Abstract
Purpose
Epidemiological studies have identified an increasing incidence of squamous cell carcinoma of the oral tongue (SCCOT) in younger patients.
Experimental Design
DNA isolated from tongue tumors of young (<45 yrs, non-smokers) and old (>45 yrs) patients at was subjected to whole-exome sequencing and copy number analysis. These data were compared to data from similar patients in the The Cancer Genome Atlas (TCGA) project.
Results
In this study, we found that gene-specific mutation and copy number alteration frequencies were similar between young and old SCCOT patients in two independent cohorts. Likewise, the types of base changes observed in the young cohort were similar to those in the old cohort even though they differed in smoking history. TCGA data also demonstrate that the genomic effects of smoking are tumor-site specific, and we find that smoking has only a minor impact on the types of mutations observed in SCCOT.
Conclusions
Overall, tumors from young SCCOT patients appear genomically similar to those of older SCCOT patients, and the cause for the increasing incidence of young SCCOT remains unknown. These data indicate that the functional impact of smoking on carcinogenesis in SCCOT is still poorly understood.
Keywords: Young tongue cancer, SCCOT, Head and neck/oral cancers, Tobacco
Introduction
Epidemiological studies have recently identified increasing incidence of squamous cell carcinoma of the oral tongue (SCCOT) in younger patients(1-3). These tumors are not HPV-related and are often found in women who are non-smokers(2, 4). Those observations contrast with most SCCOT cases which are seen in older men with a history of cigarette smoking(5). The causes for this increasing incidence in the young are unknown. We hypothesized that this epidemiologically distinct disease would also prove to be genomically distinct, especially with respect to alterations caused by smoking, and that a better understanding of the differences would identify novel opportunities for treatment and/or prevention. Hence, we undertook the sequencing and integrated genomic profiling of a cohort of younger SCCOT patients, as reported here.
Patients and Methods
Fresh-frozen surgically resected previously untreated tumor tissue and matched nonmalignant adjacent tissue were obtained from consented patients treated for head and neck squamous cell carcinoma (HNSCC) at The University of Texas MD Anderson Cancer Center (Houston, TX), under an Institutional Review Board-approved protocol. Young tongue patients were chosen based on oral tongue primary tumor site, age less than 46 and less than 1 pack-year smoking history. Old tongue patients were chosen based on oral tongue primary tumor site and age greater than 45. Patient characteristics are shown in Table S1. Exome DNA was captured with Nimblegen reagents (Nimblegen) and sequenced on a SOLiD or Illumina platform as described previously(6). SNP analysis was conducted on SNP6.0 or CytoscanHD arrays (Affymetrix), and the data were analyzed with Partek (v6.6, Partek Inc.), ASCAT (v2.1), GISTIC (v2.0.12), and R software, as described previously(6). We have previously reported sequencing and copy number data from some of these patients(6, 7). Mutation data can be found in Supplemental Table S3.
A similar cohort of patients was identified from within the TCGA HNSCC project. YT patients were chosen based on oral tongue site, age less than 46 and lifelong non-smoker or reformed smoker with unknown pack-year history. OT were chosen based on oral tongue site and age greater than 45. Patient characteristics are shown in Table S1. Mutation and copy number data were obtained from the TCGA pan-cancer project(8) and the Synapse website(9) (www.synapse.org, doi:10.7303/syn300013).
The statistical significance of mutation and copy number frequencies was determined by Fisher's exact test. Mutation type frequencies were determined for each patient, excluding indels and multiple-base mutations. Group frequencies were determined by averaging the individual patient frequencies: this prevented skewing of the data by patients with large numbers of mutations. Mutation type frequencies were compared by using the t-test on arcsine transformed data.
