, and rifampin [195]. However, outcomes of antimicrobial therapy in patients with Carrión's disease have not been as clear-cut because of several confounding factors, including patient history, immunosuppression, frequent secondary infections, and persistent or recurring bartonellosis despite therapeutic intervention.
Antimicrobial therapy for Carrión's disease is predicated on whether disease is acute or chronic. Acute disease has been treated with chloramphenicol for decades [2], [14], [193], [196]–[199], sometimes in combination with other antimicrobials [193]. One apparently effective mixture is chloramphenicol plus a beta-lactam [197]. The choice of chloramphenicol was undoubtedly made popular due to its effectiveness, low cost, and broad spectrum of activity against common co-infectious agents, such as salmonellae [37], [193]. Tetracyclines (doxycycline), beta-lactams (ampicillin, penicillin G), macrolides (erythromycin, roxithromycin), trimethoprim-sulfamethoxazole, and fluoroquinolones (norfloxacin, ciprofloxacin) have been used as alternatives to treat OF [15], [41], [196], [197]. Although ciprofloxacin holds great promise for treating acute cases, it should be used judiciously, as resistance mutants arise rapidly in vitro [200], [201], and intrinsic resistance to the drug has been reported in strains that were obtained prior to widespread use of fluoroquinolones [202]. Interestingly, in addition to antimicrobial therapy, blood transfusions have been employed for treating severe anemia of OF [36], [203].
Chronic disease is usually treated with rifampin, although streptomycin is also effective and was the drug-of-choice prior to 1975 [5], [193], [197], [199]. Of note is that VP does not respond to either chloramphenicol or penicillin treatment [15], [32]. Ciprofloxacin, azithromycin, and sultamicillin, combined with deflazacort, have been used as alternatives to treat VP [4], [5], [204].
Prevention and control measures
No vaccine is currently available for B. bacilliformis. Early work investigated a composite vaccine prepared from four strains of the bacterium that were grown axenically and formalin-inactivated (0.4% formalin in physiological saline) [205]. Immunizations were administered (one to three times subcutaneously) to 22 men stationed for one month at a Peruvian military outpost located in the “verruga zone”’ near Matucana, Peru. Nineteen of the men seroconverted during the study. Twelve men (55% of test group) became blood-culture positive over the course of exposure and eventually presented with symptoms of bartonellosis that were, for the most part, relatively mild. Unvaccinated control groups, prior to and after the study, showed approximately 75% (anecdotal) and 90% infection rates, respectively, with many incapacitated due to severity of illness [205]. Although preliminary in nature, this pilot study suggests that antibodies generated against formalin-killed B. bacilliformis reduce disease severity in humans but do not provide full protection against infection. Reasons for the partial protection are unclear, but may be due to strain-specific antigenic variation, a humoral immune response bias against the vaccine formulation, or modification of important protective antigens by formalin cross-linkage.
In the absence of a vaccine, control of the sand fly vector has been the fallback course of action in endemic areas. As early as World War II, Peruvian army personnel were provided with bed nets and given Vaseline-citronella ointments as a means of warding off sand flies [205]. Early work in endemic areas of Peru showed that DDT insecticide (5% w/v in kerosene) sprayed in and around dwellings was effective at killing sand flies and kept the insects at very low levels for up to four months [206]. The reduction of sand fly numbers “to the vanishing point” using DDT also correlated to a “virtual cessation of bartonellosis” in construction camps within endemic areas of Peru, although exact numbers were not reported [206]. More recent, unpublished results of sand fly control using residual pyrethroid insecticides in homes near Ancash, Peru are promising, as the duration of control was increased to a year (as reported in [4]). Moreover, others have reported good success with residual pyrethroids against old-world sand flies [207]. In any case, control measures using insecticides may prove to be challenging, especially considering that Lutzomyia larvae develop in largely unknown terrestrial habitats, and adult flies, when not resting in houses or animal sheds, dwell in deep cracks and crevices during daylight hours; sites that are not readily accessible to superficial application of insecticides.
In Colombia, other interventions including pyrethroid-impregnated bednets and curtains were used as practical means of controlling sand flies entering houses [208]. In Italy and Burkina Faso, permethrin-impregnated curtains reduced the numbers of endophilic phlebotomine sand flies by 90% and 95%, respectively [209], [210]. However, because peak biting activity occurs shortly after sundown and prior to the time when human inhabitants usually retire for the night, impregnated bednets and curtains may have minimal effect in interrupting sand fly–to–human transmission [211]. Furthermore, it has been shown that sand flies can pass through the mesh of an impregnated bednet and bite a host before succumbing to insecticide exposure (Personal communication, Dr. Edgar Rowton, unpublished data). Nevertheless, if the use of impregnated bednets and curtains can shorten the lives of sand flies, and thereby suppress the vector population, the risk of infection may be reduced [211]. Varying results of field studies dictate the need for trials against specific target vectors before adopting this type of intervention as a means of control for B. bacilliformis vectors.
Field trials conducted in Israel and Kenya showed that sand flies will feed on sugar sprayed on plants in the flies' natural habitats, suggesting a possible means for controlling sand flies with toxic baits [212]. This finding prompted a pilot study in Kenya to control sand flies resting in rodent burrows and eroded termitaria by spraying vegetation with sugar solution containing Bacillus sphaericus and a dye to reveal that the flies had fed on the sugar [213]. Marked flies were caught entering the rodent burrows and termitaria, and the number of flies emerging from the rodent burrows declined significantly [213]. More recently, scientists at Louisiana State University evaluated several chemical insecticide-treated sugar baits and concluded that they could be efficacious in controlling adult sand fly populations with targeted use in specific habitats [214].
In many foci, sand flies are closely associated with rodents and have been recovered entering and exiting rodent burrows (personal observation, PGL). Burrows of many rodents are the suspected habitats of the immature stages [215]. In fact, sand fly larvae have been observed feeding on rodent feces inside a burrow [216]. Both adult and immature stages benefit from the microhabitat created within rodent burrows [211]. Recently, novel research has focused on sand fly control methods that capitalize on the close ecological interaction between sand flies and rodents. Feed-through or systemic insecticides such as ivermectin or spinosad incorporated into rodent baits have been shown to cause 100% mortality of bloodfeeding sand flies for at least one week [217], [218]. In follow-on studies, it was shown that rodents fed on fipronil produced feces that were toxic to coprophagous larvae for up to 21 days. An approach using rodent baits containing fipronil could significantly suppress vector populations that originate in the vicinity of rodent populations, eliminating the portion of the adult population that feeds on the rodents and immature stages that feed on the toxic feces [219].
