SUMMARY
Objective
Infantile spasms (IS) have poor outcomes and limited treatment options, including vigabatrin, a GABA aminotransferase inactivator. Vigabatrin has been associated with retinal toxicity. A high affinity vigabatrin analogue (CPP-115, Catalyst Pharmaceutical Partners) has shown lower risk of retinal toxicity. Here, we test the efficacy of CPP-115 in reducing spasms and its tolerability in the multiple-hit rat model of IS, in which daily vigabatrin reduced spasms only for one day, but was not well tolerated.
Methods
Male rats were treated with the protocol of the multiple-hit model of IS at postnatal day 3 (PN3). Using a randomized, blinded, vehicle-controlled, dose-response study design, CPP-115 [0.1, 1, or 5 mg/kg intraperitoneally (i.p.)] or vehicle were given daily (PN4-12) or as single injection (PN7) after spasms onset. Intermittent video- or video-EEG monitoring was done. Secondary endpoints included: daily weights, survival, performance on open field activity, surface righting time, and negative geotaxis (PN3-20), horizontal bar (PN13-20), Barnes maze (PN16-19). Statistics used a linear mixed model of raw or normalized log-transformed data, taking into account the repeated observations on each animal.
Results
The lower CPP-115 doses (0.1–1 mg/kg/day, PN4-12) reduced spasms between PN6-7 without increasing mortality. CPP-115 at 5 mg/kg/day (PN4-12) reduced spasms earlier (PN5), but was eventually lethal. A single CPP-115 injection (1mg/kg i.p.) decreased electroclinical spasms acutely but transiently. CPP-115 transiently improved the probability to >50% reduction of spasms, but did not accelerate spasms cessation. CPP-115 did not alter neurodevelopmental outcomes or visuospatial learning.
Significance
We provide proof-of-concept evidence that CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of IS at considerably lower and better tolerated doses than vigabatrin did in our previous studies. Further optimization of the treatment protocol is needed. CPP-115 may be a promising new candidate treatment for IS with better tolerability than vigabatrin.
Keywords: Epilepsy, seizure, lipopolysaccharide, doxorubicin, GABA aminotransferase, neurodevelopmental reflexes, learning, memory
INTRODUCTION
Infantile spasms (IS) are age-specific seizures occurring in severe infantile epileptic encephalopathies with poor epilepsy and developmental outcomes.1, 2 The current treatments for IS, primarily adrenocorticotropic hormone (ACTH) and vigabatrin 3–5, are not always effective and may have serious side effects. Vigabatrin is an inhibitor of GABA aminotransferase, a mitochondrial enzyme that catabolizes GABA after its re-absorption from the synaptic cleft 6. Consequently, vigabatrin increases GABA availability. 6 Cessation of IS by vigabatrin occurs in 11–96% of patients with IS, with the best response rates noted for patients with IS due to tuberous sclerosis complex.3, 4, 7–9 However its use is limited by the concern of retinal toxicity 10, 11 and mixed reports of white matter vacuolization from chronic, prolonged administration of vigabatrin.12–15 A new compound, CPP-115, is an inactivator of GABA aminotransferase with higher affinity than vigabatrin, and has been proposed to have lower risk for retinal toxicity.16–18
Here we test CPP-115 for efficacy in reducing spasms in the rat multiple-hit model of IS. The multiple-hit is a chronic model which exhibits age-specific expression of flexion and extension spasms (only between PN4-13) with ictal EEG electrodecremental responses, as well as the neurodevelopmental impairment, as also seen in infants with epileptic encephalopathies with IS.2 When treatments are administered after the onset of spasms, as in clinical practice, spasms in this model of IS are responsive to carisbamate and rapamycin, resistant to ACTH, whereas phenytoin, as expected, has no efficacy on spasms.2, 19, 20 Vigabatrin transiently reduced spasms only on PN5, but was associated with significant sedation and mortality (Table 1).2
Table 1.
Comparison of vigabatrin and CPP-115 in the multiple-hit model of IS (*).
Here, we provide proof-of-concept evidence for the efficacy and tolerability of the vigabatrin analogue CPP-115, in the DLP rat model of IS, upon: the frequency of behavioral and electroclinical spasms, body weight, survival, and neurodevelopmental outcomes. CPP-115 reduced both behavioral and electroclinical spasms transiently. In addition, we observed therapeutic effects of CPP-115 across at least a 10-fold range of doses (0.1 to 1 mg/kg/dose i.p.) which were well tolerated and did not increase mortality, as vigabatrin did. These findings therefore provide the first preclinical evidence for efficacy on IS and improved tolerability of a new vigabatrin analogue that has been proposed to demonstrate lower risk for retinal toxicity.
METHODS
Animals and model induction
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine and were in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley rats (Taconic farms, Germantown, NY) were housed in litters of 10 male pups with their dam, on a 12 hour light/12 hour dark schedule. Laboratory rodent diet 5001 (Labdiet, St Louis, MO, USA) and water for the dam were provided ad libitum. All rats included in this study underwent the multiple-hit protocol for induction of spasms, described in. 2 Only the 9 controls rats used in Figure 4 underwent the daily weight and behavioral tests, without being subjected to surgery, maternal separation for video-monitoring or drug injections.
Figure 4. Effects of daily CPP-115 on learning and memory, in the Barnes maze test (PN16-19).
