Abstract
Importance
Long-term patency of human saphenous veins (HSVs) used as autologous conduits for coronary artery bypass grafting (CABG) procedures remains limited because of vein graft failure (VGF). Vein graft failure has been reported to be as high as 45% at 12 to 18 months after surgery and leads to additional surgery, myocardial infarction, recurrent angina, and death. Preparation of HSVs before implantation leads to conduit injury, which may promote VGF.
Objectives
To investigate whether pressure distension during vein graft preparation leads to endothelial injury and intimal thickening and whether limiting intraluminal pressure during pressure distension by using a pressure release valve (PRV) preserves endothelial function and prevents neointima thickening.
Design, Setting, and Participants
Segments of HSVs were collected in a university hospital from 13 patients undergoing CABG procedures immediately after harvest (unmanipulated [UM]), after pressure distension (after distension [AD]), and after typical intraoperative surgical graft preparation (after manipulation [AM]). Porcine saphenous veins (PSVs) from 7 healthy research animals were subjected to manual pressure distension with or without an in-line PRV that prevents pressures of 140 mm Hg or greater. Endothelial function of the HSVs and PSVs was determined in a muscle bath, endothelial integrity was assessed, and intimal thickening in PSVs was evaluated after 14 days in organ culture.
Main outcomes and measures
Endothelial function was measured in force, converted to stress, and defined as the percentage relaxation of maximal phenylephrine-induced contraction. Endothelial integrity was assessed by immunohistologic examination. Neointimal thickness was measured by histomorphometric analysis.
Results
Pressure distension of HSVs led to decreased mean (SEM) endothelial-dependent relaxation (5.3% [2.3%] for AD patients vs 13.7% [2.5%] for UM patients; P < .05) and denudation. In the AM group, the function of the conduits was further decreased (−3.2% [3.2%]; P < .05). Distension of the PSVs led to reduced endothelial-dependent relaxation (7.6% [4.4%] vs 61.9% [10.2%] in the control group; P < .05), denudation, and enhanced intimal thickening (15.0 [1.4] μm vs 2.2 [0.8] μm in the control group; P < .05). Distension with the PRV preserved endothelial-dependent relaxation (50.3% [9.6%]; P = .32 vs control), prevented denudation, and reduced intimal thickening (3.4 [0.8] μm; P = .56 vs controls) in PSVs.
Conclusions and Relevance
Use of a PRV during graft preparation limits intraluminal pressure generated by manual distension, preserves endothelial integrity, and reduces intimal hyperplasia. Integration of this simple device may contribute to improved long-term vein graft patency.
The human saphenous vein (HSV) is an autologous transplanted organ most commonly used for coronary artery bypass grafting (CABG) and peripheral vascular revascularization procedures. Despite advances in surgical techniques and therapeutic interventions, long-term patency of the conduits remains limited because of vein graft failure (VGF). The VGF rates have been reported to be 45% and 39% at 12 to 18 months after CABG and peripheral vascular revascularization procedures, respectively.1,2 Common causes of VGF include loss of endothelial coverage, intimal hyperplasia, and thrombosis.3 Graft patency rates are influenced by patient characteristics, intrinsic quality of the conduit, and surgical technique.4 Despite concerns about graft preparation techniques, beginning with the research by LoGerfo et al5,6 in the early 1980s, preservation of endothelial and medial integrity of the conduits during graft preparation remains suboptimal, and as such, VGF is attributable at least in part to tissue handling. Common trauma incurred to the vein during “back-table” graft preparation includes conduit storage in acidic solutions, conduit marking using surgical skin markers that injure tissue, and pressure distension by handheld syringes to identify branches and overcome vasospasm.7-10
Flushing the vein during graft preparation with uncontrolled pressure results in high intraluminal pressure, which often exceeds 600 mm Hg, leading to denudation of the endothelium that potentiates inflammatory responses.11-13 Distension also induces damage to the medial smooth muscle layer that results in apoptosis and dedifferentiation of smooth muscle cells.14-16 Given that the primary cause of graft failure is intimal hyperplasia, which represents a response to injury,17 limiting this response may influence the progression of cellular processes that lead to neointima formation and facilitate maximum readaptation of the conduit after arterialization.
The objective of this study was to investigate whether pressure distension during vein graft preparation leads to endothelial injury and intimal thickening. We hypothesized that limiting pressure during distension reduces neointima thickening and preserves the vascular functions of the grafts. We identified a pressure-limiting device that can be readily integrated into current distension techniques and reduces intraluminal pressure.