Future Prospects
In many respects, Carrión's disease is a “poster-child” for neglected tropical diseases. The illness is endemic to remote, rural areas of South America, and afflicted populations are typically indigent and lack ready access to transportation, modern means of communication, and healthcare. Not surprisingly, few people outside the Andes region are even aware of the disease and the potential public health crisis posed by its spread. Climatic change and vectoring of Carrión's disease by alternate sand fly vectors have undoubtedly contributed to the spread and re-emergence of B. bacilliformis. While immediate remediation of climate change is not realistic, improved sand fly control measures in and around homes, especially during the rainy season, and enhanced surveillance and formation of a multinational database to follow the epidemiology of Carrión's disease in endemic and proximal, nonendemic areas of South America is achievable and clearly warranted. Once identified, an adequate response to the outbreak will require more effective-yet-inexpensive diagnostics that can be done rapidly in rural settings with minimal training and modest laboratory equipment. Solid-phase immunodiagnostics (e.g., ELISA, fluorescence microscopy) seem especially well suited for development in the near-term and could be used in tandem with blood smears to rapidly diagnose Carrión's disease. Patient samples (e.g., dried blood spots as in [187]) could then be submitted to regional reference labs for confirmation of presumptive identification using blood culture and/or PCR-based techniques. Fortunately, once B. bacilliformis is diagnosed, it is susceptible to several antimicrobials and treatable. Nevertheless, in view of the logistic problems surrounding patient inaccessibility to healthcare, communication, and transportation to urban areas, the development of a protective vaccine is clearly warranted. To this end, both B- and T-cell epitopes of B. bacilliformis must be identified to allow for the generation of a protective, multivalent subunit vaccine that invokes both humoral and cellular immune responses. The human-restricted reservoir and infection bode well for the vaccine-mediated eradication of Carrión's disease. The key obstacle to overcome to realize this goal is a considerable quantity of capital for research and development.
Key Learning Points
B. bacilliformis is a sand fly–vectored bacterium responsible for an emerging infectious disease of humans that is endemic to South America.
B. bacilliformis is the most virulent Bartonella species and causes two disparate syndromes; a life-threatening, acute infection of erythrocytes (OF) and a comparatively benign, chronic infection of the vascular endothelium (VP).
The genome of B. bacilliformis has undergone extensive reduction in response to specialization related to its human-only parasitism and reservoir.
Virulence determinants are understudied but include adhesins (e.g., Bartonella repeat proteins), flagella, a deformation factor for erythrocyte membranes (deformin), an invasion-associated locus encoding two proteins (ialAB), a mitogenic protein that may facilitate angiogenesis, and two heme-acquisition systems (Hut and Hbp's).
Improved diagnostics, better sand fly control measures, enhanced monitoring and surveillance for outbreaks, and a protective vaccine are urgently needed to control Carrión's disease in South America.
Top Five Papers in Field
Foundations: Benson LA, Kar S, McLaughlin G, Ihler GM (1986) Entry of Bartonella bacilliformis into erythrocytes. Infect Immun 54: 347–353.
Angiogenesis: Minnick MF, Smitherman LS, Samuels DS (2003) Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun 71: 6933–6942.
Epidemiology: Chamberlin J, Laughlin LW, Romero S, Solórzano N, Gordon S, et al. (2002) Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis 186: 983–990.
Phylogeny: Chaloner GL, Ventosilla P, Birtles RJ (2011) Multi-locus sequence analysis reveals profound genetic diversity among isolates of the human pathogen Bartonella bacilliformis. PLoS Negl Trop Dis 5: e1248.
Control: Sanchez Clemente N, Ugarte-Gil CA, Solórzano N, Maguiña C, Pachas P, et al. (2012) Bartonella bacilliformis: a systematic review of the literature to guide the research agenda for elimination. PLoS Negl Trop Dis 6: e1819.
Funding Statement
MFM is supported by NIH grant R21 AI095804. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hertig M (1942) Phlebotomus and Carrión's Disease. Am J Trop Med Hyg S1-22: 2–81. [Google Scholar]
- 2.Weinman D (1965) The bartonella group. In: Dubos RJ, Hirsch JG, editors. Bacterial and Mycotic Infections of Man. Philadelphia: Lippincott. pp. 775–785. [Google Scholar]
- 3. Birtles RJ, Canales J, Ventosilla P, Alvarez E, Guerra H, et al. (1999) Survey of Bartonella species infecting intradomicillary animals in the Huayllacallán Valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg 60: 799–805. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez Clemente N, Ugarte-Gil CA, Solórzano N, Maguiña C, Pachas P, et al. (2012) Bartonella bacilliformis: a systematic review of the literature to guide the research agenda for elimination. PLoS Negl Trop Dis 6: e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maguiña C, Gotuzzo E (2000) Bartonellosis. New and old. Infect Dis Clin North Am 14: 1–22. [DOI] [PubMed] [Google Scholar]
- 6. Alexander B (1995) A review of bartonellosis in Ecuador and Colombia. Am J Trop Med Hyg 52: 354–359. [DOI] [PubMed] [Google Scholar]
- 7. Amano Y, Rumbea J, Knobloch J, Olson J, Kron M (1997) Bartonellosis in Ecuador: serosurvey and current status of cutaneous verrucous disease. Am J Trop Med Hyg 57: 174–179. [DOI] [PubMed] [Google Scholar]
- 8. Cooper P, Guderian R, Orellana P, Sandoval C, Olalla H, et al. (1997) An outbreak of bartonellosis in Zamora Chinchipe Province in Ecuador. Trans R Soc Trop Med Hyg 91: 544–546. [DOI] [PubMed] [Google Scholar]
- 9. Cooper P, Guderian R, Paredes W, Daniels R, Perera D, et al. (1996) Bartonellosis in Zamora Chinchipe province in Ecuador. Trans R Soc Trop Med Hyg 90: 241–243. [DOI] [PubMed] [Google Scholar]
- 10. Lydy SL, Eremeeva ME, Asnis D, Paddock CD, Nicholson WL, et al. (2008) Isolation and characterization of Bartonella bacilliformis from an expatriate Ecuadorian. J Clin Microbiol 46: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allison MJ, Pezzia A, Gerszten E, Mendoza D (1974) A case of Carrión's disease associated with human sacrifice from the Huari culture of Southern Peru. Am J Phys Anthropol 41: 295–300. [DOI] [PubMed] [Google Scholar]
- 12. Schultz MG (1968) A history of bartonellosis (Carrión's disease). Am J Trop Med Hyg 17: 503–515. [DOI] [PubMed] [Google Scholar]
- 13.Pachas P (2000) Epidemiologia de la bartonelosis en el Peru. Módulos Técnicos, Oficina General de Epidemiología-Insituto Nacional de Salud. Lima, Peru. 83 p.