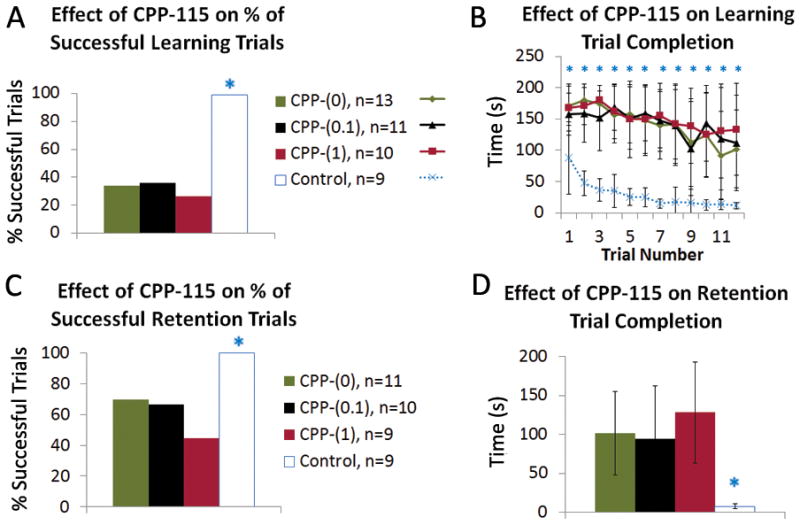
Panel A: The differences in success rate between groups are highly significant when all groups are considered (Fisher’s exact test, P<0.0001). However, this is largely due to the control rats, as examining only the DLP groups showed no difference (chi-square, P=0.26).
Panel B: DLP rats had longer times to complete the trials than controls across all 12 learning trials (asterisk marks Kruskal-Wallis test, P<0.01). Better completion times between controls and DLP groups were found also when only the successful trials were compared, to account for the fact that 60 minute is a pre-set endpoint, if pups do not fail the test earlier. Control rats (without PN3 surgery or video-monitoring) showed significantly smaller times to reach the target hole compared to DLP groups during sessions 5–10 and 12 (Kruskal-Wallis, P<0.05), whereas no differences were seen among DLP groups (data not shown). The graph shows group means by trial +/− SD. The graph shows group means by trial +/− SD.
Panel C: The differences in success rates were highly significant with all groups included (Fisher’s exact test, P<0.0001), but again all of DLP rats had similar success rates in retention of the Barnes maze (chi-square, P=0.12).
Panel D: The DLP rats were also slower to complete the trials than controls in this test; the average times among all 4 of the groups were significant (Kruskal-Wallis, P= 0.0016), but DLP groups did not differ among them (Kruskal-Wallis, P=0.99). These statistical results were maintained even when successful trials were only considered. The n of rats here is lower than in the behavioral tests because of mortality and euthanasia for histology. The graph shows group means of three trials per rat +/− SD.
In brief, PN3 rats were stereotactically infused with doxorubicin (5μg/2.5μl saline, right intracerebroventricularly), lipopolysaccharide (3μg/1.5 μl saline, right intraparietal) under isoflurane anesthesia (Aerrane, Henry Schein, Melville, NY).2, 19, 20 On PN5, rats received 200 mg/kg PCPA in saline i.p.2 We will therefore refer to this model as DLP model. Rats were perfused on PN20 for histology, according to established procedures.20 Drugs and chemicals were obtained from SIGMA-ALDRICH (St. Louis, MO).
CPP-115 administration
CPP-115 was kindly provided by Dr. Steven Miller (Catalyst Pharmaceutical Partners, Inc., Coral Gables, FL). Treatments were given after the onset of spasms as follows. Repeated daily i.p. injections of CPP-115 were given between PN4-12 at the following doses: 0.1, 1, or 5 mg/kg/day dissolved in distilled sterile water and filter-sterilized. These groups are called CPP-(5), CPP-(1), and CPP-(0.1) respectively. The CPP-(0) group received equal volume of vehicle i.p. daily (PN4-12). Different DLP rats were treated instead with a single i.p. injection of either CPP-115 (1 mg/kg, “single-CPP-(1)”) or vehicle (“single-CPP-(0)”) on PN7.
Video-monitoring and scoring for behavioral spasms
Rats were video-recorded using DVR 7.7 (AVerMedia Technologies Inc., Milpitas CA) in warmed (30–33°C) beakers with bedding. Monitoring was done daily between 10:00–12:00 and 14:00–16:00, with the following exceptions. On PN4, the rats had a 2hr morning and a 1hr afternoon session from which a baseline PN4-pre-injection spasm frequency (4PRE) was obtained. Subsequently, rats received their first CPP-115 or vehicle injection, habituated for 15 minutes, and were recorded for two more hours to obtain the PN4 post-injection spasm frequency (4POST). On PN7 and PN8 (weekend) only the afternoon sessions were done.
Video-EEG monitoring
To test the efficacy of a single CPP-115 injection and the duration of its effect on electroclinical spasms, DLP-treated rats were implanted with bilateral frontal and parietal epidural screw electrodes on PN6 (2EEG/1EMG system, Pinnacle Technology Inc., Lawrence, KS) under isoflurane anesthesia.19 Following 24 hours recovery, rats underwent intermittent video-EEG recording between PN7-12. The pre-injection baseline frequency of spasms (7PRE) was derived from the first hour of the PN7 afternoon session. Then CPP-115 (1 mg/kg i.p., “single-CPP-(1)”) or vehicle (“single-CPP-(0)”) injection was given, followed by a 15 minute habituation. The first post-injection hour (7POST) was used to evaluate the acute effects of the drug. Intermittent video-EEG monitoring continued for two hours on PN8 and for two daily two-hour sessions (PN9-12).