Results
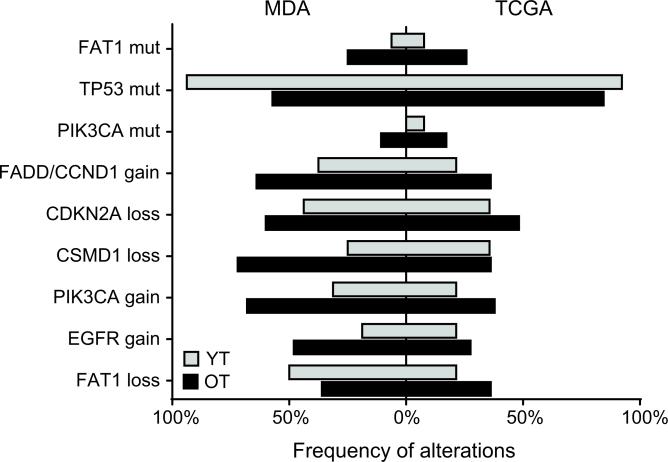
DNA from 16 young tongue (YT) and 28 old tongue (OT) patients treated at MD Anderson Cancer Center (MDA) was subjected to whole-exome sequencing (Table S1). A median of 29 and 83 mutations were identified in the YT and OT tumors, respectively. The elevated number of mutations in the OT is not surprising, since mutation number is known to be related to both age and smoking (10). This finding was validated in The Cancer Genome Atlas (TCGA) cohort, in which the median number of mutations increased from 63 in YT to 112 in OT. Figure S1 shows the relationships between mutation number and age. We next asked whether any specific genes were mutated at a different frequency in the YT cohort. Because of the low sample size we limited our initial search to the most significantly mutated genes identified by MutSig in the entire TCGA HNSCC project (HNSC). TP53 (unadjusted p=0.015) showed a slight increase in mutation frequency in the MDA YT cohort, but it was not statistically significant when adjusted for multiple testing (Table 1, Fig 1). Comparable analysis was then performed on the TCGA cohort. The TP53 mutation frequency was also elevated in the TCGA YT patients (Table 1, Fig 1). In order to increase statistical power the two cohorts were combined. Three genes showed trends toward statistical significance; FAT1, TP53, and PIK3CA (Table 1, Fig 1). However, none of those genes showed a statistically significant difference between the combined YT and OT patient cohorts. The trend of increased TP53 mutations in YT is provocative since the YT lack exposure to cigarette smoke, which has been associated with TP53 mutations. FAT1 and PIK3CA showed a lower mutation frequency in the YT cohort. Mutation frequencies for HPV-positive tumors and the entire TCGA cohort are shown for comparison (Table 1). An analysis of mutation frequencies in all genes in the combined cohorts was also performed, but no genes were found to be significantly different. Additional subset analysis for really young tongues (<30yo), OT smokers, and OT non-smokers are shown in Table S2.
Table 1.
Mutation frequencies
| Cohort (N) | MDA | TCGA | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| YT (16) | OT (28) | YT (13) | OT (58) | HPV+ (35) | All (279) | YT (29) | OT (86) | unadjusted p-value | |
| Median # mutations (range) | 28.5 (10-81) | 83 (11-251) | 63 (9-136) | 112 (11-268) | |||||
| FAT1 | 6.3% | 25.0% | 7.7% | 5.9% | 0.0% | 22.9% | 6.9% (2) | 25.6% (22) | 0.0355 |
| TP53 | 93.8% | 57.1% | 92.3% | 84.5% | 2.9% | 73.1% | 93.1% (27) | 75.6% (65) | 0.0583 |
| PIK3CA | 0.0% | 10.7% | 7.7% | 17.2% | 37.1% | 20.8% | 3.4% (1) | 15.1% (13) | 0.1140 |
| CDKN2A | 6.3% | 3.6% | 30.8% | 32.8% | 0.0% | 22.6% | |||
| CASP8 | 6.3% | 10.7% | 7.7% | 6.9% | 0.0% | 8.6% | |||
| AJUBA | 0.0% | 0.0% | 0.0% | 5.2% | 0.0% | 6.1% | |||
| NOTCH1 | 25.0% | 17.9% | 7.7% | 22.4% | 8.6% | 18.6% | |||
| MLL2 | 0.0% | 7.1% | 7.7% | 8.6% | 17.1% | 17.6% | |||
| NSD1 | 0.0% | 3.6% | 0.0% | 6.9% | 8.6% | 10.4% | |||
| HLA-A | 0.0% | 14.3% | 0.0% | 1.7% | 0.0% | 3.2% | |||
| TGFBR2 | 0.0% | 0.0% | 0.0% | 1.7% | 2.9% | 3.6% | |||
Figure 1.
Frequency of common genomic alterations in YT and OT. The frequency of each event in the MDA cohort is shown by a bar to the left of center and the frequency in the TCGA cohort is shown by a bar to the right of center.