- 14. Gray GC, Johnson AA, Thornton SA, Smith WA, Knobloch J, et al. (1990) An epidemic of Oroya fever in the Peruvian Andes. Am J Trop Med Hyg 42: 215–221. [DOI] [PubMed] [Google Scholar]
- 15.Maguiña Vargas C (1998) Bartonellosis o enfermedad de Carrión. Nuevos aspectos de una vieja enfermedad. Lima, Peru: AFA Editores Importadores. 195 p. [Google Scholar]
- 16. Kosek M, Lavarello R, Gilman RH, Delgado J, Maguiña C, et al. (2000) Natural history of infection with Bartonella bacilliformis in a nonendemic population. J Infect Dis 182: 865–872. [DOI] [PubMed] [Google Scholar]
- 17. Ellis BA, Rotz LD, Leake JA, Samalvides F, Bernable J, et al. (1999) An outbreak of acute bartonellosis (Oroya fever) in the Urubamba region of Peru, 1998. Am J Trop Med Hyg 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 18. Montoya M, Maguiña C, Vigo B, Caparo R, Briceño E, et al. (1998) Brote epidémico de enfermedad de Carrión en el Valle Sagrado de los Incas (Cuzco). Bol Soc Peru Med Int 11: 170–176. [Google Scholar]
- 19. Maco V, Maguiña C, Tirado A, Maco V, Vidal JE (2004) Carrión's disease (Bartonellosis bacilliformis) confirmed by histopathology in the high forest of Peru. Rev Inst Med Trop Sao Paulo 46: 171–174. [DOI] [PubMed] [Google Scholar]
- 20. Solano L, Morocho L, Bueno C, Cesar V, Villalta MC, et al. (1993) Investigación de Bartonelosis en el Valle de Puchka, Provincia de Huari, Ancash-Perú. Rev Per Med Trop 7: 13–25. [Google Scholar]
- 21. Huarcaya Castilla E, Chinga Alayo E, Chavez P, Juan M, Chauca Carhuajulca J, et al. (2004) Influencia del fenómeno de El Niño en la epidemiología de la bartonelosis humana en los departamentos de Ancash y Cusco entre 1996 y 1999. Rev Med Hered 15: 4–10. [Google Scholar]
- 22. Huarcaya E, Maguiña C, Torres R, Rupay J, Fuentes L (2004) Bartonelosis (Carrion's disease) in the pediatric population of Peru: an overview and update. Braz J Infect Dis 8: 331–339. [DOI] [PubMed] [Google Scholar]
- 23. Pachas P (2005) Situación de la enfermedad de Carrión en el Peru. International Conference of Human Bartonellosis March 21–23, 2005; Lima, Peru. [Google Scholar]
- 24. Chamberlin J, Laughlin LW, Romero S, Solórzano N, Gordon S, et al. (2002) Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis 186: 983–990. [DOI] [PubMed] [Google Scholar]
- 25. Chinga-Alayo E, Huarcaya E, Nasarre C, del Aguila R, Llanos-Cuentas A (2004) The influence of climate on the epidemiology of bartonellosis in Ancash, Peru. Trans R Soc Trop Med Hyg 98: 116–124. [DOI] [PubMed] [Google Scholar]
- 26. Zhou J, Lau WK-M, Masuoka PM, Andre RG, Chamberlin J, et al. (2002) El Niño helps spread bartonellosis epidemics in Peru. Eos Trans Am Geophys Union 83: 157–161. [Google Scholar]
- 27. Caceres AG (1993) Geographic distribution of Lutzomyia verrucarum (Townsend, 1913) (Diptera, Psychodidae, Phlebotaminae), vector of human bartonellosis in Peru. Rev Inst Med Trop Sao Paulo 35: 485–490. [DOI] [PubMed] [Google Scholar]
- 28. Caceres AG, Galati EA, Le Pont F, Velasquez C (1997) Possible role of Lutzomyia maranonensis and Lutzomyia robusta (Diptera: Psychodidae) as vectors of human bartonellosis in three provinces of region nor Oriental del Marañon, Peru. Rev Inst Med Trop Sao Paulo 39: 51–52. [DOI] [PubMed] [Google Scholar]
- 29. Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, et al. (2009) Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 40: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matteeli A, Castelli F, Spinetti A, Bonetti F, Graifenberghi S, et al. (1994) Short report: verruga peruana in an Italian traveler from Peru. Am J Trop Med Hyg 50: 143–144. [DOI] [PubMed] [Google Scholar]
- 31.Montoya M (2003) Características clínicas, epidemiológicas, laboratoriales y factores de riesgo en pacientes con bartonellosis aguda complicada. [Tesis de maestría]. Lima: Facultad de Medicina, Universidad Peruana Cayetano Heredia. [Google Scholar]
- 32. Maguiña C, Guerra H, Ventosilla P (2009) Bartonellosis. Clin Dermatol 27: 271–280. [DOI] [PubMed] [Google Scholar]
- 33. Reynafarje C, Ramos J (1961) The hemolytic anemia of human bartonellosis. Blood 17: 562–578. [PubMed] [Google Scholar]
- 34. Benson LA, Kar S, McLaughlin G, Ihler GM (1986) Entry of Bartonella bacilliformis into erythrocytes. Infect Immun 54: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Caceres U, Garcia FU (1991) Bartonellosis. An immunodepressive disease and the life of Daniel Alcides Carrión. Am J Clin Pathol 95: S58–66. [PubMed] [Google Scholar]
- 36. Breña Chávez JP, Maguiña Vargas CP, Hernandez Diaz HR, Castillo Diaz ME, Pozo Tovar WE (2006) Bartonelosis aguda en niños: estudio de 32 casos en el Instituto Especializado de Salud del Niño y el Hospital Nacional Cayetano Heredia (período 1993–2003). Rev Med Hered 17: 122–131. [Google Scholar]
- 37. Cuadra MS (1956) Salmonellosis complication in human bartonellosis. Tex Rep Biol Med 14: 97–113. [PubMed] [Google Scholar]
- 38.Maguiña C (1993) Estudio clinico de 145 casos de bartonelosis en el Hospital Nacional Cayetano Heredia [Tesis doctoral]. Lima: Facultad de Medicina, Universidad Peruana Cayetano Heredia. [Google Scholar]
- 39. Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, et al. (2007) Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356: 2381–2387. [DOI] [PubMed] [Google Scholar]
- 40. Arias-Stella J, Lieberman PH, Erlandson RA, Arias-Stella JJ (1986) Histology, immunohistochemistry, and ultrastructure of the verruga in Carrión's disease. Am J Surg Pathol 10: 595–610. [DOI] [PubMed] [Google Scholar]
- 41. Bass JW, Vincent JM, Person DA (1997) The expanding spectrum of Bartonella infections. I. Bartonellosis and trench fever. Pediatr Infect Dis J 16: 2–10. [DOI] [PubMed] [Google Scholar]
- 42. Blazes DL, Mullins K, Smoak BL, Jiang J, Canal E, et al. (2013) Novel Bartonella agent as cause of verruga peruana. Emerg Infect Dis 19: 1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Townsend CH (1913) A Phlebotomus the practically certain carrier of verruga. Science 38: 194–195. [DOI] [PubMed] [Google Scholar]
- 44. Townsend CH (1913) Preliminary characterization of the vector of verruga, Phlebotomus verrucarum sp. nov. Insecutor Inscitiae Menstruus 1: 107–108. [Google Scholar]
- 45. Townsend CH (1914) The Relation between lizards and Phlebotomus verrucarum as indicating the reservoir of verruga. Science 40: 212–214. [DOI] [PubMed] [Google Scholar]
- 46. Killick-Kendrick R, Ward RD (1981) Ecology of Leishmania. Workshop No. 11. Parasitology 82: 143–152. [Google Scholar]
- 47. Noguchi H, Battistini TS (1926) Etiology of Oroya fever: I. Cultivation of Bartonella bacilliformis . J Exp Med 43: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noguchi H, Shannon RC, Tilden EB, Tyler JR (1929) Etiology of Oroya fever: XIV. The insect vectors of Carrión's disease. J Exp Med 49: 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battistini TS (1927) Estudio sobre la verruga. Bol Direc Salubr Pub., 2nd Sem. 1926; Lima. pp. 191–197.
