Abstract
Background
Fat grafting has become increasingly popular for the correction of soft tissue deficits at many sites throughout the body. Long-term outcomes, however, depend on delivery of fat in the least traumatic fashion to optimize viability of the transplanted tissue. In this study, we compare the biologic properties of fat following injection using two methods.
Methods
Lipoaspiration samples were obtained from five female donors and cellular viability, proliferation, and lipolysis were evaluated following injection using either a modified Coleman technique or an automated, low shear device. Comparisons were made to minimally processed, uninjected fat. Volume retention was also measured over twelve weeks following injection of fat under the scalp of immunodeficient mice using either the modified Coleman technique or the Adipose Tissue Injector. Finally, fat grafts were analyzed histologically.
Results
Fat viability and cellular proliferation were both significantly greater with the Adipose Tissue Injector relative to injection with the modified Coleman technique. In contrast, significantly less lipolysis was noted using the automated device. In vivo fat volume retention was significantly greater than with the modified Coleman technique at 4, 6, 8, and 12 week time points. This corresponded with significantly greater histological scores for healthy fat and lower scores for injury following injection with the device.
Conclusions
Biological properties of injected tissues reflect how disruptive and harmful techniques for placement of fat may be, and our in vitro and in vivo data both support the use of the automated, low shear devices compared to the modified Coleman technique.
Keywords: adipose cells, fat transfer, tissue regeneration, fat grafting
Introduction
Fat grafting is a rapidly evolving technique in the field of Plastic and Reconstructive Surgery. While Neuber first described fat transfer to fill a depressed facial scar in 1893, the past two decades have witnessed significant refinement in this area.(1, 2) Today, autogenous fat is an attractive tissue source for soft tissue augmentation and has been applied to a wide range of facial defects from both congenital and acquired conditions.(3–6) The use of fat grafting has also been expanded to soft tissue defects throughout the body, with successful treatment of post-traumatic contour defects in the abdomen, trunk, and thighs.(7–10)
Restoration of facial contour through fat grafting can often be accomplished through small volume transfer, and Coleman and others have developed a variety of techniques and instrumentation that are now in clinical use.(5) Over the past 10 to 15 years, many surgeons have adapted specialized strategies for each step including fat harvest, processing, and graft injection.(11) In contrast to fat grafting in the face, using this approach to correct soft tissue contour abnormalities in the trunk or for breast reconstruction or augmentation can require transfer of significantly greater volumes.(7–10) And while surgeons understand the advantages of using autogenous fat for breast reconstruction or augmentation, the multiple steps required for fat preparation prior to reinjection can be tedious, requiring several assistants. Thus, while plastic surgeons in general would like to incorporate large volume fat transfer into their practices, clinical adoption hinges on more efficient techniques and improved instrumentation.
Aside from these limitations, clinical results for large volume fat grafting have been highly inconsistent.(5, 12–14) Peer postulated that the degree of fat graft survival correlated with the number of viable adipocytes following transfer, and this “cell survival theory” has been the most widely accepted explanation for variable post-transplant resorption.(15, 16) Investigations have therefore focused on minimizing trauma and optimizing viability of tissue at each of three different stages for the process of fat grafting: procurement of fat through lipoaspiration, processing of fat, and reinjection in the most “fat friendly” manner.(11) While instrumentation and technical improvements in all of these areas will likely be necessary for optimal clinical results, this present study evaluates a novel device specifically designed to minimize trauma to fat during the last stage of reinjection.
The Adipose Tissue Injector (ATI) was designed to efficiently and reproducibly inject preset volumes of fat following harvest and processing under low shear conditions. The effects of this device on adipocyte viability were compared to both injected fat using a modified Coleman technique and minimally processed fat. Both in vitro analyses on cellular metabolism and proliferation and in vivo comparisons on fat retention were performed. Based on these studies, we noted bench-top fat viability and proliferation to be significantly enhanced with the ATI relative to the modified Coleman technique. Furthermore, these results translated to significantly greater in vivo maintenance of injected fat volume with the ATI. Given these findings, fat transfer using the ATI may be more efficient and yield more reproducible results compared to the modified Coleman technique.
