Abstract
Metallothioneins are cysteine-rich metal-binding proteins. In the present study, SaMT2, a type 2 metallothionein gene, was isolated from Cd/Zn co-hyperaccumulator Sedum alfredii Hance. SaMT2 encodes a putative peptide of 79 amino acid residues including two cysteine-rich domains. The transcript level of SaMT2 was higher in shoots than in roots of S. alfredii, and was significantly induced by Cd and Zn treatments. Yeast expression assay showed SaMT2 significantly enhanced Cd tolerance and accumulation in yeast. Ectopic expression of SaMT2 in tobacco enhanced Cd and Zn tolerance and accumulation in both shoots and roots of the transgenic plants. The transgenic plants had higher antioxidant enzyme activities and accumulated less H2O2 than wild-type plants under Cd and Zn treatment. Thus, SaMT2 could significantly enhance Cd and Zn tolerance and accumulation in transgenic tobacco plants by chelating metals and improving antioxidant system.
Introduction
Heavy metals are known to cause toxic effects and inhibition of plant growth. However, rare plant species, which can accumulate and tolerate extremely high concentrations of heavy metals in their shoots without toxicity effects, have been defined as “hyperaccumulators” [1]. The elucidation of the mechanisms underlying metal hyperaccumulation may enable the phytoremediation of metal-contaminated soils and the biofortification of trace elements in food crops [2]–[4].
Higher plants have evolved various defense mechanisms to detoxify excess metals. These mechanisms contain compartmentalization in inactive tissues, chelation by metal ligands and detoxification by antioxidants [5]. Metal chelators such as organic acids, amino acids, phytochelatins and metallothioneins play important roles in metal detoxification [6]. Metallothioneins (MTs) are low-molecular-mass, cysteine-rich proteins which are broadly distributed in microorganisms, plants and animals [7]. Plant MTs can be divided into four subfamilies based on the distribution of cysteine residues in their amino- and carboxyl-terminal regions [8]. Several MT genes have been isolated and characterized from plants. There are some evidence indicating that plant MTs are involved in metal homeostasis, detoxification and reactive oxygen species (ROS) scavenging [8]–[11].
Hyperaccumulating ecotype (HE) of Sedum alfredii Hance is a Zn/Cd hyperaccumulator discovered from an old Pb/Zn mining area of China [12], [13]. It can accumulate up to 9000 µg g−1 Cd and 29000 µg g−1 Zn in its shoots without toxicity symptoms [12], [13]. This large amount of metals in plant cells needs a powerful detoxification system to protect plants from the deleterious effects of the metals. Earlier studies have demonstrated that the hyperaccumulating ecotype of Sedum alfredii has a more effective antioxidant enzyme system than non-hyperaccumulating ecotype (NHE) [14], [15]. However, the mechanism of hypertolerance of metals in this species has not been fully understood. In the present study, a metallothionein gene from hyperaccumulating ecotype of Sedum alfredii Hance, named SaMT2, was isolated and cloned The expression pattern of this gene was studied by Real Time-PCR. To analysis the function of SaMT2, its full length cDNA was cloned and expressed in yeast and tobacco. The transgenic yeast and tobacco plants were analyzed to evaluate whether SaMT2 protein played a role in Cd or Zn tolerance and accumulation.
Materials and Methods
Ethics statement
These field studies did not involve any protected species. No specific permits were required for the collection of samples in the study location.
Plant growth
The hyperaccumulating ecotype of S. alfredii Hance was collected from an old Pb/Zn mining site in Zhejiang Province, P. R. China. Plants were grown in non-polluted soils for several generations to minimize internal metal concentrations. Similar size shoot branches were cut and cultured hydroponically. After two weeks, rooted seedlings were then subjected to 4 days exposure of one-fourth, half and full strength nutrient solutions containing 2 mM Ca(NO3)2, 0.7 mM K2SO4, 0.1 mM KH2PO4, 0.1 mM KCl, 0.5 mM MgSO4, 10 µM H3BO3, 0.5 µM MnSO4, 5 µM ZnSO4, 0.2 µM CuSO4, 0.01 µM (NH4)6·Mo7O24, and 20 µM Fe-EDTA. Nutrient solution pH was adjusted daily to 5.8 with 0.1 M NaOH or HCl. Plants were grown under glasshouse conditions with natural light (day/night of 16/8 h), day/night temperature of 26/20°C and day/night humidity of 70/85%. The nutrient solution was aerated continuously and renewed every 3 d. To compare the expression of SaMT2, the precultured seedlings were treated with 100 µM CdCl2 and 500 µM ZnSO4 for 8 days.
Cloning of SaMT2 cDNA and sequence analysis
The cDNA fragment of SaMT2 was isolated from RNA-Seq data of Sedum alfredii Hance (Gao et al. 2013). The full length of SaMT2 was isolated using 3′ and 5′ RACE methods as described by the supplier (Smart RACE cDNA amplification kit; Clontech Laboratories, Inc. CA, USA). PCR was performed with the following primers: 5′-CTGGGCGTGGCTCCGAAGCAAGTGTA-3′ for 3′ RACE and 5′-CGCAACCACAGTTTCCACCACAGCA-3′ for 5′ RACE. Alignment of SaMT2 was performed by ClustalW on the internet (http://clustalw.ddbj.nig.ac.jp/). The phylogenetic tree was constructed using the neighbor-joining algorithm by MEGA 5 software (released from http://www.megasoftware.net/) after ClustalW alignment with 1000 bootstrap trials.