Whole genome copy number analysis was also performed. We compared the number and size of copy number alterations (CNA) between the YT and OT cohorts, identifing an average of 129 CNAs in YT samples and 72 in the OT samples from the MDA cohort (Table 2), and 62 and 64 CNAs in the YT and OT samples from the TCGA cohort, respectively (Table 2). These differences were not statistically significant, although the YT had a smaller mean segment length for copy number gains (p=0.00817) (Table 2) in the MDA cohort. There was no difference in segment length of losses in between YT and OT in either cohort (Table 2). The mean copy number of gains or losses was also not different for gains or losses in either cohort (Table 2). Finally, no specific genomic regions were found to be significantly different between the YT and OT patients in either the MDA cohort or the TCGA cohort. Analysis of some genes frequently gained or lost in HNSCC revealed modest differences in frequency between YT and OT (Fig 1).
Table 2.
Copy number alterations
| MDA | TCGA | |||||
|---|---|---|---|---|---|---|
| YT | OT | p-value | YT | OT | p-value | |
| Mean number | ||||||
| all CNAs | 129.00 | 71.56 | NS | 61.93 | 63.78 | NS |
| gains | 57.94 | 36.52 | NS | 29.21 | 24.91 | NS |
| losses | 71.06 | 35.04 | NS | 32.71 | 38.86 | NS |
| Mean copy number a,b | ||||||
| gains | 3.45 | 3.09 | NS | 0.77 | 0.85 | NS |
| losses | −1.14 | −1.41 | NS | −1.19 | −1.21 | NS |
| Mean segment length (Mb) | ||||||
| gains | 7.37 | 20.50 | 0.00817 | 5.61 | 8.27 | NS |
| losses | 11.39 | 15.24 | NS | 3.61 | 5.11 | NS |
MDA values are normalized absolute copy numbers
TCGA values are log2 centered at 0
For example, gains in FADD and PIK3CA were less frequent in YT, but the difference was not significant (Fig 1). Overall, the CNAs were very similar between the YT and OT cohorts, and the regions of copy number change were similar to those reported previously(6).
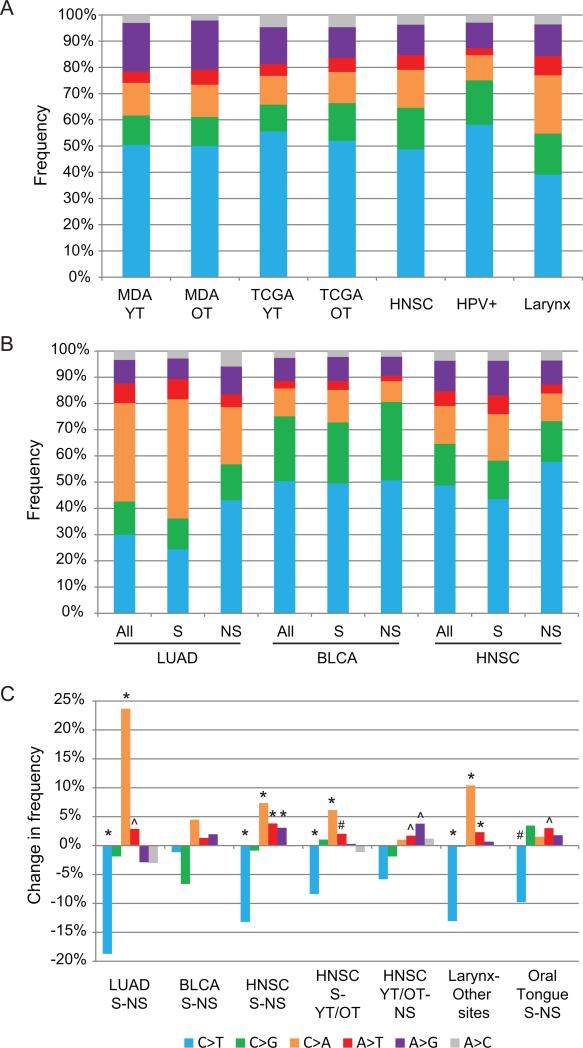
Since smoking is known to leave its mark on the genome by causing certain types of mutations, we compared mutation types in the YT and OT patients. Taking into account directional redundancy, six types of mutations can be distinguished. The frequencies of these 6 types of mutations have been shown to vary across tumor types(8, 11), but we found no significant difference in that respect for YT and OT patients in either the MDA or TCGA cohort (Fig 2A). The profiles resembled that of all head and neck tumors in the TCGA project. The profile, however, was distinct from that of HPV+ tumors or laryngeal tumors (Fig 2A). HPV+ tumors show an increase in C>T mutations (p<0.0001) and decreases in C>A, A>T (both p<0.0001) and A>G (p=0.0074) mutations when compared with HPV- HNSC tumors. Laryngeal tumors show a decrease in C>T mutations (p<0.0001) and increases in C>A and A>T mutations (both p<0.0001) when compared with non-laryngeal tumors (Fig 2A). It was expected that OT tumors would exhibit a mutation signature related to cigarette smoking when compared with the YT tumors from non-smokers. The similarity between YT and OT mutation signatures could indicate either the presence of a smoking signature in the YT tumors or a lack of a smoking signature in the OT tumors. To address those alternative possibilities, we investigated the smoking signatures in other tumor sites from the TCGA project.