Materials and Methods
Fat Harvesting and Processing
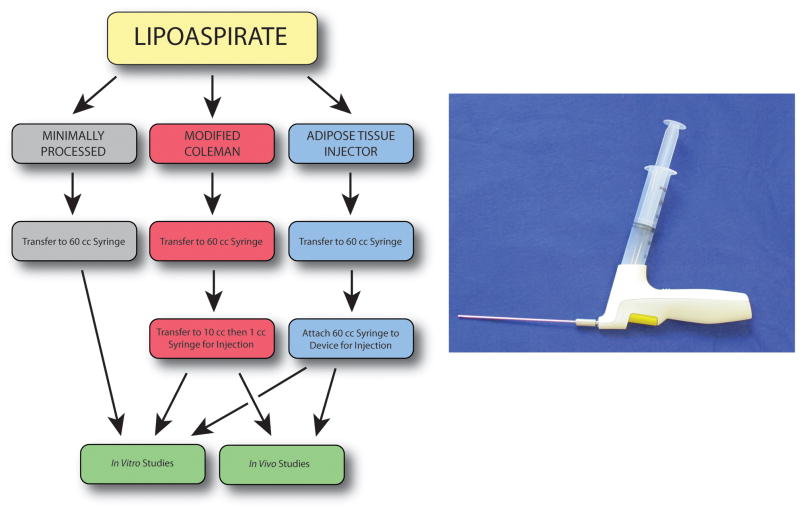
Lipoaspiration samples were obtained using suction-assisted liposuction from five healthy female donors (ages 27–47), in accordance with Stanford University Institutional Review Board guidelines. Gravity separation was performed and the fat was then separated into three experimental groups (Figure 1A). Experimental groups were applied to each patient’s fat allowing for direct comparison and elimination of differences resulting from harvest method or surgeon preferences. For the minimally processed group, fat was transferred into a 60cc syringe using a large caliber 25cc tip on a serological pipette. For the modified Coleman technique group, fat was transferred into a 60cc syringe using a large caliber 25cc tip on a serological pipette. A three-way Luer-lock stop-cock was then employed to sequentially transfer fat from the 60cc syringe into 10cc syringes and then into multiple 1cc syringes for injection. Finally, for the device group, fat was transferred into a 60cc syringe using a large caliber 25cc tip on a serological pipette. The syringe was then connected to the ATI device to deliver fat.
Figure 1.
Overview of fat processing. A) Schematic showing handling of fat for three treatment groups: minimally processed (gray), modified Coleman (red), and Adipose Tissue Injector (blue). B) Photograph depicting Adipose Tissue Injector device with attached syringe.
Adipose Injector Device
The ATI is a hand-held, sterile, single-use battery-powered disposable device designed by TauTona Group (Menlo Park, CA) to be used with off-the-shelf syringes and injection cannulas (Figure 1B). The device was set to a user defined 400 μl aliquot and the trigger was pulled to prime the system. With each subsequent pull of the trigger, precise delivery of a preset fat volume was achieved through the cannula while fat was simultaneously drawn from the syringe to refill the pump. While a single trigger pull results in delivery of a single aliquot, a sustained pull allows for continuous delivery of multiple aliquots until release of the trigger.
MTT Assay
Fat from each experimental group was placed into conical tubes in 2 ml aliquots (n=5 for all three groups). For the minimally processed group, a serological pipette was used to transfer the fat. For the modified Coleman technique group, fat from two 1cc syringes were injected into the conical tube. For the ATI device group, fat was transferred by repeated pulling of the trigger until 2 ml of fat had been transferred. To each conical tube, 1 ml of an MTT stock solution (2 mg/ml in PBS) was added and conical tubes were then incubated at 37°C on a shaking platform for 30 minutes. Following incubation, samples were sonicated for 15 seconds and corn oil was added to a final volume of 10 ml for signal ratio optimization. Tubes were then centrifuged at 10,000 RPM for 5 minutes and 150 μl of the supernatent were transferred to a 96-well plate. Absorbance was read on a microplate reader at 570 nm and 650 nm.
BrdU Assay
Fat from each of the three experimental groups was digested to isolate mature adipocytes and then transferred into separate 500 ml flasks until a final volume of 150 ml was reached. For the modified Coleman technique group, fat from 1cc syringes were injected to reach the final volume. For the ATI device group, fat was transferred by repeated pulling of the trigger. To each flask, 0.075% collagenase type I was added and the flasks were gently agitated at 37°C for 1 hour. Samples were then centrifuged at 1200 g for 5 minutes to remove adipocytes in the top layer. Adipocytes were placed into 6-well plates and at 24 and 48 hours, 200 μl of adipocytes were transferred to a 96-well plate with 20 μl of a prepared BrdU solution (Cell Signaling Technology; Boston, MA). Samples were then incubated for two hours, fixed, and treated per the manufacturer’s instructions. Absorbance was read at 450 nm.
Lipolysis Assay
Adipocytes from each group plated as described above were evaluated for lipolysis using a cultured human adipocyte lipolysis assay kit from Zen-Bio. Briefly, 150 μl of adipocyte aliquots from minimally processed, modified Coleman technique, and ATI device groups were transferred to a 96-well plate and treated with a glycerol reagent for three hours at 37°C per manufacturer’s instructions. Optical density of each well was measured at 540 nm. Absorbance values were compared to a glycerol standard curve for quantification.