Methods
Procurement of HSVs
After Vanderbilt University Medical Center Institutional Review Board approval, the HSV grafts were obtained from patients undergoing CABG procedures with informed, written consent. Segments were collected immediately after surgical harvest (unmanipulated [UM]) and after manual distension (after distension [AD]). An additional segment was collected immediately after further intraoperative manipulation according to the surgeon's discretion, such as use of skin markers and storage in a solution, before implantation (after manipulation [AM]) from the same patients. Veins were collected in heparinized (10 U/mL) Plasma-Lyte (Baxter Healthcare) solution and transported to the laboratory for immediate testing.
Collection of Clinical Demographic Variables
Demographic variables were retrospectively collected, including age, sex, race, body mass index, medical comorbidities, pre-operative laboratory values, preoperative medication regimen, and method of HSV harvest.
Procurement of Porcine Saphenous Veins
The porcine saphenous veins (PSVs) were collected from euthanized animals from the animal surgical laboratory at Vanderbilt University Medical Center. Animal procedures followed study protocols approved by the Vanderbilt Institutional Animal Care and Use Committee and adhered to National Institutes of Health guidelines for care and use of laboratory animals. The PSVs were dissected using an open harvest technique immediately after euthanasia, side branches were ligated with 3-0 silk sutures, and samples were placed in heparinized Plasma-Lyte and transported to the laboratory for immediate testing.
Physiologic Measurements of Vasocontractility and Vasorelaxation
Force measurements were obtained using the Radnoti transducer (model 159901A) interfaced with the PowerLab data acquisition system and chart software (AD Instruments) as described previously.18 All chemicals were purchased from Sigma-Aldrich unless otherwise specified. Briefly, 1-mm rings were cut from segments of saphenous veins, dissected free of fat and connective tissue, and then suspended in a muscle bath that contained bicarbonate buffer (120 mM sodium chloride, 4.7 mM potassium, 1.0 mM magnesium sulfate, 1.0 mM monosodium phosphate, 10 mM glucose, 1.5 mM calcium chloride, 25 mM sodium bicarbonate, and pH 7.4) equilibrated with 95% oxygen and 5% carbon dioxide at 37°C for 2 hours. Rings were contracted first with 110 mM potassium to determine smooth muscle functional viability. Tissues that generated 0.025 × 105N/m2 or more of stress were considered viable and were further evaluated.9 Viable tissues were then contracted with increasing doses (10−8-10−6M) of phenylephrine, a physiologic agonist. The optimal phenylephrine dose was determined as the concentration generating 70% to 80% of maximal 110 mM potassium-induced contraction. Phenylephrine precontracted tissues were then treated with 5 × 10−7M carbachol or increasing doses of sodium nitroprusside (10−8-10−5M) to determine maximal endothelial-dependent and endothelial-independent relaxation responses, respectively.19
Measurement of Intraluminal Distension Pressure in Saphenous Veins
Additional segments of HSVs obtained after typical surgical manipulation (AM group) were cannulated proximally and distally with an olive-tipped needle (Medtronic) and secured with 3-0 silk sutures on each end. A 30-mL handheld syringe was connected to the distal end, and the proximal end was connected to a manometer to record actual pressure attained during distension. For distension with limited pressure, a pressure release valve (PRV; VasoPrep Surgical) was placed in-line between the distal olive-tipped needle and the handheld syringe. Heparinized Plasma-Lyte in the syringe was then injected to distend the segments, and pressure was held for 2 minutes. For treatment of the PSVs, veins were divided into segments and randomly assigned to 1 of 3 groups: nondistended (control), distended with unlimited pressure using a handheld syringe (distended), or distended with a PRV as described for HSVs.
Histomorphometric Analysis of PSVs in an Organ Culture Model
Rings (1-2 mm in width) were cut from PSV segments before and after distension. Two rings were placed in 10% neutral buffered formalin to measure basal (preculture) intimal thickness. Two rings were placed in organ culture as described previously.20 This method of vein culture has been validated as an ex vivo model system of the changes that occur in vivo and has been used previously in our laboratory.20 After 14 days, rings were fixed in 10% formalin and sent for histologic preparation and Verhoeff-van Gieson staining at the Pathology Histochemistry Core at Vanderbilt University Medical Center. Measurements of intimal and medial thickness were made on transverse sections of each vessel using a Zeiss Axiovert 200M microscope (Carl Zeiss) with a computerized image analysis system (Zeiss software and Adobe Photoshop) as described previously.18
Immunohistochemistry
Tissue sections were stained using the avidin-biotinylated peroxidase complex method (Vector Laboratories). Antigen was retrieved using citrate buffer (pH 6) at 95°C for 5 minutes (PSVs) or 12 minutes (HSVs). Endogenous peroxidase was blocked by immersing slides in 3% hydrogen peroxide for 15 minutes. Nonspecific sites were blocked by incubating sections in 5% goat serum before incubation with primary antibodies against endothelial nitric oxide synthase (Abcam) or CD31 (Dako) for 1 hour at room temperature. Biotinylated IgG (Vector Laboratories) was used as a secondary antibody at 10 ng/mL. Negative controls were achieved by omitting incubation with the primary antibody.