Figure 2.
Analysis of mutation profiles. A) The frequency of each type of single base substitution is indicated by a different color in each annotated HNSC cohort. B) Frequency distributions by smoking status and tissue site. C) Differences in frequency between 2 cohorts. The sample name indicates which samples were compared. For example S-NS indicates that the frequency in the NS cohort was subtracted from the frequency in the S cohort, therefore a positive result is a higher frequency in the S cohort. * p<0.0001, # p<0.001, ^ p<0.01. Abbreviations: MDA – MD Anderson cohort, TCGA – The Cancer Genome Atlas cohort, YT – young tongue SCC, OT – old tongue SCC, HNSC – TCGA HNSCC cohort, HPV+ – human papilloma virus positive tumors from TCGA cohort, LUAD – TCGA lung adenocarcinoma, BLCA – TCGA bladder urothelial carcinoma, S – smokers, NS – non-smokers, YT/OT – combined TCGA young tongue and old tongue cohorts.
The mutational events linked to smoking are traditionally reported as an increase in C>A mutations and a decrease in C>T mutations. TCGA collected smoking history information for lung adenocarcinoma (LUAD), bladder urothelial carcinoma (BLCA), and HNSC. Each of these tumor types was analyzed for the presence of a smoking mutation signature. In LUAD tumors C>A mutations increased 23.7 percentage points in smokers when compared to non-smokers, and C>T mutation decreased 18.7 percentage points (Fig 2B, C). A>T mutations were also found to increase 2.9 percentage points in smokers (Fig 2B, C).
BLCA showed a very different profile, indicating the effect of tissue of origin. BLCA show a much higher frequency of C>T and C>G mutations and a lower frequency of C>A when compared with LUAD (Fig 2B). In BLCA the impact of smoking is quite different from that in LUAD. The largest change with smoking in BLCA is a decrease in C>G of 6.6 percentage points (Fig 2B, C). C>A is also increased 4.5 percentage points, but neither change is statistically significant (Fig 2B, C).
We next examined the impact of smoking on the mutation profile of HNSC. The changes in HNSC were generally similar to those in LUAD but of lower magnitude. We found a decrease in C>T of 13.2 percentage points and an increase in C>A of 7.3 percentage points that were consistent with the changes in LUAD (Fig 2B, C). HNSC also demonstrated increases in A>G and A>T of 3.8 and 3.1 percentage points, respectively (Fig 2B, C, S2A).
Having defined the smoking signature, we could then ask whether YT and OT tumors exhibit such a signature. For this analysis we combined the YT and OT tumors into one cohort (YT/OT) and compared it with the HNSC smoking and non-smoking cohorts. The YT/OT cohort had a lower C>A frequency and a higher C>T frequency when compared with the HNSC smoking cohort (Fig 2C), indicating that the YT/OT tumors lack the most definitive characteristics of the smoking signature. However, the YT/OT also demonstrated increased A>G when compared with the HNSC non-smoking tumors and an intermediate level of A>T when compared to both smoking and non-smoking cohorts (Fig 2C). Similar results were obtained when the YT and OT cohorts were separately compared to the non-smoking and smoking cohorts, although with lower statistical significance (data not shown) or when smoking and non-smoking cohorts within the OT cohort were compared to each other or YT (Fig S2B). Overall, the mutation profile of the YT/OT tumors appears most similar to the profile of non-smokers.