Behavioral Tests
Starting on PN3, body weights, surface righting time, open field activity, and negative geotaxis were assessed daily. On PN13, the horizontal bar test was added. Visuospatial learning and memory was assessed with a Barnes maze test (PN16-19). These tests are described in 2, 19, 20 and in Supplemental Methods (Supporting Information).
Blinding procedures
Each litter contained pups treated with all dose groups tested. Treatment assignment, as well as drug and vehicle dilutions, were done and given code names by ASG. All animal handling procedures, injections, data collection, inclusions and exclusions were done by SWB, blinded to treatment allocation. Unblinding followed the completion of data collection.
Inclusion and exclusion criteria
Since our goal was to treat after the onset of spasms, only DLP pups with 4PRE frequencies of at least 1 spasm/hr were included. Exclusion criteria were: (1) bilateral cortical encephalomalacia; (2) maternal neglect or accidental death (injection or surgery-related trauma) or deaths that preceded the initiation of drug treatment; (3) technical problems impeding intracerebral or i.p. drug injections (e.g., syringe malfunction).
Assessment of cortical-hippocampal injury and learning in the rats tested in the Barnes maze
Cortical injury was assessed by inspection of the extent of the cortical injury based on autopsy. Hippocampal injury was scored based on the thionin staining of coronal brain sections from PN20 rats. The scoring scale for cortical (CS) and hippocampal (HS) injury scores is described in Table 2. Because learning is significantly impaired in DLP pups, we considered as evidence for learning in the Barnes maze test the situation where rats completed trials 11 and 12 faster than trial 1.
Table 2.
Injury scoring scale for cortical (CS) and hippocampal (HS) injury.
| Injury Scores | Definition |
|---|---|
| Cortical Injury | |
| CS1 | injury along the needle track only |
| CS2 | right sided cortical linear atrophy across the parietal cortex (medial to lateral) |
| CS3 | cortical encephalomalacia of the right posterior/parietal quadrant |
| CS4 | bilateral cortical encephalomalacia (excluded, based on our exclusion criteria) |
| Hippocampal Injury | |
| HS1 | right anterior-dorsal hippocampal injury only |
| HS2 | right anterior dorsal and posterior hippocampal injury but preservation of the right ventral hippocampus |
| HS3 | complete right hippocampal injury |
| 0.5 score added | injury to the left anterior dorsal hippocampus is present |
Statistics
Statistics were done with SAS 9.3 (SAS Institute Inc., Cary, NC). Significance was set at P<0.05. Both raw frequencies of spasms and frequencies normalized to the pre-injection baselines were compared, using logarithmic transformed data, because they followed a log-normal distribution. Linear mixed models accounting for the repeated observations from each animal were used. Effects of the treatment on survival were analyzed with Log-Rank tests and Cox proportional hazard models to estimate the hazard ratios for the active treatment groups relative to the CPP-(0) group. A linear mixed model was used to compare weight gain across the four treatment groups. Reaction times in the neurodevelopmental reflexes and Barnes maze were analyzed by Kruskal-Wallis test. Comparison of failure or success rates in the behavioral tests were done by a Pearson chi-squared test (or Fisher’s exact test when the expected frequency in any cell is less than or equal to 5), both with α=0.05. Correlation of CS and HS scores with learning was assessed using Fisher’s exact test and Spearman correlation. Exact logistic regression was used to assess treatment effects on learning when the cortical and hippocampal injury is taken into account. Detailed description of the statistical methods is given in the Supplemental Methods.
RESULTS
Inclusions/exclusions
The following numbers of rats were included in our study: 33/42 CPP-(0) rats, 14/17 CPP-(0.1) rats, 25/36 CPP-(1) rats, 9/12 CPP-(5) rats, and 9/9 control rats (no surgery; underwent only daily weights/neurodevelopmental assessment and Barnes maze). The reasons for exclusion were equally distributed across treatment groups: maternal neglect (13 rats), accidental death (3 rats), technical problems with injections (4 rats), bilateral cortical encephalomalacia due to midline intracerebral injections (6 rats).
CPP-115 reduces spasms
Daily CPP-115 administration (PN4-12) transiently decreases behavioral spasms
Daily CPP-115 administration, starting after the onset of spasms on PN4, reduced behavioral spasms relative to vehicle administration (CPP-(0)) (Fig. 1; Supplemental Figure 1). None of the tested doses had an acute effect on spasms on PN4. On PN5, only CPP-(5) had raw and normalized frequencies of spasms significantly lower than CPP-(0) (P≤0.0002), whereas CPP-(0.1) showed significant reduction of raw frequencies only (P=0.04). On PN6, all tested doses reduced the raw and normalized spasms (P<0.05). On PN7, both CPP-(1) and CPP-(0.1) groups had lower raw and normalized frequencies of spasms compared to the CPP-(0). CPP-(5) was not assessed because only one CPP-(5) rat had survived till PN7. No significant differences in spasms were seen between PN8-12. CPP-115 also reduced the normalized frequencies of spasms and improved the probability to >50% reduction in spasms for specific PN days (Supplemental Figure 1), but did not affect the time to spasms-freedom (Supplemental Results and Supplemental Table 1).
Figure 1. Daily CPP-115 administration transiently reduces raw behavioral spasms frequencies in DLP pups.