Statistical Analysis
Contractile response was defined by stress, calculated using force generated by tissues as follows:
where Area = Wet Weight (mg)/Maximal Length (mm)/1.055.
Any tissue that generated stress of 0.025 × 105 N/m2 or greater was considered functionally viable.9 Data were reported as mean (SEM) responses. Paired t tests or 1-way analysis of variance was conducted to determine the significance (P value) of each experiment. P<.05 was considered statistically significant.
Results
Patient Demographics
The demographic variables for the patients from whom saphenous veins were collected are typical for patients undergoing CABG procedures (eTable in the Supplement).
Manual Distension and Physiologic Function of HSVs
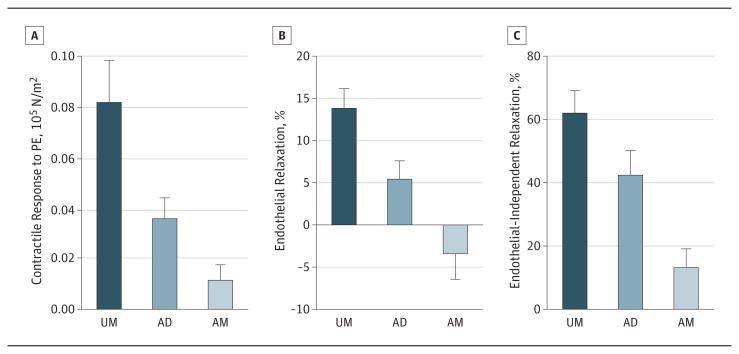
Segments of HSVs were collected as UM or AD from the same patients (n = 13). Intraoperative distension of vein grafts significantly reduced contraction to phenylephrine (1-5 × 10−6 M) in the AD segments (0.035 [0.008] × 105N/m2) compared with cognate UM segments (0.081 [0.017] × 105 N/m2; P = .02) (Figure 1A). Vascular relaxation was similarly impaired by intraoperative distension. The UM segments produced significantly greater endothelial-dependent (13.7% [2.5%] vs 5.3% [2.3%]; P = .002) (Figure 1B) and endothelial-independent (61.6% [7.5%] vs 41.9% [8.3%]; P = .005) (Figure 1C) relaxation than did the AD segments.
Figure 1. Impairment of Contractility and Vasorelaxation of Human Saphenous Vein (HSV) Graft by Intraoperative Manual Distension and Graft Preparation.
HSVs were collected after harvest (unmanipulated [UM]), after pressure distension (after distension [AD]), and after typical intraoperative surgical graft preparation (after manipulation [AM]). Phenylephrine (PE)-induced contraction (A), endothelial-dependent relaxation (B), and endothelial-independent relaxation (C) were determined in the muscle bath. Error bars indicate SEM.
Functional integrity of both endothelium and smooth muscle was further reduced in segments after additional back table manipulation, which included marking with a surgical skin marker and storage before implantation (AM group; n = 6). Phenylephrine-induced contraction was reduced to 0.011 (0.006) × 105N/m2 (Figure 1A), and endothelial-dependent and endothelial-independent relaxation further decreased to −3.2% (3.2%) and 12.8% (6.3%), respectively (Figure 1B and C). There is a statistically significant difference as determined by 1-way analysis of variance for phenylephrine contraction (F2,28 = 5.372, P = .01), endothelial-dependent relaxation (F2,29 = 8.448, P = .001), and endothelial-independent relaxation (F2,25 = 7.773, P = .002) among UM, AD, and AM segments from the same patients (n = 6).
Manual Distension and Endothelial Integrity of HSVs
Immunohistochemical examination of the UM HSV segments revealed normal venous morphologic features and intact endothelium (Figure 2A and C). In contrast, intraoperative manual distension increased luminal area and damaged the endothelium of AD segments (Figure 2B and D).Strong CD31 staining was seen along the endothelium of the UM segments (Figure 2C), whereas distension resulted in patchy staining (Figure 2D), suggesting loss of endothelial integrity after manual distension.
Figure 2. Distortion of Luminal Area and Disruption of Endothelial Integrity of Human Saphenous Veins by Intraoperative Manual Distension.