RNA Extraction and QRT-PCR
RNA was harvested from adipocytes for each experimental group using QIAzol Lysis Reagent from Qiagen (Germantown, MD). RNA was then isolated using the RNeasy lipid extraction kit and reverse transcription was performed. Quantitative reverse transcription-PCR was performed on an Applied Biosystems Prism 7900HT Sequence Detection System using a SYBR Green PCR Master Mix. Specific primers for genes examined were based on their PrimerBank sequence and listed in Table 1.
Animal Model Fat Injection and CT Analysis
Adult, 60-day-old Crl:NU-Foxn1nu male mice (Charles River Laboratories; Wilmington, MA) were used for experiments in this study to minimize effects of an immune reaction to retention of implanted human fat.(17) Fat was injected using a 2 mm cannula beneath the scalp using both the modified Coleman technique and the ATI device (n=5 mice per group). A subcutaneous tunnel was first created with the cannula and then fat was delivered in retrograde fashion.(17) With the modified Coleman technique, 400 μl was delivered by pushing on the syringe plunger. For the ATI device, fat was delivered with one pull of trigger.
Micro-computed tomography scans were performed on mice at day 3 following injection for a baseline, and then at 2, 4, 6, 8, and 12 weeks for comparison. Imaging was performed with a MicroCAT-II in vivo X-ray micro-CT scanner (Imtek, Inc.; Knoxville, TN). The imaging protocol was 9 min long with real-time reconstruction yielding a voxel resolution of 80 μm with 719 views. Three dimensional reconstruction was performed using the MicroCAT Reconstruction Software (Imtek, Inc.) and cubic-spline interpolation was used to calculate volumes. Previously performed ex-vivo imaging of fresh lipoaspirate determined voxel range values for fat between −300 and +300 HU.(17) All analyses were performed by a single person (M.T.C.).
Histological Analysis of Adipose Tissue
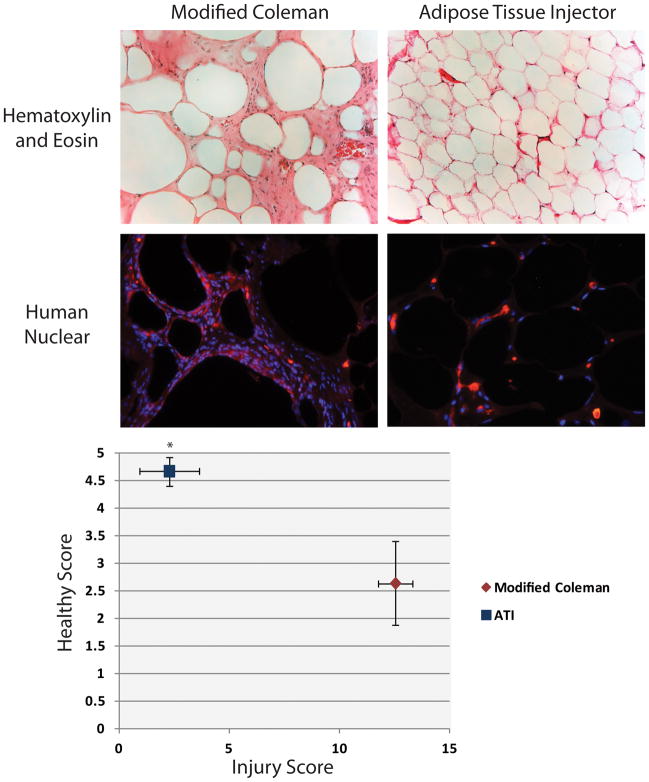
At the 12-week time point, fat was carefully dissected from the scalp, fixed, and sectioned for hematoxylin and eosin staining. Histology scores were generated by four blinded, independent investigators. The scoring method used was previously published, assessing for healthy fat, vacuoles, infiltrate, and fibrosis.(18–20) The parameters were scored as follows: 0 = absence, 1 = minimal presence, 2 = minimal to moderate presence, 3 = moderate presence, 4 = moderate to extensive presence, and 5 = extensive presence.(20) Finally, the scores for vacuoles, infiltrate, and fibrosis were combined to yield a total injury score. Immunofluorescent staining was performed for Human Nuclear Antigen (Abcam; Cambridge, MA) using an Alexa Fluor 594-conjugated secondary antibody (Invitrogen; Grand Island, NY). DAPI counterstaining was also performed.
Statistical Analysis
Statistical analysis was performed using a one-way ANOVA for multiple group comparisons and by a two-tailed Student’s t-test to directly compare two groups. A *p < 0.05 was considered statistically significant.