Real time RT-PCR analysis
The total RNA was extracted from various tissues by RNAiso plus (Takara Bio, Inc. Shiga, Japan), and then converted to cDNA using Primescript™ RT regent kit with gDNA eraser (Takara Bio, Inc. Shiga, Japan). Expression of the SaMT2 was determined by quantitative RT-PCR with the SYBR Green I reagent (SYBR Premix Ex Taq II; Takara Bio, Inc. Shiga, Japan) on an Eppendorf Mastercycler Epgradient Realplex2 (Eppendorf AG, Hamburg, Germany). A portion (10 ng) of cDNA was used for the template. The primers used for SaMT2 were forward 5′-CTGTGGTTGCGGATCTGCTT-3′ and reverse 5′- TCCATTCTCCGACACCATCT-3′. To generate standard curves for the absolute quantification for SaMT2 copy number, a series of dilutions (from 1×10−1 to 1×10−6 ng) of plasmids were made and then subjected to real-time PCR.
Plasmids construction
To express SaMT2 in Saccharomyces cerevisiae, the full ORF of SaMT2 was amplified from the cDNA of S. alfredii using primers: 5′-AGCTCGAGATGTCTTGCTGTGGTGGA-3′ contains an XhoI site and 5′-GAGGATCCTCATTTGCAAGTGCAGGG-3′ contains a BamHI site. The PCR products were then cloned into a pEASY Blunt simple vector (Transgen, Beijing, China) and its sequence confirmed. This vector was double digested with XhoI and BamHI, and the obtained fragment was cloned into pDR195 between XhoI and BamHI sites.
To construct the plant overexpression vector, the full ORF of SaMT2 was amplified using primers: 5′-AAAGATCTGATGTCTTGCTGTGGTGGA-3′ and 5′-AAGGTGACCTCATTTGCAAGTGCAGG-3′, which contained BglII and BstpI restriction sites, respectively. The obtained fragment was restricted with BglII and BstpI, and then cloned into the BglII and BstpI sites of pCAMBIA 1302 vector and its sequence was confirmed.
Yeast complementation assay
The S. cerevisiae strains BY4741 (wild type, MATα; his2Δ0; met15Δ0; ura3Δ0), Δycf1 (MATa; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0; YDR135c::kanMX4) and Δzrc1 (MATα; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0; YMR243c::kanMX4) mutants were used to investigate the role of SaMT2 in Cd and Zn tolerance. The yeast transformation was conducted using LiAc/PEG/ssDNA methods, as described by Gietz and Schiestl [16]. To obtain cells for transformation: Inoculate a single colony of the yeast strain with a sterile inoculation loop from a fresh SD (synthetic medium plus dextrose, 0.67% yeast nitrogen base, 2% D-glucose, and amino acids) plate into 5 ml of YPD medium (2% peptone, 1% yeast extracts, 2% D-glucose) and incubate overnight at 30°C. Add 2.5×108 cells to 50 ml of YPD medium in a culture flask and incubate until the cell titer is at least 2×107 cells ml−1. Cells were harvested by centrifugation at 3,000 g for 5 min and washed twice with sterilized water. Then the cells were re-suspended in 1.0 ml of sterile deionized water and pelleted by centrifugation (13 000×g for 30 sec). The supernatant was discarded and the transformation mixture {containing 240 µl PEG 3350 (50% w/v), 36 µl 1.0 M lithium acetate, 10 µl single-stranded carrier DNA (10 mg ml−1) and plasmid DNA (0.5–1 µg), and sufficient sterile deionized water to provide a final volume of 360 µl} were layered over the pellet. The mixture was vortex vigorously for 1 min and subjected to a heat shock at 42°C for 40 min. The transformation mixture was then centrifuged at 13 000×g for 30 sec to pellet the cells. After the supernatant was decanted, the cells were resuspended in 1.0 ml of sterile deionized water. Aliquots of the resuspended cells were plated onto SD-URA media. Plates were incubated for 2–3 days at 30°C until transformants were observed. Single colonies were picked from each transformant plate and established on fresh SD-URA plates.
For the metal tolerance assay, single colonies from SD-URA plates were cultured in liquid SD-URA medium until OD600 reached 1.0. After serial dilutions (OD600 = 0.1, 0.01, 0.001, 0.0001, respectively) were prepared, each dilution was spotted onto SD-URA medium with or without 5 mM ZnSO4 or 30 µM CdCl2. Plates were photographed after incubation at 30°C for 3 d.
For determination of metal concentration in yeast, transformants were grown in liquid SD-URA medium overnight. Then, cells were adjusted to OD600 = 0.2 in the presence of 10 µM CdCl2 or 100 µM ZnSO4 for Zn determination. After incubation for 48 h, the cells were harvested and washed with distilled water, 20 mM Na2EDTA and distilled water, respectively. Dry weight was determined after 3 days at 60°C. Cells were digested using 5 ml concentrated HNO3, incubated at 95°C for 2 h. The Zn and Cd concentrations were determined by using ICP-MS (Inductively Coupled Plasma Mass Spectrometry, Agilent 7500a, CA, USA).