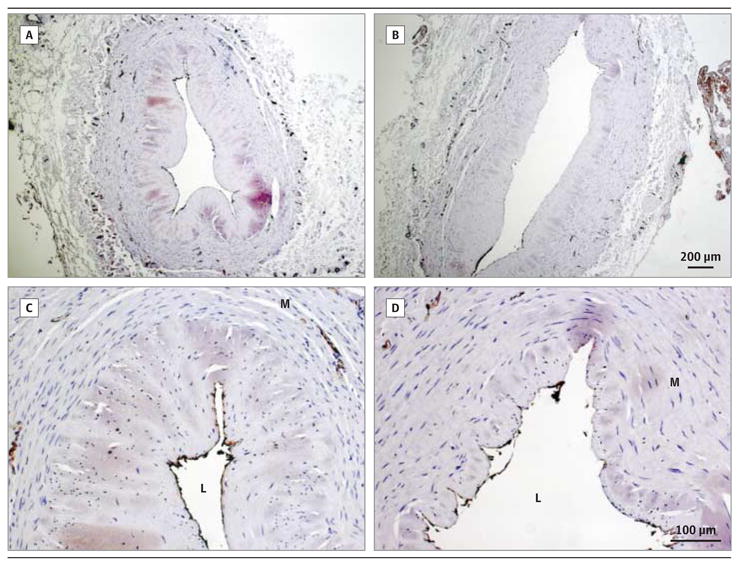
Unmanipulated (A and C) and after-distension (B and D) segments were immunostained for CD31 (original magnification ×50 [A and B] and ×200 [C and D]). L indicates lumen; M, media.
Intraluminal Pressure in HSVs Distended Using a PRV
Intraluminal pressure, as measured by a manometer at the proximal end of remnant HSVs (n = 3), was 883.7 (37.1) mm Hg when vessels were gently distended without the PRV using a 30-mL syringe. When a PRV (Figure 3) that was calibrated to open and release fluid at pressure greater than 2.5 (0.25) psi (or 130 [13] mm Hg) was used, intraluminal pressure was limited to 135.5 (1.9) mm Hg (Figure 3).
Figure 3. Reduction of Intraluminal Pressure During Manual Distension of Human Saphenous Veins With a Pressure Release Valve (PRV).
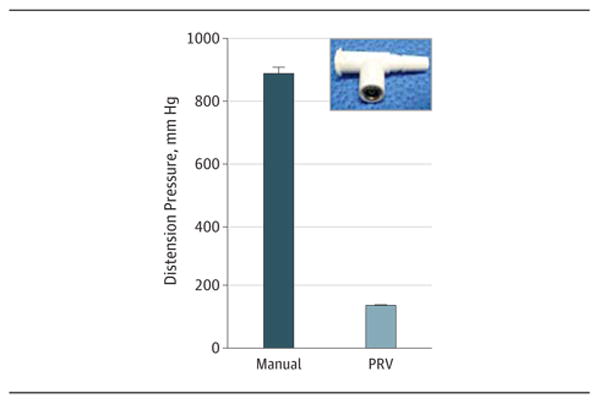
Intraluminal pressure was measured by connecting the distal end of the conduits to a manometer during manual distension in the absence (manual) or presence of the PRV. Inset: Photograph of the PRV.
Physiologic Functions of PSVs Distended With Limited Intraluminal Pressure
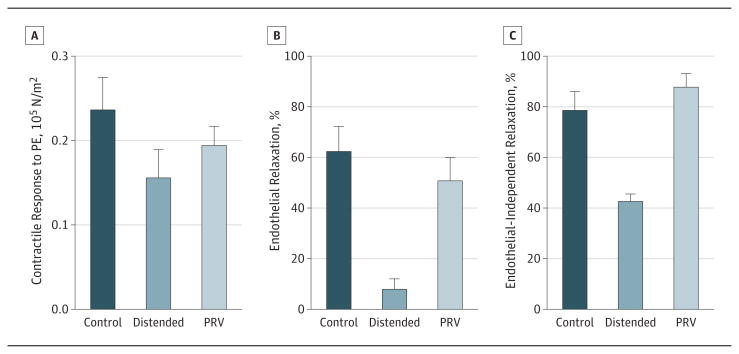
Because the length of UM HSV segments obtained for this study were insufficient for distension, PSV, a conduit of similar caliber to HSV, was used (n = 7). Manual distension reduced tissue response to phenylephrine when compared with the control segment (0.155 [0.034] × 105 N/m2 vs 0.235 [0.039] × 105 N/m2; P = .02) (Figure 4A). Contractile responses to phenylephrine did not decrease when distension pressure was limited using the PRV (0.193 [0.024] × 105 N/m2; P = .20 vs control) (Figure 4A). Manual distension reduced endothelial-dependent (7.6% [4.4%] vs 61.9% [10.2%] in the control group; P = .0005) (Figure 4B) and endothelial-independent (42.1% [3.4%] vs 78.0% [7.8%] in the control group; P = .02) (Figure 4C) relaxation in PSVs. Limiting intraluminal pressure to 140 mm Hg with the PRV preserved endothelial-dependent (50.3% [9.6%]; P = .32 vs control) (Figure 4B) and endothelial-independent (87.3% [5.8%]; P = .20 vs control) (Figure 4C) relaxation.