- 50.Battistini TS (1929) Estudios sobre la verruga peruana. La Acción Médica, Lima, Jan.
- 51. Battistini TS (1931) La verrue peruvienne: sa transmission par le Phlébotome. Rev Sud-Amer Med Chir 2: 719–724. [Google Scholar]
- 52. Tejada A, Vizcarra H, Perez J, Caceres A, Quispe J, et al. (2003) Estudio clinico epidemiológico de bartonelosis humana en el valle del Monzón, Huamalíes, Huánuco. An Fac Med 64: 211–217. [Google Scholar]
- 53. Lawyer PG, Andre RG, Fernandez R, Carrasco J, Curo JL, et al. (2005) Spatial and temporal dynamics of Lutzomyia peruensis (Diptera:Psychodidae) in an epidemic focus of human bartonellosis, Proceedings of the Fifth International Symposium on Phlebotomine Sand Flies. Archives De l'Institut Pasteur De Tunis 82 (Special Issue) 53. [Google Scholar]
- 54. Ogusuku E, Perez JE, Paz L, Nieto E, Monje J, et al. (1994) Identification of bloodmeal sources of Lutzomyia spp. in Peru. Ann Trop Med Parasitol 88: 329–335. [DOI] [PubMed] [Google Scholar]
- 55. Li H, Liu W, Zhang GZ, Sun ZZ, Bai JY, et al. (2013) Transmission and maintenance cycle of Bartonella quintana among rhesus macaques, China. Emerg Infect Dis 19: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scherer DC, DeBuron-Connors I, Minnick MF (1993) Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun 61: 4962–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hill EM, Raji A, Valenzuela MS, Garcia F, Hoover R (1992) Adhesion to and invasion of cultured human cells by Bartonella bacilliformis . Infect Immun 60: 4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Batterman HJ, Peek JA, Loutit JS, Falkow S, Tompkins LS (1995) Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun 63: 4553–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dehio C, Meyer M, Berger J, Schwarz H, Lanz C (1997) Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci 110: 2141–2154. [DOI] [PubMed] [Google Scholar]
- 60. Brouqui P, Raoult D (1996) Bartonella quintana invades and multiplies within endothelial cells in vitro and in vivo and forms intracellular blebs. Res Microbiol 147: 719–731. [DOI] [PubMed] [Google Scholar]
- 61. Musso T, Badolato R, Ravarino D, Stornello S, Panzanelli P, et al. (2001) Interaction of Bartonella henselae with the murine macrophage cell line J774: infection and proinflammatory response. Infect Immun 69: 5974–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Riess T, Andersson SG, Lupas A, Schaller M, Schäfer A, et al. (2004) Bartonella adhesin a mediates a proangiogenic host cell response. J Exp Med 200: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang P, Chomel BB, Schau MK, Goo JS, Droz S, et al. (2004) A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana . Proc Natl Acad Sci U S A 101: 13630–13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaiser PO, Riess T, O'Rourke F, Linke D, Kempf VA (2011) Bartonella spp.: throwing light on uncommon human infections. Int J Med Microbiol 301: 7–15. [DOI] [PubMed] [Google Scholar]
- 65. Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA (2006) Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol 14: 264–270. [DOI] [PubMed] [Google Scholar]
- 66. Kaiser PO, Riess T, Wagner CL, Linke D, Lupas AN, et al. (2008) The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol 10: 2223–2234. [DOI] [PubMed] [Google Scholar]
- 67. O'Rourke F, Schmidgen T, Kaiser PO, Linke D, Kempf VA (2011) Adhesins of Bartonella spp. Adv Exp Med Biol 715: 51–70. [DOI] [PubMed] [Google Scholar]
- 68. Kempf VA, Volkmann B, Schaller M, Sander CA, Alitalo K, et al. (2001) Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol 3: 623–632. [DOI] [PubMed] [Google Scholar]
- 69. Kempf VA, Lebiedziejewski M, Alitalo K, Wälzlein JH, Ehehalt U, et al. (2005) Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 70. Schulte B, Linke D, Klumpp S, Schaller M, Riess T, et al. (2006) Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect Immun 74: 5003–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Müller NF, Kaiser PO, Linke D, Schwarz H, Riess T, et al. (2011) Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect Immun 79: 2544–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walker TS, Winkler HH (1981) Bartonella bacilliformis: colonial types and erythrocyte adherence. Infect Immun 31: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iwaki-Egawa S, Ihler GM (1997) Comparison of the abilities of proteins from Bartonella bacilliformis and Bartonella henselae to deform red cell membranes and to bind to red cell ghost proteins. FEMS Microbiol Lett 157: 207–217. [DOI] [PubMed] [Google Scholar]
- 74. Dehio C (2008) Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cell Microbiol 10: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, Cescau S, et al. (2010) The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog 6: e1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deng HK, Le Rhun D, Le Naour E, Bonnet S, Vayssier-Taussat M (2012) Identification of Bartonella Trw host-specific receptor on erythrocytes. PLoS ONE 7: e41447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kreier JP, Ristic M (1981) The biology of hemotrophic bacteria. Annu Rev Microbiol 35: 325–338. [DOI] [PubMed] [Google Scholar]
- 78. Mernaugh G, Ihler GM (1992) Deformation factor: an extracellular protein synthesized by Bartonella bacilliformis that deforms erythrocyte membranes. Infect Immun 60: 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu YH, Lu ZY, Ihler GM (1995) Purification of deformin, an extracellular protein synthesized by Bartonella bacilliformis which causes deformation of erythrocyte membranes. Biochim Biophys Acta 1234: 173–183. [DOI] [PubMed] [Google Scholar]
- 80. Derrick SC, Ihler GM (2001) Deformin, a substance found in Bartonella bacilliformis culture supernatants, is a small, hydrophobic molecule with an affinity for albumin. Blood Cells Mol Dis 27: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 81. Hendrix LR, Kiss K (2003) Studies on the identification of deforming factor from Bartonella bacilliformis . Ann NY Acad Sci 990: 596–604. [DOI] [PubMed] [Google Scholar]
- 82. Mitchell SJ, Minnick MF (1995) Characterization of a two-gene locus from Bartonella bacilliformis associated with the ability to invade human erythrocytes. Infect Immun 63: 1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cartwright JL, Britton P, Minnick MF, McLennan AG (1999) The IalA invasion gene of Bartonella bacilliformis encodes a (de)nucleoside polyphosphate hydrolase of the MutT motif family and has homologs in other invasive bacteria. Biochem Biophys Res Commun 256: 474–479. [DOI] [PubMed] [Google Scholar]