Heterogeneous expression of SaMT2 in tobacco
The transformation of tobacco was constructed using the leaf disk method according to Horsch et al [17]. Surface-sterilized T1 seeds of two transgenic tobacco lines were germinated on Murashige Skoog (MS) plates containing 40 mg/L hygromycin to select hygromycin-resistance seedlings. For Zn/Cd tolerance analysis, the wild type (WT) and transgenic plants were transferred to MS plates containing 100 µM CdCl2 or 200 µM ZnSO4 for 14 d. To determine the Zn/Cd concentrations in plants, both WT and transgenic plants were transferred to hydroponic culture. One-month old plants were then treated with 50 µM CdCl2 or 100 µM ZnSO4 for one week. Cadmium and zinc concentrations in plant tissues were measured using ICP-MS as described by Yang et al. [13].
Determination of SOD, POD, CAT and H2O2
For antioxidant enzyme activity determination, a 0.5-g aliquot of plant sample was homogenized in 5 ml potassium phosphate buffer (50 mM, pH 7.8). The homogenates were then centrifuged at 12000×g for 20 min at 4 °C. The supernatants were used for the analysis of enzyme activity. Superoxide dismutase (SOD) activity was determined by the photochemical method described by Giannopotitis and Ries [18]. One unit of the enzyme activity was defined as the amount of enzyme required to result in a 50% inhibition of the rate of nitro blue tetrazolium reduction measured at 560 nm. Catalase (CAT) activity was estimated according to Cakmak et al. [19]. The reaction mixture in a total volume of 2 ml contained 25 mM sodium phosphate buffer (pH 7.0), 10 mM H2O2. The reaction was initiated by the addition of 100 µl of enzyme extract and activity was determined by measuring the initial rate of disappearance of H2O2 at 240 nm (E = 39.4 mM−1 cm−1) for 30 s. Peroxidase (POD) activity was measured as the increase of absorbance due to guaiacol oxidation [20]. The reaction mixture contained 25 mM phosphate buffer (pH 7.0), 10 mM H2O2, 0.05% guaiacol and 100 µl of enzyme extract. The reaction was initiated by the addition of H2O2. The oxidation of guaiacol was measured at 470 nm (E = 26.6 mM−1 cm−1).
H2O2 was determined according to Loreto & Velikova [21]. Leaf tissues (0.07 g) were homogenized in an ice bath with 5 ml of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000×g for 15 min and 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The absorbance of the supernatant was measured at 390 nm. The content of H2O2 was calculated by comparison with a standard calibration curve previously made by using different concentrations of H2O2.
Statistical analysis of data
All data were statistically analyzed by using the SPSS package (version 20.0). All values were performed as means of three replicates. Data was tested at significant levels of p<0.05 using one-way ANOVA (analysis of variance). The graphical works were made by using Origin software.
Results
Clone and sequence analysis of SaMT2
A cDNA fragment of metallothionein like gene was obtained from RNA-seq of S. alfredii. RT-PCR and RACE techniques were used to obtain the full length cDNA of this gene, whose sequence was identified by BLAST search (www.ncbi.nlm.nih.gov/BLAST). The obtained cDNA encoded a 79 amino acids protein, which showed certain similarity to the cDNA of AtMT2a or AtMT2b. According to the amino acid sequences, it belonged to the Type 2 MTs, and was named SaMT2 (GeneBank accession number: KJ862538).
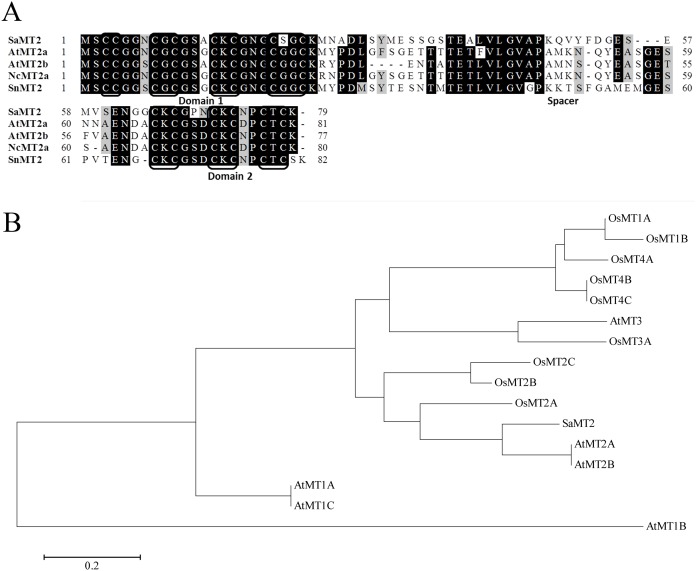
Multiple sequence alignment of the deduced amino acid sequences of SaMT2 with AtMT2a (Arabidopsis thaliana, NP_187550.1), AtMT2b (NP_195858.1), NcMT2a (Noccaea caerulescens, ACR46966.1) and SnMT2 (Solanum nigrum, ACF10396.1) were conducted (Figure 1A). SaMT2 shared 62%, 59%, 59% and 66% similarities with AtMT2a, AtMT2b, NcMT2a and SnMT2, respectively. Similar to other plant MT proteins, SaMT2 contained two cysteine-rich domain separated by a large cysteine-free domain [22]. The cysteine-rich domains in the N-terminal region is CCxxxCGCxxxCKCxxxCxGC, which was highly conserved, and that in the C-terminal region contained three CxC motifs. The spacer region between the two terminal regions contained approximately 40 amino acids.
Figure 1. Sequence alignment and phylogenic analysis of SaMT2 with other MTs.