Results
Effect of Lipoaspirate Processing / Injection Techniques on Viability
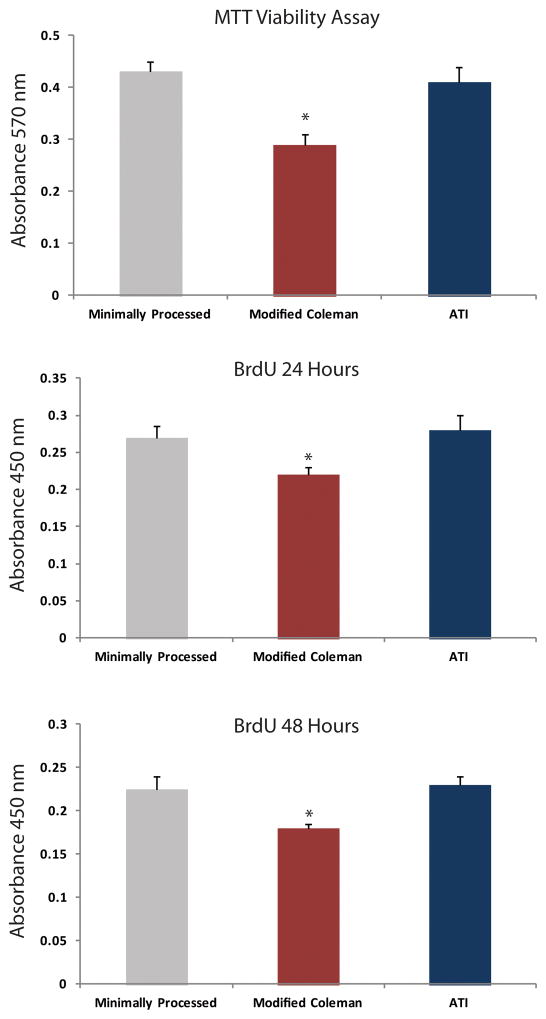
In order to evaluate the efficacy of each processing / injection technique on fat viability, we performed an MTT viability assay to determine if there were any differences in cellular survival between minimal processing, modified Coleman technique, or with the ATI device. We demonstrated that there was a significant advantage in cellular viability using the ATI device versus processing with the modified Coleman technique (*p < 0.05) (Figure 2A). In addition, there was no significant difference in cellular viability between minimally processed fat and fat passed through the ATI device (p > 0.05).
Figure 2.
Effect of processing on viability and proliferation. A) MTT assay showing significantly increased viability of fat following processing with the Adipose Tissue Injector (blue) when compared to modified Coleman (red) (*p < 0.05). BrdU proliferation assay at 24 hours (B) and 48 hours (C) also demonstrated significantly increased adipocyte proliferation following processing with the Adipose Tissue Injector relative to modified Coleman (*p < 0.05).
Effect of Lipoaspirate Processing / Injection Techniques on Proliferation
After investigating fat viability, we then determined if there was a difference in adipocyte proliferation with each of the experimental groups. At both 24 hours (Figure 2B) and 48 hours (Figure 2C), proliferation of adipocytes processed and injected with the ATI device were equivalent to minimally processed adipocytes (p > 0.05). Adipocytes from the ATI device group demonstrated significantly greater proliferation over adipocytes processed by the modified Coleman technique (*p < 0.05).
Effect of Lipoaspirate Processing / Technique on Lipolysis
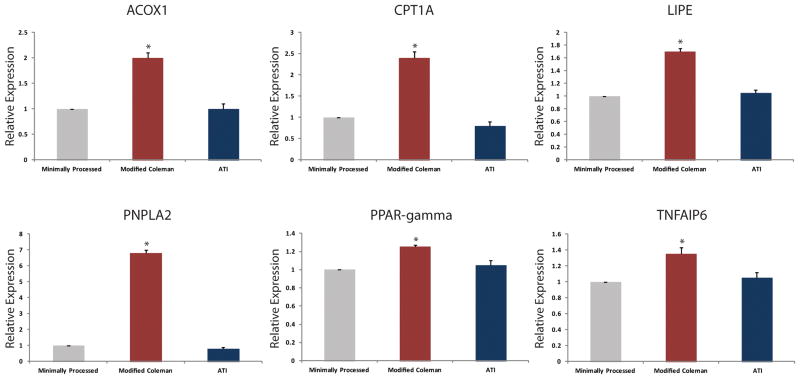
Following evaluation of fat viability and cellular proliferation, transcriptional activity of several key lipolytic genes were evaluated. Quantitative real-time PCR for acyl-CoA oxidase 1 (ACOX1), carnitine palmitoyltransferase 1A (CPT1A), hormone-sensitive lipase (LIPE), patatin-like phopholipase domain containing 2 (PNPLA2), peroxisome proliferator-activated receptor gamma (PPAR-γ), and tumor necrosis factor-alpha induced protein 6 (TNFAIP6) demonstrated significantly increased expression among adipocytes in the modified Coleman technique group compared to adipocytes from the ATI device or minimally processed groups (*p < 0.05) (Figure 3). There was no significant difference, however, between the ATI device and the minimally processed group (p > 0.05).
Figure 3.