Figure 4. Preservation of Contractility and Vasorelaxation of Porcine Saphenous Veins With the Pressure Release Valve (PRV) During Manual Distension.
The porcine saphenous veins were left undistended (control) or subjected to pressure distension in the absence (distended) or presence of the PRV. Phenylephrine (PE)-induced contraction (A), endothelial-dependent relaxation (B), and endothelial-independent relaxation (C) were determined in the muscle bath. Error bars indicate SEM.
Endothelial Integrity of PSVs Distended With Limited Intraluminal Pressure
Undistended PSV segments exhibited continuous intraluminal immunohistologic staining for the endothelial markers endothelial nitric oxide synthase and CD31 (Figure 5A). The PSV segments that were distended in the absence of the PRV revealed patchy endothelial disruption (Figure 5A), whereas the endothelium remained intact in segments distended in the presence of the PRV (Figure 5A).
Figure 5. Preservation of Endothelial Integrity and Prevention of Intimal Hyperplasia In Vitro in Porcine Saphenous Veins (PSVs) With a Pressure Release Valve (PRV) During Manual Distension.
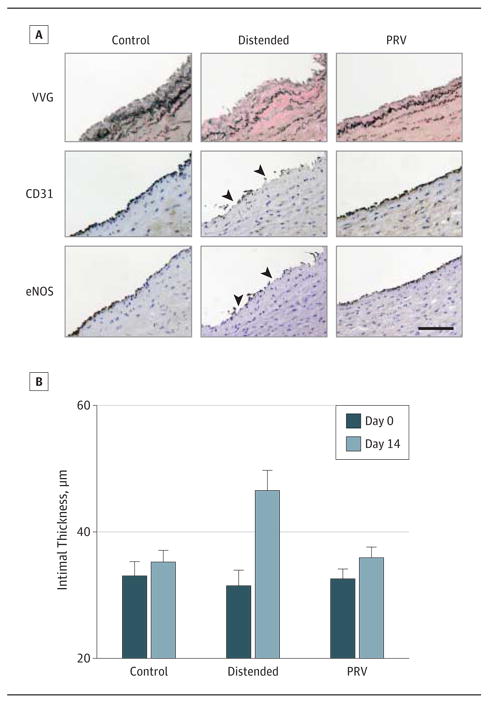
A, Immediately after distension, PSVs were fixed for endothelial nitric oxide synthase (eNOS) and CD31 immunostaining. B, Organ cultured PSVs were fixed for the Verhoeff-van Gieson stain (VVG) to measure intimal thickness. Arrowheads indicate areas of disruption. Error bars indicate SEM. Scale bar = 100 μm.
Intimal Thickening of PSVs Distended With Limited Intraluminal Pressure
Basal intimal thickness of PSVs was 31.3 (6.2) μm (n = 8) (Figure 5B). After 14 days of organ culture, intimal thickness was significantly greater in distended compared with control segments (Figure 5B). The intimal thickness increased by 2.2 (0.8) μm and 15.0 (1.4) μm in the control and distended groups, respectively. The use of the PRV prevented significant increases in neointima formation (3.4 [0.8] μm; P = .56 vs control) (Figure 5B).
fsfdDiscussion
Vein graft failure after aortocoronary bypass procedures remains a significant problem. Although it has been suggested that certain preoperative characteristics, such as endothelial coverage, wall thickness, and vein lumen diameter, may predict long-term graft patency, conduit damage in the operative arena has been widely implicated in VGF.21 Minimizing vein graft manipulation and preserving vascular integrity using a no-touch technique have reportedly resulted in improved outcomes.5,22
In the current study, HSV segments were collected from patients undergoing CABG surgeries before and after intraoperative manual distension, and the effects on vascular functions were examined. Distension under typical operating room conditions reduced contractile responses and impaired endothelial-dependent and endothelial-independent relaxation of the conduits (Figure 1), suggesting that the functional integrity of both the endothelium and medial layer is affected. Because cellular viability correlates with functional viability of veins in a muscle bath,9 our results further implicate that the number of viable cells within the AD and AM segments were diminished by graft manipulation. Intraoperative manual distension resulted in structural damage, yielding a flaccid and distended appearance of the lumen (Figure 2B) and denudation of the endothelium of HSV grafts (Figure 2D).