- 84. Miller VL, Bliska JB, Falkow S (1990) Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol 172: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heffernan EJ, Wu L, Louie J, Okamoto S, Fierer J, et al. (1994) Specificity of the complement resistance and cell association phenotypes encoded by the outer membrane protein genes rck from Salmonella typhimurium and ail from Yersinia enterocolitica . Infect Immun 62: 5183–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rhomberg TA, Truttmann MC, Guye P, Ellner Y, Dehio C (2009) A translocated protein of Bartonella henselae interferes with endocytic uptake of individual bacteria and triggers uptake of large bacterial aggregates via the invasome. Cell Microbiol 11: 927–945. [DOI] [PubMed] [Google Scholar]
- 87. Fuhrmann O, Arvand M, Göhler A, Schmid M, Krüll M, et al. (2001) Bartonella henselae induces NF-kappaB-dependent upregulation of adhesion molecules in cultured human endothelial cells: possible role of outer membrane proteins as pathogenic factors. Infect Immun 69: 5088–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmid MC, Schulein R, Dehio M, Denecker G, Carena I, et al. (2004) The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol Microbiol 52: 81–92. [DOI] [PubMed] [Google Scholar]
- 89. Scheidegger F, Ellner Y, Guye P, Rhomberg TA, Weber H, et al. (2009) Distinct activities of Bartonella henselae type IV secretion effector proteins modulate capillary-like sprout formation. Cell Microbiol 11: 1088–1101. [DOI] [PubMed] [Google Scholar]
- 90. Harms A, Dehio C (2012) Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev 25: 42–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kempf VA, Schaller M, Behrendt S, Volkmann B, Aepfelbacher M, et al. (2000) Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell Microbiol 2: 431–441. [DOI] [PubMed] [Google Scholar]
- 92. Carroll JA, Coleman SA, Smitherman LS, Minnick MF (2000) Hemin-binding surface protein from Bartonella quintana . Infect Immun 68: 6750–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Battisti JM, Sappington KN, Smitherman LS, Parrow NL, Minnick MF (2006) Environmental signals generate a differential and coordinated expression of the heme receptor gene family of Bartonella quintana . Infect Immun 74: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Minnick MF, Sappington KN, Smitherman LS, Andersson SG, Karlberg O, et al. (2003) Five-member gene family of Bartonella quintana . Infect Immun 71: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Battisti JM, Smitherman LS, Sappington KN, Parrow NL, Raghavan R, et al. (2007) Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect Immun 75: 4373–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dabo SM, Confer AW, Anderson BE, Gupta S (2006) Bartonella henselae Pap31, an extracellular matrix adhesin, binds the fibronectin repeat III13 module. Infect Immun 74: 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Parrow NL, Abbott J, Lockwood AR, Battisti JM, Minnick MF (2009) Function, regulation, and transcriptional organization of the hemin utilization locus of Bartonella quintana . Infect Immun 77: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minnick MF (1997) Virulence determinants of Bartonella bacilliformis. In: Anderson B, Bendinelli M, Friedman H, editors. Rickettsial Infection and Immunity. New York: Plenum Press. pp. 197–211. [Google Scholar]
- 99. Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, et al. (1993) Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 31: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hendrix LR (2000) Contact-dependent hemolytic activity distinct from deforming activity of Bartonella bacilliformis . FEMS Microbiol Lett 182: 119–124. [DOI] [PubMed] [Google Scholar]
- 101. Webster GF, Cockerell CJ, Friedman-Kien AE (1992) The clinical spectrum of bacillary angiomatosis. Br J Dermatol 126: 535–541. [DOI] [PubMed] [Google Scholar]
- 102. Mohle-Boetani JC, Koehler JE, Berger TG, LeBoit PE, Kemper CA, et al. (1996) Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus: clinical characteristics in a case-control study. Clin Infect Dis 22: 794–800. [DOI] [PubMed] [Google Scholar]
- 103. Garcia FU, Wojta J, Broadley KN, Davidson JM, Hoover RL (1990) Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol 136: 1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 104. Cockerell CJ, LeBoit PE (1990) Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol 22: 501–512. [DOI] [PubMed] [Google Scholar]
- 105. Noguchi H, Muller HR, Tilden EB, Tyler JR (1929) Etiology of Oroya fever: XVI. Verruga in the dog and the donkey. J Exp Med 50: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bhutto AM, Nonaka S, Hashiguchi Y, Gomez EA (1994) Histopathological and electron microscopical features of skin lesions in a patient with bartonellosis (verruga peruana). J Dermatol 21: 178–184. [DOI] [PubMed] [Google Scholar]
- 107. Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9: 653–660. [DOI] [PubMed] [Google Scholar]
- 108. Noguchi H (1926) Etiology of Oroya fever: IV. The effect of inoculation of anthropoid apes with Bartonella bacilliformis. J Exp Med 44: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noguchi H (1926) Etiology of Oroya fever: III. The behavior of Bartonella bacilliformis in Macacus rhesus . J Exp Med 44: 697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Infante B, Villar S, Palma S, Merello J, Valencia R, et al. (2008) BALB/c Mice resist infection with Bartonella bacilliformis . BMC Res Notes 1: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chiaraviglio L, Duong S, Brown DA, Birtles RJ, Kirby J E (2010) An immunocompromised murine model of Bartonella infection. Am J Pathol 176: 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Minnick MF, Smitherman LS, Samuels DS (2003) Mitogenic effect of Bartonella bacilliformis on human vascular endothelial cells and involvement of GroEL. Infect Immun 71: 6933–6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. McCord AM, Cuevas J, Anderson BE (2007) Bartonella-induced endothelial cell proliferation is mediated by release of calcium from intracellular stores. DNA Cell Biol 26: 657–663. [DOI] [PubMed] [Google Scholar]
- 114. Kirby JE, Nekorchuk DM (2002) Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc Natl Acad Sci U S A 99: 4656–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cerimele F, Brown LF, Bravo F, Ihler GM, Kouadio P, et al. (2003) Infectious angiogenesis: Bartonella bacilliformis infection results in endothelial production of angiopoetin-2 and epidermal production of vascular endothelial growth factor. Am J Pathol 163: 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684. [DOI] [PubMed] [Google Scholar]
- 117. Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Activation of the HIF pathway in cancer. Curr Opin Genet Dev 11: 293–299. [DOI] [PubMed] [Google Scholar]
- 118. Schmid MC, Scheidegger F, Dehio M, Balmelle-Devaux N, Schulein R, et al. (2006) A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog 2: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McCord AM, Burgess AW, Whaley MJ, Anderson BE (2005) Interaction of Bartonella henselae with endothelial cells promotes monocyte/macrophage chemoattractant protein 1 gene expression and protein production and triggers monocyte migration. Infect Immun 73: 5735–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ricketts WE (1949) Clinical manifestations of Carrión's disease. Arch Intern Med (Chic) 84: 751–781. [DOI] [PubMed] [Google Scholar]