(A) The deduced amino acid sequences encoded by SaMT2 were aligned with MTs from Arabidopsis thaliana, Noccaea caerulescens and Solanum nigrum. The cysteine-rich domains are boxed. (B) The phylogenic tree of SaMT2 and MTs from Arabidopsis and rice.
The phylogenetic tree of SaMT2 and MTs from Arabidopsis and rice was conducted using MEGA software (Figure 1B). These MTs were divided into several groups and SaMT2 was closely clustered with AtMT2a and AtMT2b.
Expression analysis of SaMT2 in Sedum alfredii Hance
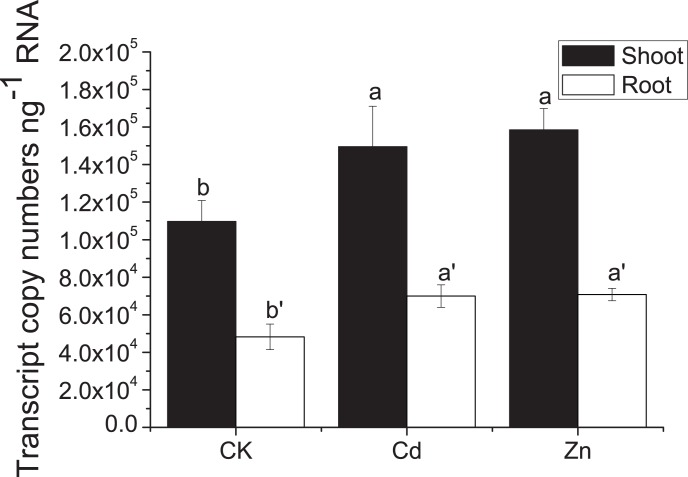
The expression of SaMT2 was investigated using absolute quantitative RT-PCR. To investigate whether Cd or Zn were involved in the regulation of SaMT2, S. alfredii seedlings were treated with 100 µM CdCl2 or 500 µM ZnSO4 and were subjected to determine the transcript level of SaMT2. The expression level of SaMT2 in roots was higher than that in shoots. The expression of SaMT2 was significantly (p<0.05) increased in both roots and shoots treated with Cd and Zn (Figure 2).
Figure 2. The expression level of SaMT2 in Sedum alfredii.
The transcript level of SaMT2 induced by Cd and Zn treatments. The different letters above the columns indicate the significant difference between the treatments (p<0.05, Tukey’s test). CK represents the control group.
SaMT2 enhanced cadmium but not zinc tolerance in yeast mutants
The Δycf1 and Δzrc1 yeast mutants and the wild type strain BY4741 were used to test the Cd and Zn tolerance ability of SaMT2. When grown in the control medium, yeasts containing either pDR195 or pDR195-SaMT2 could grow well. When grown in a medium containing 30 µM CdCl2, the growth of yeast contain pDR195 was significantly inhibited; however, the expression of SaMT2 could markedly mitigate this growth defect (Figure 3). However, the growth of yeasts in a Zn containing medium was not affected whether SaMT2 was expressed or not (Figure 3).
Figure 3. Cd and Zn tolerance of yeast cells expressing SaMT2.
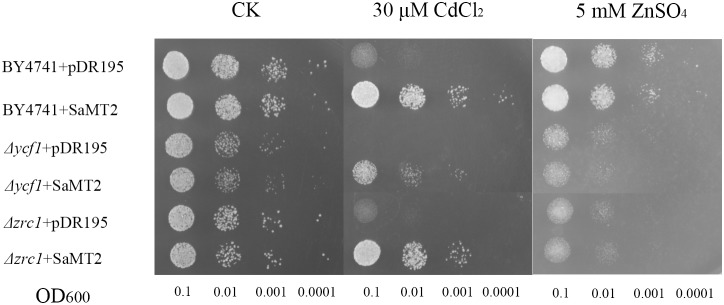
The Saccharomyces cerevisiae BY4741, Δycf1 and Δzrc1 yeast cells harboring pDR195 (vector control) or pDR195-SaMT2 were grown in liquid SD selective medium. Cultures were adjusted to OD600nm of 0.1 and serially 10-fold diluted in water. 10 µl aliquots of each dilution were spotted either on SD selective plates or on plates with 30 µM CdCl2 or 5 mM ZnSO4. After 3 days of incubation at 30°C, plates were photographed. CK represents the control group.
Similar trends were found for Cd and Zn concentrations in yeast mutants. Expression of SaMT2 significantly increased the Cd concentration in yeast Δycf1 mutant; however, it decreased the concentration of Zn in yeast Δzrc1 mutant significantly (p<0.05, Figure 4).
Figure 4. Cd and Zn concentration in Δycf1 and Δzrc1 yeast cells expressing SaMT2.
The yeast transformants containing pDR195 or pDR195-SaMT2 were grown in liquid SD selective medium with 30 µM CdCl2 and 100 µM ZnSO4 for Δycf1 and Δzrc1, respectively. Cells were incubated at 30°C for 48 h and metal contents were measured by ICP-MS. Results are averages (±S.E.) from three independent experiments done with four different colonies. The ‘*’ symbol indicates the mean values were significantly different at p<0.05 (Tukey’s test).
Overexpression of SaMT2 in tobacco enhanced Cd and Zn tolerance and accumulation
To evaluate the functions of SaMT2 in plants, transgenic tobacco plants were generated, ectopically expressing SaMT2 under the control of CaMV 35S promoter. Three independent transgenic tobacco lines over-expressing SaMT2 were selected for Cd and Zn tolerance analysis. The wild plants were used as control.