Quantitative real-time PCR of lipolytic genes. Transcript levels for A) acyl-CoA oxidase 1 (ACOX1), B) carnitine palmitoyltransferase 1A (CPT1A), C) hormone-sensitive lipase (LIPE), D) patatin-like phopholipase domain containing 2 (PNPLA2), E) peroxisome proliferator-activated receptor gamma (PPAR-γ), and F) tumor necrosis factor-alpha induced protein 6 (TNFAIP6) demonstrated significantly increased expression in adipocytes following modified Coleman technique (red) processing compared to adipocytes from the ATI device (blue) or minimally processed (gray) groups (*p < 0.05).
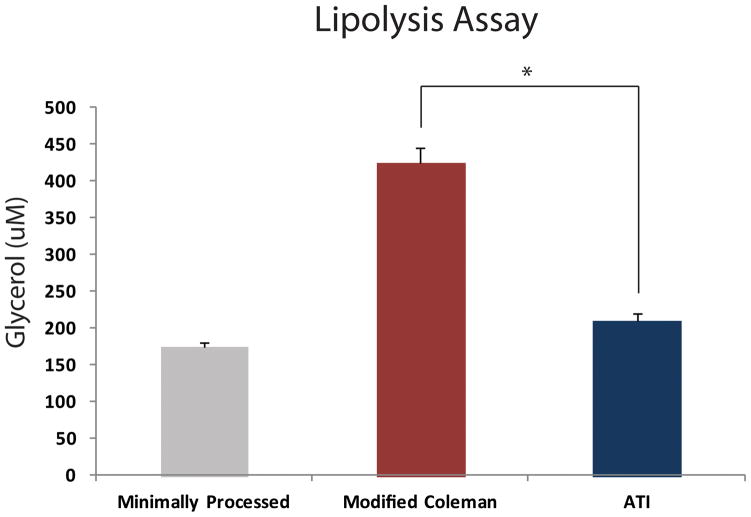
After analyzing transcriptional activity for these lipolytic genes, we then performed a lipolysis assay to detect levels of free glycerol present. After 24 hours of culture, there was significantly less free glycerol from adipocytes in the ATI device group relative to the modified Coleman technique group (*p < 0.05) (Figure 4). There was no significant difference, however, between the ATI device and the minimally processed group (p > 0.05). These findings suggest that the adipocyte breakdown and lipolysis may be greatest with the modified Coleman technique.
Figure 4.
Effect of processing on lipolysis. Fat processed using the modified Coleman technique was noted to have significantly greater free glycerol levels compared to the ATI device (*p < 0.05).
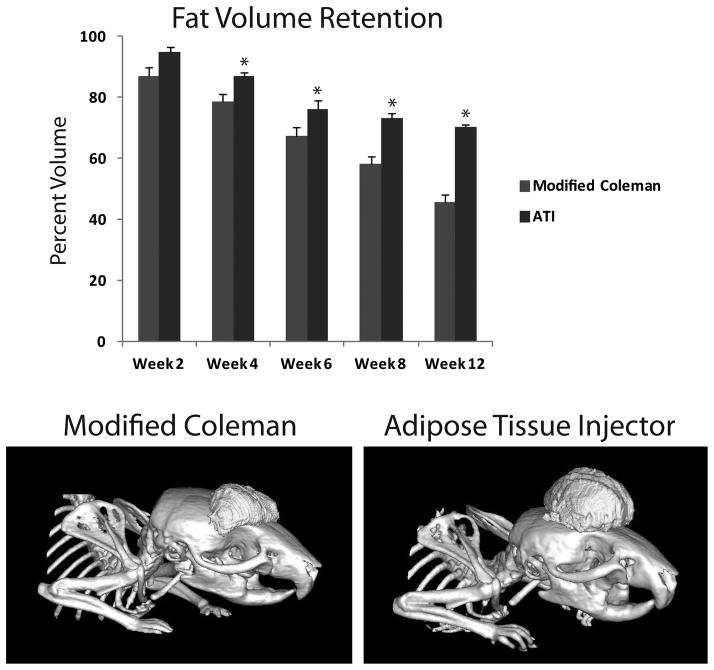
Effect of Lipoaspirate Processing / Technique on In Vivo Retention
While our in vitro data strongly support an injection advantage with the ATI device over our modified Coleman technique, these data do not necessarily translate into enhanced in vivo volume retention. To explore this question, we used our recently published nude mouse model which demonstrated CT volume measurements to accurately reflect measured volume of injected, minimally processed fat.(17) In this present study, no significant difference was appreciated in volume retention between the modified Coleman and the ATI device group at two weeks (Figure 5A). However, beginning at week four and continuing through week 12, significantly greater volume retention was appreciated with injected fat from the ATI device (*p < 0.05) (Figure 5A and B). Finally, on histological analysis, we observed significantly higher scores for healthy fat among specimens following injection with the ATI device (4.7 ± 0.3 ATI vs. 2.6 ± 0.8 modified Coleman) (*p < 0.05) (Figure 6A and B). This was also associated with significantly lower total injury scores when compared to fat injected using the modified Coleman technique (2.2 ± 1.3 ATI vs. 12.5 ± 2.3 modified Coleman) (*p < 0.05). Immunofluorescent staining for Human Nuclear Antigen confirmed the presence of human cells in our specimens (Figure 6A, bottom). However, increased cellularity of mouse origin was noted in specimens following injection with the modified Coleman technique, as demonstrated by DAPI staining in the absence of Human Nuclear Antigen staining (Figure 6B, bottom).