- 121. Knobloch J, Solano L, Alvarez O, Delgado E (1985) Antibodies to Bartonella bacilliformis as determined by fluorescence antibody test, indirect haemagglutination and ELISA. Trop Med Parasitol 36: 183–185. [PubMed] [Google Scholar]
- 122. Howe C (1943) Carrión's disease. Immunologic studies. Arch Intern Med (Chic) 72: 147–167. [Google Scholar]
- 123. Herrer A (1953) Carrión's disease. II. Presence of Bartonella bacilliformis in the peripheral blood of patients with the benign tumor form. Am J Trop Med 2: 645–649. [PubMed] [Google Scholar]
- 124. Guy L, Nystedt B, Toft C, Zaremba-Niedzwiedzka K, Berglund EC, et al. (2013) A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella . PLoS Genet 9: e1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Seubert A, Falch C, Birtles RJ, Schulein R, Dehio C (2003) Characterization of the cryptic plasmid pBGR1 from Bartonella grahamii and construction of a versatile Escherichia coli-Bartonella spp. shuttle cloning vector. Plasmid 49: 44–52. [DOI] [PubMed] [Google Scholar]
- 126. Saenz HL, Engel P, Stoeckli MC, Lanz C, Raddatz G, et al. (2007) Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet 39: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 127. Berglund EC, Frank AC, Calteau A, Vinnere Pettersson O, Granberg F, et al. (2009) Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii . PLoS Genet 5: e1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Saisongkorh W, Robert C, La Scola B, Raoult D, Rolain JM (2010) Evidence of transfer by conjugation of type IV secretion system genes between Bartonella species and Rhizobium radiobacter in amoeba. PLoS ONE 5: e12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Moran NA (2002) Microbial minimalism: genome reduction in bacterial pathogens. Cell 108: 583–586. [DOI] [PubMed] [Google Scholar]
- 130. Engel P, Salzburger W, Liesch M, Chang CC, Maruyama S, et al. (2011) Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella . PLoS Genet 7: e1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brenner DJ, O'Connor SP, Hollis DG, Weaver RE, Steigerwalt AG (1991) Molecular characterization and proposal of a neotype strain for Bartonella bacilliformis . J Clin Microbiol 29: 1299–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Birtles RJ, Harrison TG, Fry NK, Saunders NA, Taylor AG (1991) Taxonomic considerations of Bartonella bacilliformis based on phylogenetic and phenotypic characteristics. FEMS Microbiol Lett 67: 187–191. [DOI] [PubMed] [Google Scholar]
- 133. Birtles RJ, Harrison TG, Taylor AG (1991) The causative agent of bacillary angiomatosis.N Engl J Med. 325: 1447–1448. [DOI] [PubMed] [Google Scholar]
- 134. Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ (1992) Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol 30: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brenner DJ, O'Connor SP, Winkler HH, Steigerwalt AG (1993) Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol 43: 777–786. [DOI] [PubMed] [Google Scholar]
- 136. Birtles RJ, Harrison TG, Saunders NA, Molyneux DH (1995) Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol 45: 1–8. [DOI] [PubMed] [Google Scholar]
- 137. Kosoy M, Hayman DT, Chan KS (2012) Bartonella bacteria in nature: where does population variability end and a species start? Infect Genet Evol 12: 894–904. [DOI] [PubMed] [Google Scholar]
- 138. La Scola B, Zeaiter Z, Khamis A, Raoult D (2003) Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol 11: 318–321. [DOI] [PubMed] [Google Scholar]
- 139. Birtles RJ, Raoult D (1996) Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol 46: 891–897. [DOI] [PubMed] [Google Scholar]
- 140. Zeaiter Z, Fournier PE, Ogata H, Raoult D (2002) Phylogenetic classification of Bartonella species by comparing groEL sequences. Int J Syst Evol Microbiol 52: 165–171. [DOI] [PubMed] [Google Scholar]
- 141. Gundi VA, Taylor C, Raoult D, La Scola B (2009) Bartonella rattaustraliani sp. nov., Bartonella queenslandensis sp. nov. and Bartonella coopersplainsensis sp. nov., identified in Australian rats. Int J Syst Evol Microbiol 59: 2956–2961. [DOI] [PubMed] [Google Scholar]
- 142. Inoue K, Kabeya H, Shiratori H, Ueda K, Kosoy MY, et al. (2010) Bartonella japonica sp. nov. and Bartonella silvatica sp. nov., isolated from Apodemus mice. Int J Syst Evol Microbiol 60: 759–763. [DOI] [PubMed] [Google Scholar]
- 143. Alsmark CM, Frank AC, Karlberg EO, Legault BA, Ardell DH, et al. (2004) The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae . Proc Natl Acad Sci U S A 101: 9716–9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Birtles RJ, Fry NK, Ventosilla P, Cáceres AG, Sánchez E, et al. (2002) Identification of Bartonella bacilliformis genotypes and their relevance to epidemiological investigations of human bartonellosis. J Clin Microbiol 40: 3606–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hambuch TM, Handley SA, Ellis B, Chamberlin J, Romero S, et al. (2004) Population genetic analysis of Bartonella bacilliformis isolates from areas of Peru where Carrión's disease is endemic and epidemic. J Clin Microbiol 42: 3675–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chaloner GL, Ventosilla P, Birtles RJ (2011) Multi-locus sequence analysis reveals profound genetic diversity among isolates of the human pathogen Bartonella bacilliformis . PLoS Negl Trop Dis 5: e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tarazona D, Padilla C, Cáceres O, Montenegro JD, Bailón H (2013) Whole Genome Sequencing and Comparative Analysis of Bartonella bacilliformis Strain INS, the Causative Agent of Carrión's Disease. Genome Announc 1: e00053–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hurtado A, Musso JP, Merino C (1938) La anemia en la enfermedad de Carrión (verruga peruana). An Fac Med Lima 28: 154–168. [Google Scholar]
- 149. Cockerell CJ, Tierno PM, Friedman-Kien AE, Kim KS (1991) Clinical, histologic, microbiologic, and biochemical characterization of the causative agent of bacillary (epithelioid) angiomatosis: a rickettsial illness with features of bartonellosis. J Invest Dermatol 97: 812–817. [DOI] [PubMed] [Google Scholar]