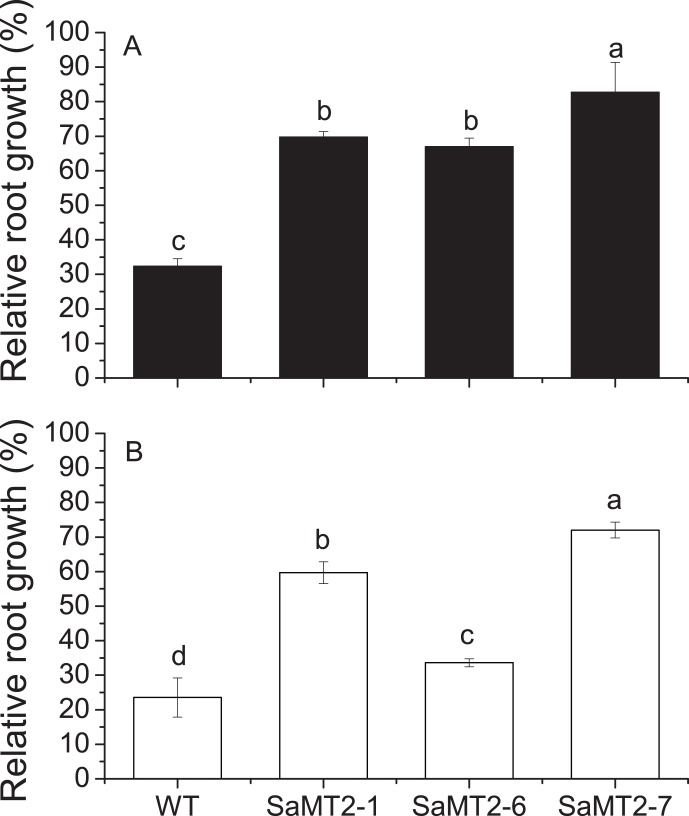
There was no difference in growth between wild type and transgenic plants under control condition. Exposure of the plants to 100 µM CdCl2 or 200 µM ZnSO4 significantly decreased root elongation and plant growth of both wild type and transgenic plants; however, the growth deficiency was less pronounced in transgenic plants (Figure 5). Under 100 µM CdCl2 or 200 µM ZnSO4 treatments, the root growth of wild type plants was decreased by 68% or 76%, compared to the control, respectively. However, the transgenic plants showed a significantly higher resistance to Cd and Zn. Compared to the control, the root growth of the transgenic plants was only decreased by 17%–33% under Cd treatment, and decreased by 28%–66% under Zn treatment (Figure 6).
Figure 5. Metal tolerance analysis of transgenic tobacco plants over-expressing SaMT2.
The figure shows the effect of 200 µM ZnSO4 or 100 µM CdCl2 on the growth of WT and transgenic plants on B5 medium. CK represents the control group.
Figure 6. Relative root growth of transgenic tobacco plants.
The relative root growth of WT and transgenic tobacco plants under Cd (A) and Zn (B) treatments. Different letters above the columns indicate a significant difference among different plant lines (p<0.05, Tukey’s test).
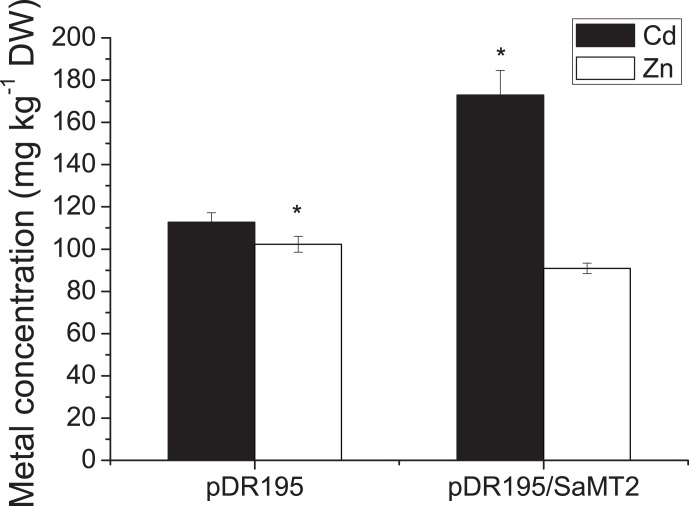
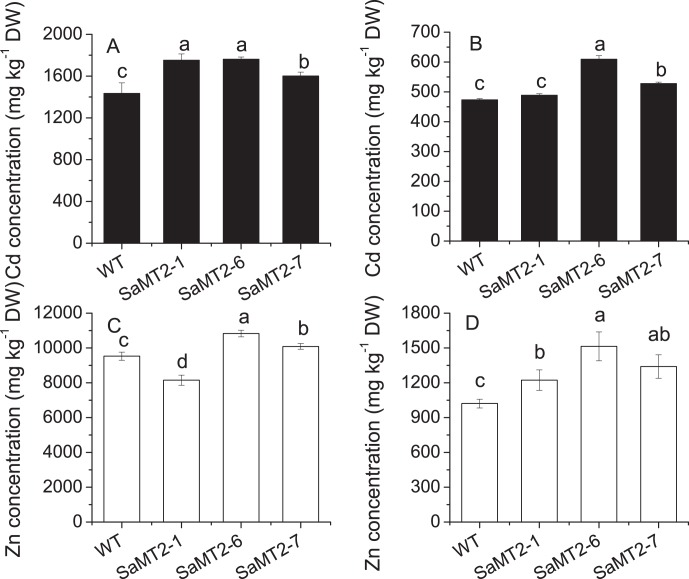
Over-expression of SaMT2 gene significantly increased both Cd and Zn concentration in transgenic tobacco plants (p<0.05) (Figure 7). Compared to the wild type plants, the Cd concentration was increased by 11–22% in the roots and by 3–28% in the shoots of the transgenic plants, respectively (Figure 7A, B). Except for the SaMT2-1 line, the Zn concentration was increased by 6–14% in roots and 20–48% in shoots of transgenic plants, respectively (Figure 7C, D). The SaMT2-6 line accumulated the highest amount of Cd and Zn among the three transgenic lines.