Intraluminal pressures greater than 600 mm Hg have been recorded with handheld syringe distension,23,24 and we routinely detected pressures of 850 mm Hg or higher when uncontrolled manual distension was performed by different surgeons (Figure 3). Thus, the pressure generated by manual distension, although broadly varied and considered gentle by most surgeons, would likely exceed pressures that have been reported to cause conduit damages.23,25-28 There was a further reduction in contractile response and virtually abolished endothelial-dependent and endothelial-independent relaxation of HSVs collected after completion of graft preparation, suggesting that manipulation after manual distension caused additional damage to the vein graft (Figure 1). Observations from a previous study suggest that this reduction was not attributable to ischemia during storage of the graft in Plasma-Lyte.7 Graft preparation techniques, such as graft handling, storage in heparinized saline solution, and marking with surgical skin markers, may contribute to this increased damage.8,29,30 Collectively, preparation of the vein graft after harvest and before implantation significantly damages conduits that are implanted into the patients undergoing CABG procedures.
Distension under uncontrolled pressure also reduced both endothelial-dependent and endothelial-independent functions (Figure 4) in the PSVs. Moreover, distension damaged the endothelial layer (Figure 5) and led to increased neointima formation in cultured PSVs (Figure 5), demonstrating a causal association among pressure distension, endothelial injury, and neointima formation. Distension pressure of 300 mm Hg has been reported to result in 50% and 200% growth of the intima in HSVs in organ culture31 and in the porcine carotid artery–jugular vein interposition bypass graft model,15 respectively.
Distension not only results in functional and morphologic changes in the vessel wall but also elicits a myriad of signaling cascades that promote neointima formation. Mechanical force induces phosphorylation of p38 mitogen-activated protein kinase.16 The acute loss of endothelial-independent function in the saphenous veins may be due to the p38 mitogen-activated protein kinase–mediated degradation of the α-actin filament in the venous smooth muscle.32 The loss of endothelial integrity also exposes the underlying medial layer to platelet aggregation and circulating growth modulators.33 Venous smooth muscle cells dedifferentiate, leading to increases in matrix metalloprotease activity and expression of cytoskeleton-associated proteins, which enable migration and proliferation of the smooth muscle cells.14 In addition, up-regulation of adhesion molecules, increased neutrophil adhesion by damaged endothelium, and the secretion of smooth muscle mitogens by inflammatory cells further extend the distension-induced damage to the medial layer.11,12,15,16
Although the no-touch harvest techniques have shown promise in reducing VGF,22 the adoption of this method is limited to a few centers. Consequently, the conventional harvest techniques dominate and manual distension remains common for CABG procedures likely because of familiarity, reliability, and limited alternatives. Currently, there are 2 pressure-controlling devices approved by the US Food and Drug Administration: the Saphenous Vein Distension System (DMC Medical), which comprises a pressure-limiting balloon connected to a syringe that relies on proper balloon inflation, vessel priming, and stopcock manipulation to deliver flushing fluid with designated pressure; and the Vasoshield Pressure Controlling Syringe (Maquet), which delivers irrigation fluid at a selectable pressure that must be secured by a dial on a specialty syringe. These devices have not been widely incorporated into routine graft preparation possibly because of high cost and difficulty of use and because, to our knowledge, the effect of these devices on vascular function and intimal hyperplasia prevention has not been reported.
We identified a simple PRV that limits distension pressure to 140 mm Hg or less (Figure 3). This valve can be placed in line with a standard syringe and vein cannula, requiring no special manipulation or change to current distension routines. When distended using the PRV, the PSV endothelium remained intact (Figure 5) and both endothelial-dependent and endothelial-independent function was preserved (Figure 4). More importantly the use of this PRV significantly reduced intimal thickening of PSVs in organ culture compared with PSVs distended manually (Figure 5). It is plausible that by protecting the endothelial and smooth muscle cells from injury, the initial cell-mediated inciting factors that lead to intimal hyperplasia, such as the release of mitogenic factors, recruitment of inflammatory factors by the endothelium, and the signaling cascades that ensues, were minimized. This is the first demonstration that limiting intraluminal pressure using a PRV prevents intimal thickening, a crucial step in the progression of graft occlusion.
Limitations of this study include the lack of feasibility to test the PRV in HSVs because only small segments of human tissue were available. The PSV represents a reasonable model system to test vein graft preparation techniques. The organ culture model lacks in vivo elements (pressure, flow, and exposure to blood components) that may influence the development of intimal hyperplasia. Further research is needed to determine the effect of limiting intraluminal pressure on development of intimal hyperplasia in vivo.