- 150. Slater LN, Welch DF, Hensel D, Coody DW (1990) A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med 323: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 151. Avidor B, Graidy M, Efrat G, Leibowitz C, Shapira G, et al. (2004) Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol 42: 3462–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Maggi RG, Duncan AW, Breitschwerdt EB (2005) Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol 43: 2651–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Probert W, Louie JK, Tucker JR, Longoria R, Hogue R, et al. (2009) Meningitis due to a “Bartonella washoensis”-like human pathogen. J Clin Microbiol 47: 2332–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Koehler JE, Quinn FD, Berger TG, Leboit PE, Tappero JW (1992) Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med 327: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 155.Battisti JM, Minnick MF (2008) Laboratory maintenance of Bartonella quintana. In: Coico R, Kowalik T, Quarles J, Stevenson B, Taylor R, editors. Current Protocols in Microbiology. New Jersey: John Wiley & Sons. Unit 3C.1.1–3C.1.13. [DOI] [PubMed] [Google Scholar]
- 156. Clarridge JE 3rd, Raich TJ, Pirwani D, Simon B, Tsai L, et al. (1995) Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J Clin Microbiol 33: 2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kreier JP, Gothe R, Ihler GM, Krampitz HE, Mernaugh G, et al. (1992) The hemotrophic bacteria: the families Bartonellaceae and Anaplasmataceae. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer K-H, editors. The Prokaryotes, A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, 2nd edition, New York: Springer-Verlag. pp. 3994–4022. [Google Scholar]
- 158. Knobloch J, Bialek R, Müller G, Asmus P (1988) Common surface epitope of Bartonella bacilliformis and Chlamydia psittaci . Am J Trop Med Hyg 39: 427–433. [DOI] [PubMed] [Google Scholar]
- 159. Minnick MF (1994) Identification of outer membrane proteins of Bartonella bacilliformis . Infect Immun 62: 2644–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liberto MD, Matera G (2000) Pathogenic mechanisms of Bartonella quintana . New Microbiol 23: 449–456. [PubMed] [Google Scholar]
- 161. Zähringer U, Lindner B, Knirel YA, van den Akker WM, Hiestand R, et al. (2004) Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J Biol Chem 279: 21046–21054. [DOI] [PubMed] [Google Scholar]
- 162. Popa C, Abdollahi-Roodsaz S, Joosten LA, Takahashi N, Sprong T, et al. (2007) Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun 75: 4831–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Burgess AWO, Anderson BE (1998) Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb Pathog 25: 157–164. [DOI] [PubMed] [Google Scholar]
- 164. Li DM, Liu QY, Zhao F, Hu Y, Xiao D, et al. (2011) Proteomic and bioinformatic analysis of outer membrane proteins of the protobacterium Bartonella henselae (Bartonellaceae). Genet Mol Res 10: 1789–1818. [DOI] [PubMed] [Google Scholar]
- 165. Rhomberg TA, Karlberg O, Mini T, Zimny-Arndt U, Wickenberg U, et al. (2004) Proteomic analysis of the sarcosine-insoluble outer membrane fraction of the bacterial pathogen Bartonella henselae . Proteomics 4: 3021–3033. [DOI] [PubMed] [Google Scholar]
- 166. Boonjakuakul JK, Gerns HL, Chen YT, Hicks LD, Minnick MF, et al. (2007) Proteomic and immunoblot analyses of Bartonella quintana total membrane proteins identify antigens recognized by sera from infected patients. Infect Immun 75: 2548–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Eberhardt C, Engelmann S, Kusch H, Albrecht D, Hecker M, et al. (2009) Proteomic analysis of the bacterial pathogen Bartonella henselae and identification of immunogenic proteins for serodiagnosis. Proteomics 9: 1967–1981. [DOI] [PubMed] [Google Scholar]
- 168. Kordick DL, Swaminathan B, Greene CE, Wilson KH, Whitney AM, et al. (1996) Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii . Int J Syst Bacteriol 46: 704–709. [DOI] [PubMed] [Google Scholar]
- 169.Weiss E, Moulder JW (1984) Order I. Rickettsiales Gieszczkiewicz 1939. In: Krieg NR, Holt JG, editors. Bergey's Manual of Systematic Bacteriology. Baltimore: Williams & Wilkins. pp. 687–729. [Google Scholar]
- 170. Welch DF, Hensel DM, Pickett DA, San-Joaquin VH, Robinson A, et al. (1993) Bacteremia due to Rochalimaea henselae in a child: practical identification of isolates in the clinical laboratory. J Clin Microbiol 31: 2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, Hegarty BC, et al. (2010) Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasit Vectors 3: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Breitschwerdt EB, Maggi RG, Sigmon B, Nicholson WL (2007) Isolation of Bartonella quintana from a woman and a cat following putative bite transmission. J Clin Microbiol 45: 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS (1990) The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N Engl J Med 323: 1573–1580. [DOI] [PubMed] [Google Scholar]
- 174. Hadfield TL, Warren R, Kass M, Brun E, Levy C (1993) Endocarditis caused by Rochalimaea henselae . Hum Pathol 24: 1140–1141. [DOI] [PubMed] [Google Scholar]
- 175. Dauga C, Miras I, Grimont PA (1996) Identification of Bartonella henselae and B. quintana 16S rDNA sequences by branch-, genus- and species-specific amplification. J Med Microbiol 45: 192–199. [DOI] [PubMed] [Google Scholar]
- 176. Anderson B, Sims K, Regnery R, Robinson L, Schmidt MJ, et al. (1994) Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol 32: 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Norman AF, Regnery R, Jameson P, Greene C, Krause DC (1995) Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol 33: 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kelly TM, Padmalayam I, Baumstark BR (1998) Use of the cell division protein FtsZ as a means of differentiating among Bartonella species. Clin Diagn Lab Immunol 5: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Bereswill S, Hinkelmann S, Kist M, Sander A (1999) Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J Clin Microbiol 37: 3159–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Colborn JM, Kosoy MY, Motin VL, Telepnev MV, Valbuena G, et al. (2010) Improved detection of Bartonella DNA in mammalian hosts and arthropod vectors by real-time PCR using the NADH dehydrogenase gamma subunit (nuoG). J Clin Microbiol 48: 4630–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Rolain JM, Franc M, Davoust B, Raoult D (2003) Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis 9: 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY (2012) Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J Clin Microbiol 50: 1645–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Renesto P, Gautheret D, Drancourt M, Raoult D (2000) Determination of the rpoB gene sequences of Bartonella henselae and Bartonella quintana for phylogenic analysis. Res Microbiol 151: 831–836. [DOI] [PubMed] [Google Scholar]
- 184. Minnick MF, Barbian KD (1997) Identification of Bartonella using PCR; genus- and species-specific primer sets. J Microbiol Methods 31: 51–57. [Google Scholar]
- 185. Buss SN, Gebhardt LL, Musser KA (2012) Real-time PCR and pyrosequencing for differentiation of medically relevant Bartonella species. J Microbiol Methods 91: 252–256. [DOI] [PubMed] [Google Scholar]
- 186. Padilla C, Ventura G (2003) Diseño y estandarización de una prueba de PCR para el diagnóstico de la Bartonelosis causada por Bartonella bacilliformis . Rev Perú Med Exp Salud Publica 20: 5–8. [Google Scholar]