Since both YT and OT tumors lack a smoking signature we next searched for the source of the smoking signature in HNSC. We found that tumors from the laryngeal subsite exhibit a smoking signature when compared with tumors from all other sites (Fig 2A, C, S2A, B) with an increase in C>A mutations and a decrease in C>T mutations. Additionally, the smoking signature within the oral tongue subsite is less recognizable than the overall HNSC smoking signature (Fig 2C). Those observations suggest that the smoking signature in HNSC is largely driven by the laryngeal subsite.
Discussion
Since the incidence of YT cancer has reportedly been increasing(1-3) we hypothesized that YT and OT tumors would prove to be genomically distinct. We found, to the contrary, that the two are genomically quite similar in their copy number alterations, mutations and types of mutations. Those conclusions were consistent for two independent patient cohorts. Although this study is the largest genomic analysis of YT tumors it is still limited by the small sample size. The rarity of these tumors has also prevented ideal matching of the YT and OT cohorts, which differ in a few parameters (Table S1), and resulted in a YT cohort that is not enriched for women as some have reported(2, 4).
The mutation frequencies of FAT1 and TP53 showed trends toward statistical significance, but would require larger cohorts for validation (estimated 170 and 189 total patients, respectively). FAT1 is inactivated in a large proportion of oral cavity tumors, either by mutation or copy number deletion(6). FAT1 may be related to differentiation, migration or Hippo signaling in HNSCC(6, 12-14), but its role is largely still unknown. The lower frequency of FAT1 mutations in YT could suggest that its inactivation is not necessary for tumor formation or that it is somehow related to smoking status. However, since older smokers are expected to have more background mutations it is possible that the difference is an artifact of the increased frequency of mutations. Since TP53 mutations are more frequent in YT patients this result is not likely to be an artifact. TP53 mutations are likely to be impactful on the tumor progression in YT, but a causative factor leading to increased TP53 mutations in YT is still unknown.
We also noted that OT tumors do not exhibit a mutation signature associated with smoking. The mutation signature from smoking appears to be site-specific, with laryngeal SCC exhibiting the strongest smoking signature among the HNSCC subsites. SCCOT did not exhibit a robust mutational smoking signature, in that regard echoing the TCGA findings in urothelial bladder cancer(15). Overall, the mutational signature that has been defined by analysis of lung tumors does not appear to reflect the effect of smoking on other tissue sites. Since smoking is clearly an epidemiological risk factor for SCCOT(5) the mechanism of carcinogenesis may differ among sites. Cigarette smoke has been shown to be both a tumor initiator and a tumor promoter(16, 17). Only the role of smoke as a tumor initiator should leave a mutational signature on the DNA. Therefore, it is clear that smoke is a tumor initiator in lung cancer and laryngeal SCC. However, it may act primarily as a tumor promoter in SCCOT and BLCA. It is also possible that epigenetic alterations are involved since we have not yet examined the DNA methylation status of these tumors.
Alcohol consumption is a known risk factor for HNSCC. In some patients alcohol is likely to contribute as an initiator. Ethanol is not mutagenic, but its metabolite, acetaldehyde, can lead to mutations, primarily C>T(18). Since C>T is the most common mutation in many tumor types(8, 11) other factors must also contribute to their frequency. Analysis of the TCGA HNSC data for an alcohol mutation signature resulted in a less dramatic version of the smoking signature with decreases in C>T and increases in C>A in heavy drinkers (data not shown). That observation contrasts with the expected increase in C>T that could be caused by acetaldehyde. Since many heavy drinkers are also heavy smokers it is likely that the smoking signature masks any alcohol signature. Therefore, the impact of alcohol use is still unclear.
These findings do not lessen the importance of smoking and drinking cessation programs to reduce SCCOT incidence. However, they suggest possible new avenues for prevention or treatment of SCCOT. If an unknown tumor initiator is acting in SCCOT, its identification could lead to new prevention strategies. Our analysis of the mutation profile does not indicate an obvious tumor initiator for SCCOT. It may, however, eliminate some candidates (e.g. many environmental mutagens) that would be expected to leave a mutation signature. It is also possible that the same initiator is acting in both YT and OT patients.