Figure 5.
In vivo fat volume retention. A) Fat volume retention was significantly greater following injection with the ATI device (blue) compared to the modified Coleman technique (red) at 4, 6, 8, and 12 weeks (*p < 0.05). B) Representative reconstructed images of injected fat following injection with modified Coleman technique (left) and ATI device (right) at 12 weeks.
Figure 6.
Histological analysis of injected fat. A) Hematoxylin and eosin staining (top) of explanted fat 12 weeks after injection with modified Coleman technique (left) and ATI device (right). Human-Nuclear Antigen immunofluorescent staining (bottom) confirms human origin of explanted fat. Note increased infiltration of mouse-derived cells with modified Coleman technique. B) Chart of histological scoring with healthy score on y-axis and combined injury score (vacuoles, infiltrate, and fibrosis) on x-axis. Injected fat with ATI device (blue) had significantly greater healthy score and significantly lower injury score compared to modified Coleman technique (red) (*p < 0.05).
Discussion
Autologous fat grafting has become an area of interest in clinical plastic surgery. Given its wide availability and ease of harvest, autologous fat possesses many of the most ideal properties desirable to address soft-tissue deficits and contour abnormalities throughout the entire body. Unfortunately, outcomes from fat grafting remain unpredictable, as survival of transplanted fat can be highly variable.(5, 12–14) Further contributing to this problem is the lack of objective analyses, as the literature in this field is rife with subjective photographic analysis or anecdotal reports.(5, 21)
Because of inconsistent results reported with fat grafting, investigators have now focused on specific interventions at three key steps during the process of transplantation. These include techniques for fat harvest, processing of tissue, and methods of fat transplantation. With respect to fat harvest, a lack of consensus still exists as to the ideal donor site.(22–25) Of likely greater importance to fat harvest is the actual technique used for removal of fat. While studies have shown no deleterious effects on fat graft retention following ultrasound-assisted liposuction, recent work from our own laboratory has observed reduced viability and proliferation of harvested cells following laser-assisted liposuction relative to SAL.(26–28) Similar to the controversy surrounding fat harvest, there is a lack of consensus regarding the optimal technique for processing harvested tissue. While Coleman described the technique of centrifugation to quickly isolate fat, subsequent studies have either shown no difference or worse survival of adipocytes after centrifugation compared to gravity separation.(5, 21, 23, 29) New devices have also been developed which simultaneously wash and filter freshly harvested lipoaspirate.(30) However, long-term clinical outcomes of fat grafting following this method of processing have yet to be reported.
With research continuing in both strategies for fat harvest and processing of tissue, recent studies have also begun to evaluate methods of fat reinjection to optimize clinical results. Erdim and colleagues investigated various needle gauges for transfer of fat and actually demonstrated no significant difference for in vitro fat viability between 14-, 16-, and 20-gauge needles.(31) One of the other key considerations is the consistent, predictable delivery of a set volume of fat in the least traumatic fashion. This represents the final common step in fat grafting. Heaton et al. described the adaptation of a foot-controlled pneumatic handpiece piston attached to a 1cc syringe for delivery of small volumes of fat into the vocal folds.(32) Importantly, while they were able to show reproducible injection of fat, the actuator on their device replicates a plunger in a 1cc syringe.(32) With the small cross-sectional area of the piston inside the syringe, similar pressures and shear stress may still be delivered to fat as that seen with the Coleman technique.
In contrast, the ATI is a low shear, automated device, and we evaluated its effects on adipocyte biology and fat retention. From a user perspective, the ATI device eliminates over-injection due to heterogeneous materials causing obstructions in the cannula which are subsequently overcome by accumulated pressure. Furthermore, imprecision from having to read graduated markings on the syringe is eliminated, and both flow rate and volume can be predefined by the surgeon. Finally, with a trigger mechanism, effort-related tremor can be minimized during operation.
We found that adipocyte viability and proliferation were both significantly greater with the ATI device compared to the modified Coleman technique and similar to minimally processed fat which had not been passed through an injection cannula. Our data also suggested that delivery of fat through the ATI device may be more “fat friendly”, as reflected by reduced lipolysis. Of greatest interest, though, we noted significantly higher fat volume retention along with healthier appearing fat on histology in our animal model following delivery of fat through the ATI device compared to the modified Coleman technique. Following injection with the modified Coleman technique, significantly higher injury scores were also noted, consistent with increased cellularity secondary to mouse-derived cellular infiltration. Collectively, these findings highlight the advantages of the ATI device.