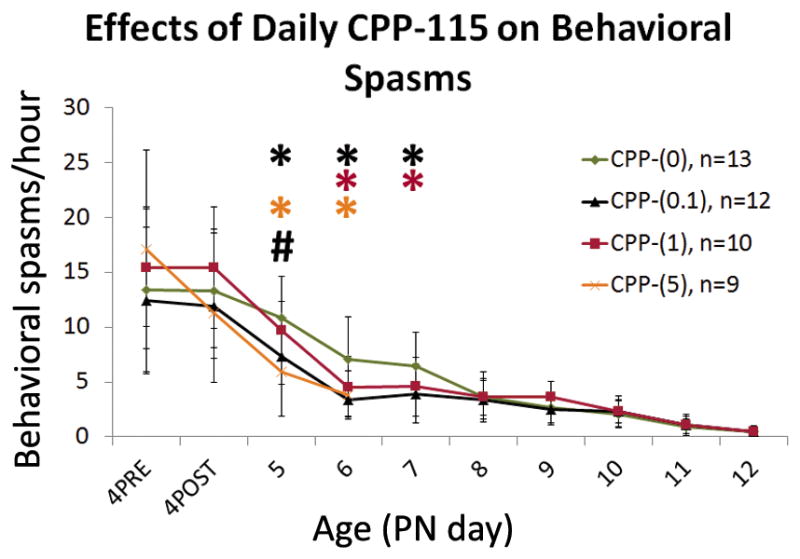
There is a significant interaction between age and treatment group for raw frequencies of spasms (P<0.01). Daily CPP-115 injections significantly reduced the raw frequencies of spasms, over several days. Comparison of log(raw frequencies of spasms) using the log(4PRE raw frequencies) as a baseline effect reveals significant reduction of raw frequencies of spasms by CPP-(5) on PN5 and PN6, by CPP-(0.1) on PN5, PN6, and PN7, and by CPP-(1) on PN6 and PN7 (P<0.05). CPP-(5) was not assessed after PN6 because of significant mortality of CPP-(5) rats (8/9 rats died by PN7). None of the tested CPP-115 doses produced an effect on spasms after PN7.
The number of rats per group is given as “n” in the figure. Graphs show group means +/− SD. Statistically significant differences in spasm frequencies versus CPP-(0) are marked with an asterisk on the graph, color coded by treatment group. (#) indicates significant differences in spasm frequencies between CPP-(5) and either of CPP-(0.1) and CPP-(1).
Acute decrease in electroclinical spasms following a single CPP-115 injection at PN7
To test whether and for how long a single CPP-115 injection reduces electroclinical spasms we administered CPP-115 (1mg/kg i.p.) once in PN7 DLP rats that had been implanted with epidural EEG electrodes on PN6. Epidural EEG electrodes were implanted on PN6 rather than on PN3, due to the small size and fragile skull. This dose was selected because it decreased behavioral spasms. Single-CPP-(1) and single-CPP-(0) groups had similar 7PRE frequencies of electroclinical spasms (p=0.92) (Fig. 2). CPP-115 injection reduced the raw (Fig. 2) and normalized frequencies of electroclinical spasms (Supplemental Figure 2) during the 1st post-injection hour (7POST) and on PN8 (p≤0.02) relative to vehicle treatment (P<0.05), but not between PN9-12. CPP-115 has therefore an acute effect in PN7 rats that was not seen on PN4. However, the single-CPP-(1) treatment did not increase the probability to spasms-freedom (Supplemental results). Interestingly, 23.5% of single-CPP-(1) showed less than 1 spasm/hr at 7POST, compared with the single-CPP-(0) group, but this trend was not statistically significant.
Figure 2. Effects of single injection of CPP-115, given at PN7, on raw electroclinical spasm frequencies.
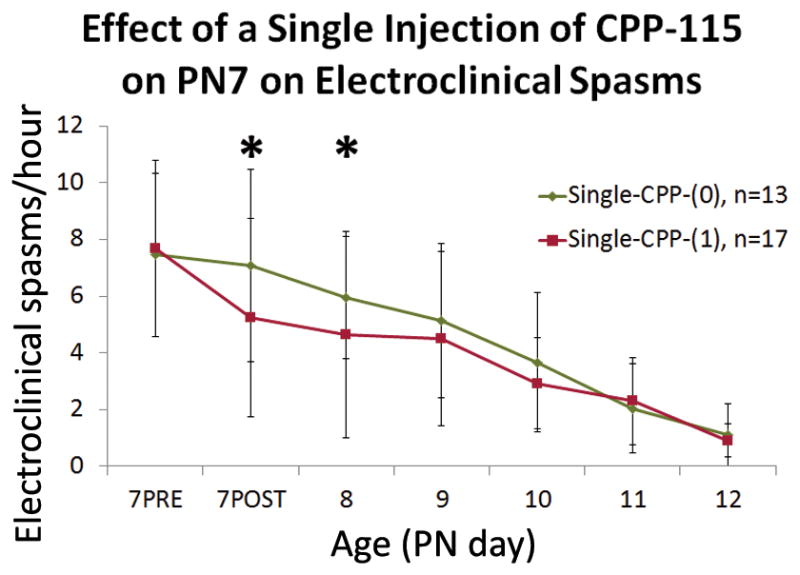
Raw electroclinical spasm frequencies were reduced by a single dose of CPP-115 (1 mg/kg i.p.) on PN7 for both the hour after CPP-115 injection (7POST) and PN8. From PN9 on, there was no significant difference. Graphs show group means +/− SD, and an asterisk indicates significant differences between single-CPP-(1) and single-CPP-(0) groups for the specified timepoint (P<0.05).