- 187. Smit PW, Peeling RW, Garcia PJ, Torres LL, Pérez-Lu JE, et al. (2013) Dried blood spots for qPCR diagnosis of acute Bartonella bacilliformis infection. Am J Trop Med Hyg 89: 988–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Westfall HN, Edman DC, Weiss E (1984) Analysis of fatty acids of the genus Rochalimaea by electron capture gas chromatography: detection of nonanoic acid. J Clin Microbiol 19: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kordick DL, Wilson KH, Sexton DJ, Hadfield TL, Berkhoff HA, et al. (1995) Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol 33: 3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Chamberlin J, Laughlin L, Gordon S, Romero S, Solórzano N, et al. (2000) Serodiagnosis of Bartonella bacilliformis infection by indirect fluorescence antibody assay: test development and application to a population in an area of bartonellosis endemicity. J Clin Microbiol 38: 4269–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Padilla C, Gallegos K, Marcelo A, Chenet S, Baldeviano C (2006) Expresión y seroreactividad de la lipoproteína recombinante de 43-kDa de Bartonella bacilliformis . Rev Peru Med Exp Salud Publica 23: 182–187. [Google Scholar]
- 192. Mallqui V, Speelmon EC, Verástegui M, Maguiña-Vargas C, Pinell-Salles P, et al. (2000) Sonicated diagnostic immunoblot for bartonellosis. Clin Diagn Lab Immunol 7: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Maguina C, Garcia PJ, Gotuzzo E, Cordero L, Spach DH (2001) Bartonellosis (Carrión's disease) in the modern era. Clin Infect Dis 33: 772–779. [DOI] [PubMed] [Google Scholar]
- 194. Maguiña C, Romero I, Soto N, Solórzano N, Tarazona A, et al. (2002) Historia natural de la fase eruptiva de la Verruga Peruana y la importancia de la prueba de western blot, reporte preliminar. Fol Dermatol Peru 13: 36–42. [Google Scholar]
- 195. Sobraquès M, Maurin M, Birtles RJ, Raoult D (1999) In vitro susceptibilities of four Bartonella bacilliformis strains to 30 antibiotic compounds. Antimicrob Agents Chermother 43: 2090–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bartlett JG (2005) 2005–2006 Pocket book of infectious disease therapy. Baltimore: Williams & Wilkins. 349 p. [Google Scholar]
- 197. Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, et al. (2004) Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 48: 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Urteaga O, Payne EH (1955) Treatment of the acute febrile phase of Carrión's disease with chloramphenicol. Am J Trop Med Hyg 4: 507–511. [DOI] [PubMed] [Google Scholar]
- 199. Arroyo A (2008) Esquemas de tratamiento para la enfermedad de Carrión no complicada en la ciudad de Caraz, Perú. An Fac Med Lima 69: 7–11. [Google Scholar]
- 200. Biswas S, Raoult D, Rolain JM (2007) Molecular mechanisms of resistance to antibiotics in Bartonella bacilliformis . J Antimicrob Chemother 59: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 201. Minnick MF, Wilson ZR, Smitherman LS, Samuels DS (2003) gyrA mutations in ciprofloxacin-resistant Bartonella bacilliformis strains obtained in vitro. Antimicrob Agents Chemother 47: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. del Valle LJ, Flores L, Vargas M, García-de-la-Guarda R, Quispe RL, et al. (2010) Bartonella bacilliformis, endemic pathogen of the Andean region, is intrinsically resistant to quinolones. Int J Infect Dis 14: e506–e510. [DOI] [PubMed] [Google Scholar]
- 203. Hodgson CH (1947) The treatment of Carrión's disease with large transfusions. Am J Trop Med Hyg 27: 69–75. [DOI] [PubMed] [Google Scholar]
- 204. Gutierrez YZ, Luna AS (1998) Verruga peruana tratada con sultamicilina y deflazacort. Dermatol Per 8: 43–46. [Google Scholar]
- 205. Howe C, Hertig M (1943) Prophylactic immunization against Carrión's disease. J Immunol 47: 471–482. [Google Scholar]
- 206. Hertig M, Fairchild GB (1948) The control of Phlebotomus in Peru with DDT. Am J Trop Med Hyg 28: 207–230. [DOI] [PubMed] [Google Scholar]
- 207. Robert LL, Perich MJ (1995) Phlebotomine sand fly (Diptera: Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc 11: 195–199. [PubMed] [Google Scholar]
- 208. Alexander B, Usma MC, Cadena H, Quesada BL, Solarte Y, et al. (1995) Evaluation of deltamethrin-impregnated bednets and curtains against phlebotomine sandflies in Valle del Cauca, Colombia. Med Vet Entomol 9: 279–283. [DOI] [PubMed] [Google Scholar]
- 209. Maroli M, Majori G (1991) Permethrin-impregnated curtains against phlebotomine sandflies (Diptera: Psychodidae); laboratory and field studies. Parassitologia 33 Suppl: 399–404. [PubMed] [Google Scholar]
- 210. Majori G, Maroli M, Sabatinelli G, Fausto AM (1989) Efficacy of permethrin-impregnated curtains against endophilic phlebotomine sandflies in Burkina Faso. Med Vet Entomol 3: 441–444. [DOI] [PubMed] [Google Scholar]
- 211. Killick-Kendrick R (1999) The biology and control of phlebotomine sand flies. Clin Dermatol 17: 279–289. [DOI] [PubMed] [Google Scholar]
- 212. Schlein Y (1987) Marking of Phlebotomus papatasi (Diptera: Psychodidae) by feeding on sprayed, coloured sugar bait: a possible means for behavioural and control studies. Trans R Soc Trop Med Hyg 81: 599. [DOI] [PubMed] [Google Scholar]
- 213. Robert LL, Perich MJ, Schlein Y, Jacobson RL, Wirtz RA, et al. (1997) Phlebotomine sand fly control using bait-fed adults to carry the larvicide Bacillus sphaericus to the larval habitat. J Am Mosq Control Assoc 13: 140–144. [PubMed] [Google Scholar]
- 214. Mascari TM, Foil LD (2010) Laboratory evaluation of insecticide-treated sugar baits for control of phlebotomine sand flies (Diptera: Psychodidae). J Am Mosq Control Assoc 26: 398–402. [DOI] [PubMed] [Google Scholar]
- 215. Yuval B, Schlein Y (1986) Leishmaniasis in the Jordan Valley. III. Nocturnal activity of Phlebotomus papatasi (Diptera: Psychodidae) in relation to nutrition and ovarian development. J Med Entomol 23: 411–415. [DOI] [PubMed] [Google Scholar]
- 216.World Health Organization (1968) WHO Interregional traveling seminar on leishmaniasis. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 217. Mascari TM, Clark J, Gordon S, Mitchell MA, Rowton ED, et al. (2011) Oral treatment of rodents with insecticides for control of sand flies (Diptera:Psychodidae) and the fluorescent tracer technique (FTT) as a tool to evaluate potential sand fly control methods. J Vector Ecol 36 Supplement 1: S132–S137. [DOI] [PubMed] [Google Scholar]
- 218. Mascari TM, Stout RW, Foil LD (2012) Laboratory evaluation of oral treatment of rodents with systemic insecticides for control of bloodfeeding sand flies (Diptera: Psychodidae). Vector Borne Zoonotic Dis 12: 699–704. [DOI] [PubMed] [Google Scholar]
- 219. Mascari TM, Stout RW, Foil LD (2013) Oral treatment of rodents with fipronil for feed-through and systemic control of sand flies (Diptera:Psychodidae). J Med Entomol 50: 122–125. [DOI] [PubMed] [Google Scholar]