Conclusions
Taken together, our findings demonstrate a causal association among manual pressure distension, endothelial and medial injury, and intimal hyperplasia. Prevention of endothelial denudation and intimal hyperplasia can be achieved in a cost- and time-efficient manner using a simple PRV that limits maximum sustained pressure during manual distension. This approach offers an effective way to mitigate the damaging effects of manual distension during bypass procedures while preserving procedural efficiency and the ability to use techniques that are familiar to surgeons. Results from this study warrant future clinical studies to determine whether this improved vein graft preparation will result in better graft patency.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by an American Heart Association grant-in-aid for design and conduct of the study and preparation of the manuscript (Dr Eagle); National Institutes of Health National Research Service Award F32HL104965 for conduct of the study and analysis of data (Dr Osgood); National Institutes of Health grant R01HL70715-09 for design and conduct of the study, collection, management, analysis, or interpretation of the data, or review and approval of the manuscript (Dr Brophy); National Center for Advancing Translational Sciences grant UL1 TR000445-06 (formerly National Center for Research Resources grant UL1 RR024975-01) for conduct of the study and National Institutes of Health grant R01HL105731-01A1 for design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation and approval of the manuscript (Dr Cheung-Flynn).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Cheung-Flynn had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Eagle, Brophy, Cheung-Flynn.
Acquisition, analysis, and interpretation of data: All authors.
Drafting of the manuscript: Eagle, Brophy, Cheung-Flynn.
Critical revision of the manuscript for important intellectual content: Li, Brophy, Hocking, Osgood, Komalavilas, Cheung-Flynn.
Statistical analysis: Li, Hocking, Osgood, Komalavilas, Cheung-Flynn.
Obtained funding: Eagle, Brophy, Cheung-Flynn.
Administrative, technical, or material support: Brophy, Cheung-Flynn.
Study supervision: Brophy.
Conflict of Interest Disclosures: Drs Brophy and Cheung-Flynn have a financial relationship with Vasoprep Surgical.
Additional Contributions: The cardiac surgical teams at Vanderbilt University Medical Center provided human specimens for this study.
Contributor Information
Fan Dong Li, General Hospital of Jinan Military District, Jinan, China.
Susan Eagle, Division of Cardiothoracic Anesthesiology, Vanderbilt University Medical Center, Nashville, Tennessee.
Colleen Brophy, Division of Vascular Surgery, Vanderbilt University Medical Center, Nashville, Tennessee.
Kyle M. Hocking, Division of Vascular Surgery, Vanderbilt University Medical Center, Nashville, Tennessee.
Michael Osgood, Division of Vascular Surgery, Vanderbilt University Medical Center, Nashville, Tennessee.
Padmini Komalavilas, Division of Vascular Surgery, Vanderbilt University Medical Center, Nashville, Tennessee.
Joyce Cheung-Flynn, Division of Vascular Surgery, Vanderbilt University Medical Center, Nashville, Tennessee.
References
- 1.Alexander JH, Hafley G, Harrington RA, et al. PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 2.Conte MS, Bandyk DF, Clowes AW, et al. PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 3.Kouzi-Koliakos K, Kanellaki-Kyparissi M, Marinov G, et al. Prebypass histological and ultrastructural evaluation of the long saphenous vein as a predictor of early graft failure. Cardiovasc Pathol. 2006;15(6):336–346. doi: 10.1016/j.carpath.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS. Technical factors in lower-extremity vein bypass surgery: how can we improve outcomes? Semin Vasc Surg. 2009;22(4):227–233. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 5.LoGerfo FW, Haudenschild CC, Quist WC. A clinical technique for prevention of spasm and preservation of endothelium in saphenous vein grafts. Arch Surg. 1984;119(10):1212–1214. doi: 10.1001/archsurg.1984.01390220086020. [DOI] [PubMed] [Google Scholar]
- 6.LoGerfo FW, Quist WC, Cantelmo NL, Haudenschild CC. Integrity of vein grafts as a function of initial intimal and medial preservation. Circulation. 1983;63(3, pt 2):II117–II124. [PubMed] [Google Scholar]
- 7.Osgood MJH, Hocking KM, Voskresensky IV, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. J Vasc Surg. doi: 10.1016/j.jvs.2013.06.004. published online July 30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagle S, Brophy CM, Komalavilas P, et al. Surgical skin markers impair human saphenous vein graft smooth muscle and endothelial function. Am Surg. 2011;77(7):922–928. [PMC free article] [PubMed] [Google Scholar]