We recognize that this study was performed using small volume fat transfer in an animal model and that translation to the clinical setting has yet to be performed. Furthermore, long-term outcomes for fat grafting using the ATI device will be necessary. Nonetheless, biologic and material properties of injected tissues offer clues as to how disruptive or harmful techniques for the procurement, processing, and placement of fat can be, and our in vitro and in vivo data of injected fat all support the “fat friendly” nature of the ATI device.
Supplementary Material
Primer sequences for lipolytic genes
Acknowledgments
Products Page
G.C.G. was supported by NIH grants R01 AG-25016, R01 DK-074095, and 1R01 HL104236-01. D.C.W. was supported by the ACS Franklin H. Martin Faculty Research Fellowship, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award. M.T.L. was supported by NIH grants R01 DE021683-01, 1 R21 DE019274-01, RC2 DE020771, the Oak Foundation, and Hagey Laboratory for Pediatric Regenerative Medicine.
J.R.R. is the Vice President of Research and Development and Chief Operating Officer at TauTona Group. G.C.G and M.T.L. have equity in the TauTona Group. M.T.L. co-authored this manuscript while on sabbatical from Stanford University.
Footnotes
Data Presented in this manuscript have not been previously presented at any meeting
Financial Disclosure
M.T.C., K.J.P., D.A.A., J.S.H., A.Mc.A., K.S.Y., E.R.Z., R.T., C.D., M.S.H., G.G.W., A.P.A., A.M., G.W.C., and D.C.W., have no financial interest or commercial association with any of the subject matter or products mentioned in our manuscript.
Author Contributions
M.T.C., K.J.P., D.A.A., J.S.H., G.C.G., D.C.W., and M.T.L. played an integral role in formulating the design of this research study and writing the manuscript. J.S.H. directed the in vitro arm of this study, and M.T.C., K.J.P., and D.A.A. headed the in vivo arm. A.Mc.A., K.S.Y., E.R.Z., R.T., C.D., M.S.H., G.G.W., A.P.A., G.W.C., and A.M. provided support in processing fat samples, analyzing results, and guiding the study. J.R.R. helped design the Adipose Device and provided the engineering expertise. G.W.C. provided the lipoaspirate used for the study material. M.T.C. and K.J.P contributed equally to the first authorship of this manuscript.
References
- 1.Neuber F. Fat transplantation. Chir Kongr Verhandl Dsch Gesellch Chir. 1893;20:66. [Google Scholar]
- 2.Klein AW, Elson ML. The history of substances for soft tissue augmentation. Dermatol Surg. 2000;26:1096–1105. [PubMed] [Google Scholar]
- 3.Guibert M, Franchi G, Ansari E, et al. Fat graft transfer in children’s facial malformations: A prospective three-dimensional evaluation. J Plast Reconstr Aesthet Surg. 2013;66:799–804. doi: 10.1016/j.bjps.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Pereira LH, Sterodimas A. Long-term fate of transplanted autologous fat in the face. J Plast Reconstr Aesthet Surg. 2010;63:e68–69. doi: 10.1016/j.bjps.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24:347–367. [PubMed] [Google Scholar]
- 6.Gamboa GM, Ross WA. Autologous fat transfer in aesthetic facial recontouring. Ann Plast Surg. 2013;70:513–516. doi: 10.1097/SAP.0b013e31827eac42. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas-Camarena L, Arenas-Quintana R, Robles-Cervantes JA. Buttocks fat grafting: 14 years of evolution and experience. Plast Reconstr Surg. 2011;128:545–555. doi: 10.1097/PRS.0b013e31821b640b. [DOI] [PubMed] [Google Scholar]
- 8.Nicareta B, Pereira LH, Sterodimas A, Illouz YG. Autologous gluteal lipograft. Aesthetic Plast Surg. 2011;35:216–224. doi: 10.1007/s00266-010-9590-y. [DOI] [PubMed] [Google Scholar]
- 9.Brongo S, Nicoletti GF, La Padula S, Mele CM, D’Andrea F. Use of lipofilling for the treatment of severe burn outcomes. Plast Reconstr Surg. 2012;130:374e–376e. doi: 10.1097/PRS.0b013e3182590387. [DOI] [PubMed] [Google Scholar]
- 10.Hoyos AE, Perez ME, Castillo L. Dynamic definition mini-lipoabdominoplasty combining multilayer liposculpture, fat grafting, and muscular plication. Aesthet Surg J. 2013;33:545–560. doi: 10.1177/1090820X13484493. [DOI] [PubMed] [Google Scholar]
- 11.Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg. 2012;130:511–522. doi: 10.1097/PRS.0b013e31825dbf8a. [DOI] [PubMed] [Google Scholar]