Figure 7. Cd and Zn concentrations in wild type amd transgenic tobacco lines overexpressing SaMT2.
Three independent SaMT2 over-expressing lines and wild-type tobacco were grown in nutrient solution containing 50 µM CdCl2, 100 µM ZnSO4 for 1 week. A: Cd concentration in roots, B: Cd concentrantion in shoots, C: Zn concentration in roots, D: Zn concentration in shoots. Results are means ± S.E. (n = 3). Different letter indicate the mean values were significantly different from WT tobacco determined by Tukey’s test (p<0.05).
Based on the tolerance and accumulation of Cd and Zn in transgenic tobacco lines, the SaMT2-7 line - having similar Cd and Zn tolerance and accumulation level- was selected, to evaluate the reason of elevated tolerance to Cd and Zn of the transgenic plants. The plants treated with different metals were used to determine the activities of SOD, POD, CAT and the content of H2O2. The transgenic plant accumulated significantly less H2O2 in both roots and shoots than the WT plants under Cd and Zn treatments. The activities of SOD and POD were significantly increased in both roots and shoots of transgenic plants compared to that of wild type plants under Cd and Zn treatments (Table 1). For CAT activity, however, no significant difference was observed between WT and transgenic plants.
Table 1. The activities of SOD, POD CAT and the content of H2O2 in the shoot and roots of wild-type and transgenic tobacco plants.
| Metal treatment | SOD (U mg−1 Protein) | POD (nanokatals mg−1 Protein) | CAT (nanokatals mg−1 Protein) | H2O2 (µg g−1 FW) | |||||
| WT | Transgenic | WT | Transgenic | WT | Transgenic | WT | Transgenic | ||
| Shoot | CK | 20.1±0.4d | 21.4±1.5d | 20190.7±205.0d | 20045.6±175.0d | 36.7±5.0b | 35.0±3.3b | 154.4±10.0d | 156.1±12.1d |
| 50 µM Cd | 23.5±0.3c | 28.6±1.2a | 29267.5±720.1b | 33821.7±688.4a | 28.3±3.3c | 45.0±1.7a | 411.2±20.7a | 167.9±10.1d | |
| 100 µM Zn | 24.3±0.5c | 25.5±0.4b | 26777.0±855.1c | 30396.0±373.4b | 25.0±1.7c | 35.0±3.3b | 267.3±9.7b | 215.2±2.0c | |
| Root | CK | 24.2±0.4e | 25.7±1.3e | 30199.3±338.4e | 31156.2±1171.9e | 5.0±1.7b | 3.3±1.7b | 131.6±6.4d | 132.0±7.4d |
| 50 µM Cd | 30.2±0.6c | 41.6±1.4a | 46077.5±606.7b | 54069.1±825.1a | 5.0±1.7b | 11.7±1.7a | 400.5±23.0a | 210.8±7.7c | |
| 100 µM Zn | 28.3±1.0d | 34.2±0.7b | 34723.6±270.0d | 38681.0±1073.5c | 6.7±1.7ab | 10.0±1.7a | 310.1±3.9b | 198.6±10.0c | |
The data presented are mean ± SD of three replicates. Different letters indicate significant differences (p<0.05) among the different treatments and different plant lines.
Discussion
Metallothioneins (MTs) are cysteine-rich proteins involved in metal tolerance of diverse living organisms. Plant metallothioneins can be divided into four subfamilies based on their sequence similarities and phylogenetic relationships [7], [8]. In the present study, the MT gene cloned from S. alfredii encoded a protein with two Cys-rich regions, showing high identity with the N- and C-terminal regions of type 2 MTs of other plants; therefore, this MT gene was named as SaMT2. S. alfredii is a Cd/Zn co-hyperaccumulator, which shows extremely high tolerance to Cd and Zn [12], [13]. Thus, It was hypothesized that SaMT2 cloned from S. alfredii might be involved in Cd or Zn tolerance.
It has been reported that different MT genes have distinct tissue specific expression patterns in plants [8]. Generally, MT1s are predominantly expressed in roots, MT2s and MT3s in shoots [22], [23]. In the present study, SaMT2 was more highly expressed in shoots of S. alfredii than in roots. Similar results have also been found in Arabidopsis, rice and other plants [22]–[25]. The expression of MT genes in plants is regulated by many factors, including metal ions, oxidative stress, and stresses such as heat, salt, wounding and so on [11]. Here, the expression of SaMT2 was significantly increased in both roots and shoots of S. alfredii treated with Cd or Zn; in contrast, it has been reported that Cd and Zn do not induce the expression of TcMT2 and TcMT3 in Thlaspi caerulescens (now Noccaea caerulescens) another Cd/Zn hyperaccumulator [29].