Assessment of CPP-115 tolerability in DLP pups
Survival
CPP-(5) resulted in lethality in all pups by PN8 (Log-Rank test, P<0.0001 versus the other DLP groups) (Fig. 3A). Lower doses (CPP-(0.1), CPP-(1)) showed non-significantly better survival relative to CPP-(0) [CPP-(1) versus CPP-(0) hazard ratio 0.573, P= 0.41; CPP-(0.1) versus CPP-(0) hazard ratio 0.264, P=0.09].
Figure 3. Effects of daily CPP-115 on survival and weight gain of DLP rats.
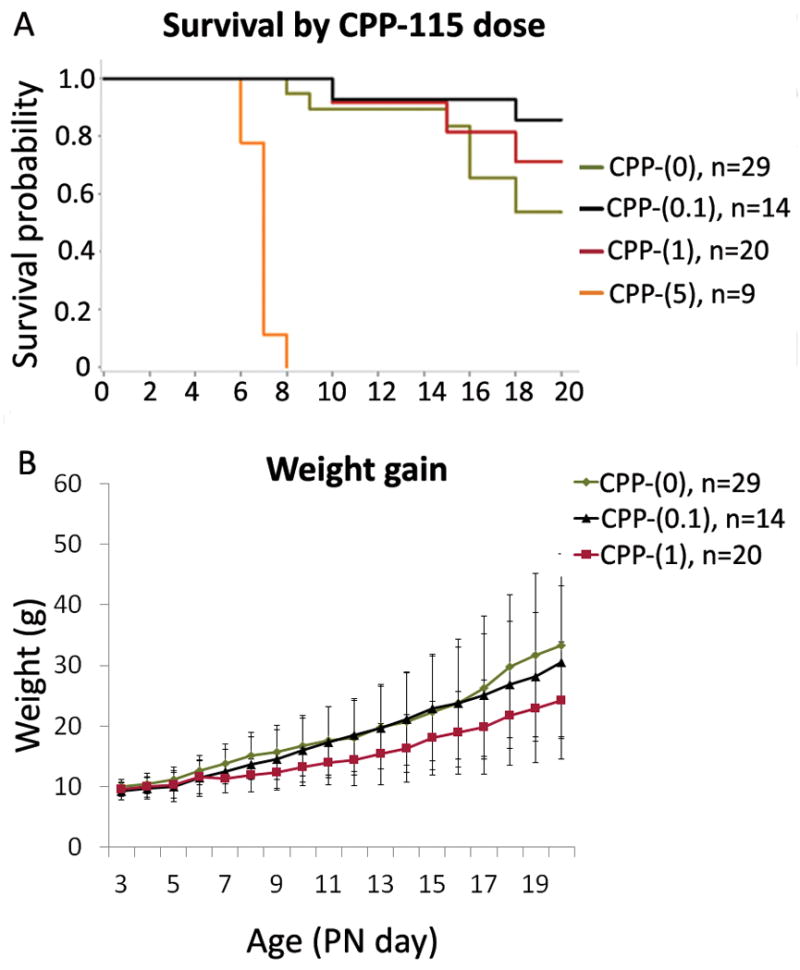
Panel A: Mortality per group was as follows by PN20: CPP-(0)=8/29, CPP-(0.1)= 2/14, CPP-(1)= 3/20, CPP-(5)= 9/9. CPP-115 doses of up to 1 mg/kg/day [CPP-(0), CPP-(0.1), and CPP-(1)] did not significantly differ in survival (Log-Rank test, P=0.17). CPP-(0.1) group showed a trend of better survival relative to CPP-(0) (hazard ratio 0.264, P=0.09). CPP-(1) group also showed better survival relative to CPP-(0) but the effect is very weak (hazard ratio 0.573, P= 0.41). CPP-(5) was uniformly lethal by PN8 (Log-Rank test, P<0.0001).
Panel B: The average weight gain rate was 0.88 g/day for the CPP-(0) group, 1.14 g/day for the CPP-(0.1) group, 0.78 g/day for the CPP-(1) group, and −0.09 g/day for the CPP-(5) group. CPP-(0.1) and CPP-(1) did not significantly affect the rate of weight gain relative to CPP-(0) (P=0.22 and 0.62 respectively). CPP-(5) rats had a lower rate weight gain than CPP-(0) (P=0.002), and they died by PN8. The graph shows group means +/− SD.
Weight
Treatment had a significant effect on weight gain (P=0.02), due to impaired weight gain in the CPP-(5) group (Fig. 3B). No significant differences were found between CPP-(0) and either CPP-(0.1) or CPP-(1).
Neurodevelopmental reflexes
CPP-(5) rats were excluded due to the high mortality in this group. No significant effect of the two lower CPP-115 doses was observed on surface righting time, open field activity, negative geotaxis and horizontal bar time across development in DLP rats treated with daily CPP-115 injections (Supplemental Results and Supplemental Figure 3).
Daily CPP-115 treatment does not affect the Barnes maze scores and learning in DLP pups, when injury is accounted for
Controls had better success rates to complete Barnes maze and shorter times to complete each trial than DLP rats, in both learning and retention sessions. No differences were seen among DLP groups (see statistics in Fig. 4).
To determine the effect on learning, we compared the times to complete each trial across the 12 sessions within each group, using a Kruskal-Wallis test. A significant improvement in time scores was observed towards the later trials in the control (P<0.0001) and CPP-(0) group (P=0.0002) but not in the CPP-(0.1) or CPP-(1) groups (P= 0.40 for each group). To clarify whether the underlying injury, rather than CPP-115, could account for the lack of learning in CPP-115 treated groups, we further correlated the extent of cortical or hippocampal injury with learning in Barnes maze.