- 12.Fontdevila J, Serra-Renom JM, Raigosa M, et al. Assessing the long-term viability of facial fat grafts: an objective measure using computed tomography. Aesthet Surg J. 2008;28:380–386. doi: 10.1016/j.asj.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez AM, Lobocki C, Kelly CP, Jackson IT. An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg. 2007;120:285–294. doi: 10.1097/01.prs.0000264401.19469.ad. [DOI] [PubMed] [Google Scholar]
- 14.Coleman SR. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg. 1995;19:421–425. doi: 10.1007/BF00453875. [DOI] [PubMed] [Google Scholar]
- 15.Rieck B, Schlaak S. Measurement in vivo of the survival rate in autologous adipocyte transplantation. Plast Reconstr Surg. 2003;111:2315–2323. doi: 10.1097/01.PRS.0000060797.59958.55. [DOI] [PubMed] [Google Scholar]
- 16.Peer LA. Cell survival theory versus replacement theory. Plast Reconstr Surg (1946) 1955;16:161–168. doi: 10.1097/00006534-195509000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chung MT, Hyun JS, Lo DD, et al. Micro-computed tomography evaluation of human fat grafts in nude mice. Tissue Eng Part C Methods. 2013;19:227–232. doi: 10.1089/ten.tec.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramon Y, Shoshani O, Peled IJ, et al. Enhancing the take of injected adipose tissue by a simple method for concentrating fat cells. Plastic and reconstructive surgery. 2005;115:197–201. discsussion 202–193. [PubMed] [Google Scholar]
- 19.Yi C, Pan Y, Zhen Y, et al. Enhancement of viability of fat grafts in nude mice by endothelial progenitor cells. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2006;32:1437–1443. doi: 10.1111/j.1524-4725.2006.32351.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG., Jr The effect of pressure and shear on autologous fat grafting. Plastic and reconstructive surgery. 2013;131:1125–1136. doi: 10.1097/PRS.0b013e3182879f4a. [DOI] [PubMed] [Google Scholar]
- 21.Thorne CHB, Aston RW, Bartlett SJSP, Gurtner GC, Spear SL. Grabb & Smith’s Plastic Surgery. 6. Philadelpha, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 22.Li K, Gao J, Zhang Z, et al. Selection of donor site for fat grafting and cell isolation. Aesthetic Plast Surg. 2013;37:153–158. doi: 10.1007/s00266-012-9991-1. [DOI] [PubMed] [Google Scholar]
- 23.Rohrich RJ, Sorokin ES, Brown SA. In search of improved fat transfer viability: a quantitative analysis of the role of centrifugation and harvest site. Plast Reconstr Surg. 2004;113:391–395. doi: 10.1097/01.PRS.0000097293.56504.00. discussion 396–397. [DOI] [PubMed] [Google Scholar]
- 24.Ullmann Y, Shoshani O, Fodor A, et al. Searching for the favorable donor site for fat injection: in vivo study using the nude mice model. Dermatol Surg. 2005;31:1304–1307. doi: 10.1111/j.1524-4725.2005.31207. [DOI] [PubMed] [Google Scholar]
- 25.Butterwick KJ, Nootheti PK, Hsu JW, Goldman MP. Autologous fat transfer: an in-depth look at varying concepts and techniques. Facial Plast Surg Clin North Am. 2007;15:99–111. viii. doi: 10.1016/j.fsc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Mordon S, Eymard-Maurin AF, Wassmer B, Ringot J. Histologic evaluation of laser lipolysis: pulsed 1064-nm Nd:YAG laser versus cw 980-nm diode laser. Aesthet Surg J. 2007;27:263–268. doi: 10.1016/j.asj.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP. Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg. 2013;132:351–361. doi: 10.1097/PRS.0b013e3182958796. [DOI] [PubMed] [Google Scholar]
- 28.Panetta NJ, Gupta DM, Kwan MD, Wan DC, Commons GW, Longaker MT. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast Reconstr Surg. 2009;124:65–73. doi: 10.1097/PRS.0b013e3181ab10cd. [DOI] [PubMed] [Google Scholar]
- 29.Conde-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381. doi: 10.1016/j.bjps.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Cohen SR, Hicok KC, et al. Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg. 2013;131:873–880. doi: 10.1097/PRS.0b013e31828276e9. [DOI] [PubMed] [Google Scholar]
- 31.Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2009;62:1210–1214. doi: 10.1016/j.bjps.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Heaton JT, Kobler JB, Ottensmeyer MP, et al. Modification and testing of a pneumatic dispensing device for controlled delivery of injectable materials. Laryngoscope. 2012;122:2023–2028. doi: 10.1002/lary.23468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for lipolytic genes