The plant MTs are suggested to be involved in metal homeostasis or tolerance, such as Cu, Cd and Zn. When expressed in yeast or E. coli, the plant MTs are able to restore Cu, Cd and Zn tolerance [9], [11], [26], [27]. In the present study, Cd and Zn induced the expression of SaMT2, suggesting its possible involvement in Cd and Zn tolerance. This was confirmed in yeast and tobacco plant overexpressing SaMT2, which exhibited increased Cd and Zn tolerance and accumulation. Previous studies also reported enhanced tolerance and accumulation of Cd or other heavy metals by over-expressing plant MT genes. For example, the over-expression of Cajanus cajan MT1 enhances Cd and Cu tolerance in E. coli and Arabidopsis [27]. The expression of Colocasia esculenta CeMT2b increases Cd tolerance and accumulation in E. coli and tobacco [28]. However, Hassinen et al. [29] have observed that MT expression and Cd accumulation are not correlated among T. caerulescens accessions. Furthermore, the overexpression of TcMT2 and TcMT3 do not increase Cd accumulation in Arabidopsis shoots. On the other hand, Lv et al. [30] have observed that the ectopic expression of either BcMT1 or BcMT2 does increase Cd tolerance, but not the Cd accumulation in Arabidopsis shoots and roots. Thus, the MT genes may have different specific functions, depending on plant species.
The plant MTs are thought to function as metal chelators or ROS scavengers in heavy metal stress [8]. On one hand, plant MT proteins are supposed to have binding activities to heavy metals, such as Cd, Zn and Cu [7], [8]. In the present study, the ectopic expression of SaMT2 in tobacco enhanced Cd tolerance and accumulation, which might be due to reduced activities of free Cd ions in the cytoplasm, by the binding of overexpressed MT protein and Cd. On the other hand, MTs can also function as ROS scavengers which can reduce the ROS induced by Cd or other metals [8]. The plants exposure to heavy metals, such as Cd, can produce ROS and oxidative stress. The present study demonstrated that overexpression of SaMT2 could significantly reduce H2O2 in tobacco exposure to excess Cd. Several studies have demonstrated that MTs can effectively scavenge ROS in plants. Over-expression of BcMT1, BcMT2 [30], EhMT1 [31], pCeMT [9] reduces ROS production in transgenic plants. Using recombinant GhMT3a protein, Xue et al. [32] have demonstrated that GhMT3a can scavenge ROS in vitro. Plants themselves have developed various antioxidant defense mechanisms to protect from deleterious effects of ROS. One of them is the enzymatic system, which includes SOD, APX, POD, and CAT. Plants overexpressing MT genes show higher antioxidant enzyme activities [8]. The present study demonstrated that tobacco plants overexpressing SaMT2 showed higher SOD and POD activities than wild type plants, indicating that SaMT2 might also act as an activator of antioxidant enzyme system.
It has been demonstrated that MTs are not related with Cd or Zn tolerance and accumulation in hyperaccumulator T. caerulescens, even though the expression of MT genes varies among T. caerulescens accessions [29]. However, in the present study, ectopic expression study in yeast and tobacco revealed that SaMT2 might play certain roles in Cd and Zn tolerance and accumulation. It is not certain whether SaMT2 is directly involved in Cd or Zn tolerance in Sedum alfredii. There are clear evidence that MTs are not directly related in Zn or Cd tolerance in T. caerulescens [29]. Data from the present study demonstrated that SaMT2 might be involved in the Cd or Zn induced antioxidant stress in Sedum alfredii. However, the exact role of SaMT2 in metal tolerance and accumulation in Sedum alfredii needs to be examined by further study in the future.
In conclusion, SaMT2 is a metallothionein gene cloned from Cd/Zn hyperaccumulator Sedum alfredii Hance. Overexpression of this gene could significantly enhance Cd tolerance and accumulation in yeasts and tobacco plants. The mechanism of the elevated Cd tolerance and accumulation by overexpressing of SaMT2 includes binding of SaMT2 with Cd and improving the antioxidant system.
Acknowledgments
The Δzrc1 and Δycf1 yeast mutant strains and the wildtype strain BY4741 were kindly supplied by Prof. Eide, University of Wisconsin-Madison, USA.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present study was supported by the Fundamental Research Funds for the Central Universities (No. 2013FZA6005), National Natural Science Foundation of China (No. 31372128; 21177107). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brooks R (1998) Geobotany and hyperaccumulators. In: Robert R. Brooks editor. Plants that Hyperaccumulate Heavy Metals. New York: CAB International. pp. 55–94.