A total of 30 rat brains were available for correlation of cortical injury with learning [11 CPP-(0) rats, 10 CPP-(0.1) rats, 9 CPP-(1) rats]. The distribution of CS scores was comparable across the three treatment groups (Fisher’s exact test, P=0.58). The majority of rats received a CS2 score (80%). Learning did not differ across treatment groups in DLP rats with a CS2 score (Fisher’s exact test, P=0.09). Cortical injury correlated with learning (Fisher’s exact test, P=0.05; Spearman correlation=−0.45, P=0.01). This was mainly due to the observation that a CS3 score was associated with no learning (n=3) whereas rats with CS1 were able to learn (n=3).
A total of 23 rat brains were evaluated histologically to assess the hippocampal injury [8 CPP-(0) rats, 10 CPP-(0.1) rats, 5 CPP-(1) rats]. HS scores were equally distributed across treatment groups (Fisher’s exact test, P=0.69). 91% of the scores ranged between HS3 and HS3.5. No significant correlation was found between HS scores and learning (Fisher’s exact test, P=0.74; Spearman correlation=−0.12, P=0.57). Compared to CPP-(0), neither CPP-(1) nor CPP-(0.1) significantly differed in learning (exact logistic regression) when taking hippocampal injury into account.
These results indicate that CPP-115 does not affect learning in the Barnes maze test, even when the underlying cortical-hippocampal injury is taken into account.
DISCUSSION
We report that CPP-115, a new vigabatrin analogue and high affinity GABA aminotransferase inactivator, proposed to have lesser risk of retinal toxicity, reduces the frequency of spasms in the DLP rat model of IS when given after the onset of spasms, as in clinical practice. CPP-115 decreases spasms in DLP rats at 400 times lower daily doses than those reported for vigabatrin, and shows improved tolerability in immature rats compared to vigabatrin (Table 1).2
Systemic CPP-115 administration reduces spasms acutely, within 1 hour, in PN7 pups but delays by 1–2 days in decreasing spasms when given on PN4. The lack of immediate response of CPP-115 on PN4 compared to PN7 could be due to the lower availability of GABA in the PN4 cortex. This could be due to the ongoing migration of GABAergic interneurons to cortical areas, during the first postnatal week of a rat, resulting in fewer interneurons in PN4 than in PN7 (our unpublished data and 21–23). Other possibilities include developmental 24 or model-specific differences in the expression of GABA aminotransferase or age-related differences in the pharmacokinetics of the drug that may render PN7 rats more sensitive to CPP-115.
The acute reduction of spasms by CPP-115 seen in PN7 pups is faster than the 1–2 days delay in vigabatrin-induced increase in GABA content in presynaptic terminals 25 or decrease in tonic seizures in adult rats.25 The high affinity of CPP-115 for GABA aminotransferase 16, 18 could explain its faster onset of action, even at 400x lower doses of CPP-115 than the vigabatrin doses that reduced DLP spasms.2 Similarly, CPP-115 inhibited cocaine-induced increase in dopamine in the nucleus accumbens at doses 300–600 times lower than vigabatrin.16 The rapid actions of CPP-115 on spasms are less likely due to off-target effects, as none has been found.16
The therapeutic doses of CPP-115 were better tolerated than vigabatrin in the DLP model (Table 1).2 CPP-(0.1) and CPP-(1) had no associated mortality, better hazard ratios in survival analyses, and did not impair neurodevelopmental reflexes or weight gain. CPP-115 has also lower risk of retinal toxicity than vigabatrin in rats.16 Between 34 to 52% of individuals with epilepsy who chronically receive vigabatrin manifest irreversible visual field deficits.26 Despite the lower (6%) risk for visual loss in vigabatrin-treated infants,27, 28 it is still important to identify safer treatments. The mechanisms of retinal toxicity from vigabatrin are unknown. Possible explanations include: 29 (a) direct toxicity from the drug or toxic byproducts of the enzymatic reaction, 16, 18 (b) elevated GABA levels (although GABA-independent retinotoxicity has been proposed 30), (c) off-target effects,16, 18 (d) photochemical injury from free radicals,30 and (e) taurine deficiency.31 The risk for visual field loss is augmented with higher daily doses of vigabatrin.26, 32 Higher affinity and more selective inactivators of GABA aminotransferase, like CPP-115, would allow for lower doses, significantly reducing the retinotoxicity stemming from the drug, its metabolites or off target effects. Indeed, long-term treatment of adult rats with maximally tolerated doses of CPP-115 produced smaller reductions in electroretinographic parameters than vigabatrin.16
CPP-115 did not improve visuospatial learning and memory in PN16-19 DLP rats, even when injury was accounted for. Clinical studies also do not support disease-modifying effect of vigabatrin in neurodevelopmental outcomes of infants with IS that are not due to tuberous sclerosis and respond with spasms cessation.33, 34 Since CPP-115 caused only transient decrease, rather than cessation, of spasms, we cannot exclude that the lack of improvement in learning could be due to the incomplete spasms control. Partial improvement in Barnes maze has been shown with pulse rapamycin that suppressed spasms early in DLP rats.20
Although our study provides proof-of-concept evidence that spasms in the DLP model respond to CPP-115, optimization of the CPP-115 treatment protocol to fully evaluate its therapeutic and disease-modifying potential will be needed. The daily administration of CPP-115 was justified because a single drug injection reduced spasms for 24 hours. However, daily CPP-115 administration had more lasting effects, reducing spasms for 2–3 days. It would be interesting to test a combination of (a) a higher starting dose (e.g., a dose between 1 and 5 mg/kg i.p. that does not impair survival) to achieve earlier spasms reduction followed by (b) lower CPP-115 doses to maintain low spasms frequencies.