- 9.Hocking KM, Brophy C, Rizvi SZ, et al. Detrimental effects of mechanical stretch on smooth muscle function in saphenous veins. J Vasc Surg. 2011;53(2):454–460. doi: 10.1016/j.jvs.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chester AH, Buttery LD, Borland JA, et al. Structural, biochemical and functional effects of distending pressure in the human saphenous vein: implications for bypass grafting. Coron Artery Dis. 1998;9(2-3):143–151. [PubMed] [Google Scholar]
- 11.Chello M, Mastroroberto P, Frati G, et al. Pressure distension stimulates the expression of endothelial adhesion molecules in the human saphenous vein graft. Ann Thorac Surg. 2003;76(2):453–458. doi: 10.1016/s0003-4975(03)00433-8. [DOI] [PubMed] [Google Scholar]
- 12.Khaleel MS, Dorheim TA, Duryee MJ, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: a potential mechanism for graft failure. Ann Thorac Surg. 2012;93(2):552–558. doi: 10.1016/j.athoracsur.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82(4):1458–1464. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21(7):1146–1151. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]
- 15.Chung AW, Rauniyar P, Luo H, et al. Pressure distention compared with pharmacologic relaxation in vein grafting upregulates matrix metalloproteinase-2 and -9. J Vasc Surg. 2005;42(4):747–756. doi: 10.1016/j.jvs.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Cornelissen J, Armstrong J, Holt CM. Mechanical stretch induces phosphorylation of p38-MAPK and apoptosis in human saphenous vein. Arterioscler Thromb Vasc Biol. 2004;24(3):451–456. doi: 10.1161/01.ATV.0000116690.17017.8b. [DOI] [PubMed] [Google Scholar]
- 17.Clowes AW. Intimal hyperplasia and graft failure. Cardiovasc Pathol. 1993;2(3 suppl):179S–186S. [Google Scholar]
- 18.Li FD, Sexton KW, Hocking KM, et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res. 2012;180(1):e55–e62. doi: 10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 20.Tessier DJ, Komalavilas P, Liu B, et al. Transduction of peptide analogs of the small heat shock-related protein HSP20 inhibits intimal hyperplasia. J Vasc Surg. 2004;40(1):106–114. doi: 10.1016/j.jvs.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Souza DS, Johansson B, Bojö L, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg. 2006;132(2):373–378. doi: 10.1016/j.jtcvs.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg. 2002;73(4):1189–1195. doi: 10.1016/s0003-4975(02)03425-2. [DOI] [PubMed] [Google Scholar]
- 23.Okon EB, Millar MJ, Crowley CM, et al. Effect of moderate pressure distention on the human saphenous vein vasomotor function. Ann Thorac Surg. 2004;77(1):108–114. doi: 10.1016/j.athoracsur.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Andreasen JJ, Yang J, et al. Manual pressure distension of the human saphenous vein changes its biomechanical properties–implication for coronary artery bypass grafting. J Biomech. 2007;40(10):2268–2276. doi: 10.1016/j.jbiomech.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Chong CF, Ong PJ, Moat N, Collins P. Effects of hydrostatic distention on in vitro vasoreactivity of radial artery conduits. J Thorac Cardiovasc Surg. 2004;128(4):609–614. doi: 10.1016/j.jtcvs.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Wendling WW, Krasner LJ, Cooper SC, et al. Effects of stretch or distention on phenylephrine-induced constriction of human coronary artery bypass grafts. J Cardiothorac Vasc Anesth. 2001;15(6):717–722. doi: 10.1053/jcan.2001.28313. [DOI] [PubMed] [Google Scholar]
- 27.Viaro F, Capellini VK, Celotto AC, et al. Immunohistochemical evaluation of three nitric oxide synthase isoforms in human saphenous vein exposed to different degrees of distension pressures. Cardiovasc Pathol. 2010;19(6):e211–e220. doi: 10.1016/j.carpath.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Angelini GD, Passani SL, Breckenridge IM, Newby AC. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21(12):902–907. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- 29.Davies MG, Hagen PO. Influence of perioperative storage solutions on long-term vein graft function and morphology. Ann Vasc Surg. 1994;8(2):150–157. doi: 10.1007/BF02018863. [DOI] [PubMed] [Google Scholar]
- 30.Dashwood MR, Savage K, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80(3):939–944. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Stigler R, Steger C, Schachner T, et al. The impact of distension pressure on acute endothelial cell loss and neointimal proliferation in saphenous vein grafts. Eur J Cardiothorac Surg. 2012;42(4):e74–e79. doi: 10.1093/ejcts/ezs402. [DOI] [PubMed] [Google Scholar]
- 32.Goldman J, Zhong L, Liu SQ. Degradation of alpha-actin filaments in venous smooth muscle cells in response to mechanical stretch. Am J Physiol Heart Circ Physiol. 2003;284(5):H1839–H1847. doi: 10.1152/ajpheart.00470.2002. [DOI] [PubMed] [Google Scholar]
- 33.Reidy MA. Factors controlling smooth-muscle cell proliferation. Arch Pathol Lab Med. 1992;116(12):1276–1280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.