- 2. Kramer U (2010) Metal hyperaccumulation in plants. Annual Review of Plant Biology 61: 517–534. [DOI] [PubMed] [Google Scholar]
- 3. McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Current Opinion in Biotechnology 14: 277–282. [DOI] [PubMed] [Google Scholar]
- 4. Zhao FJ, McGrath SP (2009) Biofortification and phytoremediation. Current Opinion in Plant Biology 12: 373–380. [DOI] [PubMed] [Google Scholar]
- 5. Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist 181: 759–776. [DOI] [PubMed] [Google Scholar]
- 6. Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany 53: 1–11. [PubMed] [Google Scholar]
- 7. Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology 53: 159–182. [DOI] [PubMed] [Google Scholar]
- 8. Hassinen VH, Tervahauta AI, Schat H, Karenlampi SO (2011) Plant metallothioneins - metal chelators with ROS scavenging activity? Plant Biology 13: 225–232. [DOI] [PubMed] [Google Scholar]
- 9. Kim YO, Jung S, Kim K, Bae HJ (2013) Role of pCeMT, a putative metallothionein from Colocasia esculenta, in response to metal stress. Plant Physiology and Biochemistry 64: 25–32. [DOI] [PubMed] [Google Scholar]
- 10. Mir G, Domenech J, Huguet G, Guo WJ, Goldsbrough P, et al. (2004) A plant type 2 metallothionein (MT) from cork tissue responds to oxidative stress. Journal of Experimental Botany 55: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 11. Xia Y, Lv Y, Yuan Y, Wang G, Chen Y, et al. (2012) Cloning and characterization of a type 1 metallothionein gene from the copper-tolerant plant Elsholtzia haichowensis . Acta Physiologiae Plantarum 34: 1819–1826. [Google Scholar]
- 12. Yang X, Long XX, Ni WZ, Fu CX (2002) Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chinese Science Bulletin 47: 1634–1637. [Google Scholar]
- 13. Yang XE, Long XX, Ye HB, He ZL, Calvert DV, et al. (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant and Soil 259: 181–189. [Google Scholar]
- 14. Jin XF, Yang X, Mahmood Q, Islam E, Liu D, et al. (2008) Response of antioxidant enzymes, ascorbate and glutathione metabolism towards cadmium in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii H. Environmental Toxicology. 23: 517–529. [DOI] [PubMed] [Google Scholar]
- 15. Jin XF, Yang XO, Islam E, Liu D, Mahmood Q (2008) Effects of cadmium on ultrastructure and antioxidative defense system in hyperaccumulator and non-hyperaccumulator ecotypes of Sedum alfredii Hance. Journal of Hazardous Materials 156: 387–397. [DOI] [PubMed] [Google Scholar]
- 16. Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nature Protocols 2: 31–34. [DOI] [PubMed] [Google Scholar]
- 17. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, et al. (1985) A simple and general method for transferring genes into plants. Science 227: 1229–1231. [DOI] [PubMed] [Google Scholar]
- 18. Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology 59: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. Journal of Experimental Botany 44: 127–132. [Google Scholar]
- 20. Zheng X, van Huystee RB (1992) Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Science 81: 47–56. [Google Scholar]
- 21. Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology 127: 1781–1787. [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou JM, Goldsbrough PB (1995) Structure, organization and expression of the metallothionein gene family in Arabidopsis. Molecular & General Genetics 248: 318–328. [DOI] [PubMed] [Google Scholar]
- 23. Guo WJ, Bundithya W, Goldsbrough PB (2003) Characterization of the Arabidopsis metallothionein gene family: tissue-specific expression and induction during senescence and in response to copper. New Phytologist 159: 369–381. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh HM, Liu WK, Chang A, Huang PC (1996) RNA expression patterns of a type 2 metallothionein-like gene from rice. Plant Molecular Biology 32: 525–529. [DOI] [PubMed] [Google Scholar]
- 25. Hsieh HM, Liu WK, Huang PC (1995) A novel stress-inducible metallothionein-like gene from rice. Plant Molecular Biology 28: 381–389. [DOI] [PubMed] [Google Scholar]
- 26. Roosens NH, Bernard C, Leplae R, Verbruggen N (2004) Evidence for copper homeostasis function metallothionein of metallothionein (MT3) in the hyperaccumulator Thlaspi caerulescens . FEBS Letters 577: 9–16. [DOI] [PubMed] [Google Scholar]
- 27. Sekhar K, Priyanka B, Reddy VD, Rao KV (2011) Metallothionein 1 (CcMT1) of pigeonpea (Cajanus cajan, L.) confers enhanced tolerance to copper and cadmium in Escherichia coli and Arabidopsis thaliana . Environmental and Experimental Botany 72: 131–139. [Google Scholar]
- 28. Kim YO, Patel DH, Lee DS, Song Y, Bae HJ (2011) High cadmium-binding ability of a novel Colocasia esculenta metallothionein increases cadmium tolerance in Escherichia coli and tobacco. Bioscience Biotechnology and Biochemistry 75: 1912–1920. [DOI] [PubMed] [Google Scholar]
- 29. Hassinen VH, Tuomainen M, Peraniemi S, Schat H, Karenlampi SO, et al. (2009) Metallothioneins 2 and 3 contribute to the metal-adapted phenotype but are not directly linked to Zn accumulation in the metal hyperaccumulator, Thlaspi caerulescens . Journal of Experimental Botany 60: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lv YY, Deng XP, Quan LT, Xia Y, Shen ZG (2013) Metallothioneins BcMT1 and BcMT2 from Brassica campestris enhance tolerance to cadmium and copper and decrease production of reactive oxygen species in Arabidopsis thaliana . Plant and Soil 367: 507–519. [Google Scholar]
- 31. Xia Y, Qi Y, Yuan YX, Wang GP, Cui J, et al. (2012) Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. Journal of Hazardous Materials 233: 65–71. [DOI] [PubMed] [Google Scholar]
- 32. Xue TT, Li XZ, Zhu W, Wu CG, Yang GG, et al. (2009) Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. Journal of Experimental Botany 60: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.