Only the extent of cortical, but not of hippocampal, injury correlated with learning, despite the known role of hippocampus in learning. This is probably because 91% of the PN20 rats had hippocampal injury scores HS3-3.5, rendering our study underpowered to detect differences in learning between DLP rats with limited (HS<2) versus more extensive (HS>3.5) hippocampal pathology. Compared to our earlier studies in PN5-8 DLP rats 19 we also observed a more extensive pathology in PN20 DLP rats, indicating a progressive course.
The daily CPP-115 administration resulted in transient only reduction of spasms (2–3 days). The loss of efficacy after PN7 could reflect (a) increased metabolism of the drug, (b) treatment or model-related downregulation of GABA receptors and/or loss of GABAergic interneurons, or (c) treatment-related loss of the drug target, GABA aminotransferase, or (d) drug tolerance, as shown for vigabatrin.35 The refractoriness is probably not developmental, since a single PN7 drug injection can still reduce spasms to PN8. Further exploration of these issues would help optimize the treatment protocol.
We used only males to reduce the variability from sex-specific differences in GABA receptor signaling.36 Future testing in females would also be important.37 Testing in other models of IS 38 or seizures could help define the therapeutic indications of the drug, although age, species, and model-specific differences have to be accounted for. Efficacy testing of CPP-115 in a second, non-human, species is less necessary, 37 especially since its target mechanism, GABA aminotransferase inhibition, has already been validated in humans for the treatment of IS and seizures, through vigabatrin.
We provide the first proof-of-principle preclinical evidence in support of CPP-115 as a candidate treatment for IS. The Food and Drug Administration has granted CPP-115 orphan drug status for treatment of IS, and it is now in phase I clinical trial (NCT01493596). As vigabatrin is particularly effective in tuberous sclerosis-related IS, these patients are likely an ideal target for this analogue that seems to be more potent and less toxic. Unfortunately, there is currently no animal model of tuberous sclerosis with documented spasms to test this possibility. The effectiveness of CPP-115 in the multiple-hit model of IS indicates that the therapeutic indications of CPP-115 may not be restricted to tuberous sclerosis.
Supplementary Material
Acknowledgments
This work was supported by NINDS grants R21NS078333 and R01NS020253, research grants from Autism Speaks, CURE, the Heffer Family Foundation, and the Siegel Family Foundation, The Einstein CTSA award (NCRR UL1-RR025750), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) Award (P30HD071593). SWB was supported in part by the NIH MSTP training grant T32-GM007288. We thank Dr Solomon L. Moshé for providing feedback on the manuscript. We thank Mrs Qianyun Li, Mrs Wei Liu, and Mrs Hong Wang for their technical assistance. We are grateful to Dr Steven Miller (Catalyst Pharmaceutical Partners, Inc., Coral Gables, FL) for kindly providing us the CPP-115.
Footnotes
DISCLOSURE
The authors have no financial conflicts of interest to disclose in relevance to the current work. Dr Galanopoulou has received speaker’s honorarium from Novartis and royalties from Morgan & Claypool Publishers and John Libbey Eurotext for publications. The Albert Einstein College of Medicine has a patent on the multiple-hit model of infantile spasms (# 7863499). The authors have not received any royalties associated with this patent. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Hrachovy RA, Frost JD., Jr Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J Clin Neurophysiol. 2003;20:408–25. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Scantlebury MH, Galanopoulou AS, Chudomelova L, et al. A model of symptomatic infantile spasms syndrome. Neurobiology of disease. 2010;37:604–12. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–81. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–89. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 5.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–80. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolman JA, Faulkner MA. Vigabatrin: a comprehensive review of drug properties including clinical updates following recent FDA approval. Expert opinion on pharmacotherapy. 2009;10:3077–89. doi: 10.1517/14656560903451690. [DOI] [PubMed] [Google Scholar]
- 7.Lerner JT, Salamon N, Sankar R. Clinical profile of vigabatrin as monotherapy for treatment of infantile spasms. Neuropsychiatric disease and treatment. 2010;6:731–40. doi: 10.2147/NDT.S5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jambaque I, Chiron C, Dumas C, et al. Mental and behavioural outcome of infantile epilepsy treated by vigabatrin in tuberous sclerosis patients. Epilepsy Res. 2000;38:151–60. doi: 10.1016/s0920-1211(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Carmant L. Vigabatrin therapy for infantile spasms: review of major trials in Europe, Canada, and the United States; and recommendations for dosing. Acta neurologica Scandinavica Supplementum. 2011:36–47. doi: 10.1111/j.1600-0404.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 10.Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ. 1997;314:180–1. doi: 10.1136/bmj.314.7075.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–8. doi: 10.1212/wnl.50.3.614. [DOI] [PubMed] [Google Scholar]
- 12.John RA, Rimmer EM, Williams J, et al. Micro-vacuolation in rat brains after long term administration of GABA-transaminase inhibitors. Comparison of effects of ethanolamine-O-sulphate and vigabatrin. Biochemical pharmacology. 1987;36:1467–73. doi: 10.1016/0006-2952(87)90112-2. [DOI] [PubMed] [Google Scholar]