If smoking is indeed a tumor promoter in SCCOT, it will be important to determine the mechanism. In lung cancer, smoke has been shown to function as a tumor promoter by increasing inflammation(16), and that might also be the case in SCCOT. Inflammation from other causes promotes many tumor types including; esophageal, pancreatic, and colorectal(19). That connection suggests that inflammation is an important factor in non-smoking related SCCOT, as previously reported(20, 21). Prevention strategies that include anti-inflammatory agents may prove useful. Although the results of clinical trials of COX inhibitors for prevention of SCCOT have not generally been positive(20, 22), agents that target other inflammatory pathways (such as the NFκ-B pathway) still look very promising(20).
Another factor that could be promoting SCCOT is the oral microbiome. The oral cavity is home to a diverse population of microorganisms(23, 24), even in healthy individuals, and some of those microorganisms could also play a role in carcinogenesis(21, 25). They may produce carcinogenic compounds such as acetaldehyde, inducing local inflammation, or they may act through still-undiscovered mechanisms(21, 24, 25). Numerous microorganisms have already been associated with the development of oral cancer(21, 24, 25). Included are species of Streptococcus, Neisseria, and Candida. Conversely, the “normal” microbiome may protect against carcinogenesis and a disrupted microbiome may cause it to no longer be protective(23, 24). Finally, it is unlikely that microorganisms are directly oncogenic in the way that HPV is oncogenic in oropharyngeal cancer. HPV is the only virus detected in SCCOT, and it is found in only 5% of cases. Additionally, we found that HPV leaves a distinct mutation signature and expect that other pathogens would also leave distinct mutation signatures. However, we detect no such signature. In sum, the oral microbiome is likely to be involved in SCCOT in some way, but very little is understood about its causal role or the specific organisms involved.
Finally, YT cancer is troubling in that it defies the stereotype of HNSCC, which generally occurs in older men with long histories of heavy smoking and drinking(5). That observation is even more disturbing because the incidence of YT cancer is increasing(1-3). Hence, there is a strong interest in identifying the cause. Clinically, the cancer-specific outcomes for YT patients are similar to those for OT patients(4, 26-29), and we have shown here that the genomic alterations in YT tumors are also similar to those in OT tumors. Therefore, the only evidence suggesting that they are a unique cohort is epidemiological(1-3). Although statistically significant, the epidemiological findings are based on a low number of patients, since YT tumors are still quite rare, and the apparent difference could be a statistical anomaly. Additional epidemiological studies will be necessary to validate the original reports. Our data indicate that the mechanistic cause of YT cancer is likely to be similar to the mechanistic cause of OT cancer. For example, if smoking causes inflammation to promote OT perhaps another factor that also induces inflammation is promoting YT, resulting in a genomically and clinically similar disease. Similarly a factor that disrupts the oral microbiome in YT could promote carcinogenesis in a way similar to the mechanism in OT. However, the identity of a causative factor in YT cancer, if it exists, is still unknown.
Supplementary Material
Statement of Translational Relevance.
Genomic alterations do not explain the increasing incidence of oral tongue cancer in young patients, and smoking does not dramatically alter the genome of tongue cancer at any age. Therefore, the causes of tongue cancer are still largely unknown, and their identification could provide novel avenues for therapeutic intervention.
Acknowledgements
We thank our patients for their courage and generosity. We thank the members of the Myers lab for moral support, technical support, and helpful discussions. This work was supported by the Cancer Prevention Research Institute of Texas grant RP100233 (to J.N. Myers); National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research grant RC2DE020958 (to J.N. Myers); NIH Specialized Program of Research Excellence grants P50CA097007; Cancer Center Support Grant P30CA16672; an Institutional Research Grant from the University of Texas M.D. Anderson Cancer Center (to M.J. Frederick) and the Pantheon Program.
Financial support: This work was supported by the Cancer Prevention Research Institute of Texas grant RP100233; National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research grant RC2DE020958; NIH Specialized Program of Research Excellence grants P50CA097007; Cancer Center Support Grant P30CA16672 and the Pantheon Program.
Abbreviations
- SCCOT
squamous cell carcinoma of the oral tongue
- HNSCC
head and neck squamous cell carcinoma
- YT
young tongue
- OT
old tongue
- MDA
MD Anderson Cancer Center
- HNSC
TCGA HNSCC project
- CNA
copy number alterations
- LUAD
lung adenocarcinoma
- BLCA
bladder urothelial carcinoma
Footnotes
Conflicts: The authors disclose no potential conflicts of interest.