- 13.Hammond EJ, Ballinger WE, Jr, Lu L, et al. Absence of cortical white matter changes in three patients undergoing long-term vigabatrin therapy. Epilepsy Res. 1992;12:261–5. doi: 10.1016/0920-1211(92)90080-d. [DOI] [PubMed] [Google Scholar]
- 14.Agosti R, Yasargil G, Egli M, et al. Neuropathology of a human hippocampus following long-term treatment with vigabatrin: lack of microvacuoles. Epilepsy Res. 1990;6:166–70. doi: 10.1016/0920-1211(90)90092-a. [DOI] [PubMed] [Google Scholar]
- 15.Horton M, Rafay M, Del Bigio MR. Pathological evidence of vacuolar myelinopathy in a child following vigabatrin administration. Journal of child neurology. 2009;24:1543–6. doi: 10.1177/0883073809348796. [DOI] [PubMed] [Google Scholar]
- 16.Pan Y, Gerasimov MR, Kvist T, et al. (1S, 3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a potent gamma-aminobutyric acid aminotransferase inactivator for the treatment of cocaine addiction. Journal of medicinal chemistry. 2012;55:357–66. doi: 10.1021/jm201231w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Y, Qiu J, Silverman RB. Design, synthesis, and biological activity of a difluoro-substituted, conformationally rigid vigabatrin analogue as a potent gamma-aminobutyric acid aminotransferase inhibitor. Journal of medicinal chemistry. 2003;46:5292–3. doi: 10.1021/jm034162s. [DOI] [PubMed] [Google Scholar]
- 18.Silverman RB. The 2011 E. B. Hershberg award for important discoveries in medicinally active substances: (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms. Journal of medicinal chemistry. 2012;55:567–75. doi: 10.1021/jm201650r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono T, Moshe SL, Galanopoulou AS. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia. 2011;52:1678–84. doi: 10.1111/j.1528-1167.2011.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raffo E, Coppola A, Ono T, et al. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiology of disease. 2011;43:322–9. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16:1032–60. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- 22.Balcar VJ, Zetzsche T, Wolff JR. Glutamate decarboxylase in developing rat neocortex: does it correlate with the differentiation of GABAergic neurons and synapses? Neurochemical research. 1992;17:253–60. doi: 10.1007/BF00966667. [DOI] [PubMed] [Google Scholar]
- 23.Wong PT, McGeer EG. Postnatal changes of GABAergic and glutamatergic parameters. Brain research. 1981;227:519–29. doi: 10.1016/0165-3806(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 24.Kristt DA, Waldman JV. Late postnatal changes in rat somatosensory cortex. Temporal and spatial relationships of GABA-T and AChE histochemical reactivity. Anat Embryol (Berl) 1986;174:115–22. doi: 10.1007/BF00318343. [DOI] [PubMed] [Google Scholar]
- 25.Gale K, Iadarola MJ. Seizure protection and increased nerve-terminal GABA: delayed effects of GABA transaminase inhibition. Science. 1980;208:288–91. doi: 10.1126/science.6768130. [DOI] [PubMed] [Google Scholar]
- 26.Maguire MJ, Hemming K, Wild JM, et al. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia. 2010;51:2423–31. doi: 10.1111/j.1528-1167.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 27.Gaily E, Jonsson H, Lappi M. Visual fields at school-age in children treated with vigabatrin in infancy. Epilepsia. 2009;50:206–16. doi: 10.1111/j.1528-1167.2008.01961.x. [DOI] [PubMed] [Google Scholar]
- 28.Camposano SE, Major P, Halpern E, et al. Vigabatrin in the treatment of childhood epilepsy: a retrospective chart review of efficacy and safety profile. Epilepsia. 2008;49:1186–91. doi: 10.1111/j.1528-1167.2008.01589.x. [DOI] [PubMed] [Google Scholar]
- 29.Heim MK, Gidal BE. Vigabatrin-associated retinal damage: potential biochemical mechanisms. Acta neurologica Scandinavica. 2012;126:219–28. doi: 10.1111/j.1600-0404.2012.01684.x. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Ishikawa M, Benz AM, et al. Acute vigabatrin retinotoxicity in albino rats depends on light but not GABA. Epilepsia. 2004;45:1043–8. doi: 10.1111/j.0013-9580.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 31.Jammoul F, Wang Q, Nabbout R, et al. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann Neurol. 2009;65:98–107. doi: 10.1002/ana.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wild JM, Chiron C, Ahn H, et al. Visual field loss in patients with refractory partial epilepsy treated with vigabatrin: final results from an open-label, observational, multicentre study. CNS drugs. 2009;23:965–82. doi: 10.2165/11317650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Darke K, Edwards SW, Hancock E, et al. Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: a multi-centre randomised trial. Archives of disease in childhood. 2010;95:382–6. doi: 10.1136/adc.2009.160606. [DOI] [PubMed] [Google Scholar]
- 34.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet neurology. 2005;4:712–7. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- 35.Rundfeldt C, Loscher W. Development of tolerance to the anticonvulsant effect of vigabatrin in amygdala-kindled rats. European journal of pharmacology. 1992;213:351–66. doi: 10.1016/0014-2999(92)90624-d. [DOI] [PubMed] [Google Scholar]
- 36.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galanopoulou AS, Kokaia M, Loeb JA, et al. Epilepsy therapy development: Technical and methodologic issues in studies with animal models. Epilepsia. 2013;54 (Suppl 4):13–23. doi: 10.1111/epi.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galanopoulou AS. Basic mechanisms of catastrophic epilepsy - Overview from animal models. Brain & development. 2013;35:748–56. doi: 10.1016/j.braindev.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