References
- 1.Brown LM, Check DP, Devesa SS. Oral cavity and pharynx cancer incidence trends by subsite in the United States: changing gender patterns. J Oncol. 2012;2012:649498. doi: 10.1155/2012/649498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–94. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 3.Myers JN, Elkins T, Roberts D, Byers RM. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122:44–51. doi: 10.1016/S0194-5998(00)70142-2. [DOI] [PubMed] [Google Scholar]
- 4.Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never-smokers, never-drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010;32:499–503. doi: 10.1002/hed.21220. [DOI] [PubMed] [Google Scholar]
- 5.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004;31:726–33. doi: 10.1053/j.seminoncol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omberg L, Ellrott K, Yuan Y, Kandoth C, Wong C, Kellen MR, et al. Enabling transparent and collaborative computational analysis of 12 tumor types within The Cancer Genome Atlas. Nat Genet. 2013;45:1121–6. doi: 10.1038/ng.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijg J, Suh Y. Genome instability and aging. Annual review of physiology. 2013;75:645–68. doi: 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- 11.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris LG, Kaufman AM, Gong Y, Ramaswami D, Walsh LA, Turcan S, et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat Genet. 2013;45:253–61. doi: 10.1038/ng.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa Y, Miyazaki T, Nakashiro K, Yamagata H, Isokane M, Goda H, et al. Human FAT1 cadherin controls cell migration and invasion of oral squamous cell carcinoma through the localization of beta-catenin. Oncol Rep. 2011;26:587–92. doi: 10.3892/or.2011.1324. [DOI] [PubMed] [Google Scholar]
- 14.Sopko R, McNeill H. The skinny on Fat: an enormous cadherin that regulates cell adhesion, tissue growth, and planar cell polarity. Curr Opin Cell Biol. 2009;21:717–23. doi: 10.1016/j.ceb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Research N Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014 doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Duuren BL, Sivak A, Katz C, Melchionne S. Cigarette smoke carcinogenesis: importance of tumor promoters. J Natl Cancer Inst. 1971;47:235–40. [PubMed] [Google Scholar]
- 18.Noori P, Hou SM. Mutational spectrum induced by acetaldehyde in the HPRT gene of human T lymphocytes resembles that in the p53 gene of esophageal cancers. Carcinogenesis. 2001;22:1825–30. doi: 10.1093/carcin/22.11.1825. [DOI] [PubMed] [Google Scholar]
- 19.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 20.Vander Broek R, Snow GE, Chen Z, Van Waes C. Chemoprevention of head and neck squamous cell carcinoma through inhibition of NF-kappaB signaling. Oral Oncol. 2013 doi: 10.1016/j.oraloncology.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bektas-Kayhan K. Role of Inflammation in Oral Squamous Cell Carcinoma. In: Li X, editor. Squamous Cell Carcinoma. InTech; 2012. pp. 195–212. [Google Scholar]
- 22.Papadimitrakopoulou VA, William WN, Jr., Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095–101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 23.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009;31:1228–39. doi: 10.1002/hed.21140. [DOI] [PubMed] [Google Scholar]
- 26.Atula S, Grenman R, Laippala P, Syrjanen S. Cancer of the tongue in patients younger than 40 years. A distinct entity? Arch Otolaryngol Head Neck Surg. 1996;122:1313–9. doi: 10.1001/archotol.1996.01890240021006. [DOI] [PubMed] [Google Scholar]
- 27.Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20:363–8. doi: 10.1002/(sici)1097-0347(199808)20:5<363::aid-hed1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Granizo R, Rodriguez-Campo F, Naval L, Diaz Gonzalez FJ. Squamous cell carcinoma of the oral cavity in patients younger than 40 years. Otolaryngol Head Neck Surg. 1997;117:268–75. doi: 10.1016/s0194-5998(97)70185-2. [DOI] [PubMed] [Google Scholar]
- 29.Siegelmann-Danieli N, Hanlon A, Ridge JA, Padmore R, Fein DA, Langer CJ. Oral tongue cancer in patients less than 45 years old: institutional experience and comparison with older patients. J Clin Oncol. 1998;16:745–53. doi: 10.1200/JCO.1998.16.2.745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.